Black Liquor and Wood Char-Derived Nitrogen-Doped Carbon Materials for Supercapacitors
Abstract
1. Introduction
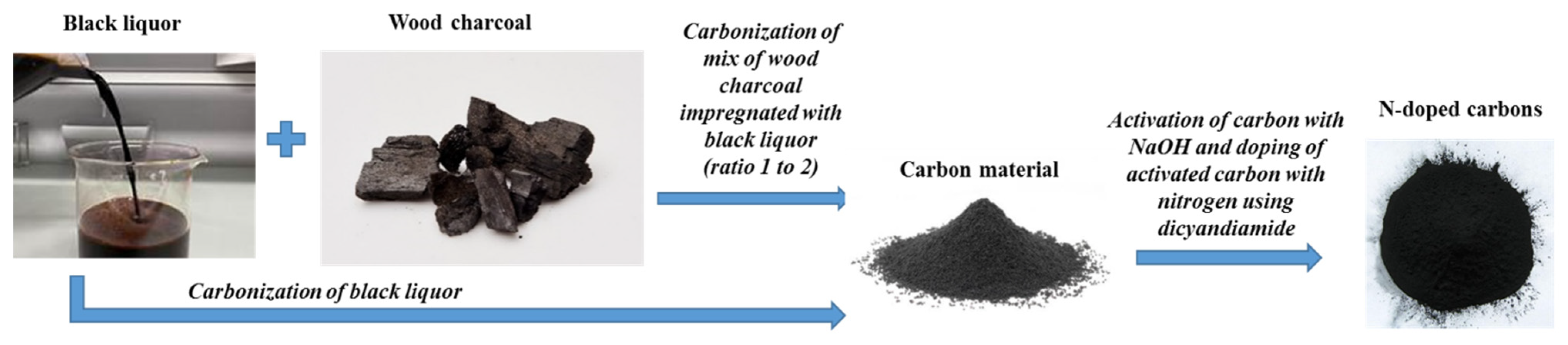
2. Materials and Methods
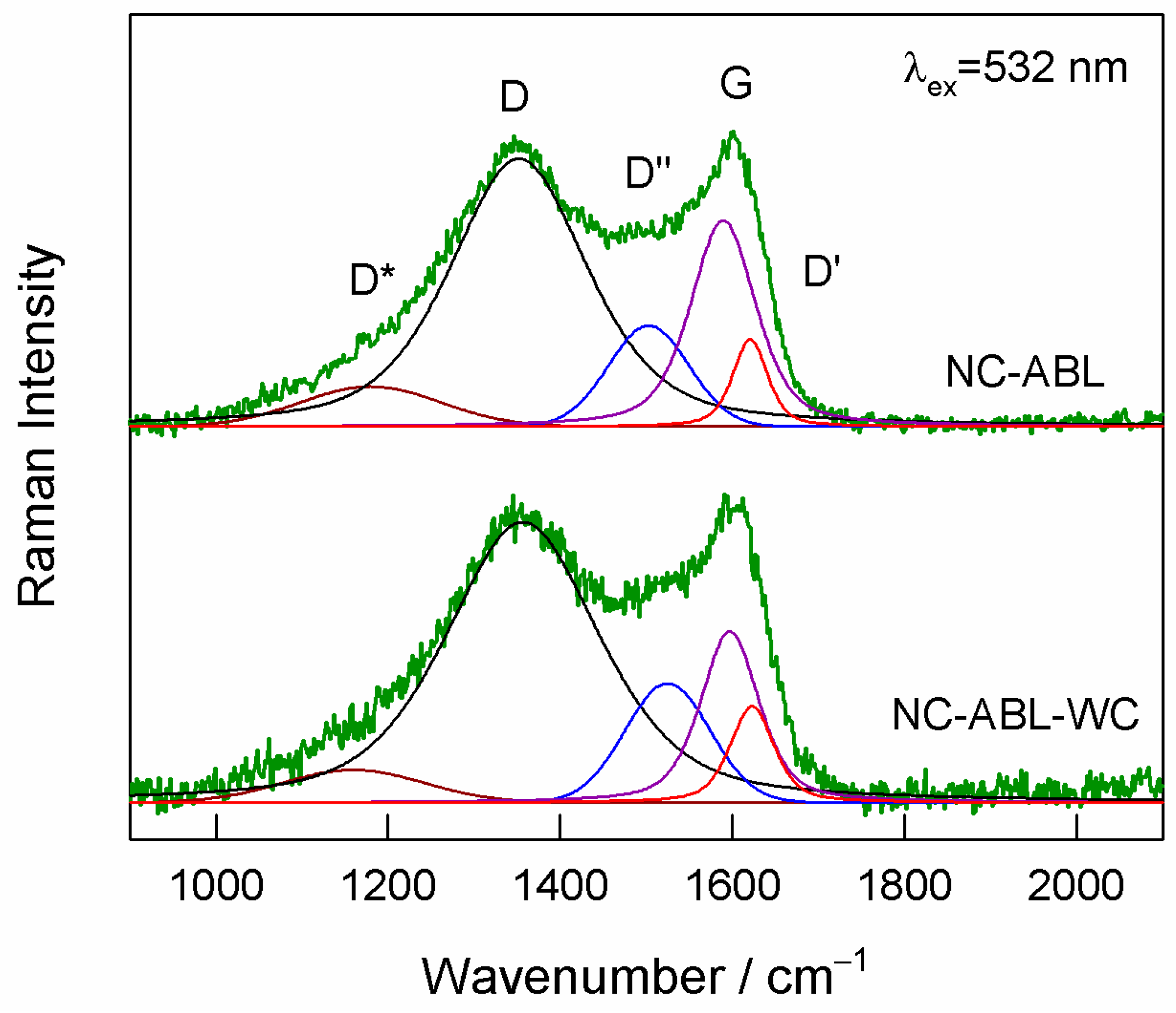
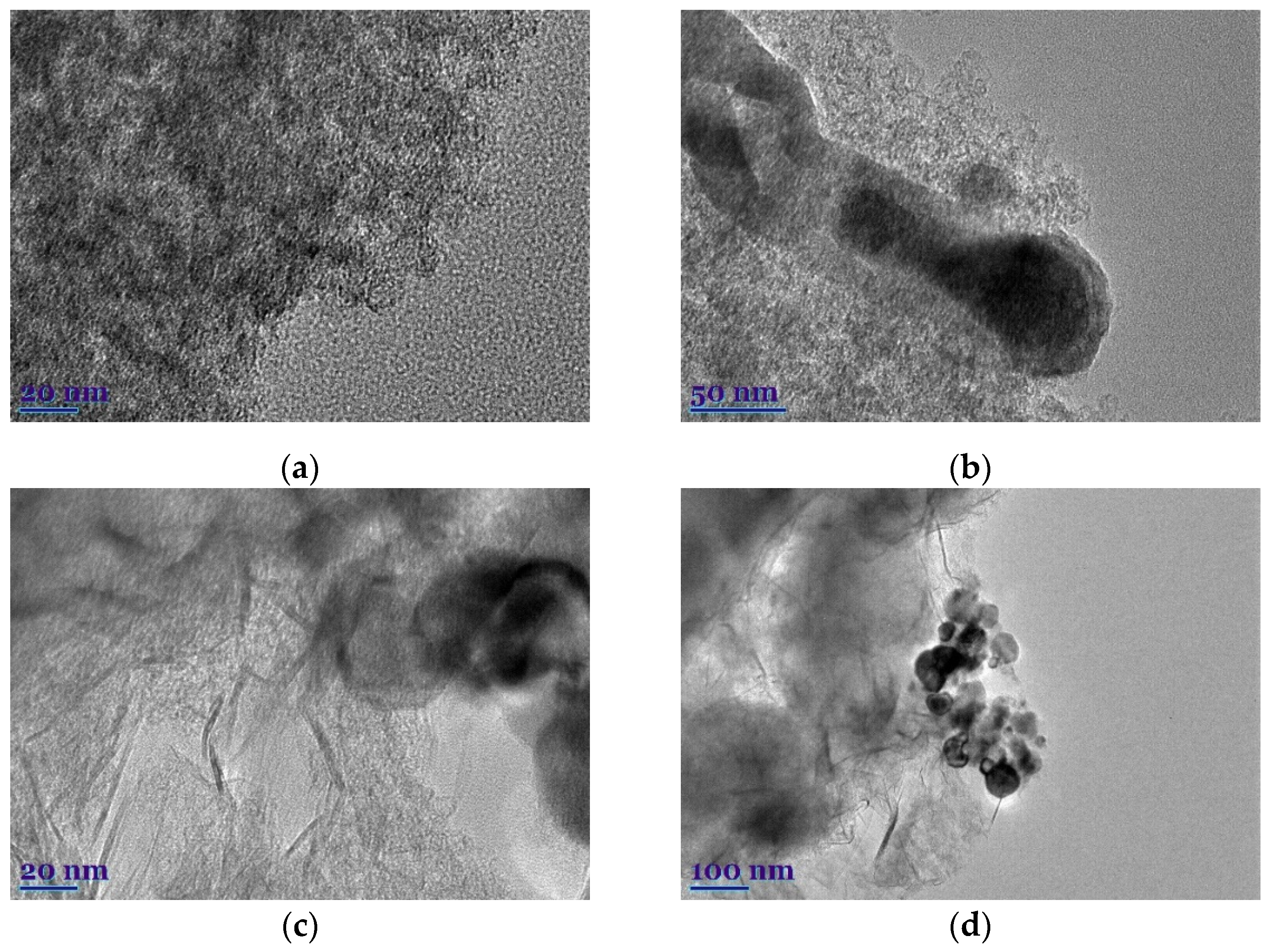
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, 6468. [Google Scholar] [CrossRef]
- Chuhadiya, S.; Himanshu; Suthar, D.; Patel, S.L.; Dhaka, M.S. Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review. Coord. Chem. Rev. 2021, 446, 214115. [Google Scholar] [CrossRef]
- Zhou, H.; Li, H.; Li, L.; Liu, T.; Chen, G.; Zhu, Y.; Zhou, L.; Huang, H. Structural composite energy storage devices—A review. Mater. Today Energy 2022, 24, 100924. [Google Scholar] [CrossRef]
- Patel, K.K.; Singhal, T.; Pandey, V.; Sumangala, T.P.; Sreekanth, M.S. Evolution and recent developments of high performance electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103366. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Sakib, M.N.; Ahmed, S.; Sultan Mahmud Rahat, S.M.; Shuchi, S.B. A review of recent advances in manganese-based supercapacitors. J. Energy Storage 2021, 44, 103322. [Google Scholar] [CrossRef]
- Yue, T.; Shen, B.; Gao, P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: A review. Renew. Sustain. Energy Rev. 2022, 158, 112131. [Google Scholar] [CrossRef]
- Lakra, R.; Kumar, R.; Sahoo, P.K.; Thatoi, D.; Soam, A. A mini-review: Graphene based composites for supercapacitor application. Inorg. Chem. Commun. 2021, 133, 108929. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Li, B.; Yu, C.; Wang, H.; Li, Q. Construction of heteroatom-doped and three-dimensional graphene materials for the applications in supercapacitors: A review. J. Energy Storage 2021, 44, 103437. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Kim, H.-J. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J. Energy Storage 2020, 27, 101035. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukha, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123. [Google Scholar] [CrossRef]
- Burke, A.; Liu, Z.; Zhao, H. Present and future applications of supercapacitors in electric and hybrid vehicles. In Proceedings of the 2014 IEEE International Electric Vehicle Conference (IEVC), Florence, Italy, 17–19 December 2014; pp. 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797. [Google Scholar] [CrossRef]
- Bai, Y.; Shen, B.; Zhang, S.; Zhu, Z.; Sun, S.; Gao, J.; Li, B.; Wang, Y.; Zhang, R.; Wei, F. Storage of mechanical energy based on carbon nanotubes with high energy density and power density. Adv. Mater. 2018, 31, 1800680. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Azad, A.T.; Zaini, J.; Islam, A.; Azad, A. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Abdul Rahman, N.; Sulaiman, Y. Ternary functionalised carbon nanofibers/polypyrrole/manganese oxide as high specific energy electrode for supercapacitor. Ceram. Int. 2019, 45, 8433–8439. [Google Scholar] [CrossRef]
- Akbar, A.R.; Tian, W.; Qadir, M.B.; Khaliq, Z.; Liu, Z.; Tahir, M.; Hu, Y.; Xiong, C.; Yang, Q. A novel ternary composite aerogel for high-performance supercapacitor. Colloids Surf. A 2021, 610, 125644. [Google Scholar] [CrossRef]
- Hareesh, K.; Shateesh, B.; Joshi, R.P.; Williams, J.F.; Phase, D.M.; Haram, S.K.; Dhole, S.D. Ultra high stable supercapacitance performance of conducting polymer coated MnO2 nanorods/rGO nanocomposites. RSC Adv. 2017, 7, 20027. [Google Scholar] [CrossRef]
- Pandolfo, A.; Hollenkamp, A. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Rawat, S.; Mishra, R.K.; Bhaskar, T. Biomass derived functional carbon materials for supercapacitor applications. Chemosphere 2022, 286, 131961. [Google Scholar] [CrossRef]
- Feng, T.; Wang, S.; Hua, Y.; Zhou, P.; Liu, G.; Ji, K.; Lin, Z.; Shi, S.; Jiang, X.; Zhang, R. Synthesis of biomass-derived N,O-codoped hierarchical porous carbon with large surface area for high-performance supercapacitor. J. Energy Storage 2021, 44, 103286. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renew. Sustain. Energ. Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Pham, H.D.; Mishra, S.; Deep, A.; Dubal, D.P. Advances in bio-waste derived activated carbon for supercapacitors: Trends, challenges and prospective. Resour. Conserv. Recycl. 2021, 169, 105548. [Google Scholar] [CrossRef]
- Chaparro-Garnica, J.; Salinas-Torres, D.; Mostazo-López, M.J.; Morallón, E.; Cazorla-Amoró, D. Biomass waste conversion into low-cost carbon-based materials for supercapacitors: A sustainable approach for the energy scenario. J. Electroanal. Chem. 2021, 880, 114899. [Google Scholar] [CrossRef]
- Wu, J.; Xia, M.; Zhang, X.; Chen, Y.; Sun, F.; Wang, X.; Yang, H.; Chen, H. Hierarchical porous carbon derived from wood tar using crab as the template: Performance on supercapacitor. J. Power Sources 2020, 455, 227982. [Google Scholar] [CrossRef]
- Jiang, C.; Yakaboylu, G.A.; Yumak, T.; Zondlo, J.W.; Sabolsky, E.M.; Wang, J. Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew. Energy 2020, 155, 38–52. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, Z.; Wang, L.; Guo, Y.; Zhou, C.; Li, X.; Du, K.; Luo, Y. Nickel-cobalt layered double hydroxide nanosheets anchored to the inner wall of wood carbon tracheids by nitrogen-doped atoms for high performance supercapacitors. J. Colloid Interface Sci. 2022, 608, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Nirmaladevi, S.; Boopathiraja, R.; Kandasamy, S.K.; Sathishkumar, S.; Parthibavarman, M. Wood based biochar supported MnO2 nanorods for high energy asymmetric supercapacitor application. Surf. Interface 2021, 27, 101548. [Google Scholar] [CrossRef]
- Shan, X.; Wu, J.; Zhang, X.; Wang, L.; Yang, J.; Chen, Z.; Yu, J.; Wang, X. Wood for application in electrochemical energy storage devices. Cell Rep. Phys. Sci. 2021, 2, 100654. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources 2020, 480, 228830. [Google Scholar] [CrossRef]
- Plavniece, A.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Kaare, K.; Kruusenberg, I.; Kaprans, K.; Knoks, A.; Kleperis, J. Wood and black liquor-based N-doped activated carbon for energy application. Sustainability 2021, 13, 9237. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass based activated carbons for fuel cells. Renew. Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Jablonskiene, J.; Simkunaite, D.; Vaiciuniene, J.; Stalnionis, G.; Drabavicius, A.; Jasulaitiene, V.; Pakstas, V.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Synthesis of carbon-supported MnO2 nanocomposites for supercapacitors application. Crystals 2021, 11, 784. [Google Scholar] [CrossRef]
- Nacys, A.; Šimkūnaitė, D.; Balčiūnaitė, A.; Zabielaitė, A.; Upskuvienė, D.; Šebeka, B.; Jasulaitienė, V.; Kovalevskij, K.; Norkus, E.; Tamašauskaitė-Tamašiūnaitė, L. An enhanced oxidation of formate on PtNi/Ni foam catalyst in an alkaline medium. Crystals 2022, 12, 362. [Google Scholar] [CrossRef]
- Kaare, K.; Yu, E.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Dyck, A.; Niaura, G.; Tamasauskaite-Tamasiunaite, L.; Norkus, E.; Andrulevičius, M.; et al. Highly active wood-derived nitrogen-doped carbon catalyst for the oxygen reduction reaction. ACS Omega 2020, 5, 23578–23587. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Zhang, H.; Zhang, D.; Ma, Y. Microwave-assisted reflux rapid synthesis of MnO2 nanostructures and their application in supercapacitors Electrochim. Acta 2013, 87, 637. [Google Scholar] [CrossRef]
- Ferrari, A.C. and Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Trusovas, R.; Račiukaitis, G.; Niaura, G.; Barkauskas, J.; Valušis, G.; Pauliukaite, R. Recent advances in laser utilization in the chemical modification of graphene oxide and its applications. Adv. Opt. Mater. 2016, 4, 37–65. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The importance of interbands on the interpretation of the Raman spectrum of graphene oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Trusovas, R.; Ratautas, K.; Račiukaitis, G.; Niaura, G. Graphene layer formation in pinewood by nanosecond and picosecond laser irradiation. Appl. Surf. Sci. 2019, 471, 154–161. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascón, J.M.D. Comparative performance of X-ray diffraction and Raman microprobe techniques for the study of carbon materials. J. Mater. Chem. 1998, 8, 2875–2879. [Google Scholar] [CrossRef]
- Baccile, N.; Laurent, G.; Babonneau, F.; Fayon, F.; Titirici, M.M.; Antonietti, M. Structural characterization of hydrothermal carbon spheres by advanced solid-state MAS 13C NMR investigations. J. Phys. Chem. C 2009, 113, 9644–9654. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Zeng, L.; Cui, X.; Shi, J. Engineering crystalline CoOOH anchored on an N-doped carbon support as a durable electrocatalyst for the oxygen reduction reaction. Dalt. Trans. 2018, 47, 6069–6074. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Sosenkin, V.E.; Bagotsky, V.S. Structural and wetting properties of fuel cell components. J. Power Sources 2010, 195, 5429–5441. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Huang, Y.; Li, N.; Hu, Y.; Zhao, C. Soybean root-derived N, O co-doped hierarchical porous carbon for supercapacitors. Appl. Surf. Sci. 2021, 555, 149726. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Y.; Tang, J.A.; Guo, S.H.; Gao, Z.M.; Wei, Y.J.; Chen, G.; Gao, Y. A high-performance supercapacitor based on activated carbon fibers with an optimized pore structure and oxygen-containing functional groups. Mater. Chem. Front. 2017, 1, 958–966. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Szkoda, M.; Zarach, Z.; Kamedulski, P.; Zielinski, W.; Lukaszewicz, J.P. Combined effect of nitrogen-doped functional groups and porosity of porous carbons on electrochemical performance of supercapacitors. Sci. Rep. 2021, 11, 18387. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhang, Y.; He, J.; Wang, Y.; Wang, K.; Xu, Y.; Li, H.; Wang, Y. Biomass-derived microporous carbon with large micropore size for high-performance supercapacitors. J. Power Sources 2020, 448, 227396. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Chen, H.; Wang, S.; Kong, F.; Liu, S. Facile control of the porous structure of Larch-derived mesoporous carbons via self-assembly for supercapacitors. Materials 2017, 10, 1330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.M.; He, Y.; Liu, N.; Tan, T.; Liang, C. Biomass derived nitrogen-doped highly porous carbon material with a hierarchical porous structure for high-performance lithium/sulfur batteries. Materials 2017, 10, 1158. [Google Scholar] [CrossRef]
- Ma, X.; Gan, L.; Liu, M.; Tripathi, P.K.; Chen, L. Mesoporous size controllable carbon microspheres and their electrochemical performances for supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 8407–8415. [Google Scholar] [CrossRef]
- Chen, H.; Sha, L.; Zhang, Y.; Wang, S.; Kong, F.; Muench, F.; Zhao, X. Larch-derived hierarchical nitrogen-doped carbon with echinus-like architecture for supercapacitor applications. Holzforschung 2020, 74, 529–538. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Hu, P.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Nitrogen-doped mesoporous carbon thin film for binder-free supercapacitor. Appl. Materials Today 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Fischer, J.; Pohle, B.; Dmitrieva, E.; Thummler, K.; Fischer, S. Symmetric supercapacitors with cellulose-derived carbons and Na2SO4 electrolytes operating in a wide temperature range. J. Energy Storage 2022, 55, 105725. [Google Scholar] [CrossRef]
- Demarconnay, L.; Raymundo-Pinero, E.; Beguin, F. A symmetric carbon/carbon supercapacitor operating at 1.6 V by using a neutral aqueous solution. Electrochem. Commun. 2010, 12, 1275–1278. [Google Scholar] [CrossRef]
- Bichat, M.P.; Raymundo-Pinero, E.; Beguin, F. High voltage supercapacitor built with seaweed carbons in neutral aqueous electrolyte. Carbon 2010, 48, 4351–4361. [Google Scholar] [CrossRef]
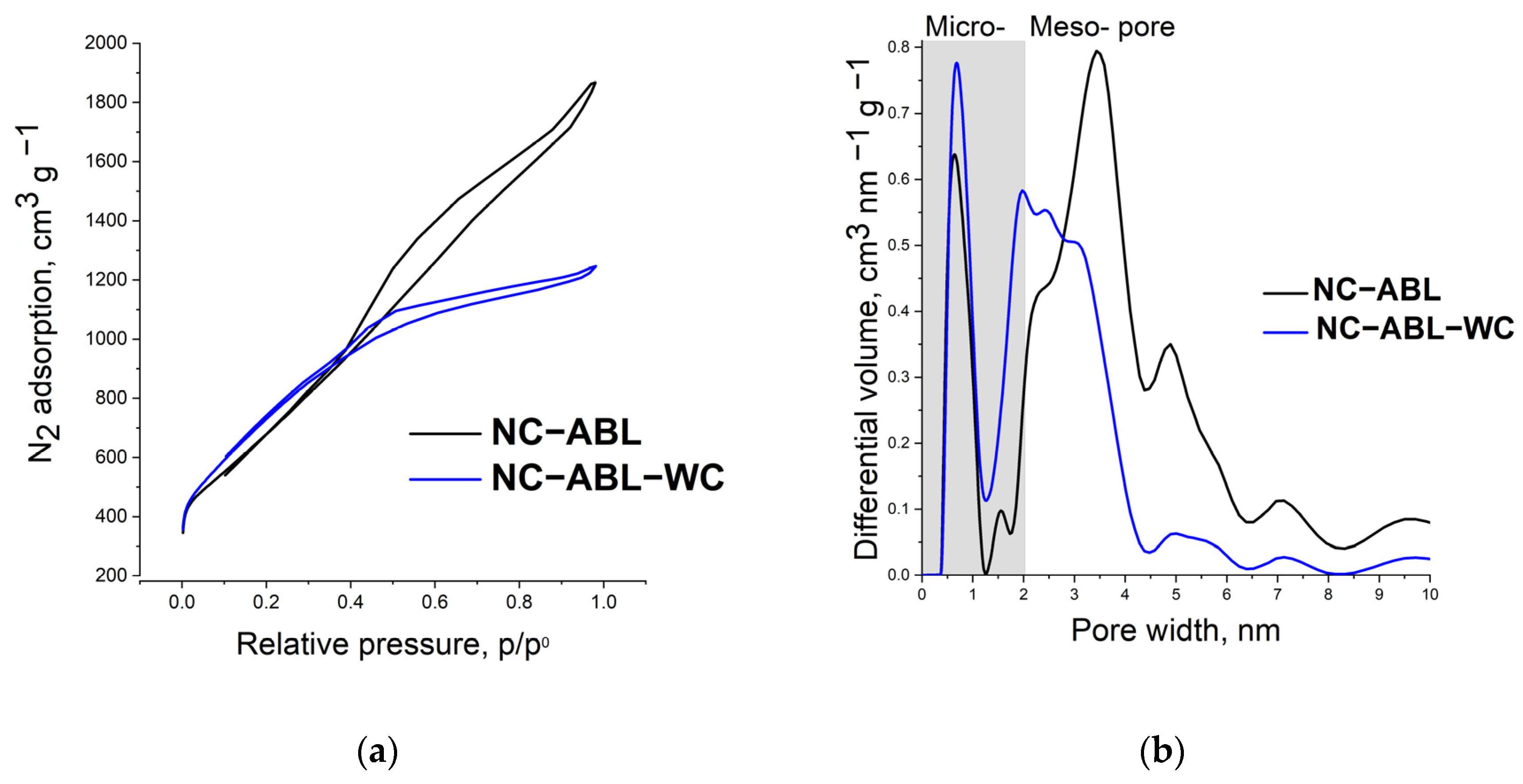
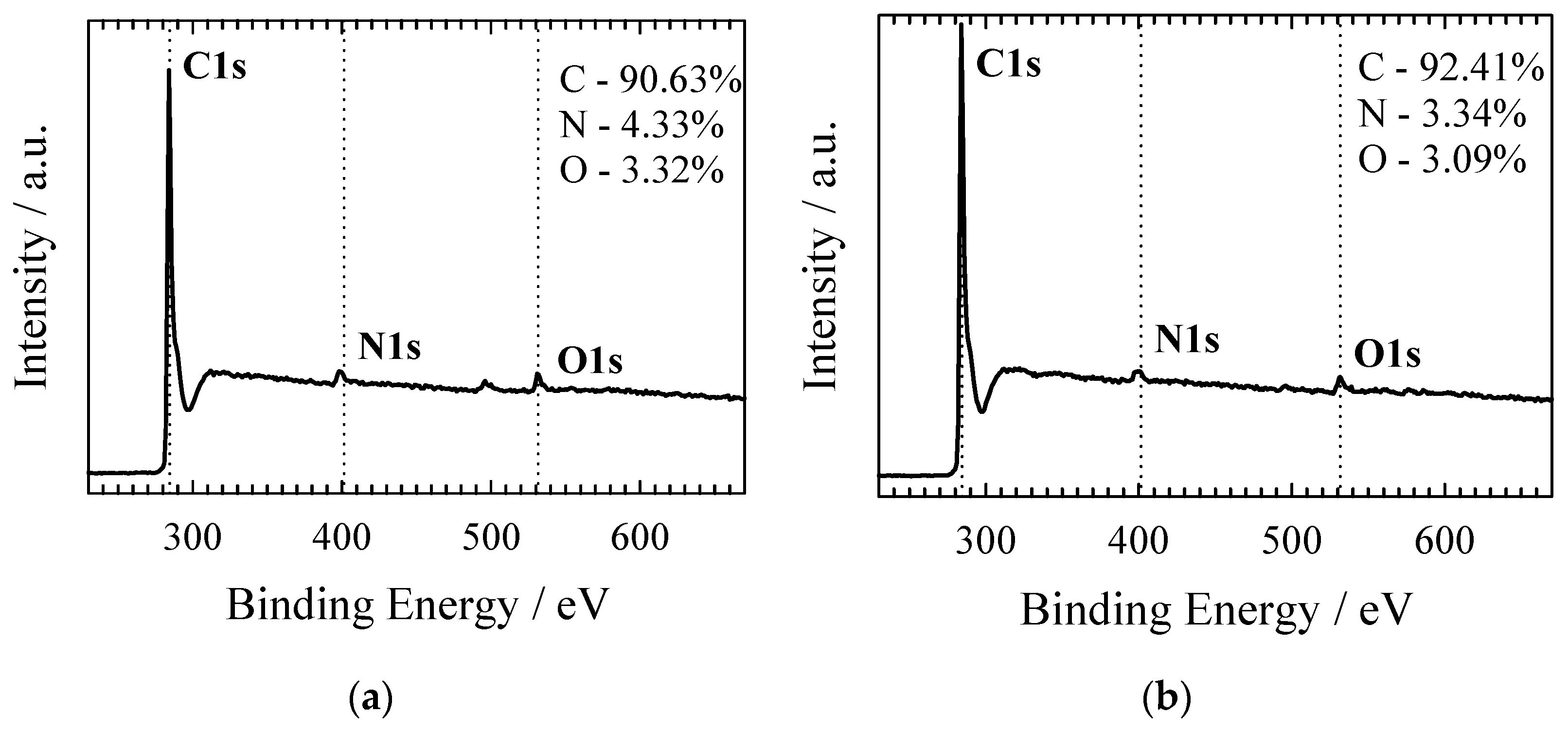
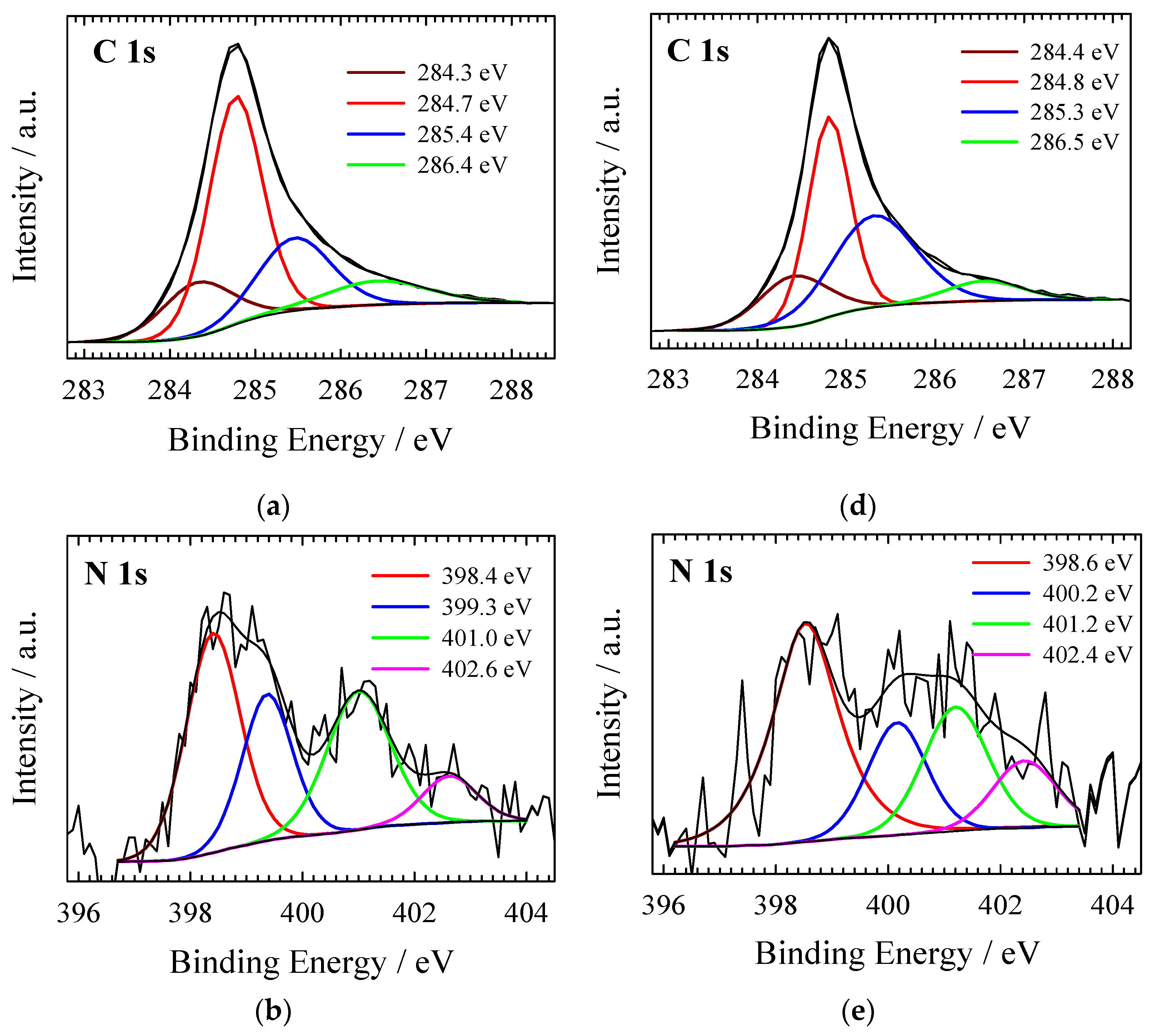
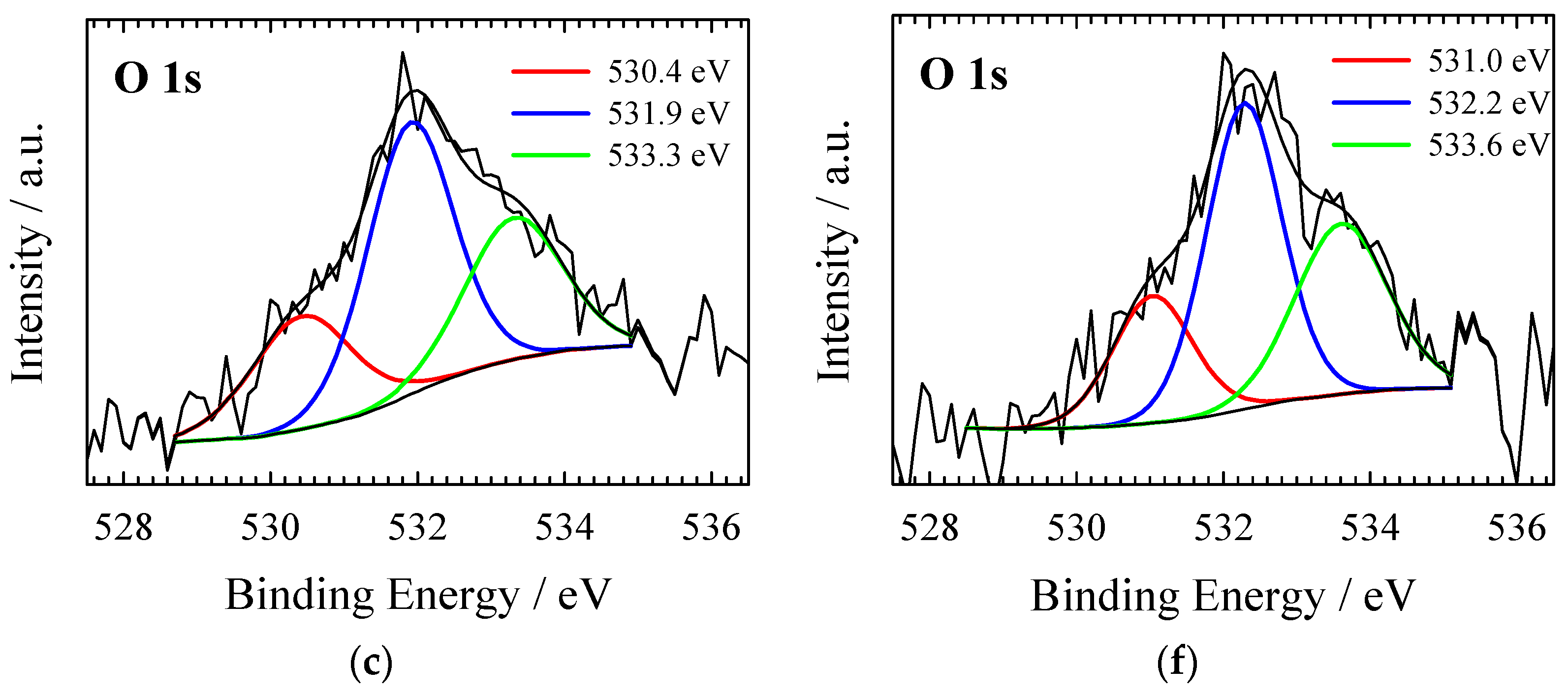
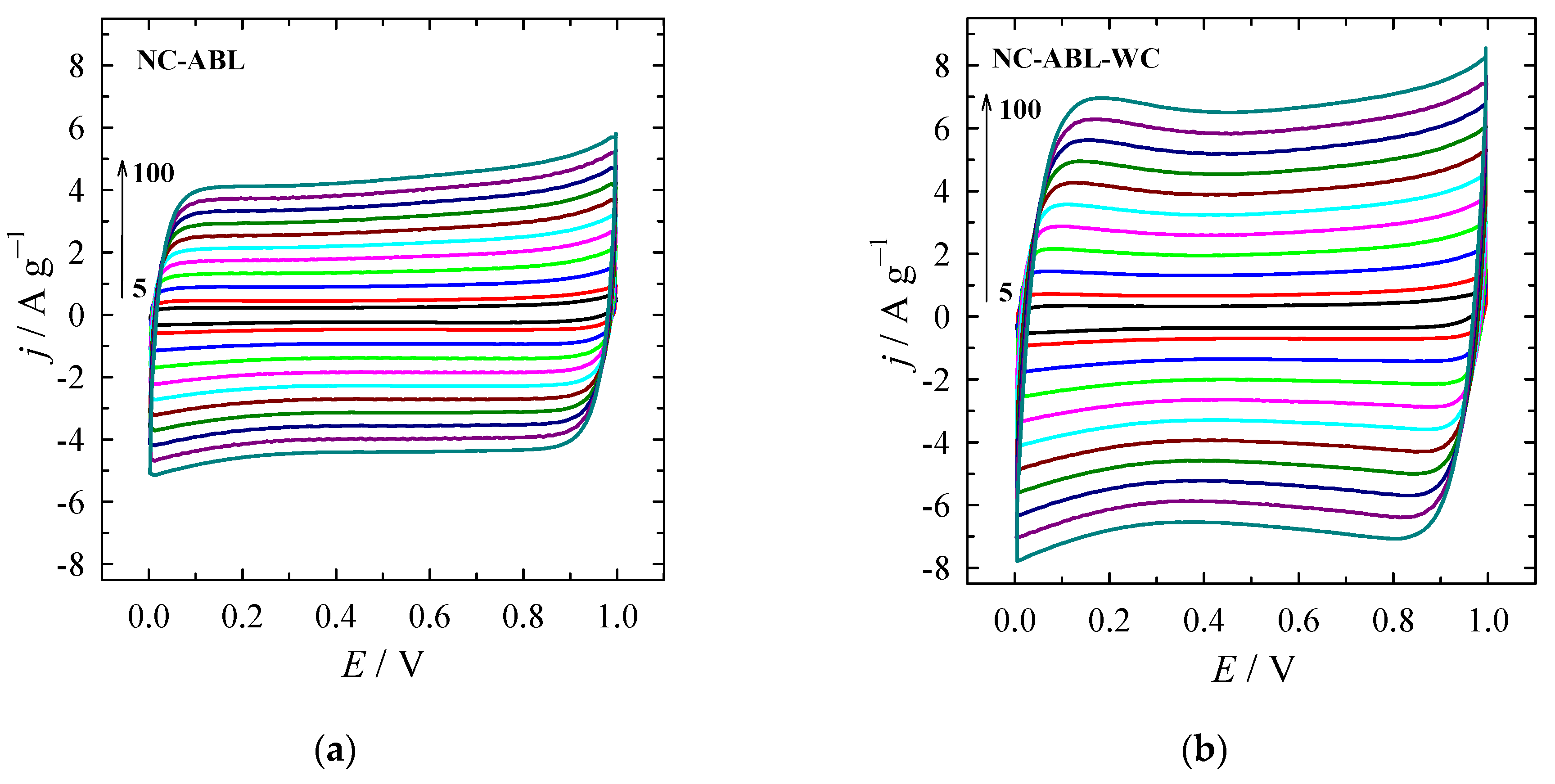
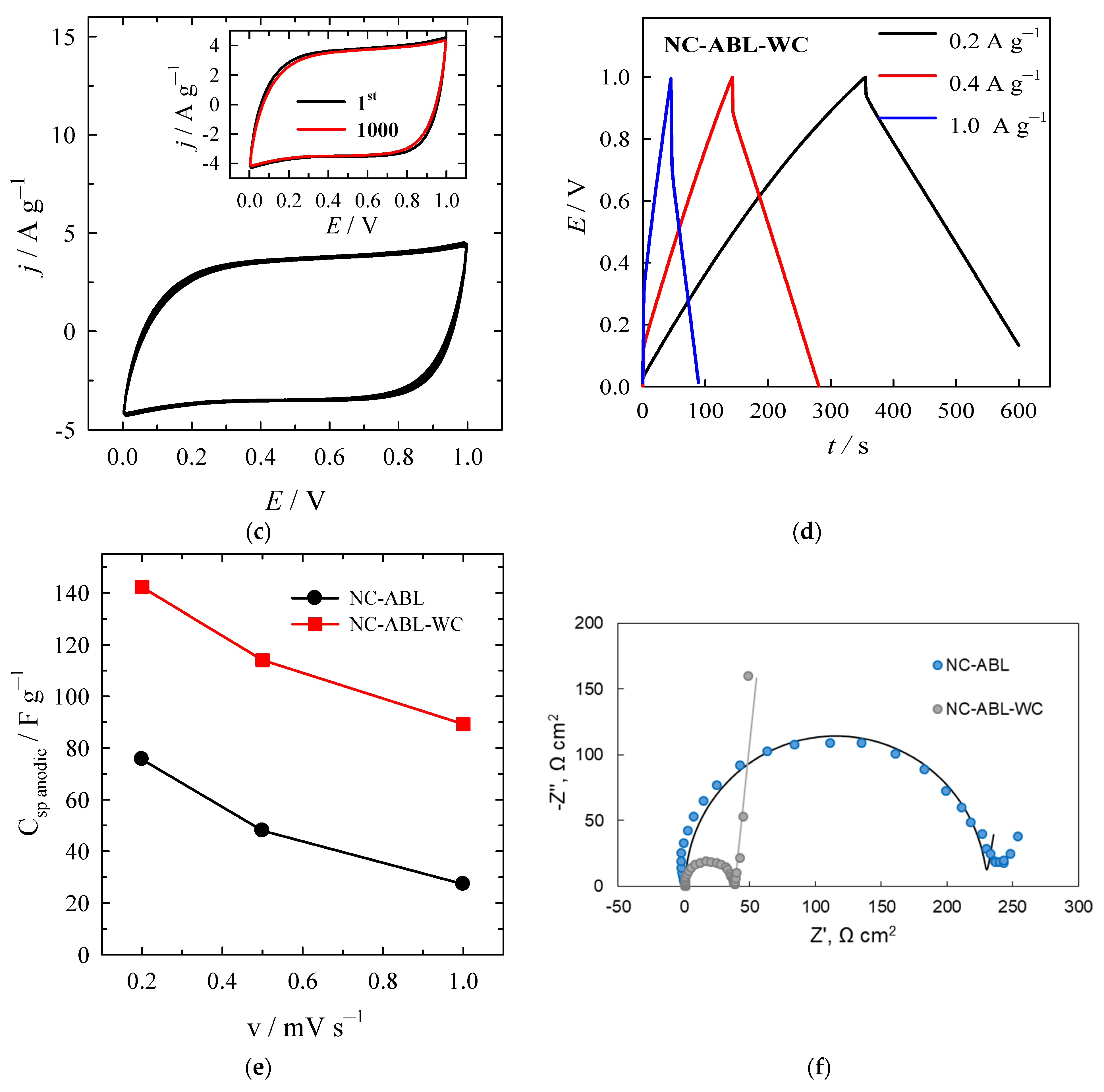
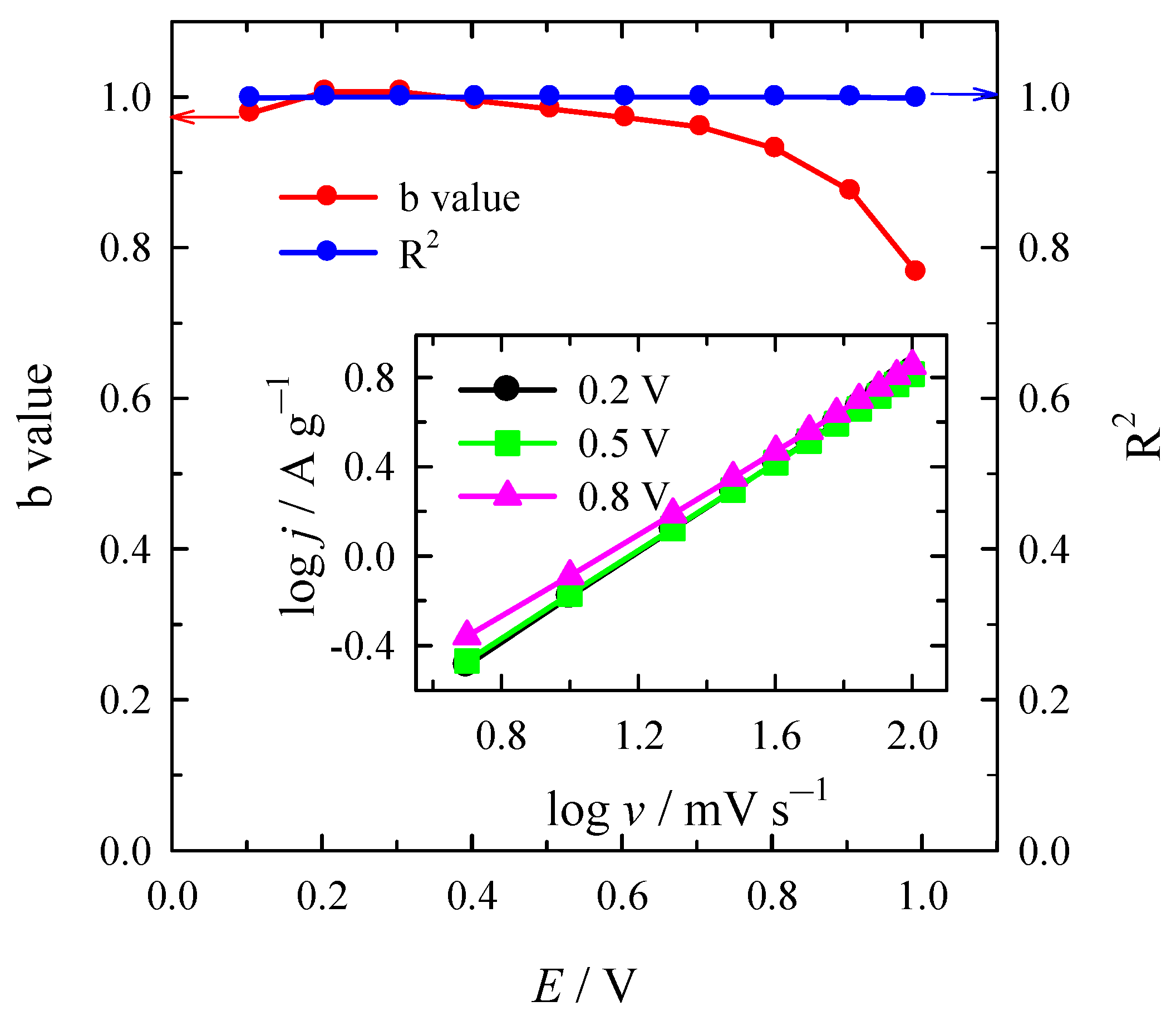
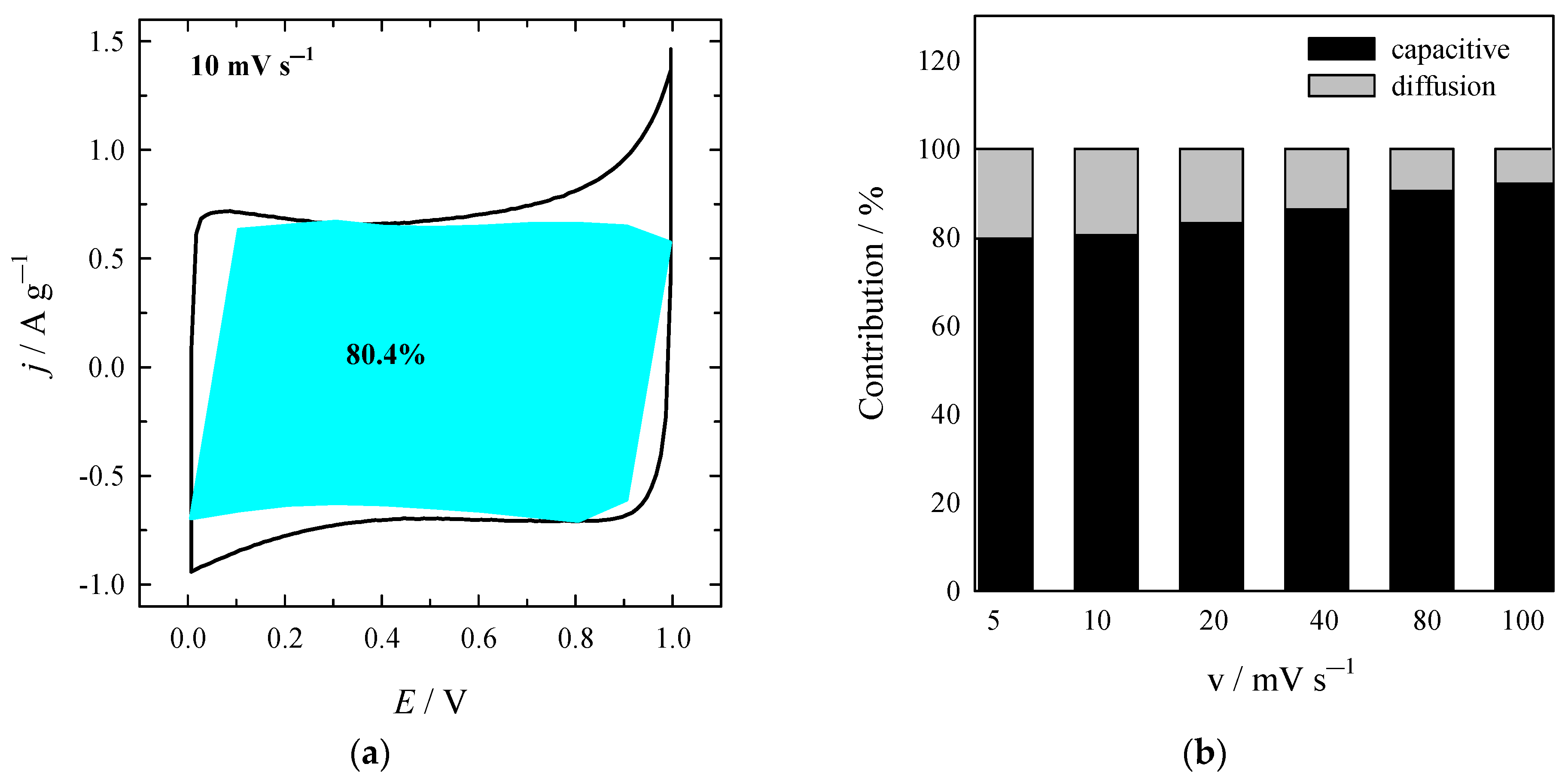
| Material | SSA (BET), m2 g−1 | Total Pore Volume (Vt), cm3 g−1 | Micropore Volume, cm3 g−1 | Mesopore Volume, cm3 g−1 | Mesopores from Vt, % | Average Pore Width, (nm) |
|---|---|---|---|---|---|---|
| NC-ABL | 2480 | 2.9 | 0.8 | 2.1 | 71.6 | 4.7 |
| NC-ABL-WC | 2690 | 1.9 | 0.8 | 1.1 | 56.5 | 2.9 |
| Material | Rs, Ω cm2 | Cdl, mF cm2 | Rct, Ω cm2 |
|---|---|---|---|
| NC-ABL-WC | 0.83 | 32.3 | 37.7 |
| NC-ABL | 0.88 | 26.2 | 228.4 |
| Material | Specific Surface Area, m2 g−1 | Electrolyte | Specific Capacitance, F g−1 | Current Density, A g−1 | Ref. |
|---|---|---|---|---|---|
| NC-ABL | 2481 | 1 M Na2SO4 | 142.23 | 0.2 | This work |
| NC-ABL-WC | 2690 | 1 M Na2SO4 | 80.93 | 0.2 | This work |
| N-APC-800 | 623 | 0.2 M K2SO4 | 231 | 0.1 | [55] |
| Mp-NCF-900 | 60.8 | 1 M Na2SO4 | 316 | 0.5 | [62] |
| 168 | 5.0 | ||||
| AC | 1198 | 1 M Na2SO4 | 93 | 1.0 | [63] |
| AC | 2250 | 1 M Na2SO4 | 135 | 0.2 | [64] |
| Seaweed carbons LN600 | 746 | 0.5 M Na2SO4 | 125 | 0.2 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamasauskaite-Tamasiunaite, L.; Jablonskienė, J.; Šimkūnaitė, D.; Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Jasulaitiene, V.; Niaura, G.; Drabavicius, A.; et al. Black Liquor and Wood Char-Derived Nitrogen-Doped Carbon Materials for Supercapacitors. Materials 2023, 16, 2551. https://doi.org/10.3390/ma16072551
Tamasauskaite-Tamasiunaite L, Jablonskienė J, Šimkūnaitė D, Volperts A, Plavniece A, Dobele G, Zhurinsh A, Jasulaitiene V, Niaura G, Drabavicius A, et al. Black Liquor and Wood Char-Derived Nitrogen-Doped Carbon Materials for Supercapacitors. Materials. 2023; 16(7):2551. https://doi.org/10.3390/ma16072551
Chicago/Turabian StyleTamasauskaite-Tamasiunaite, Loreta, Jolita Jablonskienė, Dijana Šimkūnaitė, Aleksandrs Volperts, Ance Plavniece, Galina Dobele, Aivars Zhurinsh, Vitalija Jasulaitiene, Gediminas Niaura, Audrius Drabavicius, and et al. 2023. "Black Liquor and Wood Char-Derived Nitrogen-Doped Carbon Materials for Supercapacitors" Materials 16, no. 7: 2551. https://doi.org/10.3390/ma16072551
APA StyleTamasauskaite-Tamasiunaite, L., Jablonskienė, J., Šimkūnaitė, D., Volperts, A., Plavniece, A., Dobele, G., Zhurinsh, A., Jasulaitiene, V., Niaura, G., Drabavicius, A., Juel, M., Colmenares-Rausseo, L., Kruusenberg, I., Kaare, K., & Norkus, E. (2023). Black Liquor and Wood Char-Derived Nitrogen-Doped Carbon Materials for Supercapacitors. Materials, 16(7), 2551. https://doi.org/10.3390/ma16072551









