The Synergistic Effects of Ultrafine Slag Powder and Limestone on the Rheology Behavior, Microstructure, and Fractal Features of Ultra-High Performance Concrete (UHPC)
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Test Method
3. Result and Discussion
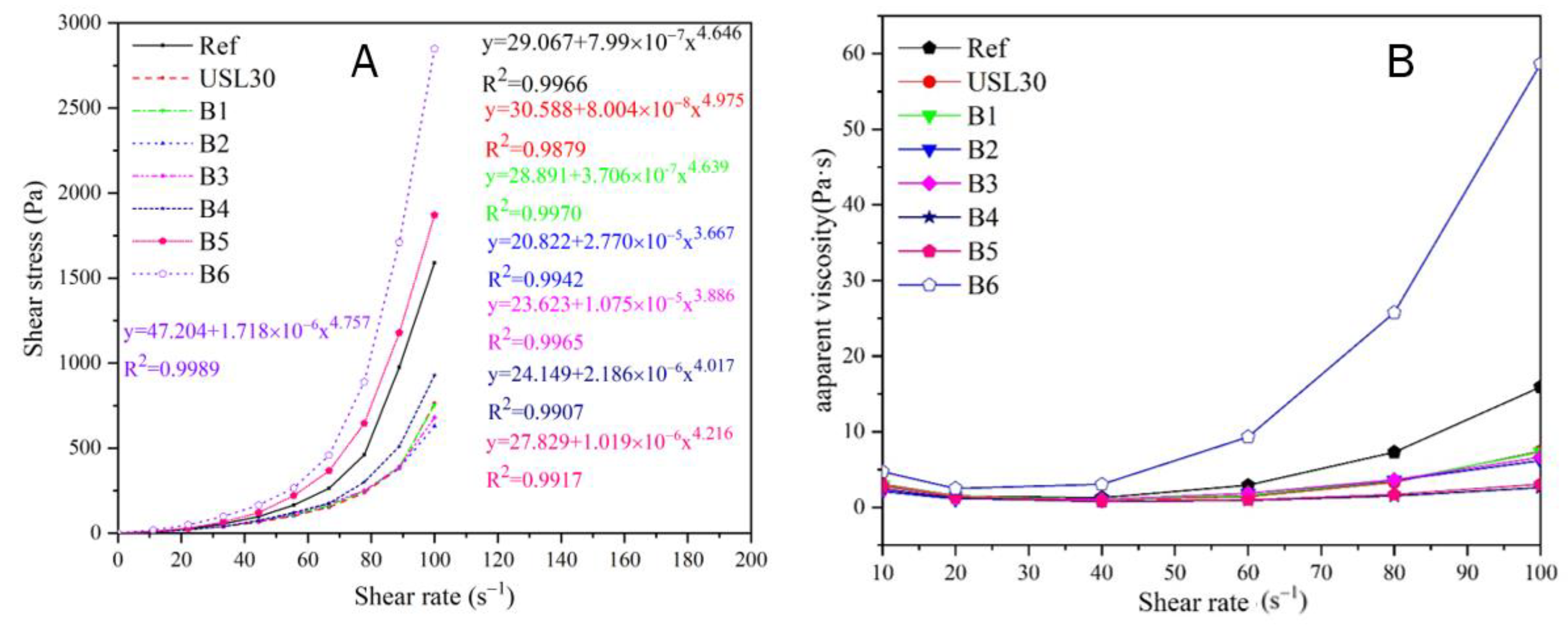
3.1. Rheological Properties
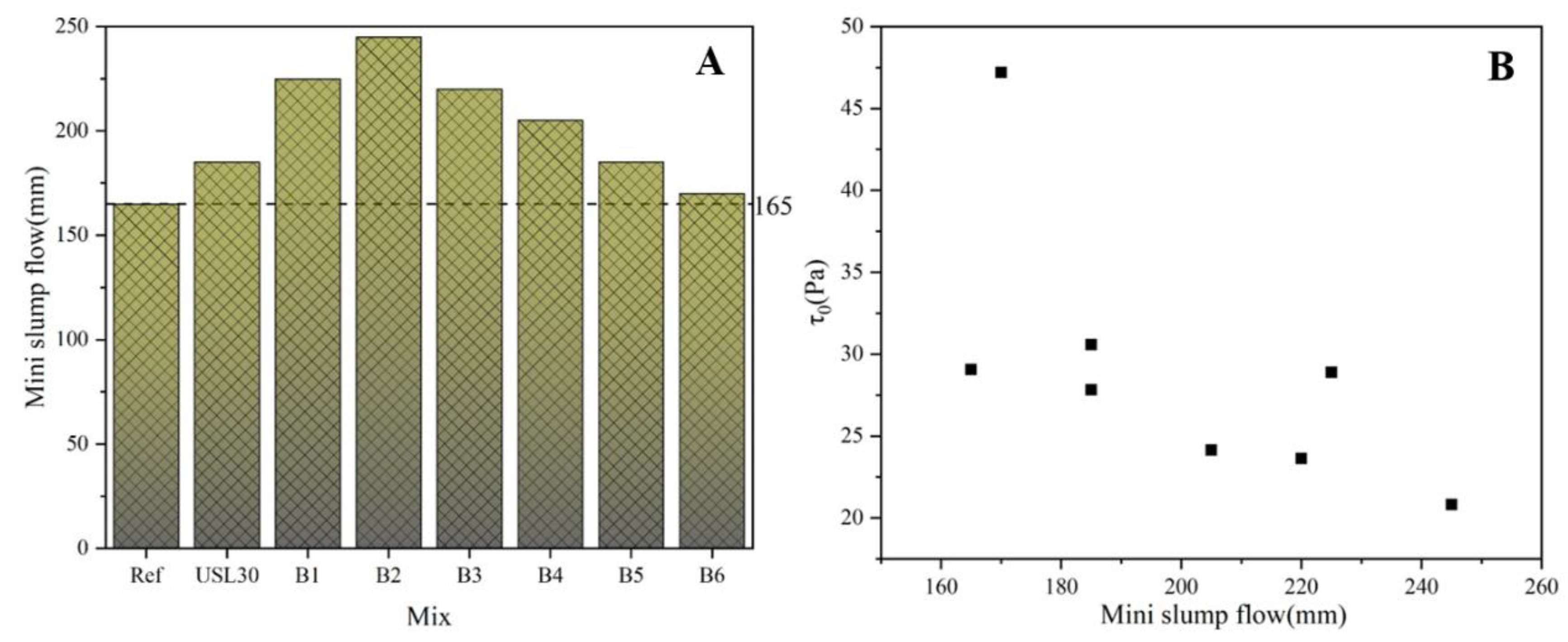
3.2. Flowability
3.3. Compressive Strength
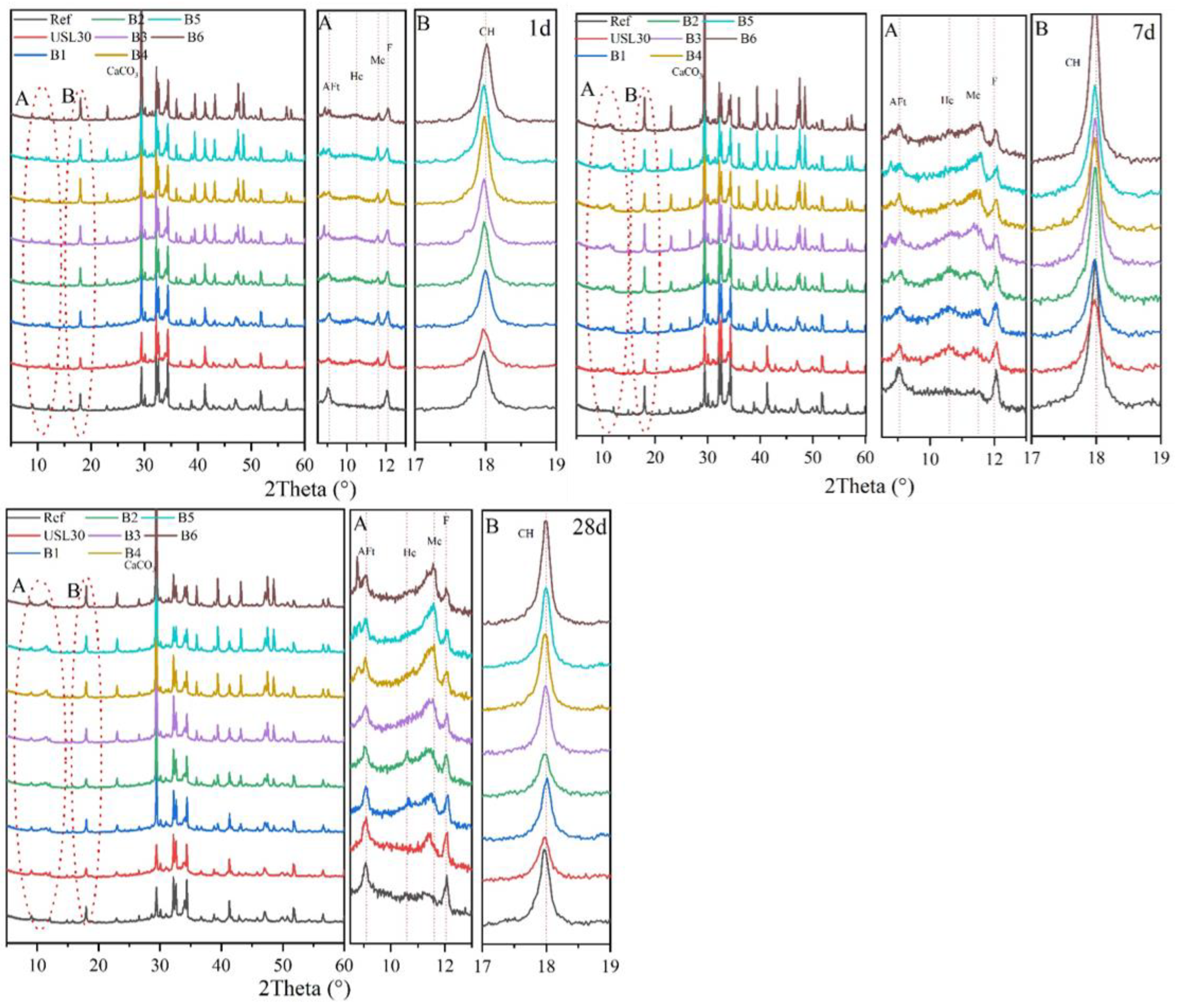
3.4. XRD Patterns
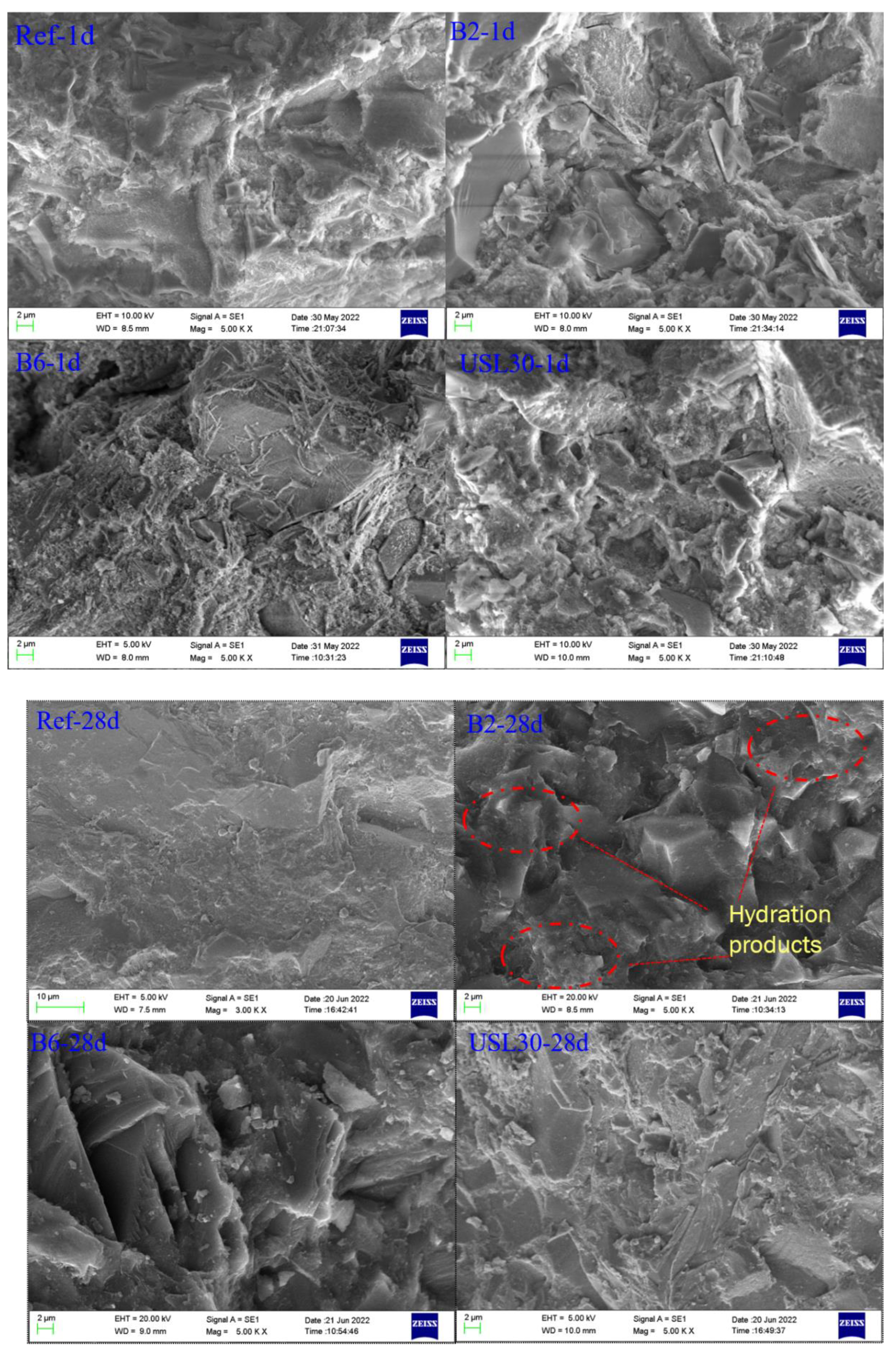
3.5. Hydration Products
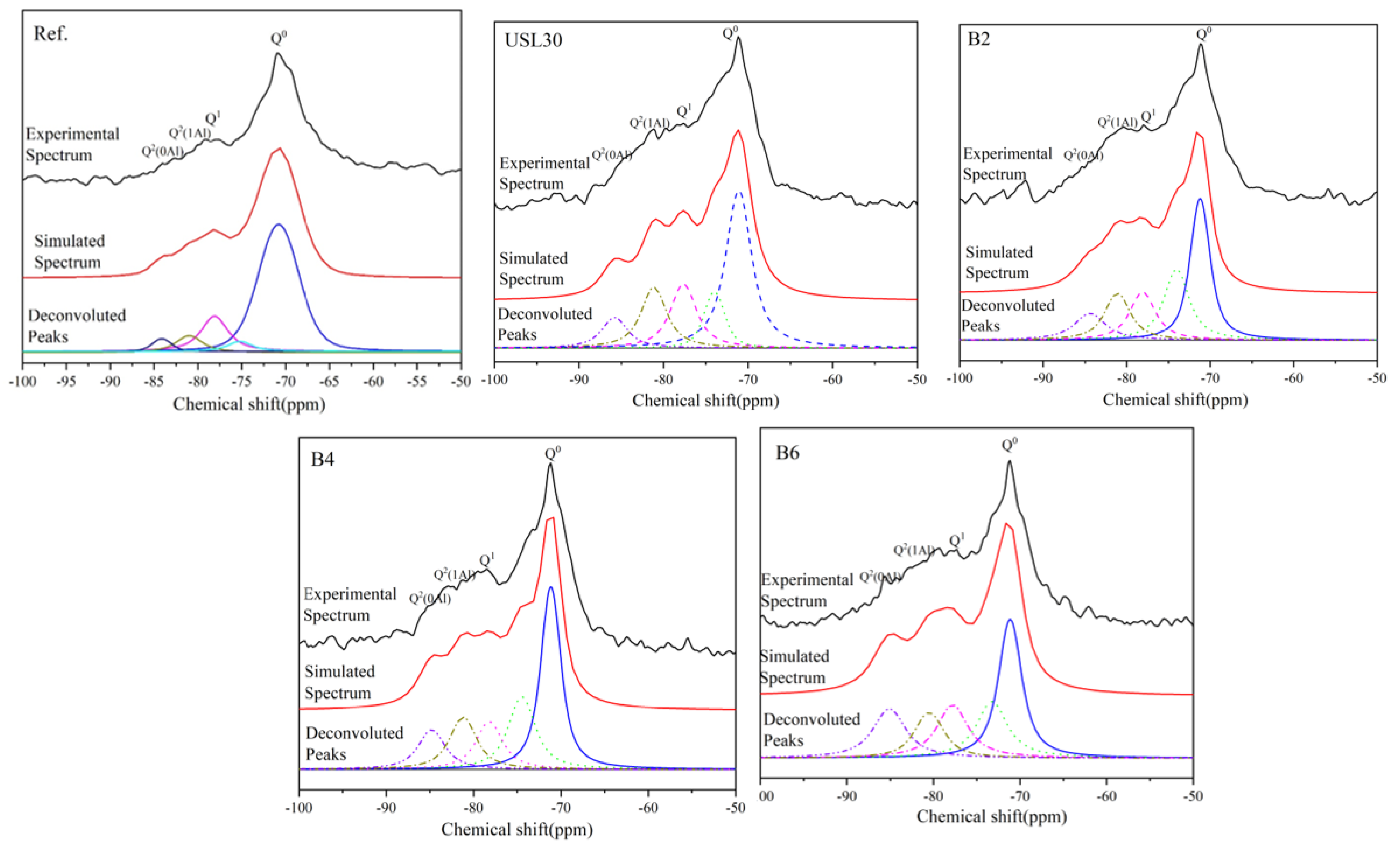
3.6. Structure of the C–S–H
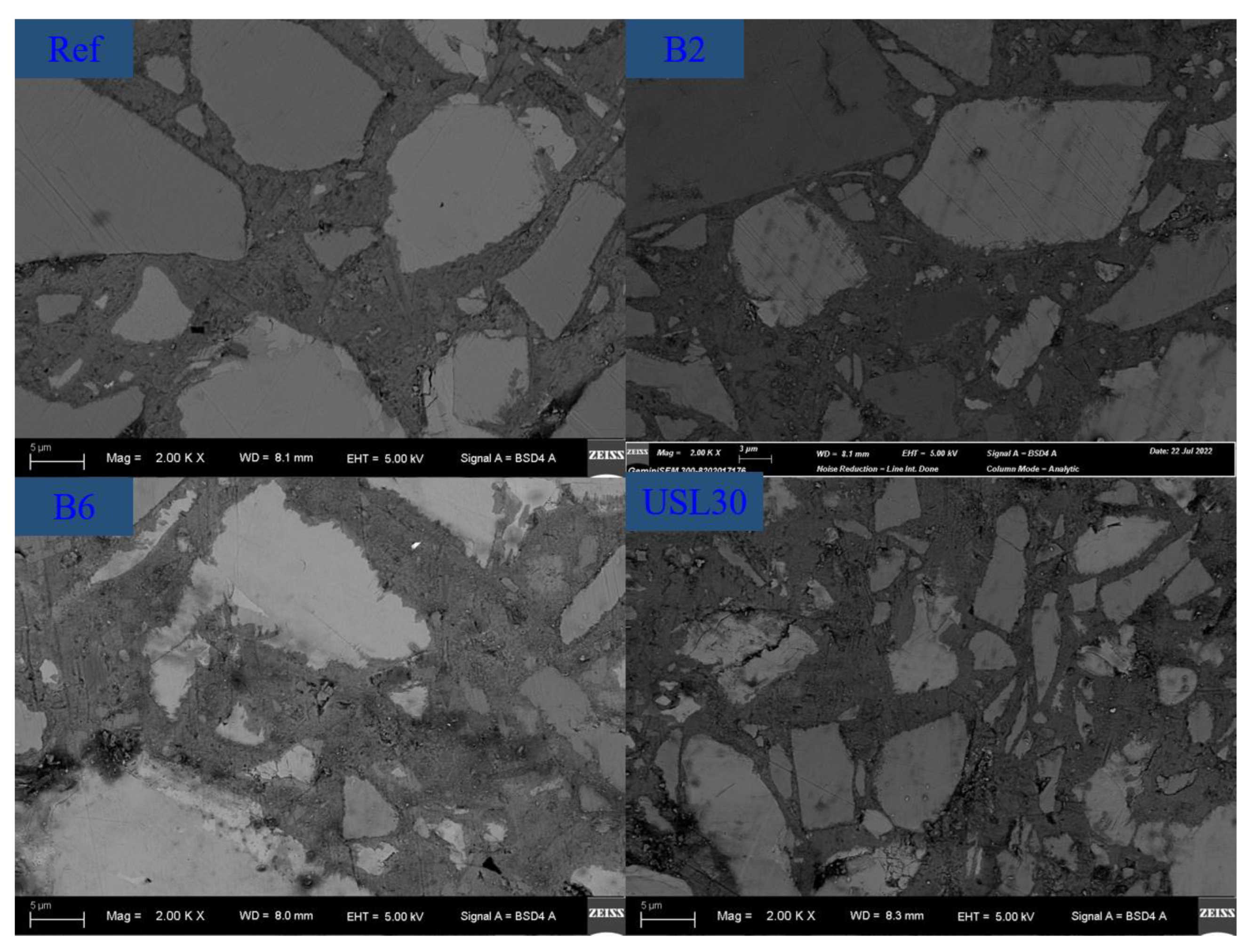
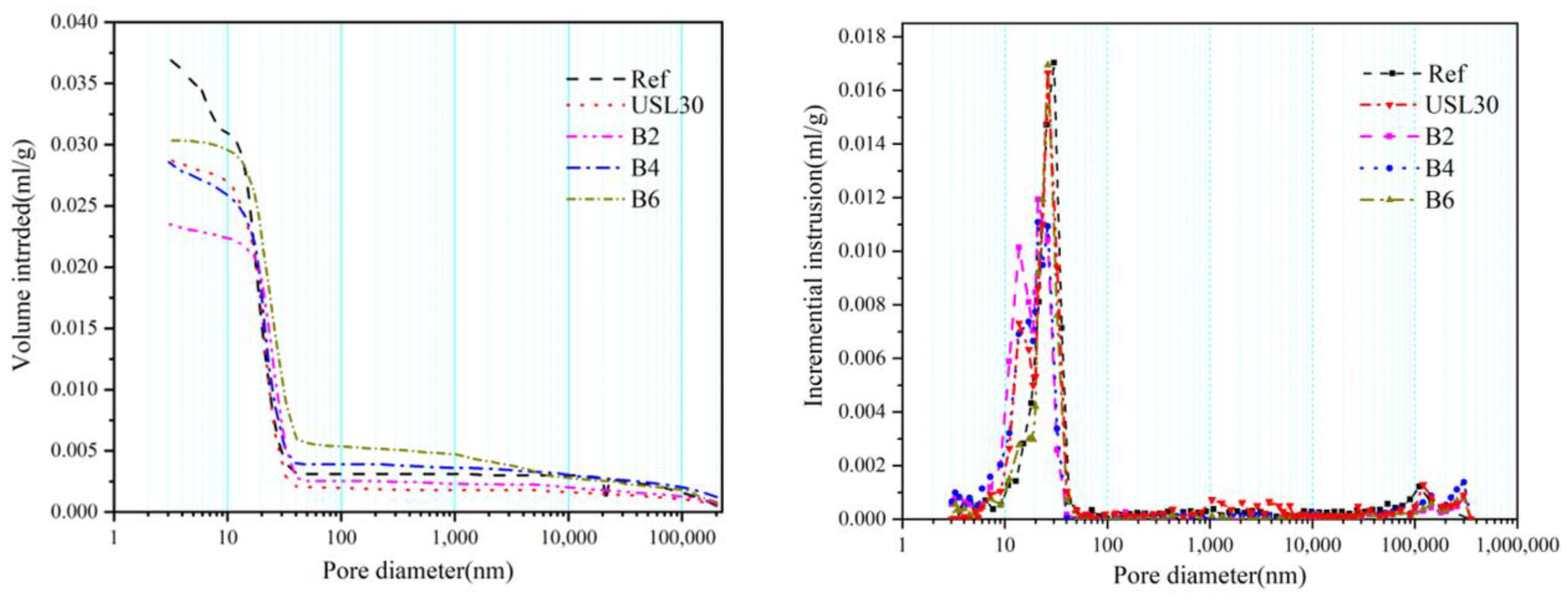
3.7. Pore Structure
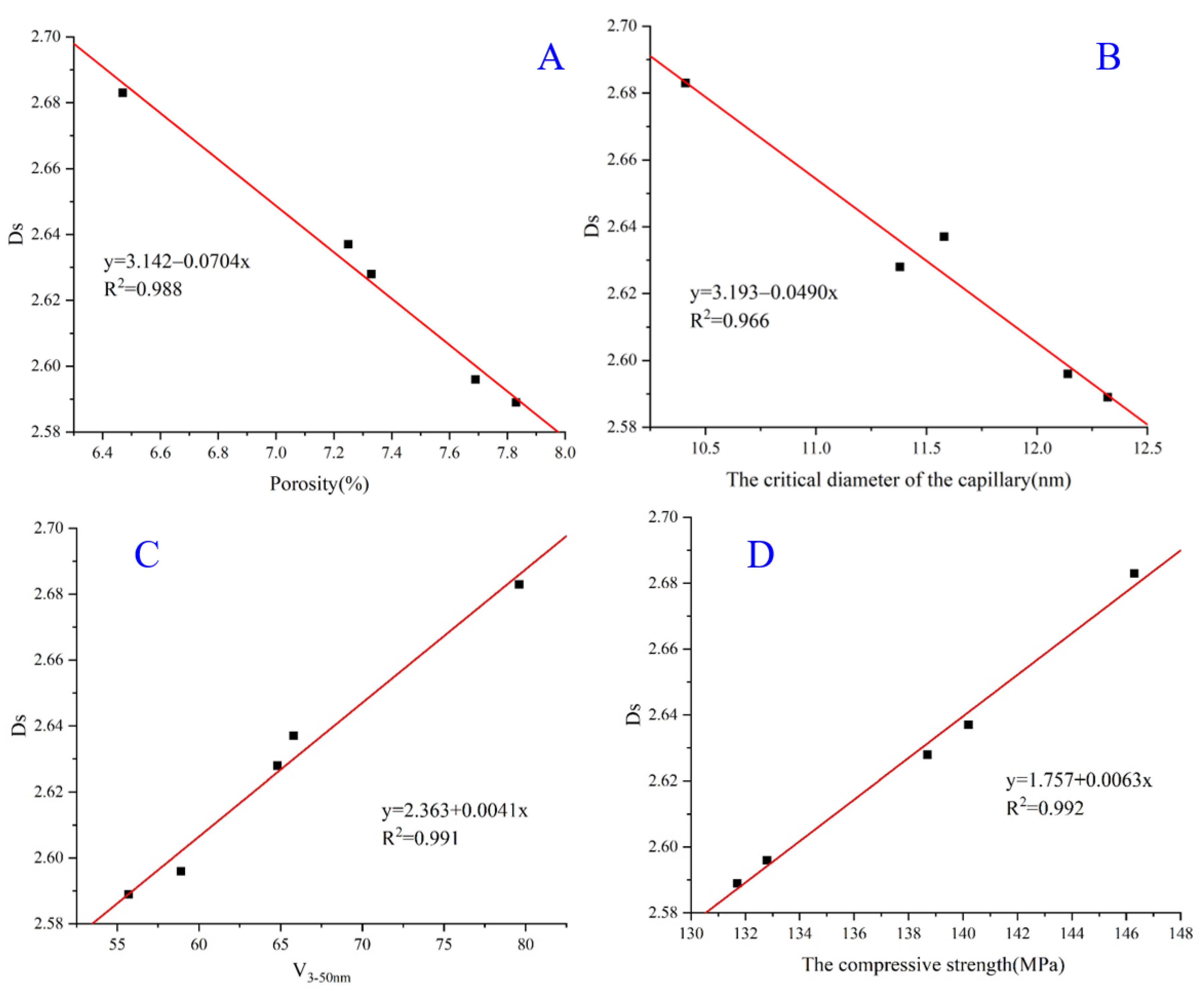
3.8. Pore Surface Fractal Dimension (Ds)
4. Conclusions
- The synergistic effect between the USL and LS in the UHPC resulted in a decrease in the yield stress and fluid flow index, and an increase in the workability and compressive strength. Among the different USL to LS mass ratios tested, B2 with a ratio of 2:1 obtained the highest compressive strength, maximum slump flowability, and lowest yield stress.
- The synergistic interaction between the USL and LS improved the homogeneity of the paste, promoted cement hydration, facilitated the formation of the ettringite and monosulfate phases, and increased the chain length of the calcium-silicate-hydrate (C–S–H) gel. These effects refined the pore structure and led to a denser microstructure in the UHPC.
- The fractal dimension (Ds) of the ultra-high performance concrete (UHPC) was found to be strongly correlated with its pore structure and compressive strength, suggesting that the fractal theory can be used to better understand the synergistic effect between the USL and LS on the properties of the UHPC. Therefore, the Ds value may be used as an indicator to assess the synergistic effect of the USL and LS in the UHPC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| USL | ultrafine slag powder |
| LS | limestone |
| UHPC | ultra-high performance concrete |
| Mc | monocarboaluminate |
| Ds | fractal dimension |
| CH | portlandite |
| SCM | supplementary cementitious material |
| Hc | hemi-carboaluminate |
| MK | metakaolin |
| XRD | X-ray diffraction |
| MIP | mercury intrusion porosimeter |
| SEM | scanning electron microscope |
| C–S–H | calcium silicate hydrate |
| MCL | the mean chain length |
| SP | superplasticizer |
| 29Si NMR | 29Si NMR nuclear magnetic resonance |
| AFt | ettringite |
| CDC | the critical diameter of the capillary |
References
- Shah, H.A.; Yuan, Q.; Photwichai, N. Use of materials to lower the cost of ultra-high-performance concrete—A review. Constr. Build. Mater. 2022, 327, 127045. [Google Scholar] [CrossRef]
- Tang, J.; Wei, S.; Li, W.; Ma, S.; Ji, P.; Shen, X. Synergistic effect of metakaolin and limestone on the hydration properties of Portland cement. Constr. Build. Mater. 2019, 223, 177–184. [Google Scholar] [CrossRef]
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H.; Ou, Z. A review on use of limestone powder in cement-based materials: Mechanism, hydration and microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- Shirdam, R.; Amini, M.; Bakhshi, N. Investigating the Effects of Copper Slag and Silica Fume on Durability, Strength, and Workability of Concrete. Int. J. Environ. Res. 2019, 13, 909–924. [Google Scholar] [CrossRef]
- Joudi-Bahri, I.; Lecomte, A.; Ouezdou, M.B.; Achour, T. Use of limestone sands and fillers in concrete without superplasticizer. Cem. Concr. Compos. 2012, 34, 771–780. [Google Scholar] [CrossRef]
- Yaşar, E.; Erdoğan, Y.; Kılıç, A. Effect of limestone aggregate type and water–cement ratio on concrete strength. Mater. Lett. 2004, 58, 772–777. [Google Scholar] [CrossRef]
- Arora, A.; Sant, G.; Neithalath, N. Ternary blends containing slag and interground/blended limestone: Hydration, strength, and pore structure. Constr. Build. Mater. 2016, 102, 113–124. [Google Scholar] [CrossRef]
- Aquino, C.; Inoue, M.; Miura, H.; Mizuta, M.; Okamoto, T. The effects of limestone aggregate on concrete properties. Constr. Build. Mater. 2010, 24, 2363–2368. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Zhou, M. Effect of limestone fines content in manufactured sand on durability of low- and high-strength concretes. Constr. Build. Mater. 2009, 23, 2846–2850. [Google Scholar] [CrossRef]
- Menadi, B.; Kenai, S.; Khatib, J.; Aït-Mokhtar, A. Strength and durability of concrete incorporating crushed limestone sand. Constr. Build. Mater. 2009, 23, 625–633. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, T.-C.; Wang, M.; Wu, Y.-Y. Synergic performance of low-kaolinite calcined coal gangue blended with limestone in cement mortars. Constr. Build. Mater. 2021, 300, 124012. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Zajac, M.; Stabler, C.; Lothenbach, B.; Black, L. Influence of limestone on the hydration of ternary slag cements. Cem. Concr. Res. 2017, 100, 96–109. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Song, J. Study on the grinding kinetics of copper tailing powder. Powder Technol. 2018, 330, 105–113. [Google Scholar] [CrossRef]
- Teng, S.; Lim, T.Y.D.; Sabet Divsholi, B. Durability and mechanical properties of high strength concrete incorporating ultra fine Ground Granulated Blast-furnace Slag. Constr. Build. Mater. 2013, 40, 875–881. [Google Scholar] [CrossRef]
- Luan, C.; Wang, J.; Gao, J.; Wang, J.; Du, P.; Zhou, Z.; Huang, Y.; Du, S. Changes in fractal dimension and durability of ultra-high performance concrete (UHPC) with silica fume content. Arch. Civ. Mech. Eng. 2022, 22, 123. [Google Scholar] [CrossRef]
- Lü, Q.; Qiu, Q.; Zheng, J.; Wang, J.; Zeng, Q. Fractal dimension of concrete incorporating silica fume and its correlations to pore structure, strength and permeability. Constr. Build. Mater. 2019, 228, 116986. [Google Scholar] [CrossRef]
- Wang, L.E.I.; Luo, R.; Zhang, W.E.I.; Jin, M.; Tang, S. Effects of Fineness and Content of Phosphorus Slag on Cement Hydration, Permeability, Pore Structure and Fractal Dimension of Concrete. Fractals 2021, 29, 2140004. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Wu, Y.; Zhou, Y.; Tang, S. Hydration, shrinkage, pore structure and fractal dimension of silica fume modified low heat Portland cement-based materials. Constr. Build. Mater. 2021, 272, 121952. [Google Scholar] [CrossRef]
- Tang, S.; Huang, J.; Duan, L.; Yu, P.; Chen, E. A review on fractal footprint of cement-based materials. Powder Technol. 2020, 370, 237–250. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Huang, M.; Yin, H.; Jiang, K.; Xiao, K.; Tang, S. Influence of Different Alkali Sulfates on the Shrinkage, Hydration, Pore Structure, Fractal Dimension and Microstructure of Low-Heat Portland Cement, Medium-Heat Portland Cement and Ordinary Portland Cement. Fractal Fract. 2021, 5, 79. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, J.; Han, S. Fractal analysis of relation between strength and pore structure of hardened mortar. Constr. Build. Mater. 2017, 135, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Yang, H.; Mu, R.; Chen, H. Rheological Properties of Nanosilica-Modified Cement Paste at Different Temperatures and Hydration Times. J. Mater. Civ. Eng. 2021, 33. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J. Fractal characteristics of pore structure of hardened cement paste prepared by pressurized compact molding. Constr. Build. Mater. 2020, 259, 119856. [Google Scholar] [CrossRef]
- Zeng, Q.; Luo, M.; Pang, X.; Li, L.; Li, K. Surface fractal dimension: An indicator to characterize the microstructure of cement-based porous materials. Appl. Surf. Sci. 2013, 282, 302–307. [Google Scholar] [CrossRef]
- Chen, M.; Yang, L.; Zheng, Y.; Huang, Y.; Li, L.; Zhao, P.; Wang, S.; Lu, L.; Cheng, X. Yield stress and thixotropy control of 3D-printed calcium sulfoaluminate cement composites with metakaolin related to structural build-up. Constr. Build. Mater. 2020, 252, 119090. [Google Scholar] [CrossRef]
- Peng, J.; Deng, D.; Liu, Z.; Yuan, Q.; Ye, T. Rheological models for fresh cement asphalt paste. Constr. Build. Mater. 2014, 71, 254–262. [Google Scholar] [CrossRef]
- Jiao, D.; Shi, C.; Yuan, Q. Time-dependent rheological behavior of cementitious paste under continuous shear mixing. Constr. Build. Mater. 2019, 226, 591–600. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, W.; Li, Z.; Liu, J.; Ran, Q.; Li, X.; Tang, J.; Shu, X. An approach to describe the shear-thickening viscosity of cement paste incorporating microfines of manufactured sand. Constr. Build. Mater. 2022, 340, 127743. [Google Scholar] [CrossRef]
- Rojo-López, G.; González-Fonteboa, B.; Martínez-Abella, F.; González-Taboada, I. Rheology, durability, and mechanical performance of sustainable self-compacting concrete with metakaolin and limestone filler. Case Stud. Constr. Mater. 2022, 17, e01143. [Google Scholar] [CrossRef]
- Benaicha, M.; Belcaid, A.; Alaoui, A.H.; Jalbaud, O.; Burtschell, Y. Effects of limestone filler and silica fume on rheology and strength of self-compacting concrete. Struct. Concr. 2019, 20, 1702–1709. [Google Scholar] [CrossRef]
- Agostinho, L.B.; Alexandre, d.C.P.; da Silva, E.F.; Toledo Filho, R.D. Rheological study of Portland cement pastes modified with superabsorbent polymer and nanosilica. J. Build. Eng. 2021, 34, 102024. [Google Scholar] [CrossRef]
- Benjeddou, O.; Soussi, C.; Jedidi, M.; Benali, M. Experimental and theoretical study of the effect of the particle size of limestone fillers on the rheology of self-compacting concrete. J. Build. Eng. 2017, 10, 32–41. [Google Scholar] [CrossRef]
- Khayat, K.H.; Meng, W.; Vallurupalli, K.; Teng, L. Rheological properties of ultra-high-performance concrete—An overview. Cem. Concr. Res. 2019, 124, 105828. [Google Scholar] [CrossRef]
- Roussel, N.; Spangenberg, J.; Wallevik, J.; Wolfs, R. Numerical simulations of concrete processing: From standard formative casting to additive manufacturing. Cem. Concr. Res. 2020, 135, 106075. [Google Scholar] [CrossRef]
- Li, W.G.; Huang, Z.Y.; Zu, T.Y.; Shi, C.J.; Duan, W.H.; Shah, S.P. Influence of Nanolimestone on the Hydration, Mechanical Strength, and Autogenous Shrinkage of Ultrahigh-Performance Concrete. J. Mater. Civ. Eng. 2016, 28, 04015068. [Google Scholar] [CrossRef]
- Irassar, E.F. Sulfate attack on cementitious materials containing limestone filler—A review. Cem. Concr. Res. 2009, 39, 241–254. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K.; Scherer, G. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Yalçınkaya, Ç.; Yazıcı, H. Effects of ambient temperature and relative humidity on early-age shrinkage of UHPC with high-volume mineral admixtures. Constr. Build. Mater. 2017, 144, 252–259. [Google Scholar] [CrossRef]
- Chang, W.; Zheng, W. Effects of key parameters on fluidity and compressive strength of ultra-high performance concrete. Struct. Concr. 2019, 21, 747–760. [Google Scholar] [CrossRef]
- De Weerdt, K.; Kjellsen, K.O.; Sellevold, E.; Justnes, H. Synergy between fly ash and limestone powder in ternary cements. Cem. Concr. Compos. 2011, 33, 30–38. [Google Scholar] [CrossRef]
- Prakash, S.; Kumar, S.; Biswas, R.; Rai, B. Influence of silica fume and ground granulated blast furnace slag on the engineering properties of ultra-high-performance concrete. Innov. Infrastruct. Solut. 2021, 7, 117. [Google Scholar] [CrossRef]
- Sujjavanich, S.; Suwanvitaya, P.; Chaysuwan, D.; Heness, G. Synergistic effect of metakaolin and fly ash on properties of concrete. Constr. Build. Mater. 2017, 155, 830–837. [Google Scholar] [CrossRef]
- Moon, J.; Yoon, S.; Wentzcovitch, R.M.; Monteiro, P.J.M. First-principles elasticity of monocarboaluminate hydrates. Am. Mineral. 2014, 99, 1360–1368. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service life and life cycle assessment of reinforced concrete systems with limestone calcined clay cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]
- Guo, M.; Gong, G.; Yue, Y.; Xing, F.; Zhou, Y.; Hu, B. Performance evaluation of recycled aggregate concrete incorporating limestone calcined clay cement (LC3). J. Clean. Prod. 2022, 366, 132820. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Zhou, S.; Tang, S.; Lu, X. Investigation of microstructure of C-S-H and micro-mechanics of cement pastes under NH4NO3 dissolution by 29Si MAS NMR and microhardness. Measurement 2021, 185, 110019. [Google Scholar] [CrossRef]
- Luan, C.; Zhou, Y.; Liu, Y.; Ren, Z.; Wang, J.; Yuan, L.; Du, S.; Zhou, Z.; Huang, Y. Effects of nano-SiO2, nano-CaCO3 and nano-TiO2 on properties and microstructure of the high content calcium silicate phase cement (HCSC). Constr. Build. Mater. 2022, 314, 125377. [Google Scholar] [CrossRef]
- Sun, G.K.; Young, J.F.; Kirkpatrick, R.J. The role of Al in C–S–H: NMR, XRD, and compositional results for precipitated samples. Cem. Concr. Res. 2006, 36, 18–29. [Google Scholar] [CrossRef]
- Wang, L.; Guo, F.; Lin, Y.; Yang, H.; Tang, S.W. Comparison between the effects of phosphorous slag and fly ash on the C-S-H structure, long-term hydration heat and volume deformation of cement-based materials. Constr. Build. Mater. 2020, 250, 118807. [Google Scholar] [CrossRef]
- L’Hôpital, E.; Lothenbach, B.; Kulik, D.A.; Scrivener, K. Influence of calcium to silica ratio on aluminium uptake in calcium silicate hydrate. Cem. Concr. Res. 2016, 85, 111–121. [Google Scholar] [CrossRef]
- Ma, B.; Li, H.; Li, X.; Mei, J.; Lv, Y. Influence of nano-TiO2 on physical and hydration characteristics of fly ash–cement systems. Constr. Build. Mater. 2016, 122, 242–253. [Google Scholar] [CrossRef]
- Ting, L.; Qiang, W.; Yuqi, Z. Influence of ultra-fine slag and silica fume on properties of high-strength concrete. Mag. Concr. Res. 2020, 72, 610–621. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Z.; Liu, B.; Zhao, F.; Tang, S.; Jin, M. Effects of Fly Ash Dosage on Shrinkage, Crack Resistance and Fractal Characteristics of Face Slab Concrete. Fractal Fract. 2022, 6, 335. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Surface fractal analysis of pore structure of high-volume fly-ash cement pastes. Appl. Surf. Sci. 2010, 257, 762–768. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, S.; Shi, Y.; Huang, Y.; Zhao, F.; Huo, T.; Tang, S. The Influence of Fly Ash Dosages on the Permeability, Pore Structure and Fractal Features of Face Slab Concrete. Fractal Fract. 2022, 6, 476. [Google Scholar] [CrossRef]
- Zhang, W.; Hama, Y.; Na, S.H. Drying shrinkage and microstructure characteristics of mortar incorporating ground granulated blast furnace slag and shrinkage reducing admixture. Constr. Build. Mater. 2015, 93, 267–277. [Google Scholar] [CrossRef]
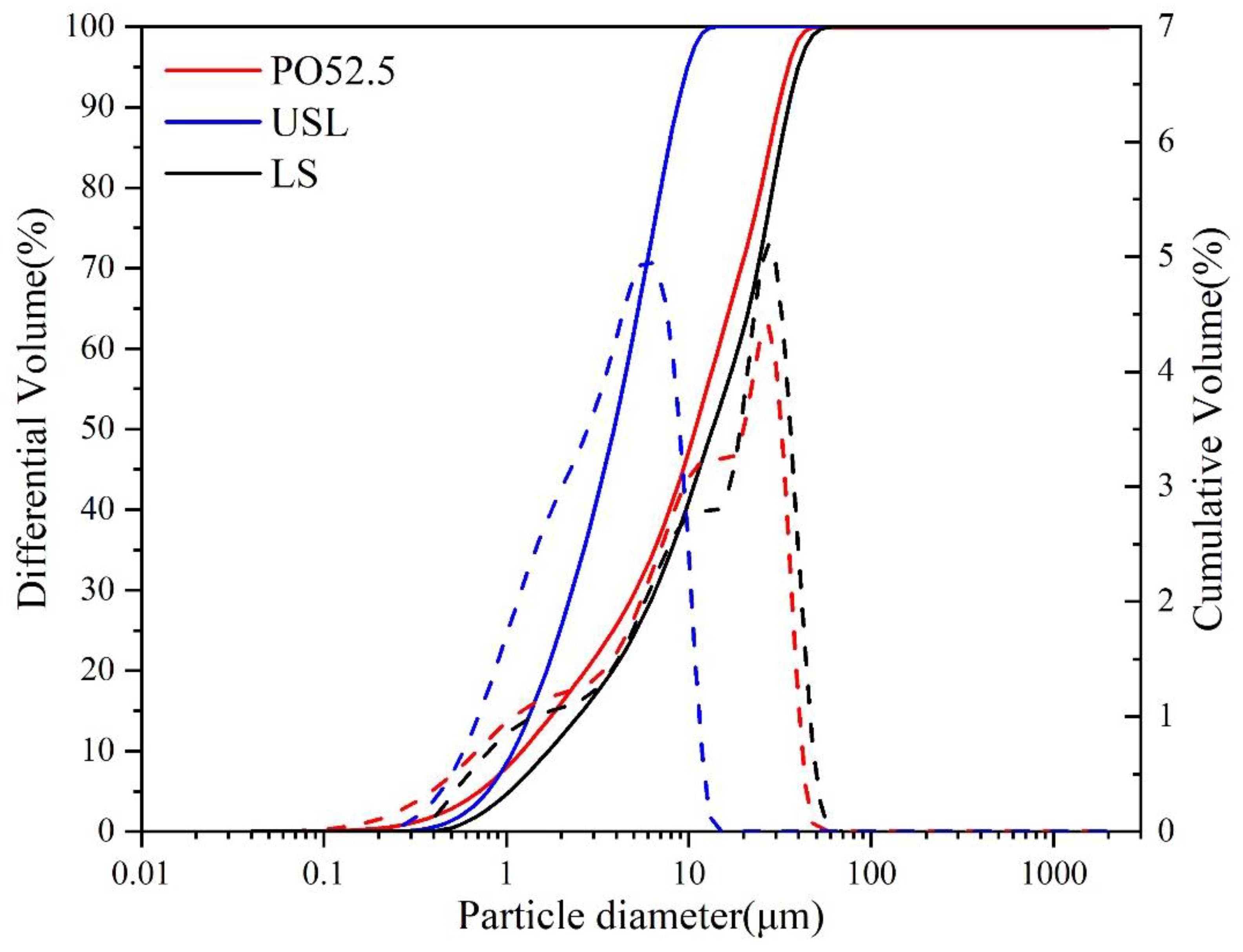
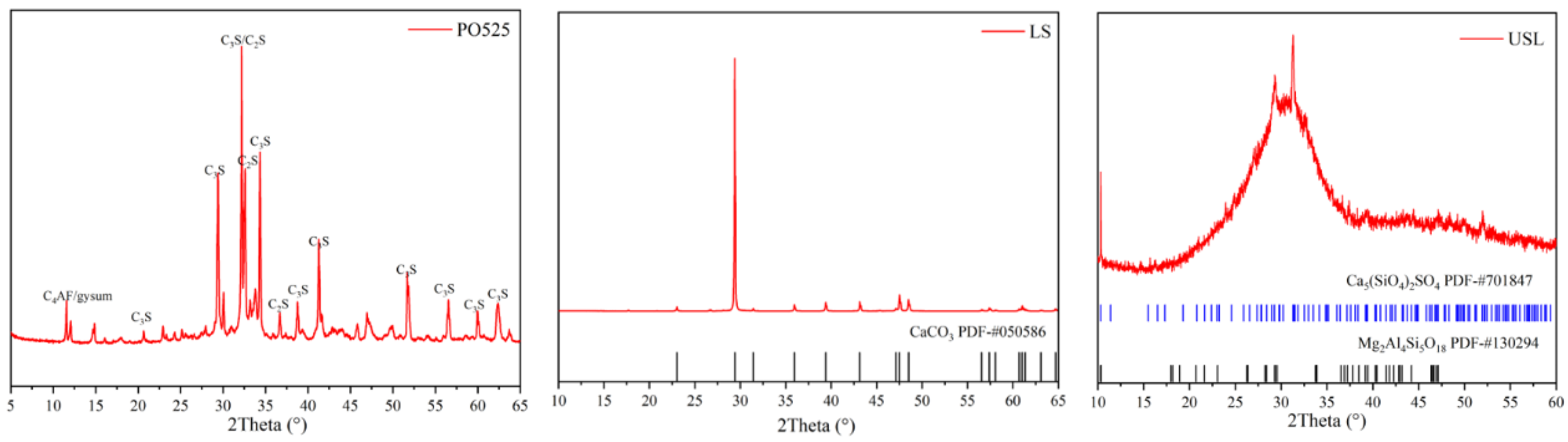



| Oxide Formula | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl− | K2O | CaO | TiO2 | MnO | Fe2O3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USL | 0.66 | 9.37 | 16.82 | 27.39 | 0 | 2.60 | 0.07 | 0.32 | 36.31 | 0.91 | 0.37 | 0.33 | 1.20 |
| LS | 0.16 | 1.11 | 1.48 | 3.19 | 0.06 | 0.08 | 0.02 | 0.29 | 59.42 | 0 | 0.07 | 1.22 | 40.58 |
| P·O52.5 | 0.50 | 4.94 | 8.06 | 22.89 | 0.10 | 3.55 | 0.11 | 0.73 | 52.84 | 0.38 | 0.13 | 2.39 | 3.27 |
| P·O 52.5 | LS | USL | Water | SP | Sand | Steel Fiber | |
|---|---|---|---|---|---|---|---|
| Ref | 1150 | 0 | 0 | 207 | 17.25 | 1150 | 152 |
| USL30 | 805 | 0 | 345 | 207 | 17.25 | 1150 | 152 |
| B1 | 805 | 57.5 | 287.5 | 207 | 17.25 | 1150 | 152 |
| B2 | 805 | 115 | 230 | 207 | 17.25 | 1150 | 152 |
| B3 | 805 | 172.5 | 172.5 | 207 | 17.25 | 1150 | 152 |
| B4 | 805 | 230 | 115 | 207 | 17.25 | 1150 | 152 |
| B5 | 805 | 287.5 | 57.5 | 207 | 17.25 | 1150 | 152 |
| B6 | 805 | 345 | 0 | 207 | 17.25 | 1150 | 152 |
| Herschel–Bulkley Parameters | R2 | Plastic Viscosity (Pa·s) | |||
|---|---|---|---|---|---|
| τ0/Pa | k | n | |||
| Ref | 29.067 | 7.99 × 10−7 | 4.646 | 0.9966 | 7.065 |
| USL30 | 30.588 | 8.00 × 10−7 | 4.975 | 0.9879 | 3.067 |
| B1 | 28.891 | 3.71 × 10−7 | 4.639 | 0.9970 | 3.180 |
| B2 | 20.822 | 2.77 × 10−5 | 3.667 | 0.9942 | 0.0316 |
| B3 | 23.623 | 1.08 × 10−5 | 3.886 | 0.9965 | 0.0326 |
| B4 | 24.149 | 2.19 × 10−6 | 4.017 | 0.9907 | 0.118 |
| B5 | 27.829 | 1.019 × 10−6 | 4.216 | 0.9917 | 0.133 |
| B6 | 47.204 | 1.7818 × 10−6 | 4.757 | 0.9989 | 2.584 |
| NO. | Q0 | Q1 | Q2(1Al) | Q2(0Al) | Al/Si | MCL | |
|---|---|---|---|---|---|---|---|
| Ref | Peak (ppm) | −70.8 | −78.1 | −81.0 | −84.1 | 0.107 | 3.25 |
| Area (%) | 67.18 | 21.53 | 6.79 | 3.31 | |||
| USL30 | Peak (ppm) | −71.1 | −77.7 | −81.2 | −85.8 | 0.196 | 5.80 |
| Area (%) | 55.93 | 18.16 | 17.22 | 8.69 | |||
| B2 | Peak (ppm) | −71.2 | −78.1 | −81.1 | −84.4 | 0.182 | 6.34 |
| Area (%) | 57.64 | 15.79 | 15.41 | 11.15 | |||
| B4 | Peak (ppm) | −71.15 | −78.2 | −81.2 | −84.2 | 0.167 | 5.89 |
| Area (%) | 57.58 | 16.82 | 14.16 | 11.44 | |||
| B6 | Peak (ppm) | −71.15 | −77.8 | −80.5 | −85.1 | 0.140 | 5.68 |
| Area (%) | 55.85 | 17.73 | 12.40 | 14.03 |
| Samples | Average Pore Diameter (nm) | Median Pore Diameter (nm) | Critical Diameter of the Capillary (nm) | Porosity (by Volume) (%) | Pore Distribution (%) | |||
|---|---|---|---|---|---|---|---|---|
| By Area Method | By Volume Method | <10 nm | 10–100 nm | >100 nm | ||||
| Ref | 20.16 | 19.63 | 17.76 | 12.32 | 7.83 | 5.52 | 63.68 | 29.8 |
| USL30 | 17.87 | 17.77 | 20.80 | 11.58 | 7.25 | 6.51 | 86.2 | 7.29 |
| B2 | 18.1 | 17.25 | 21.63 | 10.41 | 6.47 | 8.41 | 78.21 | 13.38 |
| B4 | 22.63 | 21.62 | 25.49 | 11.38 | 7.33 | 5.64 | 83.49 | 10.87 |
| B6 | 23.80 | 20.59 | 25.65 | 12.14 | 7.69 | 2.56 | 79.82 | 17.62 |
| No. | Ds | R2 |
|---|---|---|
| Ref | 2.589 | 0.996 |
| USL30 | 2.637 | 0.994 |
| B2 | 2.683 | 0.997 |
| B4 | 2.628 | 0.992 |
| B6 | 2.596 | 0.989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, C.; Yang, Q.; Lin, X.; Gao, X.; Cheng, H.; Huang, Y.; Du, P.; Zhou, Z.; Wang, J. The Synergistic Effects of Ultrafine Slag Powder and Limestone on the Rheology Behavior, Microstructure, and Fractal Features of Ultra-High Performance Concrete (UHPC). Materials 2023, 16, 2281. https://doi.org/10.3390/ma16062281
Luan C, Yang Q, Lin X, Gao X, Cheng H, Huang Y, Du P, Zhou Z, Wang J. The Synergistic Effects of Ultrafine Slag Powder and Limestone on the Rheology Behavior, Microstructure, and Fractal Features of Ultra-High Performance Concrete (UHPC). Materials. 2023; 16(6):2281. https://doi.org/10.3390/ma16062281
Chicago/Turabian StyleLuan, Congqi, Qingchun Yang, Xinru Lin, Xin Gao, Heng Cheng, Yongbo Huang, Peng Du, Zonghui Zhou, and Jinbang Wang. 2023. "The Synergistic Effects of Ultrafine Slag Powder and Limestone on the Rheology Behavior, Microstructure, and Fractal Features of Ultra-High Performance Concrete (UHPC)" Materials 16, no. 6: 2281. https://doi.org/10.3390/ma16062281
APA StyleLuan, C., Yang, Q., Lin, X., Gao, X., Cheng, H., Huang, Y., Du, P., Zhou, Z., & Wang, J. (2023). The Synergistic Effects of Ultrafine Slag Powder and Limestone on the Rheology Behavior, Microstructure, and Fractal Features of Ultra-High Performance Concrete (UHPC). Materials, 16(6), 2281. https://doi.org/10.3390/ma16062281





