Influence of Trabecular Geometry on Scaffold Mechanical Behavior and MG-63 Cell Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Scaffold Generative Design
2.2. Scaffold Manufacturing
2.3. Structural Characterization
2.4. Mechanical Compression Tests
2.5. Biological Tests
2.5.1. Cell Culture
2.5.2. Sterilization and Conditioning
2.5.3. MG-63 Seeding
2.5.4. MTT Assay
2.5.5. Cell Morphology
2.5.6. Statistical Analysis
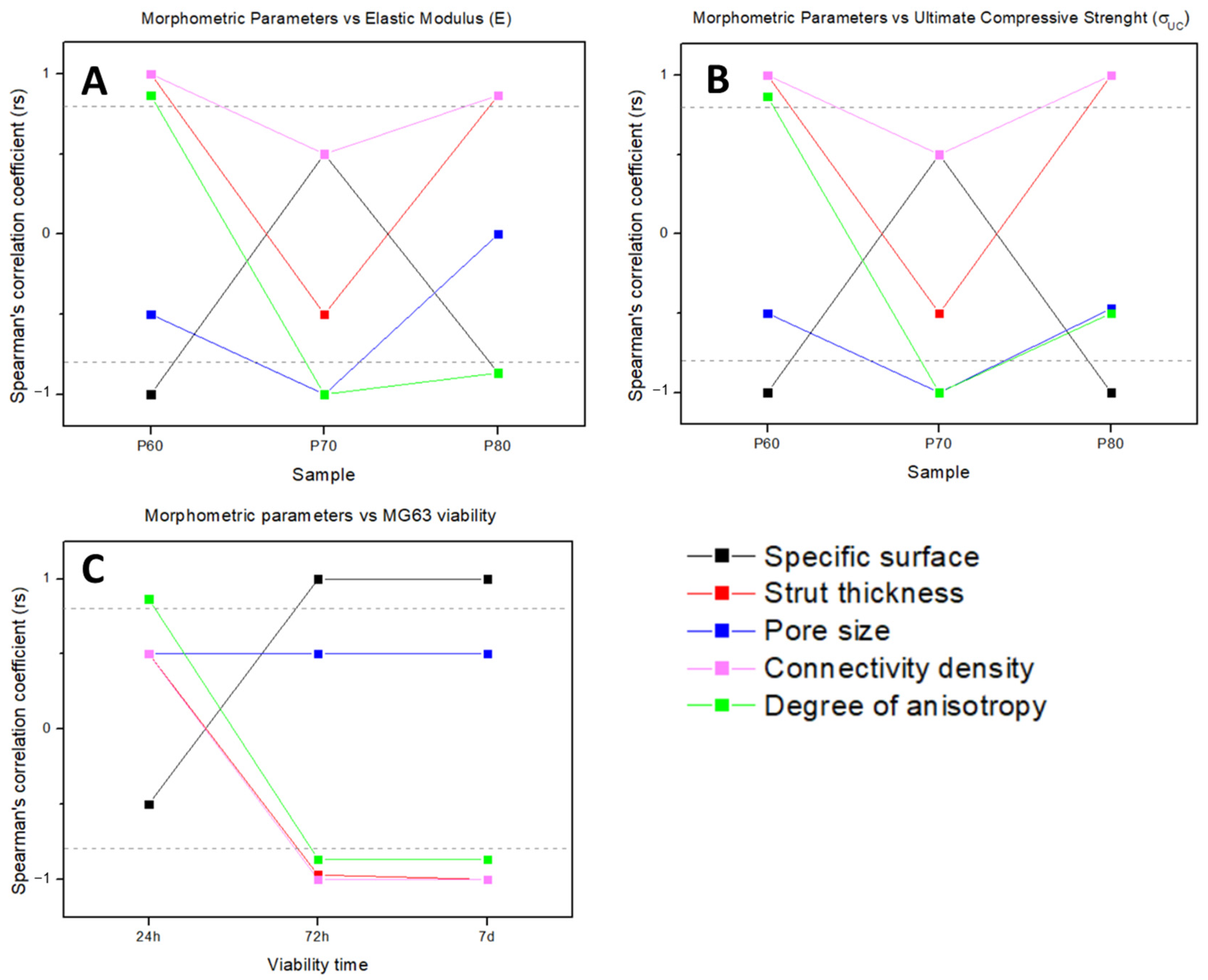
2.6. Correlation between Morphometry and Biomechanical Properties
3. Results
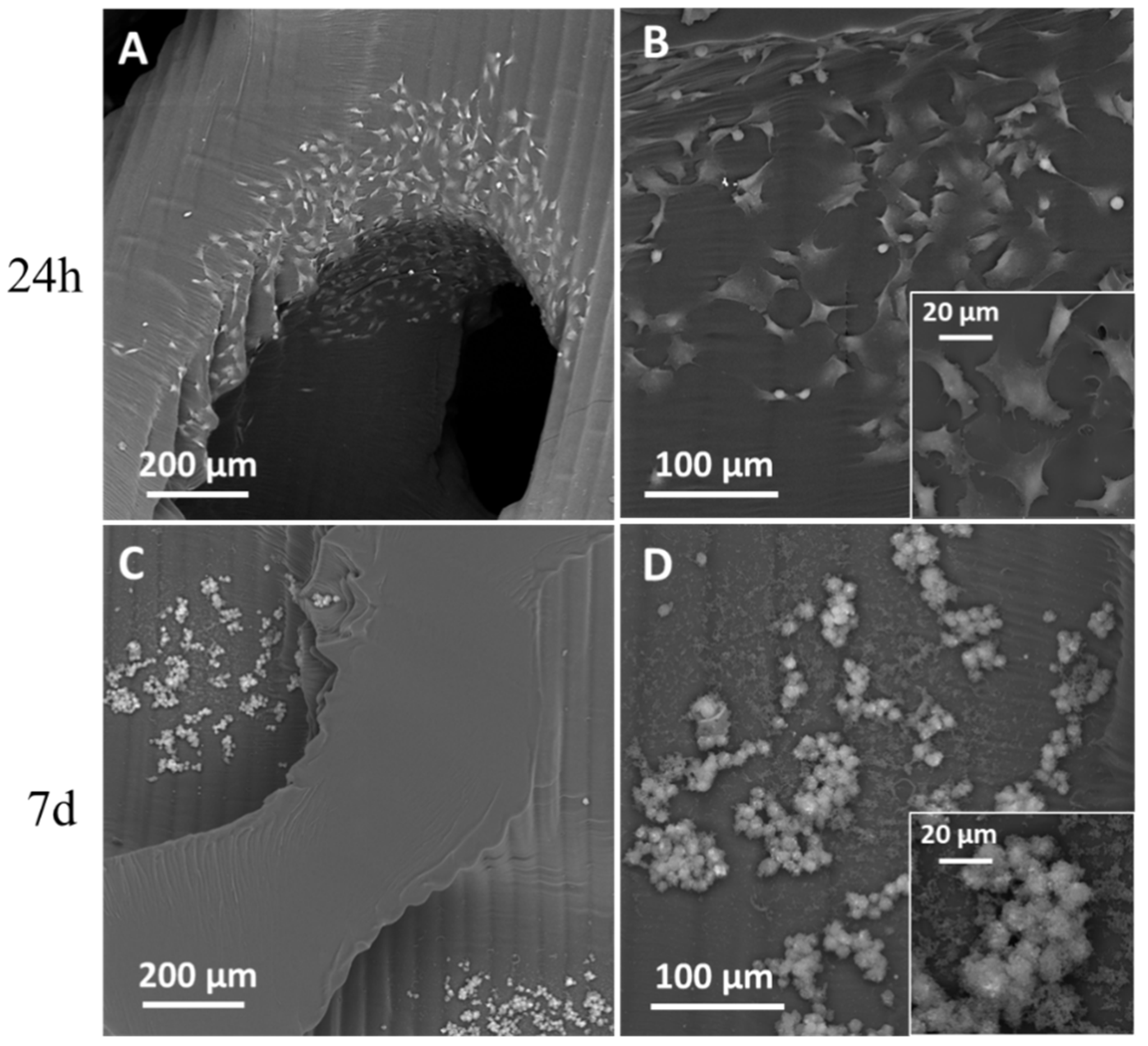
3.1. SEM Analysis
3.2. Mechanical Tests
| Scaffold Geometry | ES [Mpa] | σUCS [MPa] |
|---|---|---|
| P60 | 8.5 ± 0.6 | 1.4 ± 0.1 |
| P70 | 4.0 ± 0.5 | 0.50 ± 0.03 |
| P80 | 1.6 ± 0.4 | 0.17 ± 0.03 |
| Middle region including premolars of human mandible [22] | - | 1 ÷ 4 |
| Distal region including molars of human mandible [22] | - | 0.5 ÷ 2.5 |
3.3. XµCT Analysis
3.4. Biological Tests
3.5. PhC-XµCT Analysis at Synchrotron
3.6. Correlation between Morphometry and Biomechanical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Codrea, C.I.; Croitoru, A.-M.; Baciu, C.C.; Melinescu, A.; Ficai, D.; Fruth, V.; Ficai, A. Advances in Osteoporotic Bone Tissue Engineering. J. Clin. Med. 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.L.; Groppo, R.; Bloise, N.; Fassina, L.; Visai, L.; Galati, M.; Iuliano, L.; Mengucci, P. Topological, Mechanical and Biological Properties of Ti6Al4V Scaffolds for Bone Tissue Regeneration Fabricated with Reused Powders via Electron Beam Melting. Materials 2021, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.L.; Furlani, M.; Giuliani, A.; Bloise, N.; Fassina, L.; Visai, L.; Mengucci, P. Biomechanical performances of PCL/HA micro- and macro-porous lattice scaffolds fabricated via laser powder bed fusion for bone tissue engineering. Mater. Sci. Eng. C 2021, 128, 112300. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A review on fabricating tissue scaffolds using vat photopolymerization. Acta Biomater. 2018, 74, 90–111. [Google Scholar] [CrossRef]
- Fantini, M.; Curto, M.; De Crescenzio, F. A Method to Design Biomimetic Scaffolds for Bone Tissue Engineering Based on Voronoi Lattices. Virtual Phys. Prototyp. 2016, 11, 77–90. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Wang, C.; Zhang, A.; Chen, B.; Han, Q.; Wang, J. Design and Properties of Biomimetic Irregular Scaffolds for Bone Tissue Engineering. Comput. Biol. Med. 2021, 130, 104241. [Google Scholar] [CrossRef]
- Maia, F.R.; Bastos, A.R.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Recent Approaches towards Bone Tissue Engineering. Bone 2022, 154, 116256. [Google Scholar] [CrossRef]
- Kanwar, S.; Vijayavenkataraman, S. Design of 3D Printed Scaffolds for Bone Tissue Engineering: A Review. Bioprinting 2021, 24, e00167. [Google Scholar] [CrossRef]
- Klinger, S.; Greinwald, M.; Augat, P.; Hollensteiner, M. Mechanical and morphometric characterization of custom-made trabecular bone surrogates. J. Mech. Behav. Biomed. Mater. 2022, 129, 105146. [Google Scholar] [CrossRef]
- Uchiyama, T.; Tanizawa, T.; Muramatsu, H.; Endo, N.; Takahashi, H.E.; Hara, T. Three-dimensional microstructural analysis of human trabecular bone in relation to its mechanical properties. Bone 1999, 25, 487–491. [Google Scholar] [CrossRef]
- Liang, H.; Chao, L.; Xie, D.; Yang, Y.; Shi, J.; Zhang, Y.; Xue, B.; Shen, L.; Tian, Z.; Li, L.; et al. Trabecular-like Ti–6Al–4V scaffold for bone repair: A diversified mechanical stimulation environment for bone regeneration. Compos. Part B Eng. 2022, 241, 110057. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Xie, D.; Li, L.; Mao, N.; Wang, C.; Tian, Z.; Jiang, Q.; Shen, L. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: Fabrication by selective laser melting and in vitro biocompatibility. J. Mater. Sci. Technol. 2019, 35, 1284–1297. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The recent development of vat photopolymerization: A review. Addit. Manuf. 2021, 48, 102423. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Barone, M.; De Stefani, A.; Baciliero, U.; Bruno, G.; Gracco, A. The Accuracy of Jaws Repositioning in Bimaxillary Orthognathic Surgery with Traditional Surgical Planning Compared to Digital Surgical Planning in Skeletal Class III Patients: A Retrospective Observational Study. J. Clin. Med. 2020, 9, 1840. [Google Scholar] [CrossRef]
- Mangano, F.G.; Margiani, B.; Solop, I.; Latuta, N.; Admakin, O. An Experimental Strategy for Capturing the Margins of Prepared Single Teeth with an Intraoral Scanner: A Prospective Clinical Study on 30 Patients. Int. J. Environ. Res. Public Health 2020, 17, 392. [Google Scholar] [CrossRef]
- Han, W.; El Botty, R.; Montaudon, E.; Malaquin, L.; Deschaseaux, F.; Espagnolle, N.; Marangoni, E.; Cottu, P.; Zalcman, G.; Parrini, M.C.; et al. In vitro bone metastasis dwelling in a 3D bioengineered niche. Biomaterials 2021, 269, 120624. [Google Scholar] [CrossRef]
- Accardo, A.; Courson, R.; Riesco, R.; Raimbault, V.; Malaquin, L. Direct laser fabrication of meso-scale 2D and 3D architectures with micrometric feature resolution. Addit. Manuf. 2018, 22, 440–446. [Google Scholar] [CrossRef]
- Fantini, M.; Curto, M. Interactive design and manufacturing of a Voronoi-based biomimetic bone scaffold for morphological characterization. Int. J. Interact. Des. Manuf. 2018, 12, 585–596. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Cedola, A. (Eds.) Advanced High-Resolution Tomography in Regenerative Medicine: Three-Dimensional Exploration into the Interactions between Tissues, Cells, and Biomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Du, Y.; Liang, H.; Xie, D.; Mao, N.; Zhao, J.; Tian, Z.; Wang, C.; Shen, L. Design and statistical analysis of irregular porous scaffolds for orthopedic reconstruction based on voronoi tessellation and fabricated via selective laser melting (SLM). Mater. Chem. Phys. 2020, 239, 121968. [Google Scholar] [CrossRef]
- Alvarez, R.R. Study of 3D Cellular Microenvironments for the Development of Bone Marrow In-Vitro Models. Ph.D. Dissertation, INSA de Toulouse, Toulouse, France, 2021. [Google Scholar]
- Amirikia, M.; Shariatzadeh, S.M.A.; Jorsaraei, S.G.A.; Mehranjani, M.S. Impact of pre-incubation time of silk fibroin scaffolds in culture medium on cell proliferation and attachment. Tissue Cell 2017, 49, 657–663. [Google Scholar] [CrossRef]
- Babi, M.; Riesco, R.; Boyer, L.; Fatona, A.; Accardo, A.; Malaquin, L.; Moran-Mirabal, J. Tuning the Nanotopography and Chemical Functionality of 3D Printed Scaffolds through Cellulose Nanocrystal Coatings. ACS Appl. Bio Mater. 2021, 4, 8443–8455. [Google Scholar] [CrossRef]
- Baptista, R.; Guedes, M. Porosity and pore design influence on fatigue behavior of 3D printed scaffolds for trabecular bone replacement. J. Mech. Behav. Biomed. Mater. 2021, 117, 104378. [Google Scholar] [CrossRef]
- Vafaeefar, M.; Moerman, K.M.; Kavousi, M.; Vaughan, T.J. A morphological, topological and mechanical investigation of gyroid, spinodoid and dual-lattice algorithms as structural models of trabecular bone. J. Mech. Behav. Biomed. Mater. 2023, 138, 105584. [Google Scholar] [CrossRef]



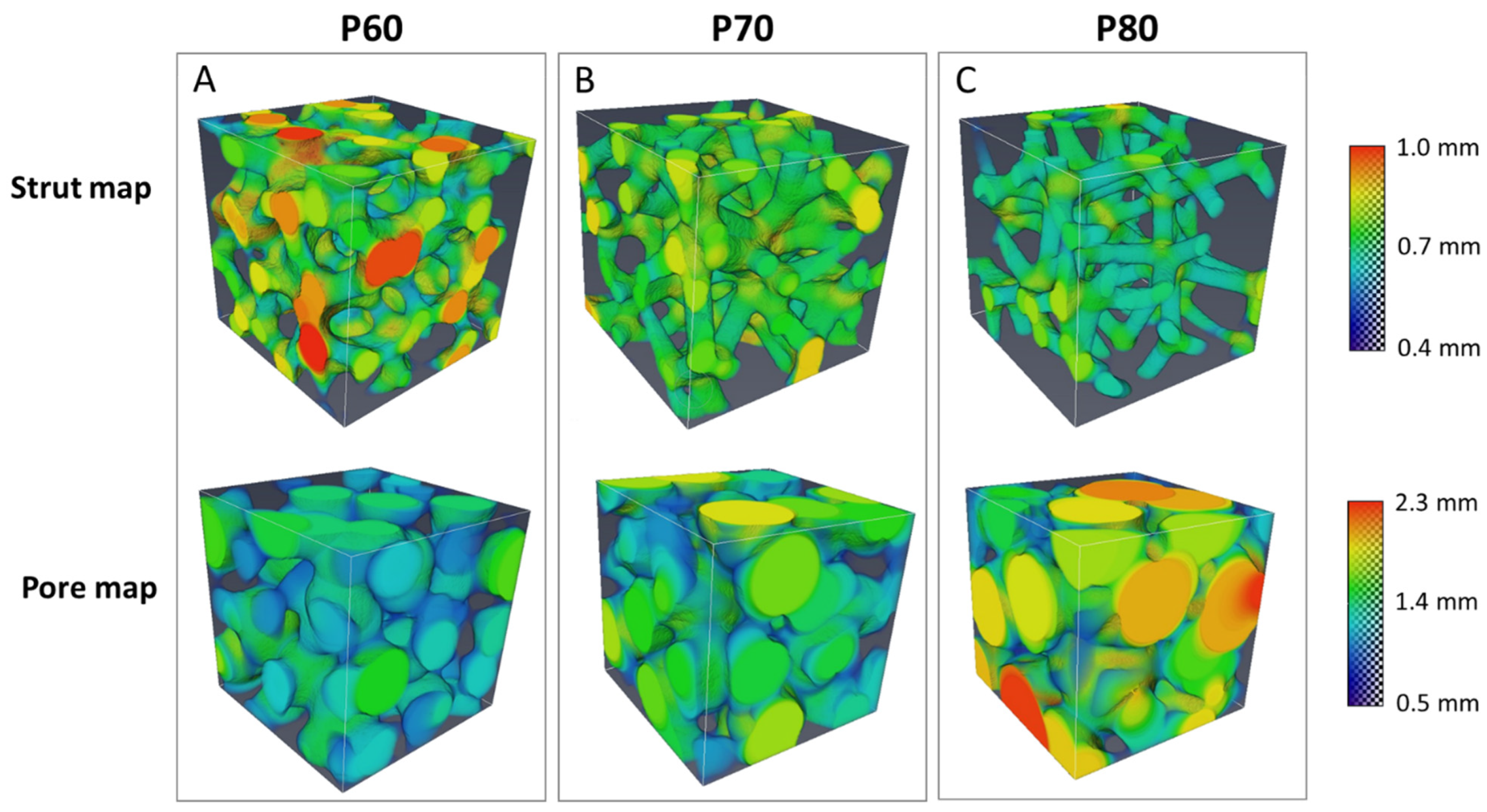



| Parameter | P60 | P70 | P80 |
|---|---|---|---|
| Target porosity [%] | 60 | 70 | 80 |
| Target mean pore size [mm] | 0.8 | 1 | 1.5 |
| Number of seeds | 2544 | 1397 | 759 |
| Strut thickness [mm] | 0.6 | ||
| Average strut length [mm] | 0.6 | 0.8 | 1 |
| Parameter | Value |
|---|---|
| Contours [n] | 3 |
| Hatching [mm] | 0.07 |
| Laser speed [mm/s] | 5800 |
| Laser power [mW] | 86 |
| Laser spot [mm] | 0.04 |
| Slicing [mm] | 0.05 |
| Morphometric Parameters | Before Mechanical Testing | After Mechanical Testing | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P60 | P70 | P80 | P60 | P70 | P80 | ||||||||
| AV | SD | AV | SD | AV | SD | AV | SD | AV | SD | AV | SD | ||
| Specific surface | mm−1 | 4.50 | 0.14 | 5.54 | 0.06 | 6.18 | 0.06 | 4.65 | 0.13 | 5.48 | 0.11 | 5.96 | 0.05 |
| Strut thickness | mm | 0.75 | 0.02 | 0.65 | 0.01 | 0.61 | 0.02 | 0.74 | 0.01 | 0.66 | 0.02 | 0.63 | 0.01 |
| Pore size | mm | 1.11 | 0.03 | 1.29 | 0.01 | 1.69 | 0.05 | 1.08 | 0.04 | 1.31 | 0.02 | 1.62 | 0.06 |
| Total porosity | % | 54 | 1 | 66 | 1 | 78 | 2 | 54 | 0 | 66 | 0 | 75 | 1 |
| Sample | As-Built | 7 d Culture Medium | 7 d Cells | |||
|---|---|---|---|---|---|---|
| AV | SD | AV | SD | AV | SD | |
| Segment length (µm) | 34.1 | 5.5 | 26.4 | 1.5 | 25.2 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, M.L.; Cerqueni, G.; Furlani, M.; Riberti, N.; Tognoli, E.; Denti, L.; Leonardi, F.; Giuliani, A.; Mattioli-Belmonte, M.; Mengucci, P. Influence of Trabecular Geometry on Scaffold Mechanical Behavior and MG-63 Cell Viability. Materials 2023, 16, 2342. https://doi.org/10.3390/ma16062342
Gatto ML, Cerqueni G, Furlani M, Riberti N, Tognoli E, Denti L, Leonardi F, Giuliani A, Mattioli-Belmonte M, Mengucci P. Influence of Trabecular Geometry on Scaffold Mechanical Behavior and MG-63 Cell Viability. Materials. 2023; 16(6):2342. https://doi.org/10.3390/ma16062342
Chicago/Turabian StyleGatto, Maria Laura, Giorgia Cerqueni, Michele Furlani, Nicole Riberti, Emanuele Tognoli, Lucia Denti, Francesco Leonardi, Alessandra Giuliani, Monica Mattioli-Belmonte, and Paolo Mengucci. 2023. "Influence of Trabecular Geometry on Scaffold Mechanical Behavior and MG-63 Cell Viability" Materials 16, no. 6: 2342. https://doi.org/10.3390/ma16062342
APA StyleGatto, M. L., Cerqueni, G., Furlani, M., Riberti, N., Tognoli, E., Denti, L., Leonardi, F., Giuliani, A., Mattioli-Belmonte, M., & Mengucci, P. (2023). Influence of Trabecular Geometry on Scaffold Mechanical Behavior and MG-63 Cell Viability. Materials, 16(6), 2342. https://doi.org/10.3390/ma16062342










