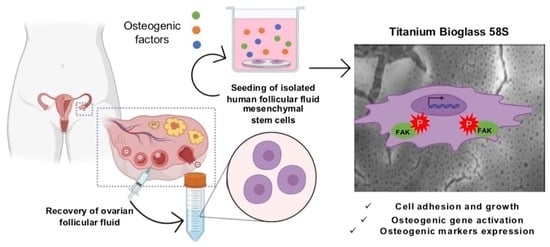
Human Ovarian Follicular Fluid Mesenchymal Stem Cells Express Osteogenic Markers When Cultured on Bioglass 58S-Coated Titanium Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium and Bioglass Coating Preparation and Characterization
2.2. Multipotent Follicular Mesenchymal Stem Cells (FF-MSC) Culture
2.3. Immunostaining and Fluorescence Microscopy Analysis
2.4. Experimental Design
2.5. Analysis by Confocal Laser Scanning Microscope (CLSM)
2.6. Western Blot Analysis
2.7. Cell Viability Assay
2.8. Scanning Electron Microscopy (SEM) Analysis
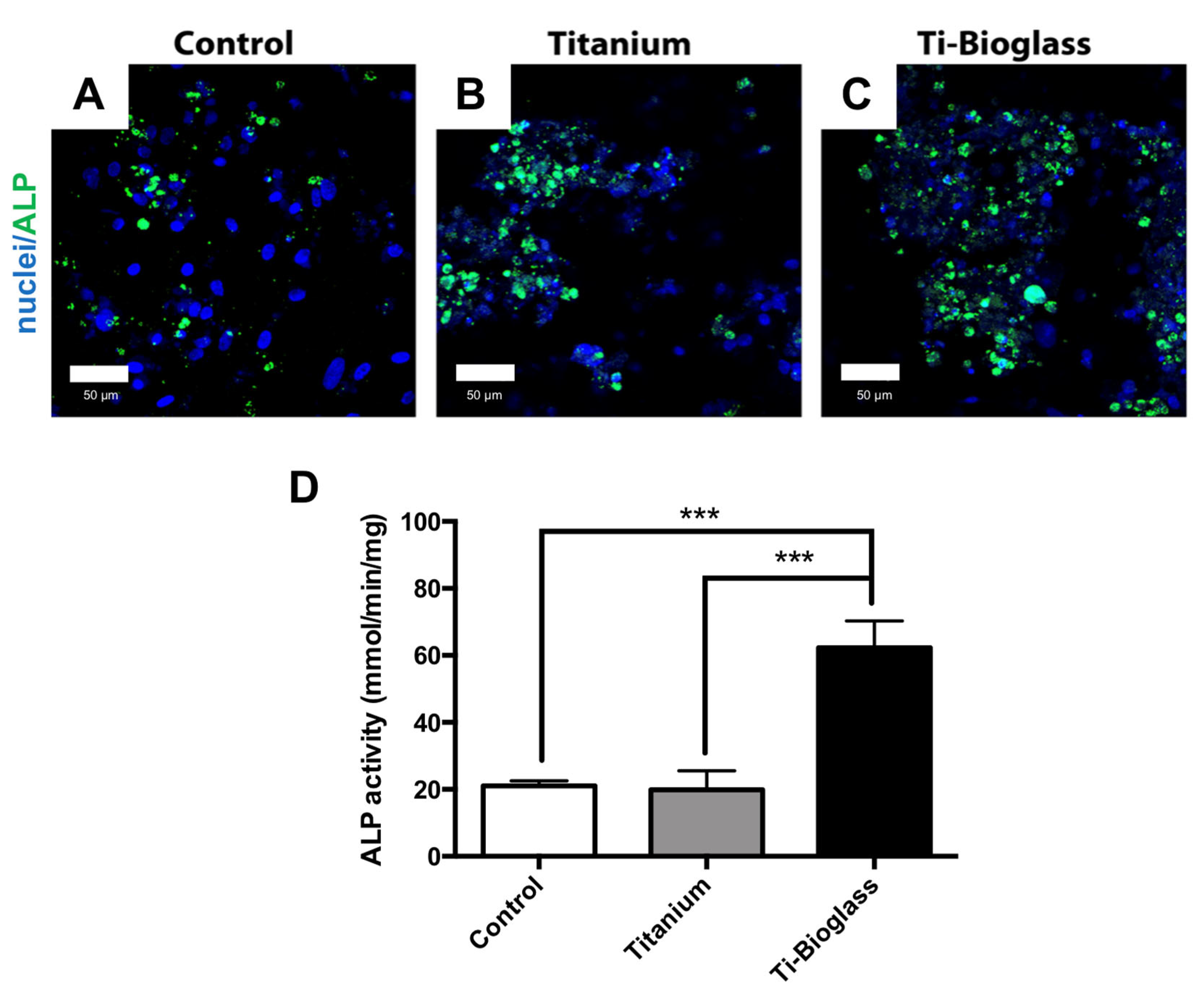
2.9. Alkaline Phosphatase (ALP) Assay
2.10. Calcium–Cresolphthalein Complexone Method
2.11. Gene Expression Analysis
2.12. Extraction of ECM Proteins and Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Statistical Analysis
3. Results
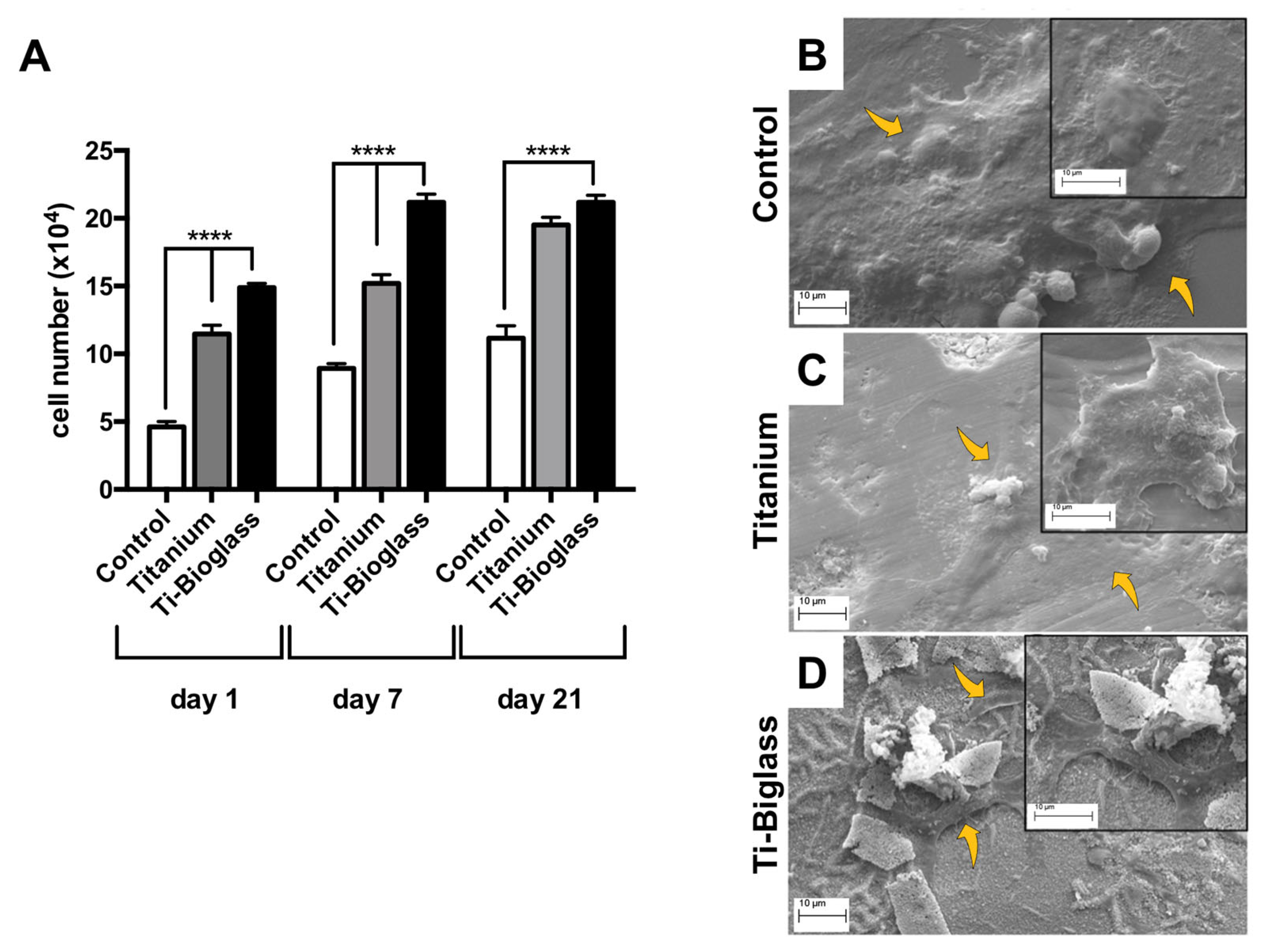
3.1. Chemical and Morphological Characterization of the Scaffolds
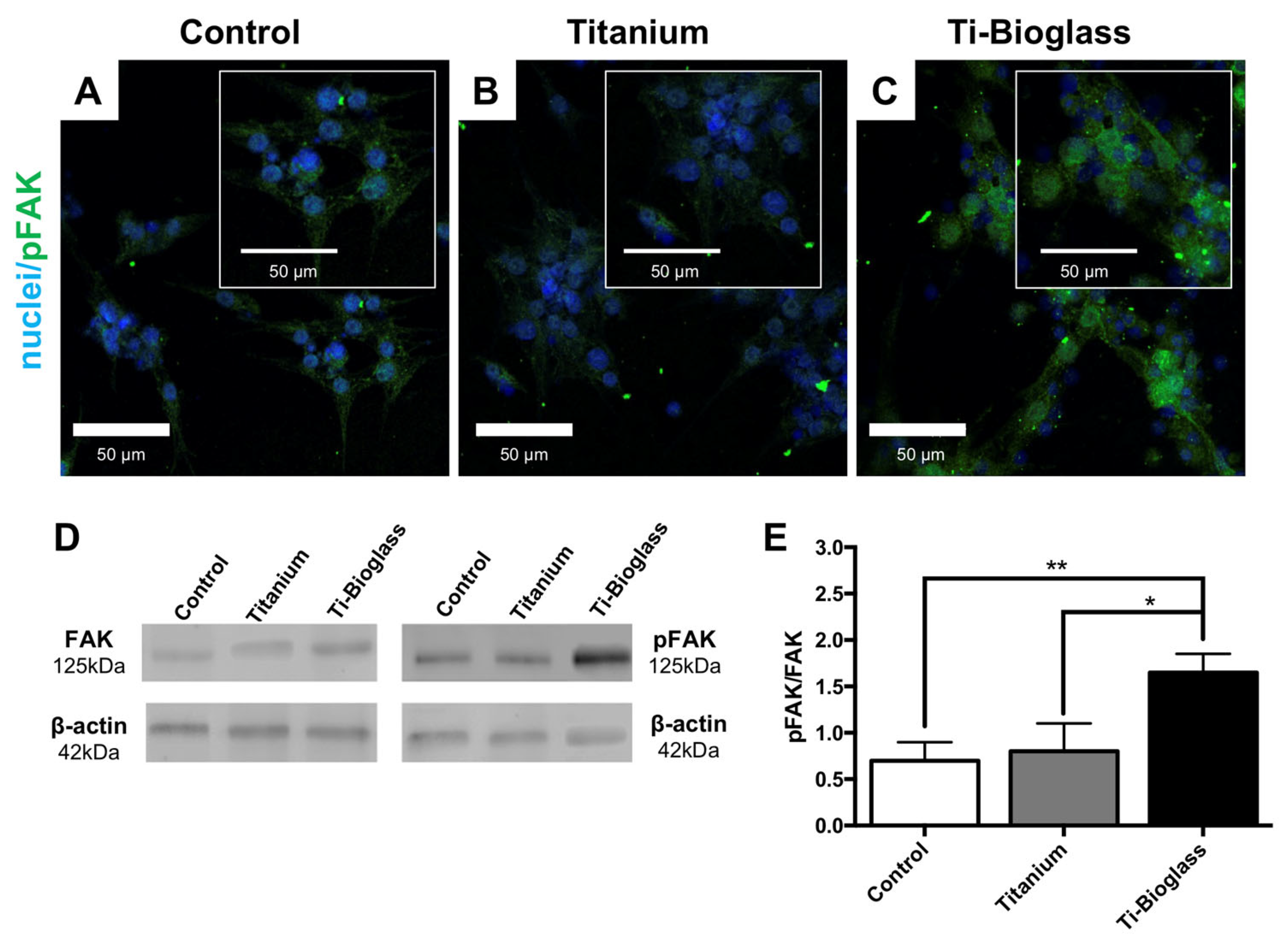
3.2. hFF-MSC Cell Adhesion and Viability
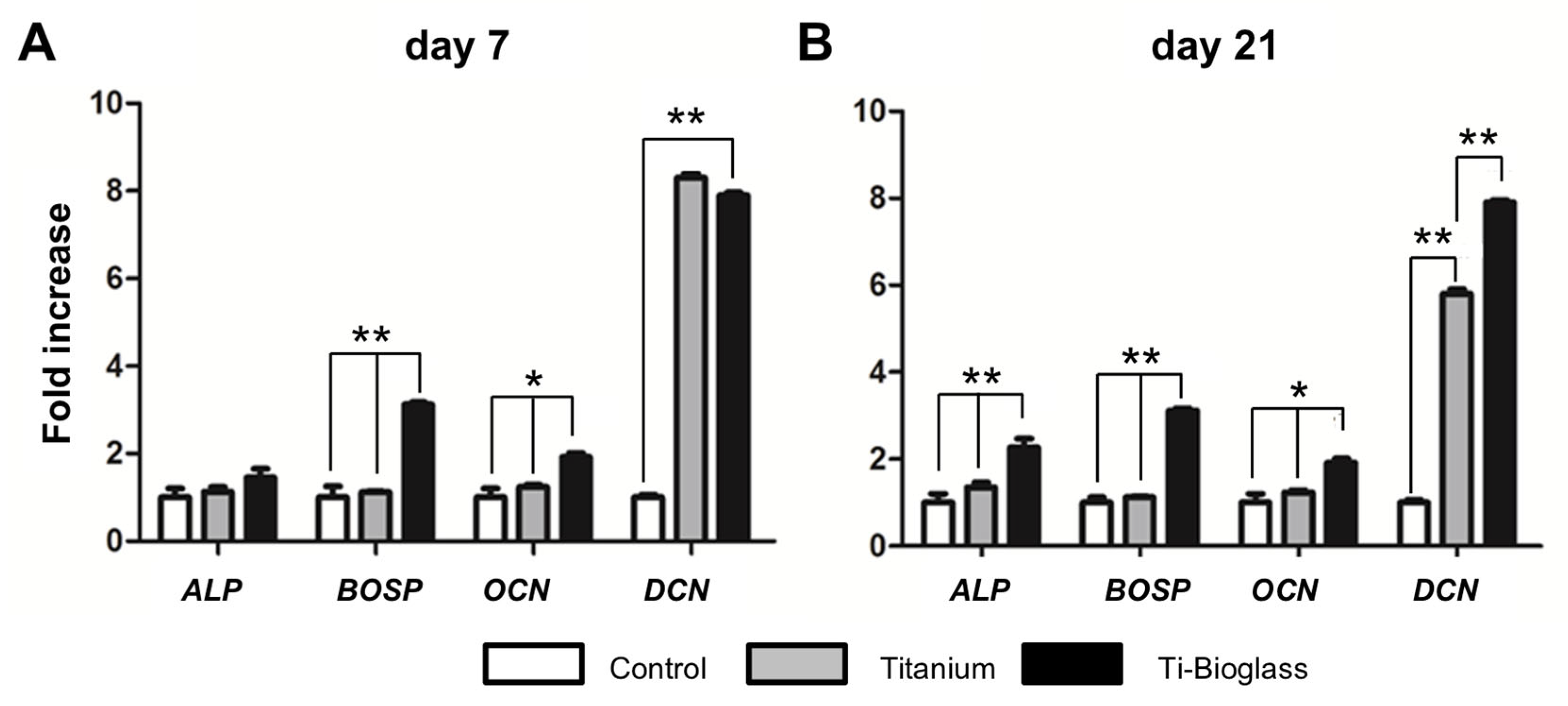
3.3. hFF-MSC Osteogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nosé, Y.; Okubo, H. Artificial Organs versus Regenerative Medicine: Is It True? Artif. Organs 2003, 27, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J. Bone Tissue Regeneration—Application of Mesenchymal Stem Cells and Cellular and Molecular Mechanisms. Curr. Stem Cell Res. Ther. 2017, 12, 357–364. [Google Scholar] [CrossRef]
- Moreira, C.A.; Dempster, D.W.; Baron, R. Anatomy and Ultrastructure of Bone—Histogenesis, Growth and Remodeling. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the Art and New Perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Visai, L.; Avanzini, M.A.; Cusella De Angelis, M.G.; Benazzo, F.; Van Vlierberghe, S.; Dubruel, P.; Magenes, G. Use of a Gelatin Cryogel as Biomaterial Scaffold in the Differentiation Process of Human Bone Marrow Stromal Cells. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 247–250. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Ashraf, R.; Beigh, M.A.; Sheikh, F.A. Scaffolds Fabricated from Natural Polymers/Composites by Electrospinning for Bone Tissue Regeneration. Adv. Exp. Med. Biol. 2018, 1078, 49–78. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A State-of-the-Art Review of the Fabrication and Characteristics of Titanium and Its Alloys for Biomedical Applications. Biodes. Manuf. 2022, 5, 371–395. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating Bone with Bioactive Glass Scaffolds: A Review of in Vivo Studies in Bone Defect Models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive Glasses Incorporating Less-Common Ions to Improve Biological and Physical Properties. J. Mater. Sci. Mater. Med. 2021, 33, 3. [Google Scholar] [CrossRef]
- Saino, E.; Grandi, S.; Quartarone, E.; Maliardi, V.; Galli, D.; Bloise, N.; Fassina, L.; De Angelis, M.G.C.; Mustarelli, P.; Imbriani, M.; et al. In Vitro Calcified Matrix Deposition by Human Osteoblasts onto a Zinc-Containing Bioactive Glass. Eur. Cell Mater. 2011, 21, 59–72. [Google Scholar] [CrossRef]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal Stem Cells: Cell Therapy and Regeneration Potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]
- Bukovsky, A.; Svetlikova, M.; Caudle, M.R. Oogenesis in Cultures Derived from Adult Human Ovaries. Reprod. Biol. Endocrinol. 2005, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Bukovsky, A. Ovarian Stem Cell Niche and Follicular Renewal in Mammals. Anat. Rec. 2011, 294, 1284–1306. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Omes, C.; Bassani, R.; Nappi, R.E.; Mazzini, G.; Icaro Cornaglia, A.; Casasco, A. In-Vitro Culture System for Mesenchymal Progenitor Cells Derived from Waste Human Ovarian Follicular Fluid. Reprod. Biomed. Online 2014, 29, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Rungsiwiwut, R.; Numchaisrika, P.; Thuwanut, P.; Pruksananonda, K. Characterization of Stem Cells from Human Ovarian Follicular Fluid; a Potential Source of Autologous Stem Cell for Cell-Based Therapy. Hum. Cell 2021, 34, 300–309. [Google Scholar] [CrossRef]
- Lai, D.; Xu, M.; Zhang, Q.; Chen, Y.; Li, T.; Wang, Q.; Gao, Y.; Wei, C. Identification and Characterization of Epithelial Cells Derived from Human Ovarian Follicular Fluid. Stem Cell Res. Ther. 2015, 6, 13. [Google Scholar] [CrossRef]
- Taheri, M.; Saki, G.; Nikbakht, R.; Eftekhari, A.R. Bone Morphogenetic Protein 15 Induces Differentiation of Mesenchymal Stem Cells Derived from Human Follicular Fluid to Oocyte-like Cell. Cell Biol. Int. 2021, 45, 127–139. [Google Scholar] [CrossRef]
- Vernet, D.; Nolazco, G.; Cantini, L.; Magee, T.R.; Qian, A.; Rajfer, J.; Gonzalez-Cadavid, N.F. Evidence That Osteogenic Progenitor Cells in the Human Tunica Albuginea May Originate from Stem Cells: Implications for Peyronie Disease. Biol. Reprod. 2005, 73, 1199–1210. [Google Scholar] [CrossRef]
- Omes, C.; Fassina, L.; Van Vlierberghe, S.; Magenes, G.; Dubruel, P.; Vaghi, P.; Reguzzoni, M.; Riva, F. A Case of Successful Interaction between Cells Derived from Human Ovarian Follicular Liquid and Gelatin Cryogel for Biotech and Medical Applications. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6240–6243. [Google Scholar] [CrossRef]
- Grandi, S.; Cassinelli, V.; Bini, M.; Saino, E.; Mustarelli, P.; Arciola, C.R.; Imbriani, M.; Visai, L. Bone Reconstruction: Au Nanocomposite Bioglasses with Antibacterial Properties. Int. J. Artif. Organs 2011, 34, 920–928. [Google Scholar] [CrossRef]
- Kossowska-Tomaszczuk, K.; De Geyter, C.; De Geyter, M.; Martin, I.; Holzgreve, W.; Scherberich, A.; Zhang, H. The Multipotency of Luteinizing Granulosa Cells Collected from Mature Ovarian Follicles. Stem Cells 2009, 27, 210–219. [Google Scholar] [CrossRef]
- Bloise, N.; Patrucco, A.; Bruni, G.; Montagna, G.; Caringella, R.; Fassina, L.; Tonin, C.; Visai, L. In Vitro Production of Calcified Bone Matrix onto Wool Keratin Scaffolds via Osteogenic Factors and Electromagnetic Stimulus. Materials 2020, 13, 3052. [Google Scholar] [CrossRef]
- Rath, S.N.; Strobel, L.A.; Arkudas, A.; Beier, J.P.; Maier, A.-K.; Greil, P.; Horch, R.E.; Kneser, U. Osteoinduction and Survival of Osteoblasts and Bone-Marrow Stromal Cells in 3D Biphasic Calcium Phosphate Scaffolds under Static and Dynamic Culture Conditions. J. Cell Mol. Med. 2012, 16, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, M.; Ceccarelli, G.; Cristofaro, F.; Balli, M.; Bertoglio, F.; Bruni, G.; Benedetti, L.; Avanzini, M.A.; Imbriani, M.; Visai, L. Nanostructured TiO₂ Surfaces Promote Human Bone Marrow Mesenchymal Stem Cells Differentiation to Osteoblasts. Nanomaterials 2016, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Kossowska-Tomaszczuk, K.; Pelczar, P.; Güven, S.; Kowalski, J.; Volpi, E.; De Geyter, C.; Scherberich, A. A Novel Three-Dimensional Culture System Allows Prolonged Culture of Functional Human Granulosa Cells and Mimics the Ovarian Environment. Tissue Eng. Part A 2010, 16, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-H.; Jin, X.; Shankar, P.; Lee, J.H.; Jo, K.; Lim, K.-I. Molecular-Level Interactions between Engineered Materials and Cells. Int. J. Mol. Sci. 2019, 20, 4142. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Versaevel, M.; Bruyère, C.; Alaimo, L.; Luciano, M.; Vercruysse, E.; Procès, A.; Gabriele, S. Innovative Tools for Mechanobiology: Unraveling Outside-In and Inside-Out Mechanotransduction. Front. Bioeng. Biotechnol. 2019, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Jell, G.; Stevens, M.M. Gene Activation by Bioactive Glasses. J. Mater. Sci. Mater. Med. 2006, 17, 997–1002. [Google Scholar] [CrossRef]
- Saino, E.; Maliardi, V.; Quartarone, E.; Fassina, L.; Benedetti, L.; De Angelis, M.G.C.; Mustarelli, P.; Facchini, A.; Visai, L. In Vitro Enhancement of SAOS-2 Cell Calcified Matrix Deposition onto Radio Frequency Magnetron Sputtered Bioglass-Coated Titanium Scaffolds. Tissue Eng. Part A 2010, 16, 995–1008. [Google Scholar] [CrossRef]
- Ojansivu, M.; Hyväri, L.; Kellomäki, M.; Hupa, L.; Vanhatupa, S.; Miettinen, S. Bioactive Glass Induced Osteogenic Differentiation of Human Adipose Stem Cells Is Dependent on Cell Attachment Mechanism and Mitogen-Activated Protein Kinases. Eur. Cell Mater. 2018, 35, 54–72. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Y.; Dai, K.; Chen, X.; Read, H.M.; Zeng, L.; Hang, F. Three Dimensional Printed Bioglass/Gelatin/Alginate Composite Scaffolds with Promoted Mechanical Strength, Biomineralization, Cell Responses and Osteogenesis. J. Mater. Sci. Mater. Med. 2020, 31, 77. [Google Scholar] [CrossRef]
- Chandramohan, Y.; Jeganathan, K.; Sivanesan, S.; Koka, P.; Amritha, T.M.S.; Vimalraj, S.; Dhanasekaran, A. Assessment of Human Ovarian Follicular Fluid Derived Mesenchymal Stem Cells in Chitosan/PCL/Zn Scaffold for Bone Tissue Regeneration. Life Sci. 2021, 264, 118502. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-Regulated FAK-Src Signaling in Normal and Cancer Cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of Dexamethasone, Ascorbic Acid and β-Glycerophosphate on the Osteogenic Differentiation of Stem Cells in Vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Bloise, N.; Petecchia, L.; Ceccarelli, G.; Fassina, L.; Usai, C.; Bertoglio, F.; Balli, M.; Vassalli, M.; Cusella De Angelis, M.G.; Gavazzo, P.; et al. The Effect of Pulsed Electromagnetic Field Exposure on Osteoinduction of Human Mesenchymal Stem Cells Cultured on Nano-TiO2 Surfaces. PLoS ONE 2018, 13, e0199046. [Google Scholar] [CrossRef] [PubMed]
- Cristofaro, F.; Gigli, M.; Bloise, N.; Chen, H.; Bruni, G.; Munari, A.; Moroni, L.; Lotti, N.; Visai, L. Influence of the Nanofiber Chemistry and Orientation of Biodegradable Poly(Butylene Succinate)-Based Scaffolds on Osteoblast Differentiation for Bone Tissue Regeneration. Nanoscale 2018, 10, 8689–8703. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Choi, J.H.; Jun, Y.; Lim, S.M.; Park, S.; Paek, J.-Y.; Lee, S.-H.; Hwang, J.-Y.; Kim, G.J. 3D-Cultured Human Placenta-Derived Mesenchymal Stem Cell Spheroids Enhance Ovary Function by Inducing Folliculogenesis. Sci. Rep. 2018, 8, 15313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Z.; Wang, Y.; Wu, Z.; Yu, B. Dynamic Expression Profiles of Marker Genes in Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Chin. Med. Sci. J. 2015, 30, 108–113. [Google Scholar] [CrossRef]
- Gong, X.; Li, H.; Zhao, Y. The Improvement and Clinical Application of Human Oocyte In Vitro Maturation (IVM). Reprod. Sci. 2022, 29, 2127–2135. [Google Scholar] [CrossRef]
- Telfer, E.E.; Andersen, C.Y. In Vitro Growth and Maturation of Primordial Follicles and Immature Oocytes. Fertil. Steril. 2021, 115, 1116–1125. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-Secreted Factors: Regulators of Cumulus Cell Function and Oocyte Quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Sittadjody, S.; Enck, K.M.; Wells, A.; Yoo, J.J.; Atala, A.; Saul, J.M.; Opara, E.C. Encapsulation of Mesenchymal Stem Cells in 3D Ovarian Cell Constructs Promotes Stable and Long-Term Hormone Secretion with Improved Physiological Outcomes in a Syngeneic Rat Model. Ann. Biomed. Eng. 2020, 48, 1058–1070. [Google Scholar] [CrossRef]
- Kalehoei, E.; Moradi, M.; Azadbakht, M.; Zhaleh, H.; Parvini, M.; Cheraghbaeigi, S.; Saghari, S. In Vitro Maturation Medium Supplementation: Utilization of Repaglinide, L-Carnitine, and Mesenchymal Stem Cell-Conditioned Medium to Improve Developmental Competence of Oocytes Derived from Endometriosis Mouse Models. Braz. J. Med. Biol. Res. 2022, 55, e11948. [Google Scholar] [CrossRef] [PubMed]
- Toosi, S.; Naderi-Meshkin, H.; Esmailzadeh, Z.; Behravan, G.; Ramakrishna, S.; Behravan, J. Bioactive Glass-Collagen/Poly (Glycolic Acid) Scaffold Nanoparticles Exhibit Improved Biological Properties and Enhance Osteogenic Lineage Differentiation of Mesenchymal Stem Cells. Front. Bioeng. Biotechnol. 2022, 10, 963996. [Google Scholar] [CrossRef] [PubMed]
- Malekahmadi, B.; Esfahanian, V.; Ejeian, F.; Dastgurdi, M.E.; Agheb, M.; Kaveian, F.; Rafienia, M.; Nasr-Esfahani, M.H. In Vitro Study of the Recruitment and Expansion of Mesenchymal Stem Cells at the Interface of a Cu-Doped PCL-Bioglass Scaffold. Biomimetics 2022, 7, 19. [Google Scholar] [CrossRef] [PubMed]


| Control (pg/Cell) | Titanium (pg/Cell) | Ratio Related to Control | Ti-Bioglass (pg/Cell) | Ratio Related to Control | Ratio Ti-Bioglass/ Titanium | |
|---|---|---|---|---|---|---|
| ALP | 20.60 ± 3.28 | 29.82 ± 1.88 | 1.45 * | 45.70 ± 19.97 | 2.22 *** | 1.53 |
| FN | 1.25 ± 0.19 | 2.42 ± 1.00 | 1.93 ** | 2.96 ± 0.11 | 2.36 *** | 1.22 |
| OCN | 3.19 ± 0.02 | 8.18 ± 0.08 | 2.56 ** | 13.40 ± 1.14 | 4.20 *** | 1.63 ** |
| ON | 2.15 ± 001 | 4.30 ± 0.11 | 2.00 ** | 6.40 ± 0.08 | 2.98 *** | 1.49 **** |
| OPN | 36.95 ± 2.64 | 47.71 ± 1.37 | 1.29 | 71.81 ± 3.02 | 1.95 ** | 1.50 *** |
| COL-I | 100.40 ± 1.46 | 155.07 ± 1.24 | 1.54 * | 242.13 ± 27.35 | 2.41 *** | 1.56 ** |
| COL-III | 84.50 ± 3.36 | 113.74 ± 3.79 | 1.35 * | 175.31 ± 15.14 | 2.07 *** | 1.54 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, F.; Bloise, N.; Omes, C.; Ceccarelli, G.; Fassina, L.; Nappi, R.E.; Visai, L. Human Ovarian Follicular Fluid Mesenchymal Stem Cells Express Osteogenic Markers When Cultured on Bioglass 58S-Coated Titanium Scaffolds. Materials 2023, 16, 3676. https://doi.org/10.3390/ma16103676
Riva F, Bloise N, Omes C, Ceccarelli G, Fassina L, Nappi RE, Visai L. Human Ovarian Follicular Fluid Mesenchymal Stem Cells Express Osteogenic Markers When Cultured on Bioglass 58S-Coated Titanium Scaffolds. Materials. 2023; 16(10):3676. https://doi.org/10.3390/ma16103676
Chicago/Turabian StyleRiva, Federica, Nora Bloise, Claudia Omes, Gabriele Ceccarelli, Lorenzo Fassina, Rossella Elena Nappi, and Livia Visai. 2023. "Human Ovarian Follicular Fluid Mesenchymal Stem Cells Express Osteogenic Markers When Cultured on Bioglass 58S-Coated Titanium Scaffolds" Materials 16, no. 10: 3676. https://doi.org/10.3390/ma16103676
APA StyleRiva, F., Bloise, N., Omes, C., Ceccarelli, G., Fassina, L., Nappi, R. E., & Visai, L. (2023). Human Ovarian Follicular Fluid Mesenchymal Stem Cells Express Osteogenic Markers When Cultured on Bioglass 58S-Coated Titanium Scaffolds. Materials, 16(10), 3676. https://doi.org/10.3390/ma16103676