Preparation of Spherical Ultrafine Silver Particles Using Y-Type Microjet Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
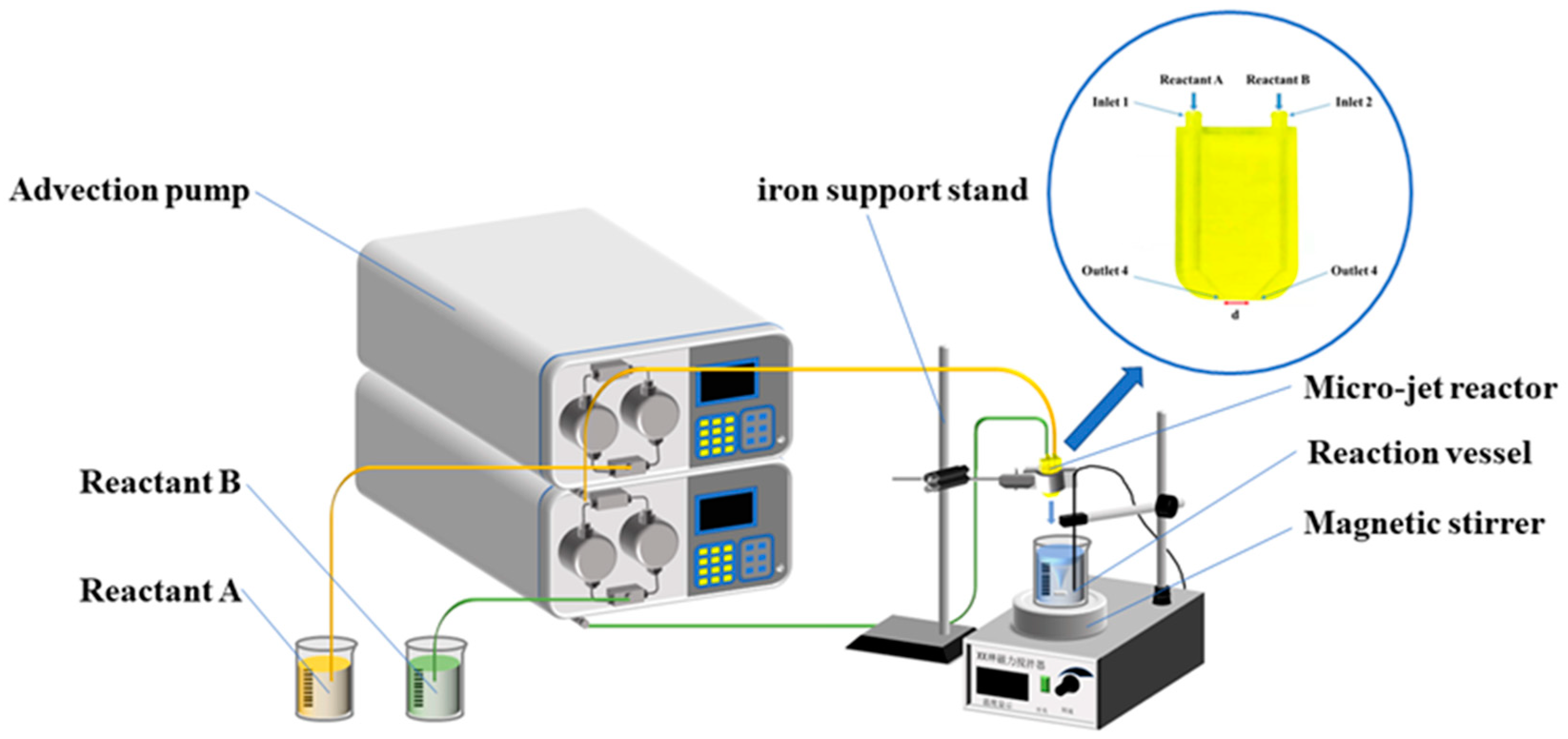
2.2. Experimental Methods
2.3. Characterization Testing
3. Results and Discussion
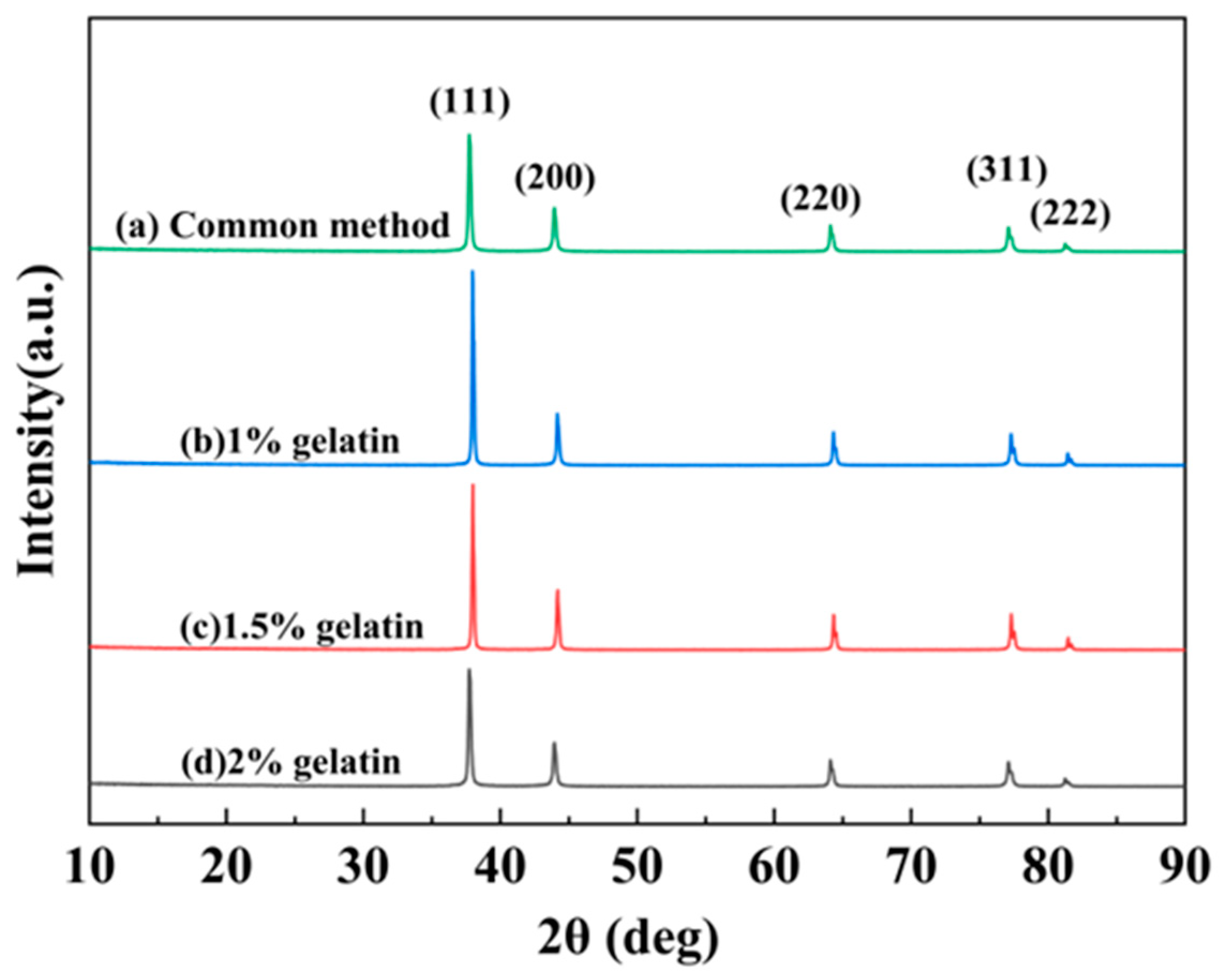
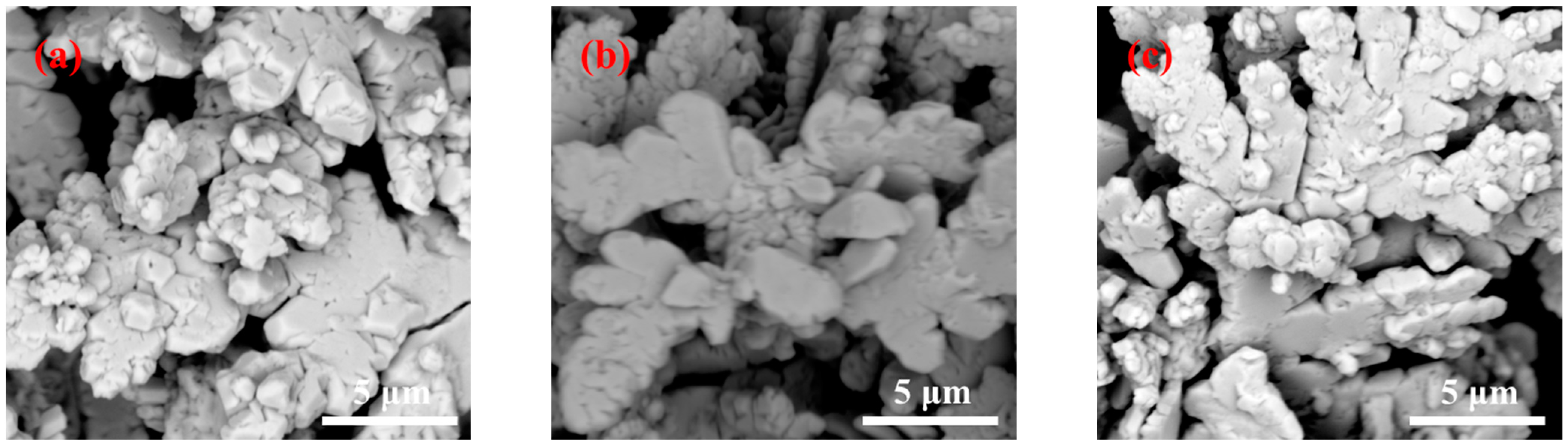
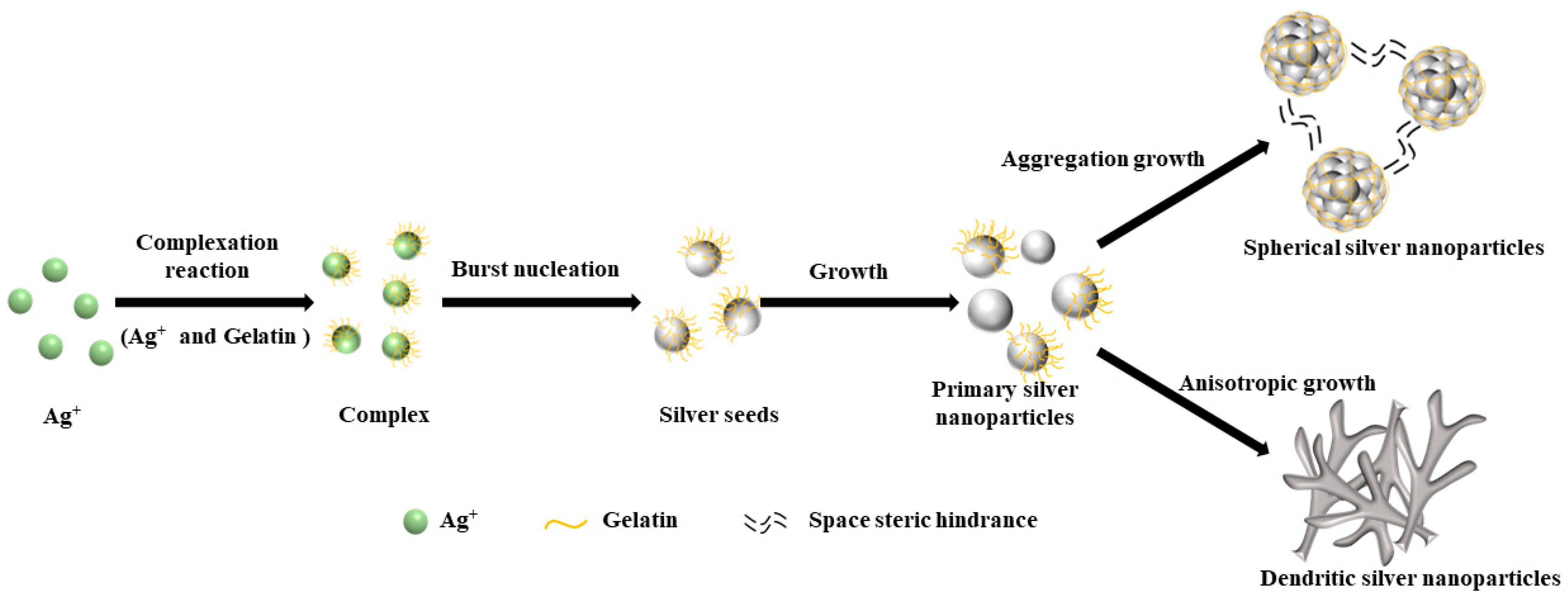
3.1. Effect of Preparation Method on the Morphology of Silver Powders
3.2. Effect of Dispersant Dosage on the Morphology under Microjet Conditions
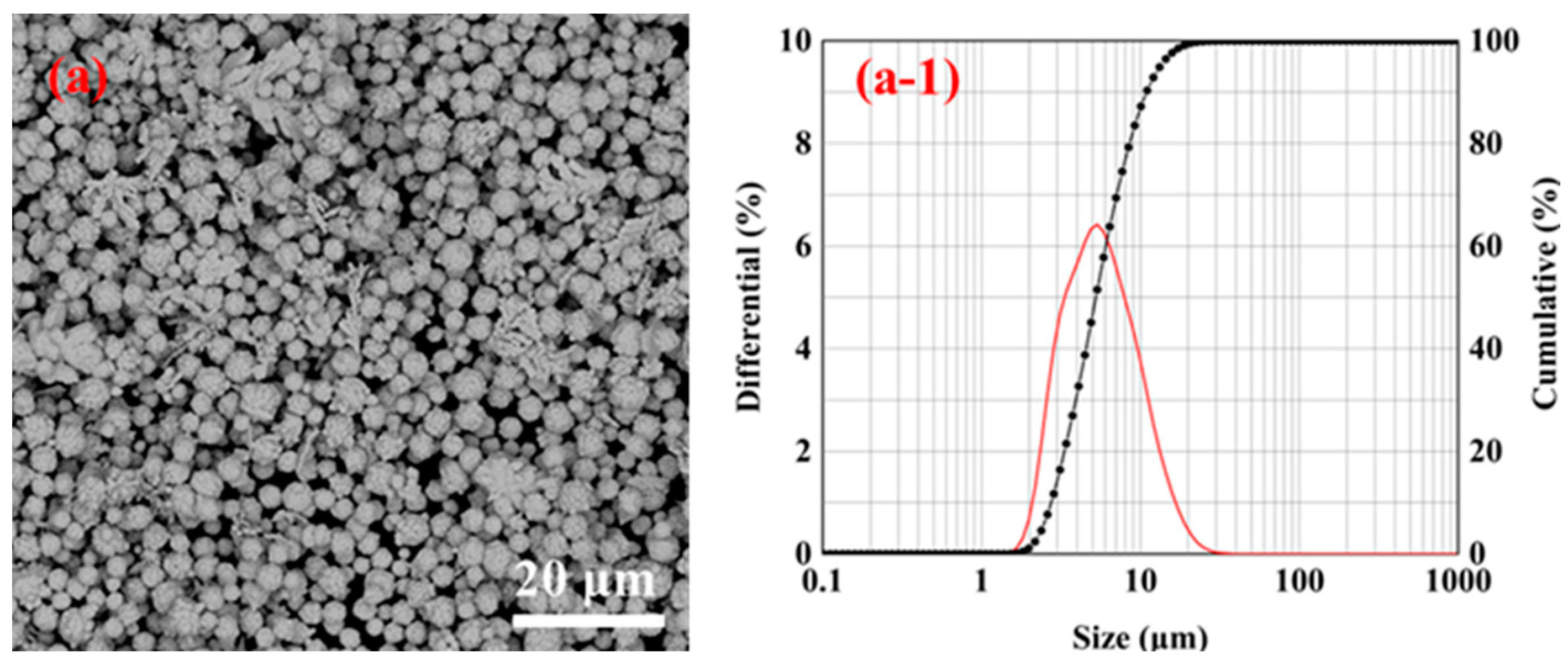
3.3. Effect of Dispersant Dosage on the Particle Size of Silver Powder under Microjet Conditions
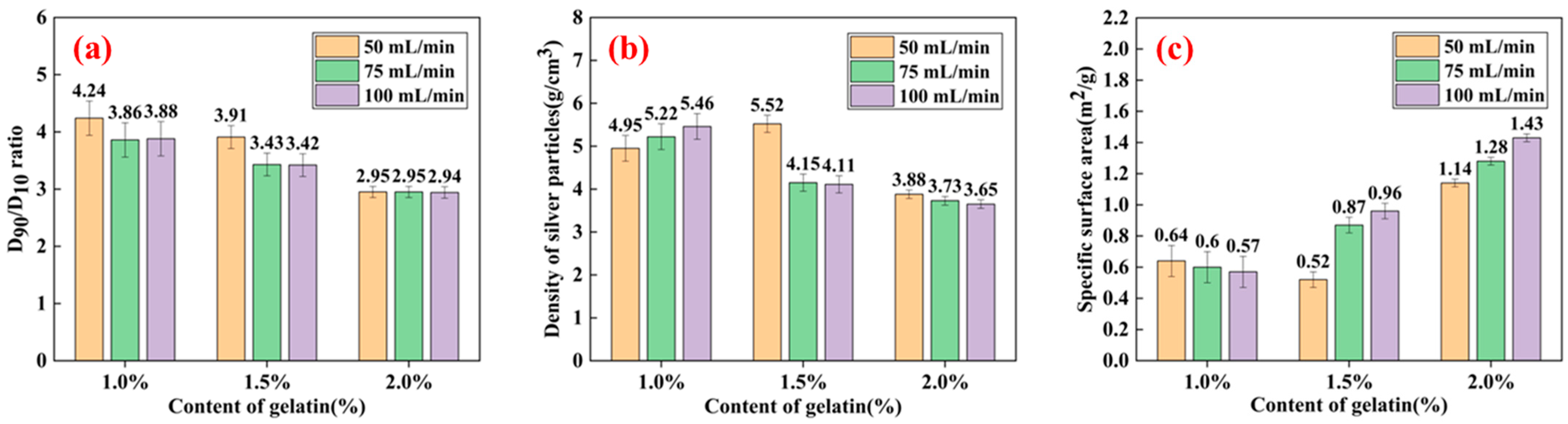
3.4. Effect of Dispersant Addition on Silver Powder Parameters
4. Conclusions
- (1)
- The outcomes of this study demonstrate that under specific circumstances and within a specific range, the Y-type microjet reactor may be utilized to regulate silver particles’ morphology and particle size.
- (2)
- By changing the experimental conditions, spherical and dendritic silver particles can be obtained using a Y-type microjet reactor. When the microjet flow rate was 75 mL/min, and the gelatin content was 1% of the AgNO3 mass, the ultrafine spherical silver powder with a particle size of 4.84 μm and a tap density of 5.22 g/cm3 could be synthesized using the microreactor at room temperature.
- (3)
- Compared to conventional stirred reactors, the Y-type microjet reactor can quickly and efficiently mix reactant solutions, and the process is controllable. This reactor, unlike other microchemical reactors, does not have synthetic product deposition or channel blockage problems. The controlled synthesis of silver nanoparticles offers a potential future application for the microjet reactor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.W.; Kwon, S.N.; Na, S.I. Stretchable and electrically conductive polyurethane-silver/graphene composite fibers prepared by wet-spinning process. Compos. B Eng. 2019, 167, 573–581. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, H.; Jang, H.J.; Lee, N.; Nam, K.H.; Chung, D.; Lee, S. Improvement of the thermal stability of dendritic silver-coated copper microparticles by surface modification based on molecular self-assembly. Nano Converg. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Chen, B.; Ma, Y.; Huang, Y.; Tang, S.; Liu, W. Tuning the electrical resistivity of conductive silver paste prepared by blending multi-morphologies and micro-nanometers silver powder. J. Mater. Sci. Mater. Electron. 2021, 32, 13777–13786. [Google Scholar] [CrossRef]
- Gulyaev, A.I.; Besednov, K.L.; Petrova, A.P.; Antyufeeva, N.V. The Influence of Silver Powder Filler on the Curing Process and Structure of Conducting Adhesives. Polym. Sci. Ser. D 2022, 15, 389–393. [Google Scholar] [CrossRef]
- Yaseen, B.; Gangwar, C.; Kumar, I.; Sarkar, J.; Naik, R.M. Detailed Kinetic and Mechanistic Study for the Preparation of Silver Nanoparticles by a Chemical Reduction Method in the Presence of a Neuroleptic Agent (Gabapentin) at an Alkaline pH and its Characterization. ACS Omega 2022, 7, 5739–5750. [Google Scholar] [CrossRef]
- Hu, J.Q.; Chen, Q.; Xie, Z.X.; Han, G.B.; Wang, R.H.; Ren, B.; Zhang, Y.; Yang, Z.L.; Tian, Z.Q. A Simple and Effective Route for the Synthesis of Crystalline Silver Nanorods and Nanowires. Adv. Funct. Mater. 2004, 14, 183–189. [Google Scholar] [CrossRef]
- Sinha, A.; Sharma, B.P. Preparation of silver powder through glycerol process. Bull. Mater. Sci. 2005, 28, 213–217. [Google Scholar] [CrossRef]
- Khayati, G.R.; Janghorban, K. The nanostructure evolution of Ag powder synthesized by high energy ball milling. Adv. Powder Technol. 2012, 23, 393–397. [Google Scholar] [CrossRef]
- Yang, S.Y.; Kim, S.G. Characterization of silver and silver/nickel composite particles prepared by spray pyrolysis. Powder Technol. 2004, 146, 185–192. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, S.M.; Park, D.W. Preparation of silver nanopowder by thermal plasma. Mater. Sci. Eng. C 2007, 27, 1286–1290. [Google Scholar] [CrossRef]
- Tian, Q.H.; Deng, D.; Li, Y.; Guo, X.Y. Preparation of ultrafine silver powders with controllable size and morphology. Trans. Nonferrous Met. Soc. China 2018, 28, 524–533. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, J.H.; Son, H.T.; Won, C.W.; Maeng, D.Y. A new and effective chemical reduction method for preparation of nanosized silver powder and colloid dispersion. Mater. Res. Bull. 2003, 38, 949–956. [Google Scholar] [CrossRef]
- Salabat, A.; Saydi, H. Microemulsion route to fabrication of silver and platinum-polymer nanocomposites. Polym. Compos. 2014, 35, 2023–2028. [Google Scholar] [CrossRef]
- Pedroza-Toscano, M.A.; Rabelero-Velasco, M.; Díaz de León, R.; Saade, H.; López, R.G.; Mendizábal, E.; Puig, J.E. Preparation of Silver Nanostructures from Bicontinuous Microemulsions. J. Nanomater. 2012, 2012, 975106. [Google Scholar] [CrossRef]
- Cui, G.H.; Qi, S.; Wang, X.; Tian, G.; Sun, G.; Liu, W.; Yan, X.; Wu, D.; Wu, Z.; Zhang, L. Interfacial Growth of Controllable Morphology of Silver Patterns on Plastic Substrates. J. Phys. Chem. B 2012, 116, 12349–12356. [Google Scholar] [CrossRef]
- Aziz, N.; Faraz, M.; Pandey, R.; Shakir, M.; Fatma, T.; Varma, A.; Barman, I.; Prasad, R. Facile Algae-Derived Route to Biogenic Silver Nanoparticles: Synthesis, Antibacterial, and Photocatalytic Properties. Langmuir 2015, 31, 11605–11612. [Google Scholar] [CrossRef]
- Lim, J.-M.; Bertrand, N.; Valencia, P.M.; Rhee, M.; Langer, R.; Jon, S.; Farokhzad, O.C.; Karnik, R. Parallel microfluidic synthesis of size-tunable polymeric nanoparticles using 3D flow focusing towards in vivo study. Nanomedicine 2014, 10, 401–409. [Google Scholar] [CrossRef]
- Ying, Y.; Chen, G.; Zhao, Y.; Li, S.; Yuan, Q. A high throughput methodology for continuous preparation of monodispersed nanocrystals in microfluidic reactors. Chem. Eng. J. 2008, 135, 209–215. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, Z.; Wang, X.; Goncalves, D.; Zhang, B.; Hierlemann, A.; Hunziker, P. Microfluidics-based single-step preparation of injection-ready polymeric nanosystems for medical imaging and drug delivery. Nanoscale 2015, 7, 16983–16993. [Google Scholar] [CrossRef]
- Elvira, K.S.; Casadevall i Solvas, X.; Wootton, R.C.; deMello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Ortiz de Solorzano, I.; Uson, L.; Larrea, A.; Miana, M.; Sebastian, V.; Arruebo, M. Continuous synthesis of drug-loaded nanoparticles using microchannel emulsification and numerical modeling: Effect of passive mixing. Int. J. Nanomed. 2016, 11, 3397–3416. [Google Scholar] [CrossRef]
- Feng, H.; Zheng, T.; Li, M.; Wu, J.; Ji, H.; Zhang, J.; Zhao, W.; Guo, J. Droplet-based microfluidics systems in biomedical applications. Electrophoresis 2019, 40, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Schoenitz, M.; Grundemann, L.; Augustin, W.; Scholl, S. Fouling in microstructured devices: A review. Chem. Commun. 2015, 51, 8213–8228. [Google Scholar] [CrossRef] [PubMed]
- Rathi, N.; Gaikar, V.G. Optimization of Continuous Synthesis of Cross-Linked Chitosan Nanoparticles Using Microreactors. Chem. Eng. Technol. 2017, 40, 506–513. [Google Scholar] [CrossRef]
- Lim, J.-M.; Swami, A.; Gilson, L.M.; Chopra, S.; Choi, S.; Wu, J.; Langer, R.; Karnik, R.; Farokhzad, O.C. Ultra-High Throughput Synthesis of Nanoparticles with Homogeneous Size Distribution Using a Coaxial Turbulent Jet Mixer. ACS Nano 2014, 8, 6056–6065. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, V.; Jensen, K.F. Nanoengineering a library of metallic nanostructures using a single microfluidic reactor. Nanoscale 2016, 8, 15288–15295. [Google Scholar] [CrossRef]
- Baber, R.; Mazzei, L.; Thanh, N.T.K.; Gavriilidis, A. Synthesis of silver nanoparticles using a microfluidic impinging jet reactor. J. Flow Chem. 2016, 6, 268–278. [Google Scholar] [CrossRef]
- Sahoo, K.; Kumar, S. Green synthesis of sub 10 nm silver nanoparticles in gram scale using free impinging jet reactor. Chem. Eng. Process. Process Intensif. 2021, 165, 108439. [Google Scholar] [CrossRef]
- Chen, Z.W.; Gan, G.Y.; Yan, J.K.; Liu, J. Preparation of Spherical Ultra-fine Silver Powder Using Gelatin as Dispersant. Rare Met. Mater. Eng. 2011, 40, 741–744. [Google Scholar]
- Sannohe, K.; Ma, T.; Hayase, S. Synthesis of monodispersed silver particles: Synthetic techniques to control shapes, particle size distribution and lightness of silver particles. Adv. Powder Technol. 2019, 30, 3088–3098. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, X.; Wang, H. Synthesis and characterization of spherical and mono-disperse micro-silver powder used for silicon solar cell electronic paste. Adv. Powder Technol. 2012, 23, 250–255. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Li, J.; Li, N.; Zhang, J.; Gu, Y.; Chen, G.; Ju, S. Preparation of Spherical Ultrafine Silver Particles Using Y-Type Microjet Reactor. Materials 2023, 16, 2217. https://doi.org/10.3390/ma16062217
Wan X, Li J, Li N, Zhang J, Gu Y, Chen G, Ju S. Preparation of Spherical Ultrafine Silver Particles Using Y-Type Microjet Reactor. Materials. 2023; 16(6):2217. https://doi.org/10.3390/ma16062217
Chicago/Turabian StyleWan, Xiaoxi, Jun Li, Na Li, Jingxi Zhang, Yongwan Gu, Guo Chen, and Shaohua Ju. 2023. "Preparation of Spherical Ultrafine Silver Particles Using Y-Type Microjet Reactor" Materials 16, no. 6: 2217. https://doi.org/10.3390/ma16062217
APA StyleWan, X., Li, J., Li, N., Zhang, J., Gu, Y., Chen, G., & Ju, S. (2023). Preparation of Spherical Ultrafine Silver Particles Using Y-Type Microjet Reactor. Materials, 16(6), 2217. https://doi.org/10.3390/ma16062217





