PCL and DMSO2 Composites for Bio-Scaffold Materials
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Melting Temperature
2.3. Hydrophilicity
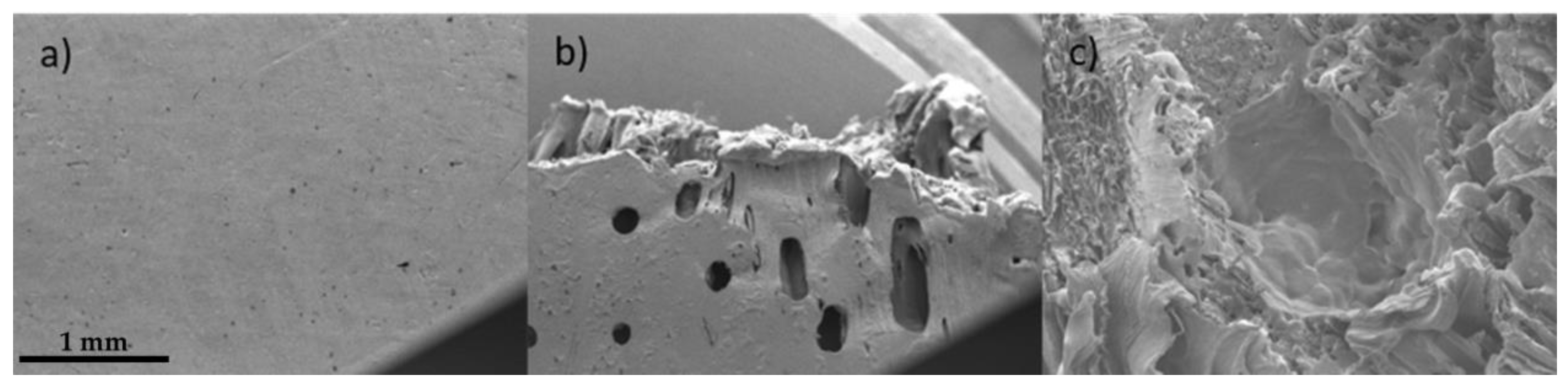
2.4. Mechanical Test and Failure Analysis
2.5. Degradation Test
3. Results
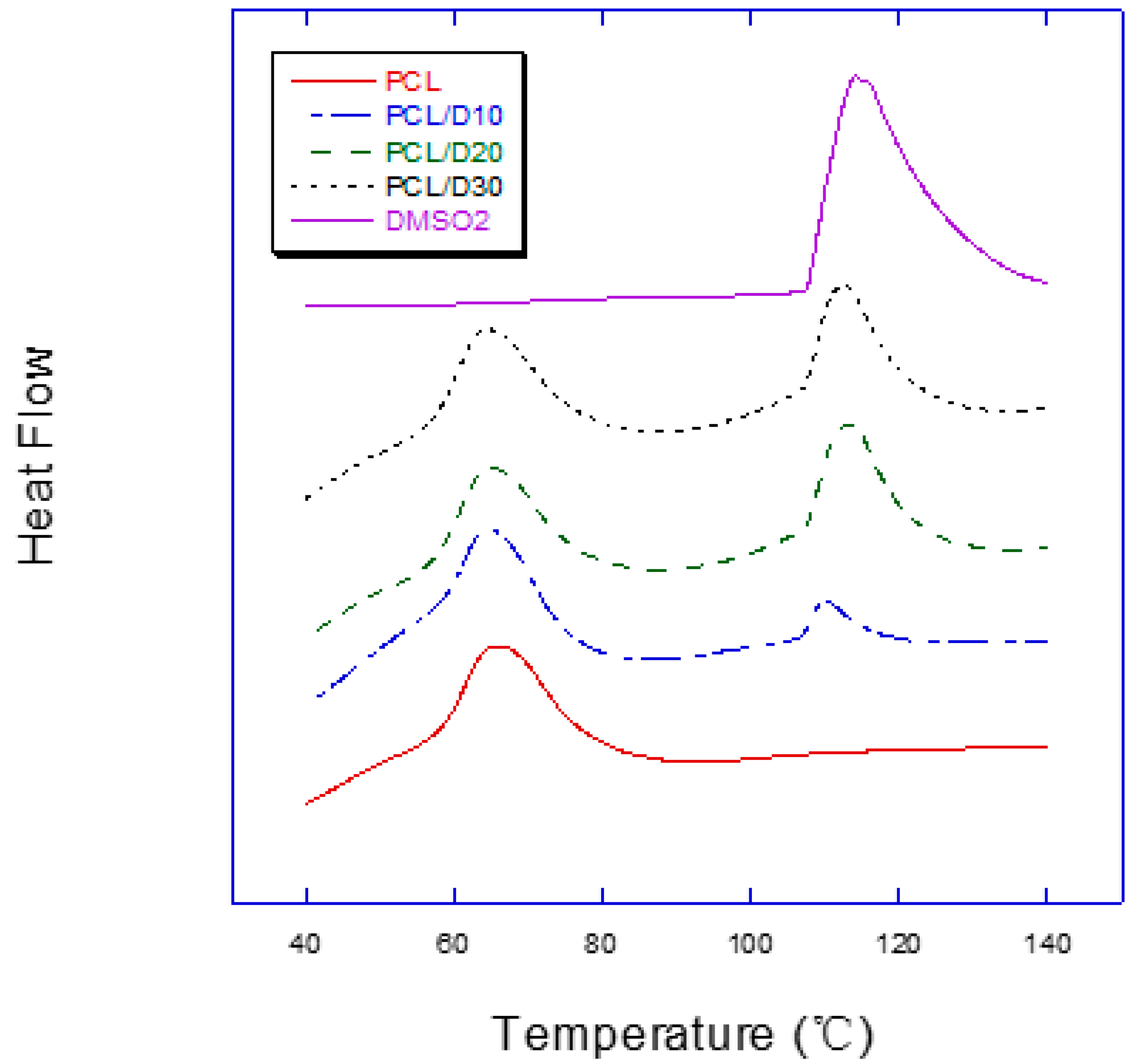
3.1. Melting Temperature
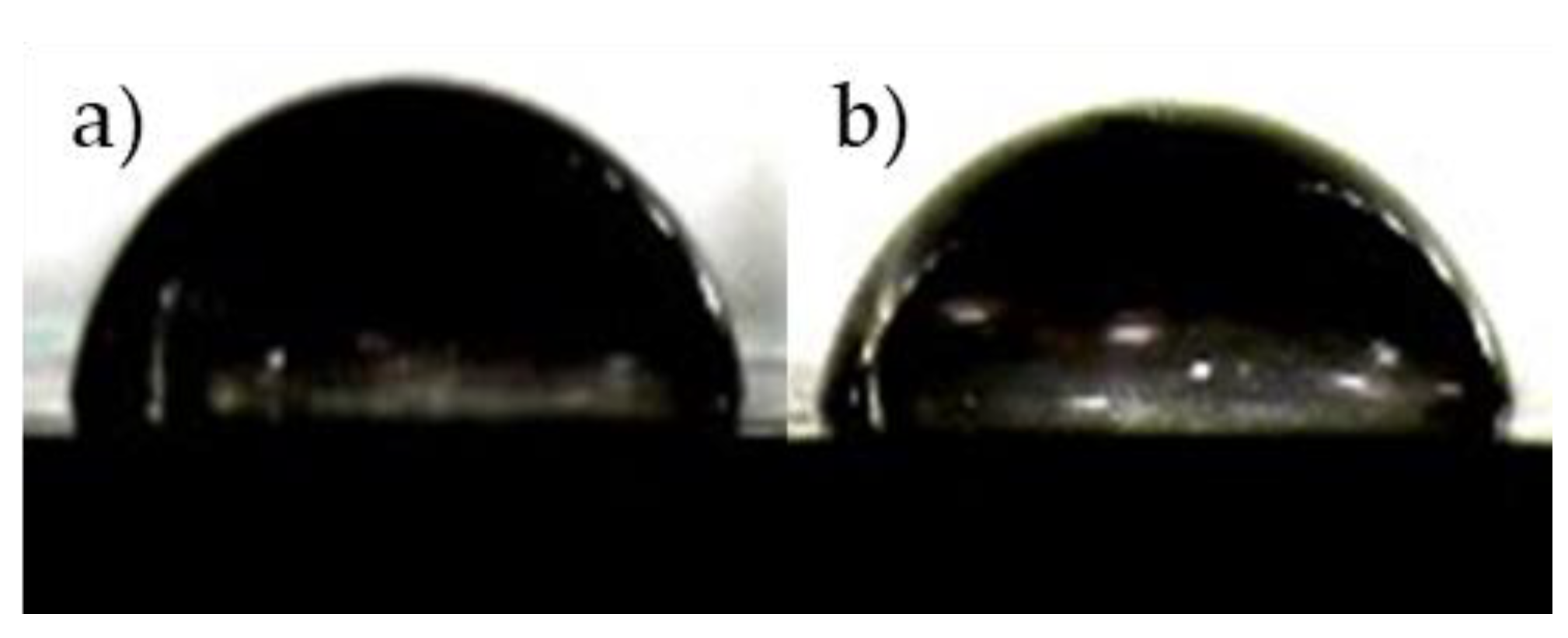
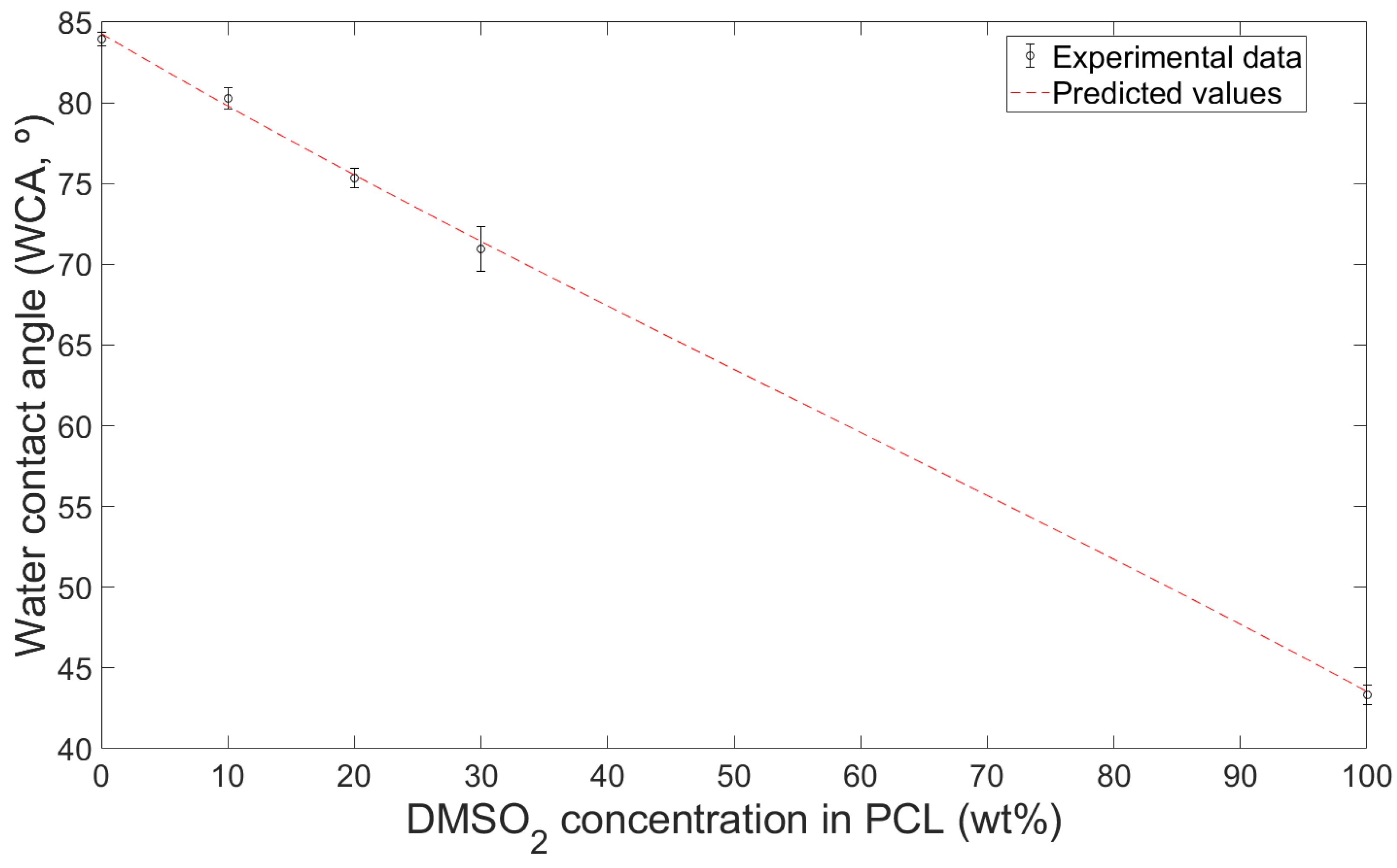
3.2. Hydrophilicity of Composites
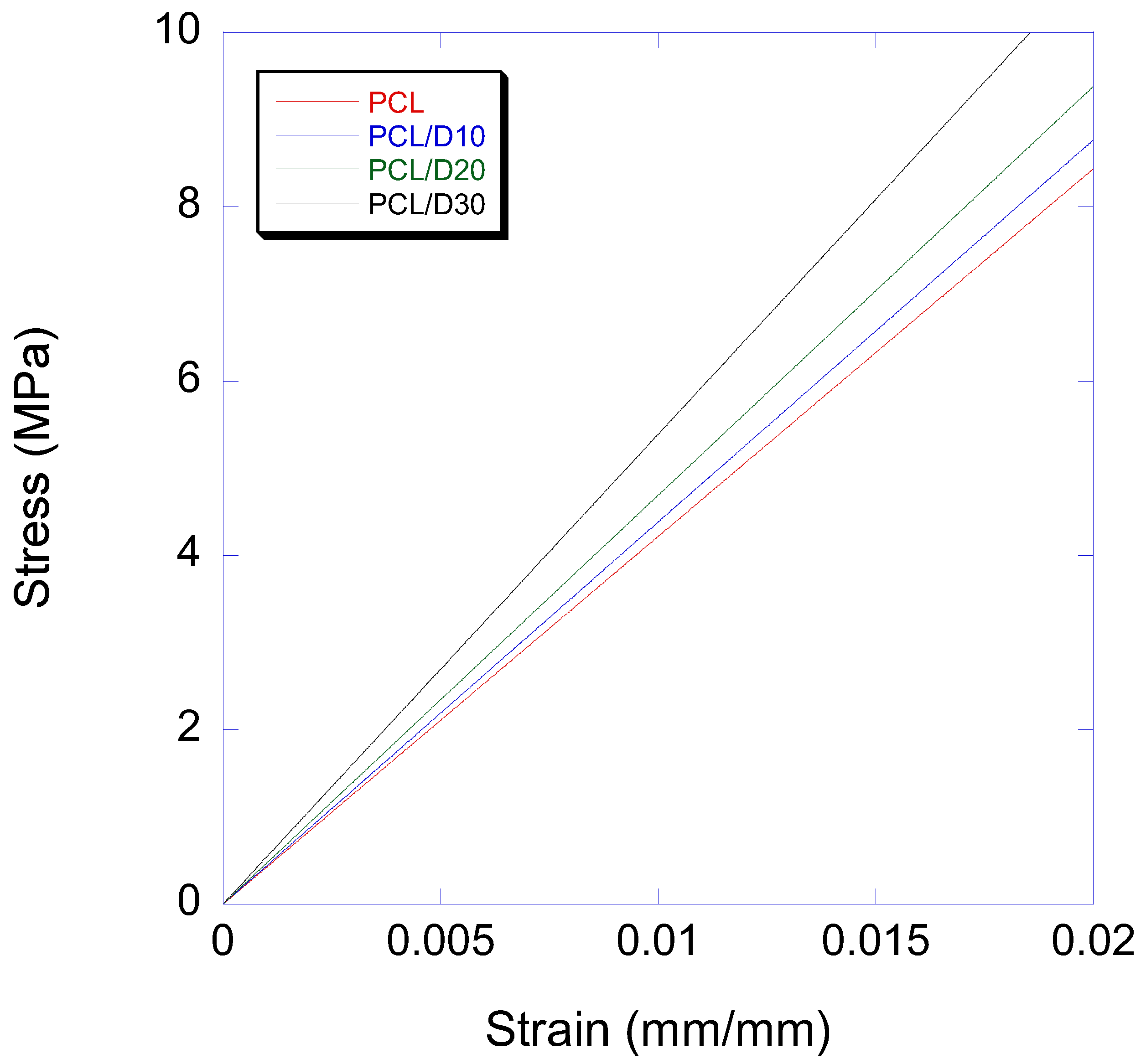
3.3. Mechanical Properties
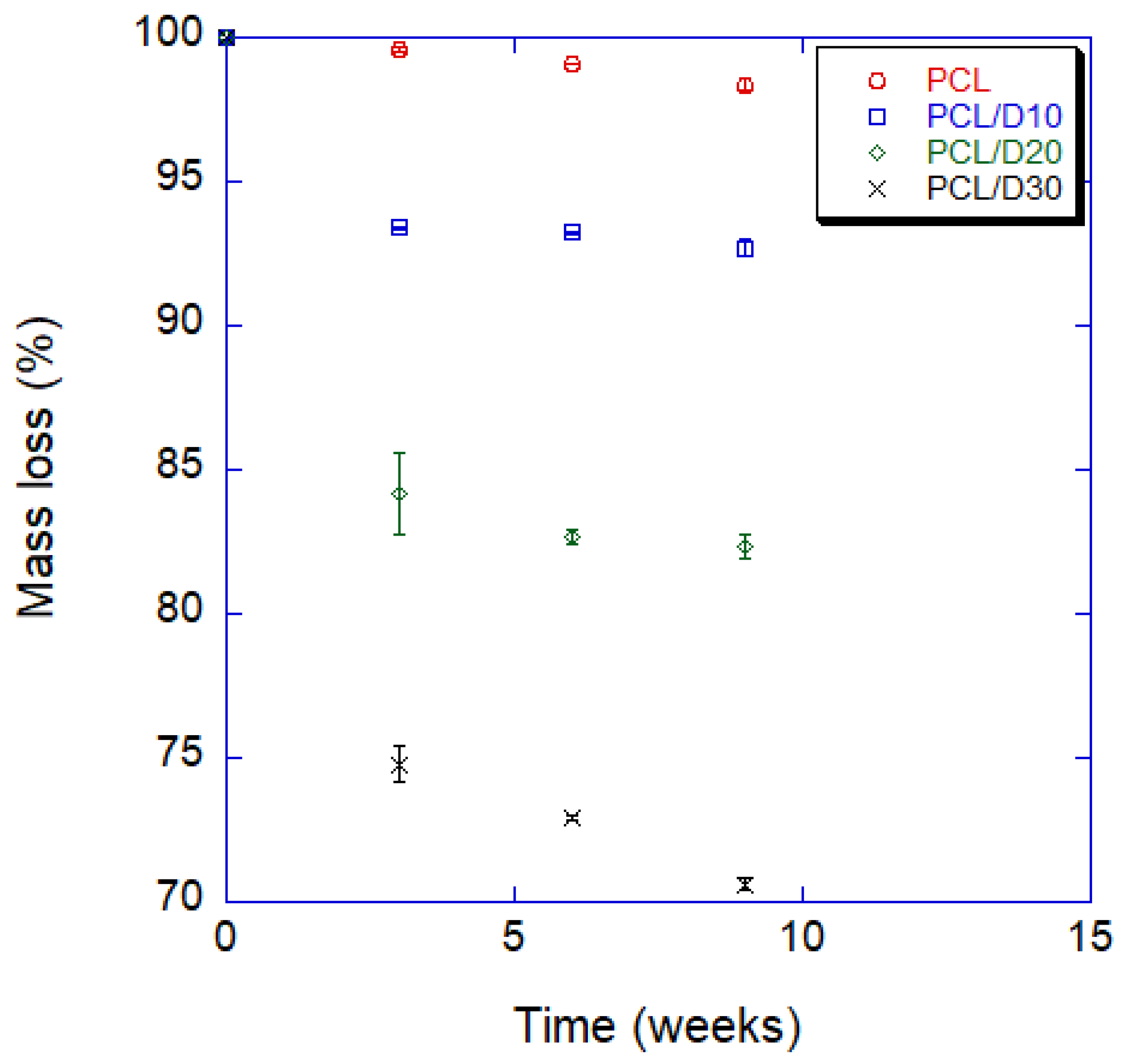
3.4. Degradation Property In Vitro
4. Discussion
5. Conclusions
- The water contact angle decreased by 4.4%, 10.2%, and 15.5% with 10%, 20%, and 30 wt%, respectively, while the contact angle with PBS solution decreases 3.0%, 8.7%, and 18.5% with those. The water contact angle of the composites can be predicted using the surface tension of each material.
- Adding DMSO2 to the PCL matrix increased the elastic modulus with increasing DMSO2 concentration rate. However, the 0.2% offset yield strength decreased with increasing DMSO2 ratio due to poor interfacial adhesion between PCL and DMSO2, which occurred more frequently with micro-sized particles than with nano-sized particles. The addition of extra additives, such as a binder, can be used to improve the yield strengths of the composites. The degradation rate should be regulated for specific conditions.
- The degradation time of the composite with 30 wt% of DMSO2 was 18 times faster than that of pure PCL in a 9 week test. PCL and DMSO2 composites can tailor the degraded rate with DMSO2 ratio, and a wide range of degradation time can increase the selection for applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Black, C.R.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O. Bone tissue engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Burr, D.B. Basic biomechanical measurements of bone: A tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, H.; Zhao, Y.; Luo, J.; Yang, L.; Liu, W.; He, Q. Functionalized cell-free scaffolds for bone defect repair inspired by self-healing of bone fractures: A review and new perspectives. Mater. Sci. Eng. C 2019, 98, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone: The influence of processing on safety and performance. Orthop. Clin. 1999, 30, 571–581. [Google Scholar]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Migliorini, F.; La Padula, G.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 118. [Google Scholar] [CrossRef]
- Khush, K.K.; Menza, R.; Nguyen, J.; Zaroff, J.G.; Goldstein, B.A. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ. Heart Fail. 2013, 6, 300–309. [Google Scholar] [CrossRef]
- Marew, T.; Birhanu, G. Three dimensional printed nanostructure biomaterials for bone tissue engineering. Regen. Ther. 2021, 18, 102–111. [Google Scholar] [CrossRef]
- Ren, J.; Xu, Y.; Zhiyi, G.; Ren, T.; Ren, J.; Wang, K.; Luo, Y.; Zhu, M.; Tan, Q. Reconstruction of the trachea and carina: Surgical reconstruction, autologous tissue transplantation, allograft transplantation, and bioengineering. Thorac. Cancer 2022, 13, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Organ Donation Statistics. 2021. Available online: https://www.organdonor.gov/learn/organ-donation-statistics (accessed on 9 January 2022).
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Woodfield, T.B.; Dalton, P.D. Scaffold design and fabrication. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 311–346. [Google Scholar]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Tathe, A.; Ghodke, M.; Nikalje, A.P. A brief review: Biomaterials and their application. Int. J. Pharm. Pharm. Sci. 2010, 2, 19–23. [Google Scholar]
- Black, J.; Hastings, G. Handbook of Biomaterial Properties; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Sampath, U.G.T.; Ching, Y.C.; Chuah, C.H.; Sabariah, J.J.; Lin, P.-C. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials 2016, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic biopolymers and their composites: Advantages and limitations—An overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Patrício, T.; Domingos, M.; Gloria, A.; D’Amora, U.; Coelho, J.; Bártolo, P. Fabrication and characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Rapid Prototyp. J. 2014, 20, 145–156. [Google Scholar] [CrossRef]
- Carmona, V.B.; Corrêa, A.C.; Marconcini, J.M.; Mattoso, L.H.C. Properties of a biodegradable ternary blend of thermoplastic starch (TPS), poly (ε-caprolactone)(PCL) and poly (lactic acid)(PLA). J. Polym. Environ. 2015, 23, 83–89. [Google Scholar] [CrossRef]
- Agrawal, G.; Negi, Y.S.; Pradhan, S.; Dash, M.; Samal, S. Wettability and contact angle of polymeric biomaterials. In Characterization of Polymeric Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–81. [Google Scholar]
- Udofia, E.N.; Zhou, W. Microextrusion based 3D printing—A review. In proceeding of the 2018 International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 13–15 August 2018. [Google Scholar]
- Wong, T.; Bloomer, R.J.; Benjamin, R.L.; Buddington, R.K. Small intestinal absorption of methylsulfonylmethane (MSM) and accumulation of the sulfur moiety in selected tissues of mice. Nutrients 2017, 10, 19. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Sulfur in human health. EC Nutr. 2019, 14, 785–791. [Google Scholar]
- Marañón, G.; Muñoz-Escassi, B.; Manley, W.; García, C.; Cayado, P.; De la Muela, M.S.; Olábarri, B.; León, R.; Vara, E. The effect of methyl sulphonyl methane supplementation on biomarkers of oxidative stress in sport horses following jumping exercise. Acta Vet. Scand. 2008, 50, 45. [Google Scholar] [CrossRef]
- Kim, L.S.; Axelrod, L.; Howard, P.; Buratovich, N.; Waters, R. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: A pilot clinical trial. Osteoarthr. Cartil. 2006, 14, 286–294. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic science of articular cartilage. Clin. Sport. Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, J.; Hashimoto, M.; Hosokawa, Y.; Ishimi, Y. Assessment of safety and efficacy of methylsulfonylmethane on bone and knee joints in osteoarthritis animal model. J. Bone Miner. Metab. 2013, 31, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rafei, M.K.; Thabet, N.M. Modulatory effect of methylsulfonylmethane against BPA/γ-radiation induced neurodegenerative alterations in rats: Influence of TREM-2/DAP-12/Syk pathway. Life Sci. 2020, 260, 118410. [Google Scholar] [CrossRef]
- Máca, J.; Vondrák, J.; Sedlaříková, M. Use of Dimethyl Sulfone as Additive in Aprotic Electrolytes. ECS Trans. 2014, 48, 135. [Google Scholar] [CrossRef]
- Markaryan, S.A.; Aznauryan, M.; Kazoyan, E. Physicochemical properties of aqueous solutions of dimethyl-and diethylsulfones. Russ. J. Phys. Chem. A 2011, 85, 2138–2141. [Google Scholar] [CrossRef]
- Markuniene, I.; Rabiei, M.; Nasiri, S.; Urbaite, S.; Palevicius, A.; Janusas, G. Biocompatible Piezoelectric PVDF/HA/AgNO3 Thin Film Prepared by the Solvent Casting Method. Sensors 2022, 23, 289. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Gelatin-polycaprolactone-nanohydroxyapatite electrospun nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2021, 119, 111588. [Google Scholar] [CrossRef]
- Murugan, S.; Parcha, S.R. Fabrication techniques involved in developing the composite scaffolds PCL/HA nanoparticles for bone tissue engineering applications. J. Mater. Sci. Mater. Med. 2021, 32, 93. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Desando, G.; Cavallo, C.; Bartolotti, I.; Shelyakova, T.; Goranov, V.; Brucale, M.; Dediu, V.A.; Fini, M. Multifunctional 3D-printed magnetic polycaprolactone/hydroxyapatite scaffolds for bone tissue engineering. Polymers 2021, 13, 3825. [Google Scholar] [CrossRef]
- Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Märtin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and characterization of PCL/HA filament as a 3D printing material using thermal extrusion technology for bone tissue engineering. Polymers 2022, 14, 669. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ying, W.S.; Razzaghi, M.; Sharif, S.; Ismail, A.F.; Berto, F. Improved bacteriostatic and anticorrosion effects of polycaprolactone/chitosan coated magnesium via incorporation of zinc oxide. Materials 2021, 14, 1930. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Drug release and biodegradability of electrospun cellulose nanocrystal reinforced polycaprolactone. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 929–937. [Google Scholar] [CrossRef]
- Min, K.-E.; Kim, H.-Y.; Bang, J.-H.; Kim, J.-H.; Kim, J.-K. Effects of Hardeners and Catalysts on the Reliability of Copper to Copper Adhesive Joint. Korean J. Mater. Res. 2011, 21, 283–287. [Google Scholar] [CrossRef]
- Min, K.-E.; Jang, J.-W.; Kim, J.-K.; Wern, C.; Yi, S. Thermophysical Properties of Inorganic Phase-Change Materials Based on MnCl2· 4H2O. Appl. Sci. 2022, 12, 6338. [Google Scholar] [CrossRef]
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. Annual Book of ASTM Standards: West Conshohocken, PA, USA, 1997. Available online: https://www.astm.org/d0790-17.html (accessed on 15 December 2022).
- ASTM D7334-08; ASTM Standards. Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. Annual Book of ASTM Standards: West Conshohocken, PA, USA, 2013. Available online: https://www.astm.org/d7334-08r22.html (accessed on 15 December 2022).
- Haibach, K.; Menner, A.; Powell, R.; Bismarck, A. Tailoring mechanical properties of highly porous polymer foams: Silica particle reinforced polymer foams via emulsion templating. Polymer 2006, 47, 4513–4519. [Google Scholar] [CrossRef]
- Robertson, C.G.; Lin, C.; Rackaitis, M.; Roland, C. Influence of particle size and polymer− filler coupling on viscoelastic glass transition of particle-reinforced polymers. Macromolecules 2008, 41, 2727–2731. [Google Scholar] [CrossRef]
- Chandramohan, D.; Rajesh, S. Study of machining parameters on natural fiber particle reinforced polymer composite material. Acad. J. Manuf. Eng. 2014, 12, 72–77. [Google Scholar]
- Chandramohan, D.; Kumar, A.J.P. Experimental data on the properties of natural fiber particle reinforced polymer composite material. Data Brief 2017, 13, 460–468. [Google Scholar] [CrossRef]
- Jose, A.S.; Athijayamani, A.; Jani, S. A review on the mechanical properties of bio waste particulate reinforced polymer composites. Mater. Today Proc. 2021, 37, 1757–1760. [Google Scholar] [CrossRef]
- Kuan, H.T.N.; Tan, M.Y.; Shen, Y.; Yahya, M.Y. Mechanical properties of particulate organic natural filler-reinforced polymer composite: A review. Compos. Adv. Mater. 2021, 30, 1–17. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Chawla, N.; Sidhu, R.; Ganesh, V. Three-dimensional visualization and microstructure-based modeling of deformation in particle-reinforced composites. Acta Mater. 2006, 54, 1541–1548. [Google Scholar] [CrossRef]
- Hendrikson, W.; Rouwkema, J.; Van Blitterswijk, C.; Moroni, L. Influence of PCL molecular weight on mesenchymal stromal cell differentiation. RSC Adv. 2015, 5, 54510–54516. [Google Scholar] [CrossRef]
- Kołbuk, D.; Ciechomska, M.; Jeznach, O.; Sajkiewicz, P. Effect of crystallinity and related surface properties on gene expression of primary fibroblasts. RSC Adv. 2022, 12, 4016–4028. [Google Scholar] [CrossRef]
- Ayyoob, M.; Kim, Y.J. Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers 2018, 10, 1132. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Cheon, K.H.; Park, C.; Kang, M.H.; Kang, I.G.; Lee, M.K.; Lee, H.; Kim, H.E.; Jung, H.D.; Jang, T.S. Construction of tantalum/poly(ether imide) coatings on magnesium implants with both corrosion protection and osseointegration properties. Bioact. Mater. 2021, 6, 1189–1200. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wen, C. Fabrication and Characterization of Nanoporous Niobia, and Nanotubular Tantala, Titania and Zirconia via Anodization. J. Funct. Biomater. 2015, 6, 153–170. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, Y.; Zhou, Q.; Ding, Z.; Wu, Y.; Zhu, Y.; Shi, W.; He, Q. The Preparation and Properties of Multilayer Cu-MTa2O5 Composite Coatings on Ti6Al4V for Biomedical Applications. Nanomaterials 2019, 9, 1498. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Jeon, H.; Yi, C.; Park, S.; Ryu, S.; Kim, S.B. Mutual Interaction between Plasma Characteristics and Liquid Properties in AC-driven Pin-to-Liquid Discharge. Sci. Rep. 2018, 8, 12037. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Wei, C.K.; Ho, C.C.; Ding, S.J. Enhanced Hydrophilicity and Biocompatibility of Dental Zirconia Ceramics by Oxygen Plasma Treatment. Materials 2015, 8, 684–699. [Google Scholar] [CrossRef]
- Chen, X.; Meng, J.; Xu, H.; Shinoda, M.; Kishimoto, M.; Sakurai, S.; Yamane, H. Fabrication and Properties of Electrospun Collagen Tubular Scaffold Crosslinked by Physical and Chemical Treatments. Polymers 2021, 13, 755. [Google Scholar] [CrossRef]
- Li, Y.; Ceylan, M.; Shrestha, B.; Wang, H.; Lu, Q.R.; Asmatulu, R.; Yao, L. Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromolecules 2014, 15, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Gholami, N.; Jaleh, B.; Golbedaghi, R.; Larijani, M.M.; Wanichapichart, P.; Nasrollahzadeh, M.; Varma, R.S. Modification of Chitosan Membranes via Methane Ion Beam. Molecules 2020, 25, 2292. [Google Scholar] [CrossRef]
- Ercan, U.K.; İbiş, F.; Dikyol, C.; Horzum, N.; Karaman, O.; Yıldırım, Ç.; Çukur, E.; Demirci, E.A. Prevention of bacterial colonization on nonthermal atmospheric plasma treated surgical sutures for control and prevention of surgical site infections. PLoS ONE 2018, 13, e0202703. [Google Scholar] [CrossRef]
- Aghdam, R.M.; Najarian, S.; Shakhesi, S.; Khanlari, S.; Shaabani, K.; Sharifi, S. Investigating the effect of PGA on physical and mechanical properties of electrospun PCL/PGA blend nanofibers. J. Appl. Polym. Sci. 2011, 124, 123–131. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Villa, F.; Marengo, M.; De Coninck, J. A new model to predict the influence of surface temperature on contact angle. Sci. Rep. 2018, 8, 6549. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Strnad, G.; Chirila, N.; Petrovan, C.; Russu, O. Contact Angle Measurement on Medical Implant Titanium Based Biomaterials. Procedia Technol. 2016, 22, 946–953. [Google Scholar] [CrossRef]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Peponi, L.; Navarro-Baena, I.; Báez, J.E.; Kenny, J.M.; Marcos-Fernández, A. Effect of the molecular weight on the crystallinity of PCL-b-PLLA di-block copolymers. Polymer 2012, 53, 4561–4568. [Google Scholar] [CrossRef]
- Olubamiji, A.D.; Izadifar, Z.; Si, J.L.; Cooper, D.M.; Eames, B.F.; Chen, D.X. Modulating mechanical behaviour of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight and pore geometry. Biofabrication 2016, 8, 025020. [Google Scholar] [CrossRef] [PubMed]
- Perstorp. CAPA 6000 Series Datasheet. Available online: http://www.rapstrap.com/TDS-CAPA6500.pdf (accessed on 11 August 2022).
- Liu, H.; Webster, T.J. Bioinspired nanocomposites for orthopedic applications. In Nanotechnology for the Regeneration of Hard and Soft Tissues; World Scientific: Singapore, 2007; pp. 1–51. ISBN 978-981-270-615-7. [Google Scholar]
- Doyle, S.E.; Henry, L.; McGennisken, E.; Onofrillo, C.; Bella, C.D.; Duchi, S.; O’Connell, C.D.; Pirogova, E. Characterization of Polycaprolactone Nanohydroxyapatite Composites with Tunable Degradability Suitable for Indirect Printing. Polymers 2021, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Germiniani, L.G.L.; da Silva, L.C.E.; Plivelic, T.S.; Gonçalves, M.C. Poly(ε-caprolactone)/cellulose nanocrystal nanocomposite mechanical reinforcement and morphology: The role of nanocrystal pre-dispersion. J. Mater. Sci. 2018, 54, 414–426. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Fang, H.Y.; Hsu, T.T.; Lin, C.Y.; Shie, M.Y. The Characteristics of Mineral Trioxide Aggregate/Polycaprolactone 3-dimensional Scaffold with Osteogenesis Properties for Tissue Regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef]
- Bellani, C.F.; Pollet, E.; Hebraud, A.; Pereira, F.V.; Schlatter, G.; Avérous, L.; Bretas, R.E.S.; Branciforti, M.C. Morphological, thermal, and mechanical properties of poly(ε-caprolactone)/poly(ε-caprolactone)-grafted-cellulose nanocrystals mats produced by electrospinning. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Gągol, M.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs). Ultrason. Sonochemistry 2019, 58, 104610. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Pountos, I. Tissue regeneration: The past, the present and the future. Injury 2005, 36, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef] [PubMed]
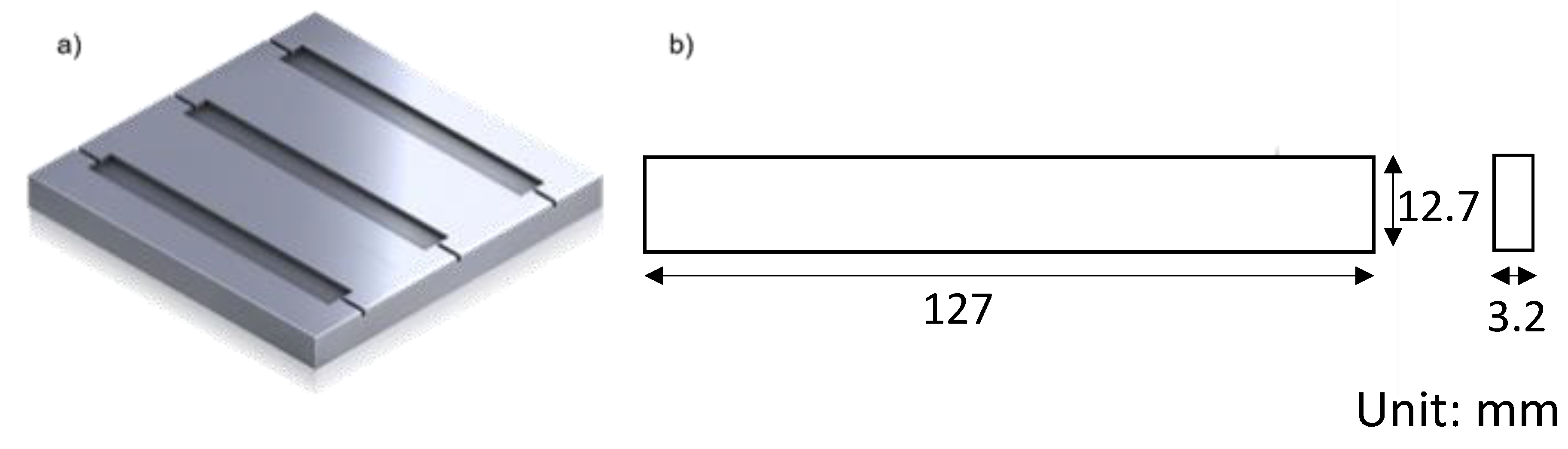
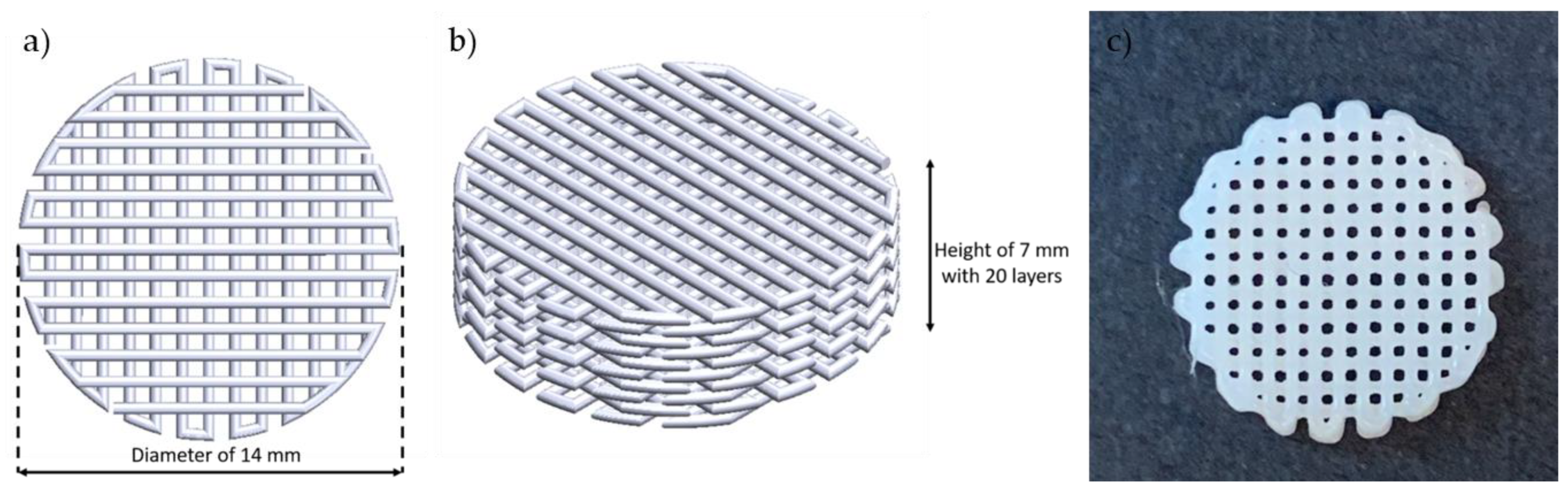

| Material | Appearance | Molecular Weight (g/mol) | Density (kg/m3) | Flash Point (°C) | Surface Tension (mN/m) | Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| PCL | Powder | 50,000 | 275 | |||
| DMSO2 | Powder | 94.13 | 1450 | 143 | 60.15 | 1.14 |
| Material | Melting Temperature (°C) | |||
|---|---|---|---|---|
| Peak 1 | Peak 2 | |||
| Mean | SD | Mean | SD | |
| PCL | 56.59 | 0.16 | ||
| PCL/D10 | 55.39 | 0.52 | 106.82 | 0.30 |
| PCL/D20 | 56.40 | 0.20 | 106.67 | 0.31 |
| PCL/D30 | 56.27 | 0.07 | 106.73 | 0.31 |
| DMSO2 | 106.85 | 0.46 | ||
| Modulus in Elasticity (MPa) | 0.2% Offset Yield Strength (MPa) | |||||||
|---|---|---|---|---|---|---|---|---|
| Material | PCL | PCL/D10 | PCL/D20 | PCL/D30 | PCL | PCL/D10 | PCL/D20 | PCL/D30 |
| Mean | 424 | 440 | 469 | 532 | 13.70 | 11.08 | 9.67 | 8.73 |
| SD | 2.94 | 1.41 | 5.35 | 5.35 | 0.44 | 0.25 | 0.41 | 0.22 |
| Weeks | PCL | PCL/D10 | PCL/D20 | PCL/D30 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 0 | 100 | 100 | 100 | 100 | ||||
| 3 | 99.60 | 0.07 | 93.40 | 0.02 | 84.19 | 1.43 | 74.79 | 0.60 |
| 6 | 99.09 | 0.01 | 93.23 | 0.06 | 82.67 | 0.23 | 72.91 | 0.10 |
| 9 | 98.38 | 0.17 | 92.71 | 0.26 | 82.33 | 0.45 | 70.60 | 0.21 |
| Group | Materials | Water Contact Angle (°) | Refs. | |
|---|---|---|---|---|
| Metal | Magnesium | 40.8 | [66] | |
| Tantalum | 61 | [67] | ||
| Titanium | 73 | [68] | ||
| Ceramic | Alumina | 64.74 | [69] | |
| Zirconia | 65 | [70] | ||
| Polymer | Natural Polymer | Collagen | 62.17 | [71] |
| Gelatin | 78.6 | [72] | ||
| Chitosan | 80 | [73] | ||
| Synthetic Polymer | PLA 1 | 87.2 | [74] | |
| PCL | 83.9 | Current study | ||
| 118 | [75] | |||
| PGA 2 | 109.8 | [76] | ||
| PLGA 3 | 124.9 | [64] | ||
| Polymer Matrix | Additive | Additive Ratio (%) | Specimen | Test Method | Modulus (Mpa) | Refs. |
|---|---|---|---|---|---|---|
| PCL | - | - | Solid | Tensile test | 440 | [83] |
| - | - | Solid | Three-point bending | 414 | [83] | |
| - | - | Solid | Compressive test | 455 | [83] | |
| - | - | Scaffold | Compressive test | 10 | [83] | |
| DMSO2 | 10 | Molded bar | Three-point bending | 440 | Current study | |
| DMSO2 | 20 | Molded bar | Three-point bending | 469 | Current study | |
| DMSO2 | 30 | Molded bar | Three-point bending | 532 | Current study | |
| nHA 1 | 0 | Cylindrical disk | Compressive test | 71.72 | [85] | |
| nHA | 10 | Cylindrical disk | Compressive test | 67.65 | [85] | |
| nHA | 30 | Cylindrical disk | Compressive test | 68.55 | [85] | |
| CNC 2 | 0 | nano fiber | - | 23.4 | [49] | |
| CNC | 0 | nano fiber | - | 33.1 | [49] | |
| CNC | 1 | nano fiber | - | 43.8 | [49] | |
| CNC | 1.5 | nano fiber | - | 39 | [49] | |
| CNC | 2.5 | nano fiber | - | 39.6 | [49] | |
| CNC | 4 | nano fiber | - | 27.8 | [49] | |
| CNC | 0 | - | 246.45 | [86] | ||
| CNC | 5 | - | 205.95 | [86] | ||
| CNC | 10 | - | 313.55 | [86] | ||
| CNC | 15 | - | 460.5 | [86] | ||
| CNC | 20 | - | 500.99 | [86] | ||
| CNC | 25 | - | 629.42 | [86] | ||
| - | - | Scaffold | - | 3.58 | [87] | |
| MTA 3 | Scaffold | - | 4.07 | [87] | ||
| PCL grafted CNC | 0 | nano fiber | - | 4.09 | [88] | |
| PCL grafted CNC | 1 | nano fiber | - | 4.49 | [88] | |
| PCL grafted CNC | 3 | nano fiber | - | 6.01 | [88] | |
| PCL grafted CNC | 5 | nano fiber | - | 6.94 | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.-W.; Min, K.-E.; Kim, C.; Wern, C.; Yi, S. PCL and DMSO2 Composites for Bio-Scaffold Materials. Materials 2023, 16, 2481. https://doi.org/10.3390/ma16062481
Jang J-W, Min K-E, Kim C, Wern C, Yi S. PCL and DMSO2 Composites for Bio-Scaffold Materials. Materials. 2023; 16(6):2481. https://doi.org/10.3390/ma16062481
Chicago/Turabian StyleJang, Jae-Won, Kyung-Eun Min, Cheolhee Kim, Chien Wern, and Sung Yi. 2023. "PCL and DMSO2 Composites for Bio-Scaffold Materials" Materials 16, no. 6: 2481. https://doi.org/10.3390/ma16062481
APA StyleJang, J.-W., Min, K.-E., Kim, C., Wern, C., & Yi, S. (2023). PCL and DMSO2 Composites for Bio-Scaffold Materials. Materials, 16(6), 2481. https://doi.org/10.3390/ma16062481








