Hierarchical CoNiO2 Microflowers Assembled by Mesoporous Nanosheets as Efficient Electrocatalysts for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experimental
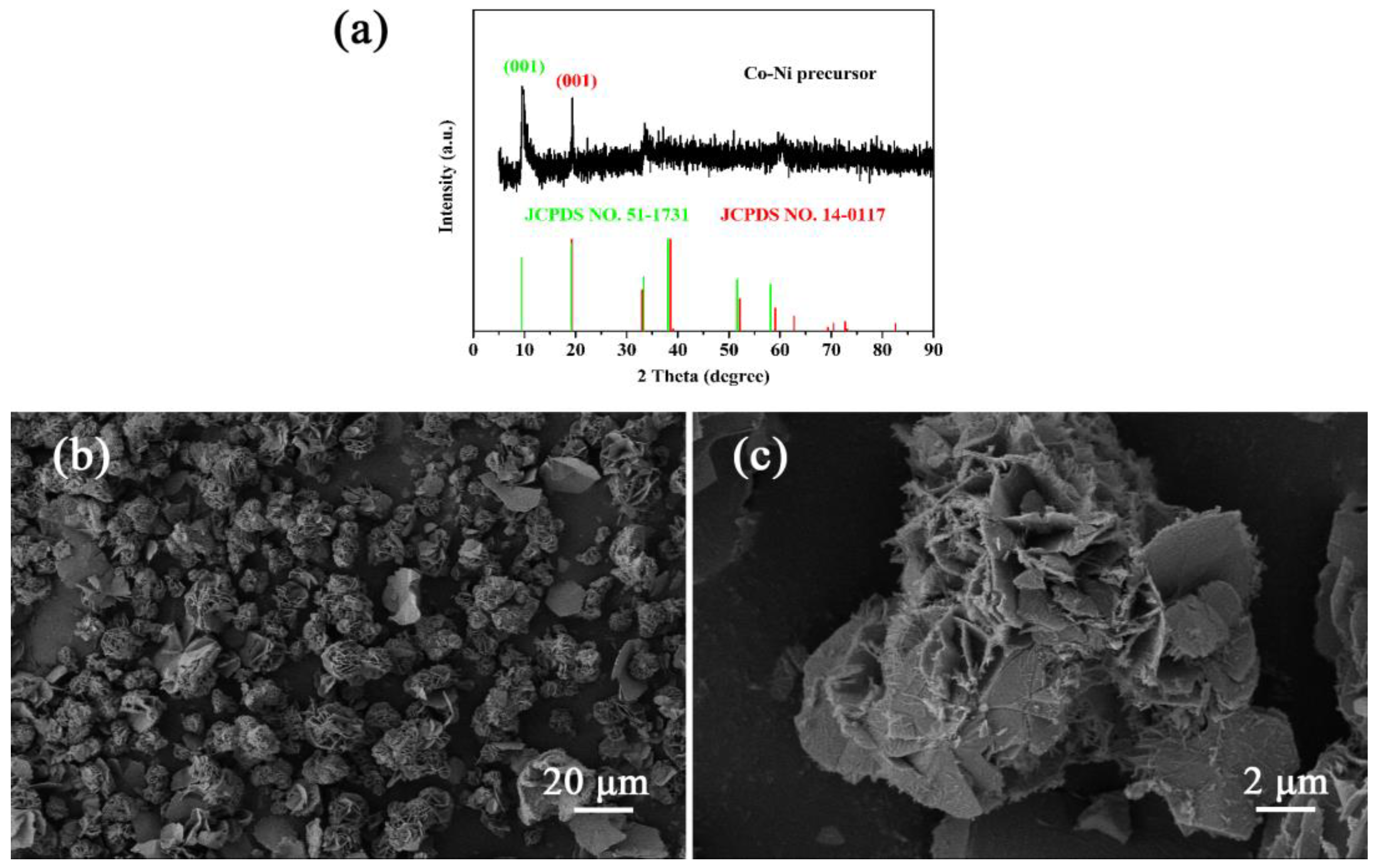
2.1. Synthesis of CoNiO2
2.2. Characterizations
2.3. Electrochemical Measurements
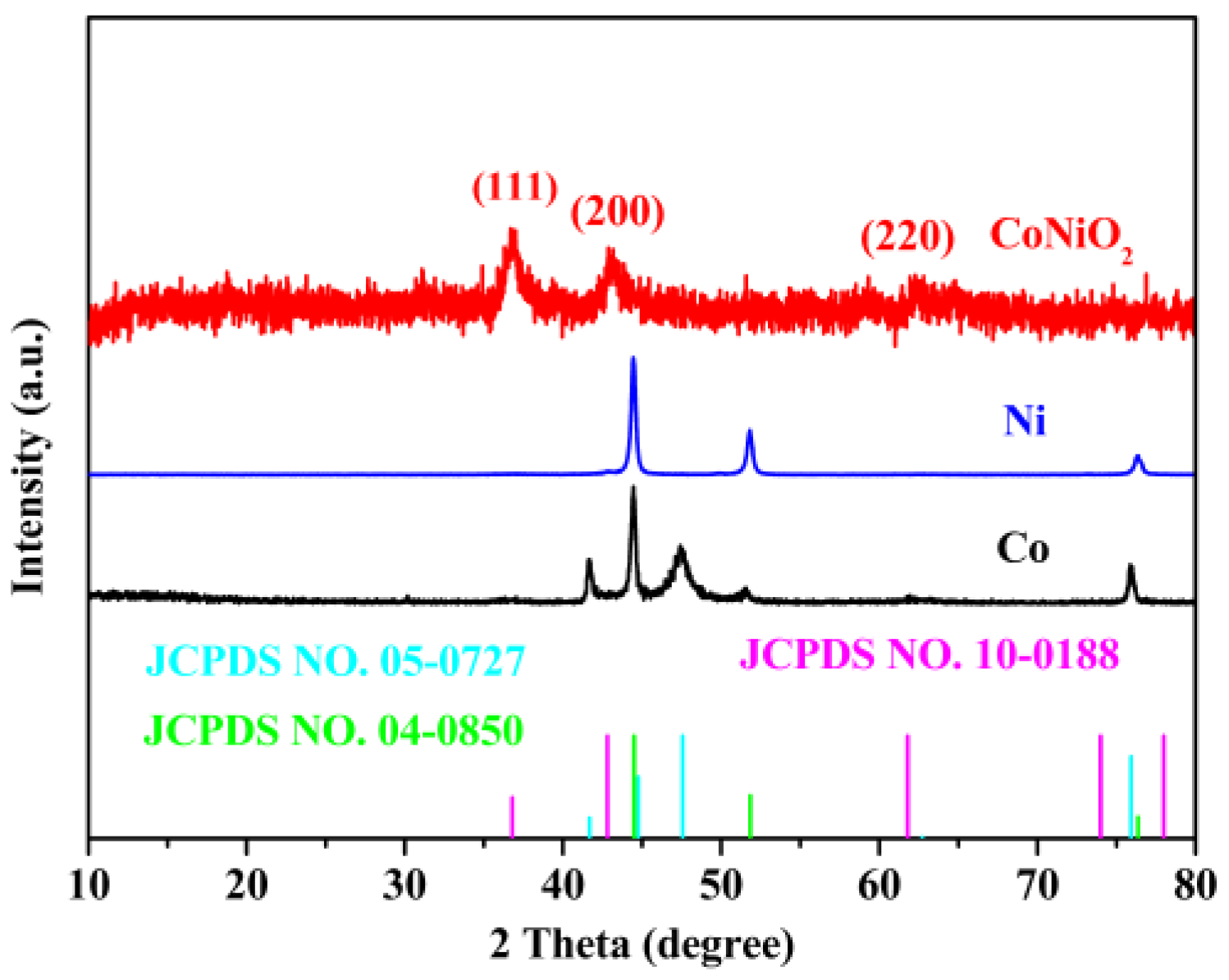
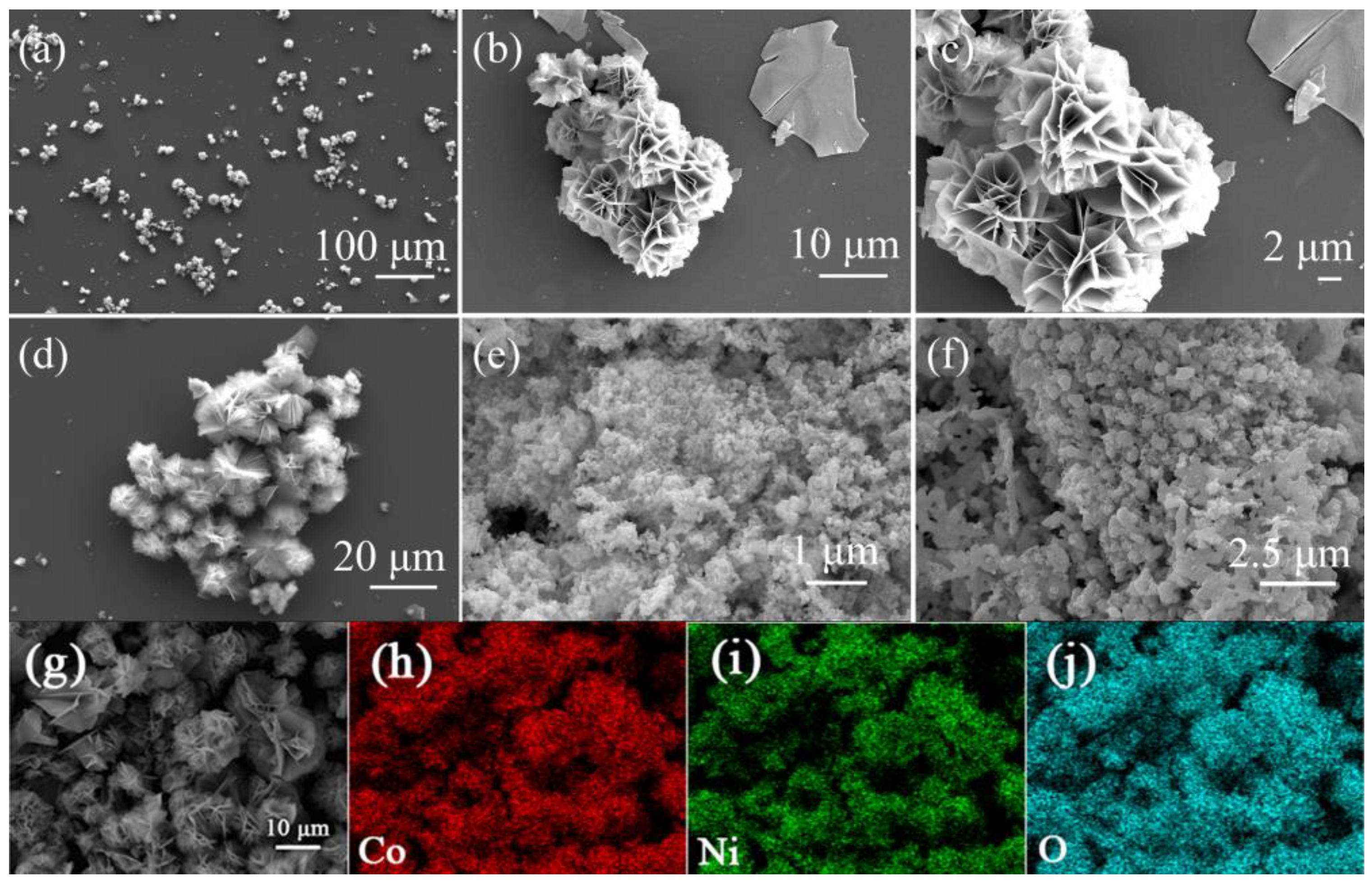
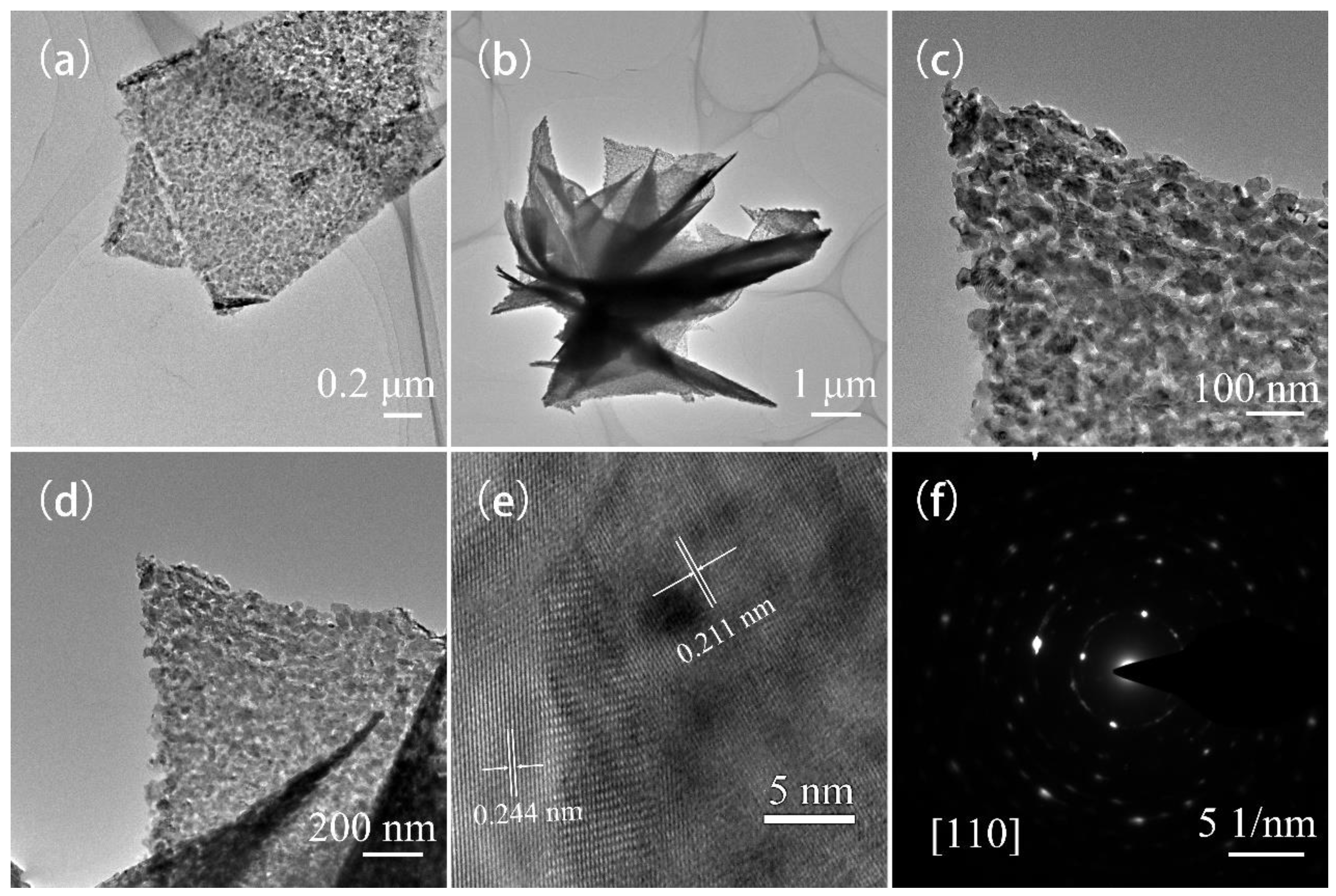
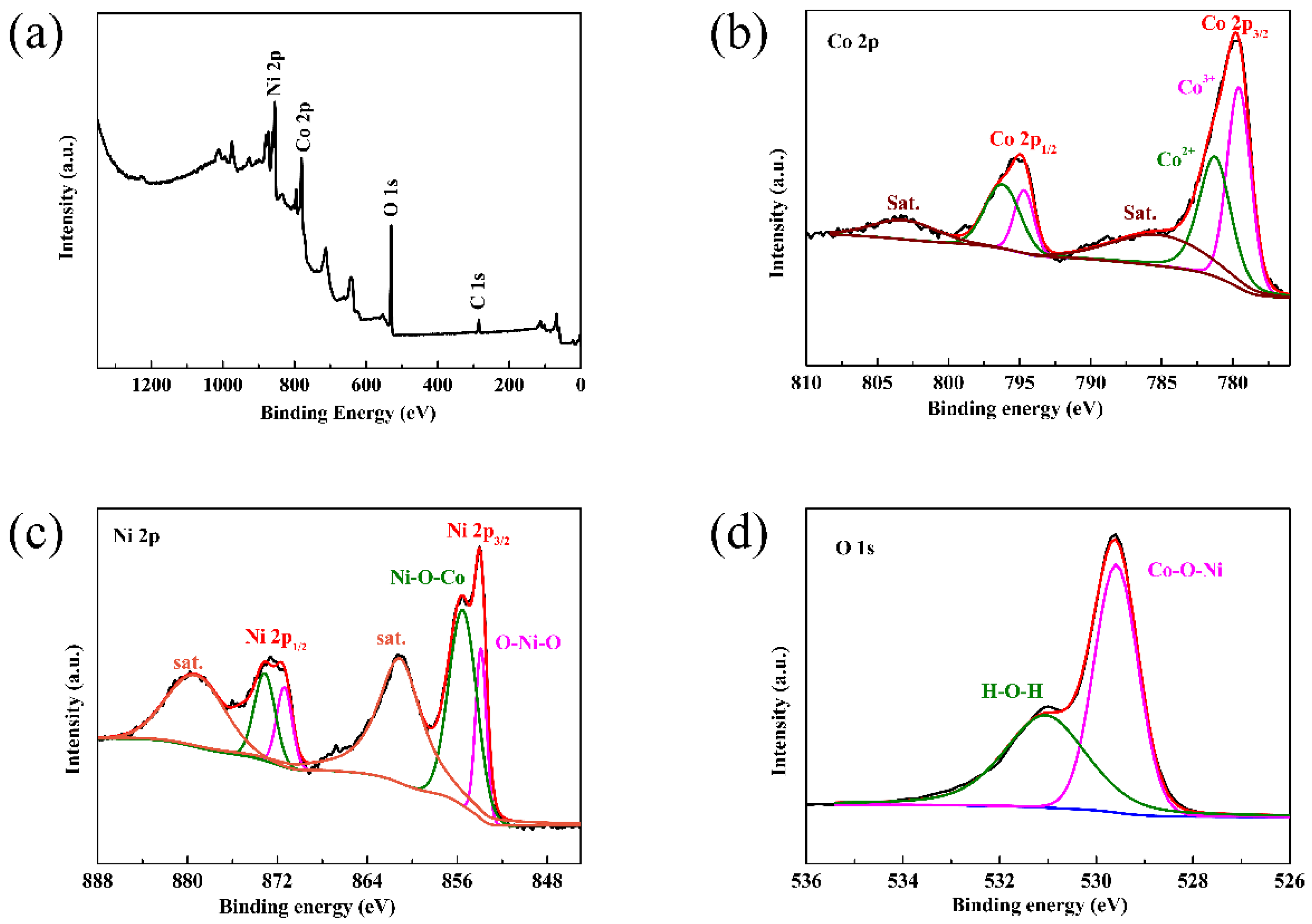
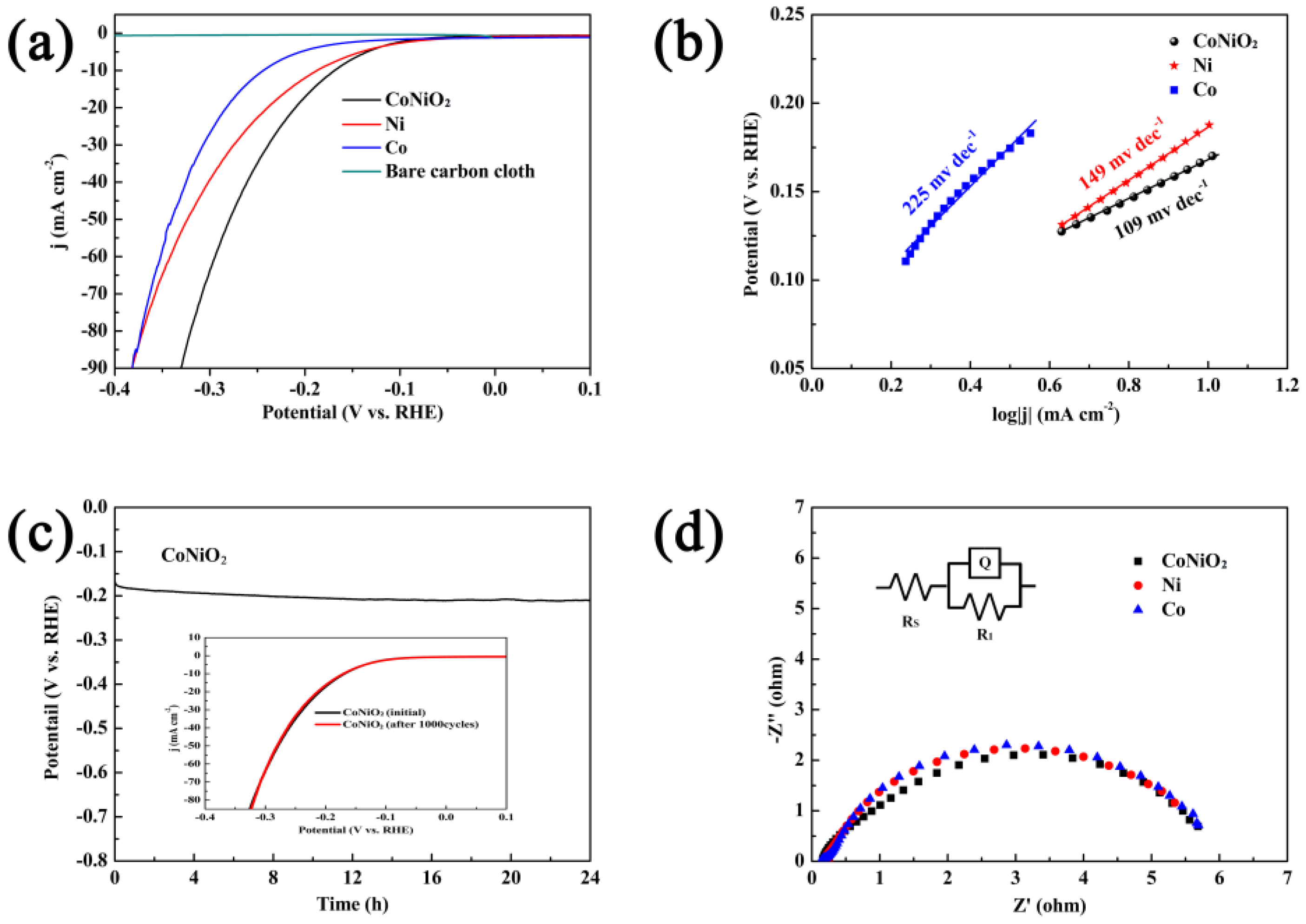
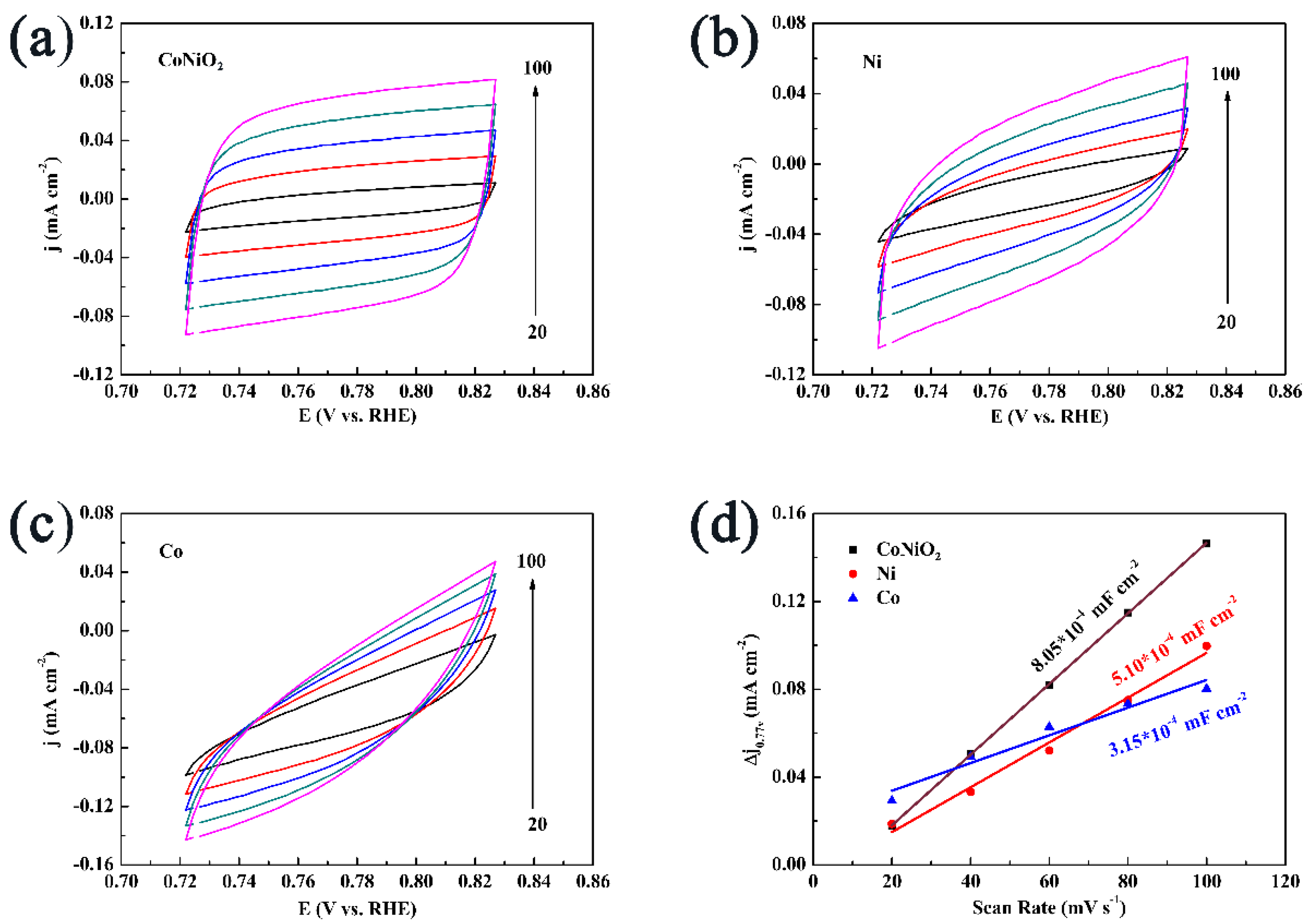
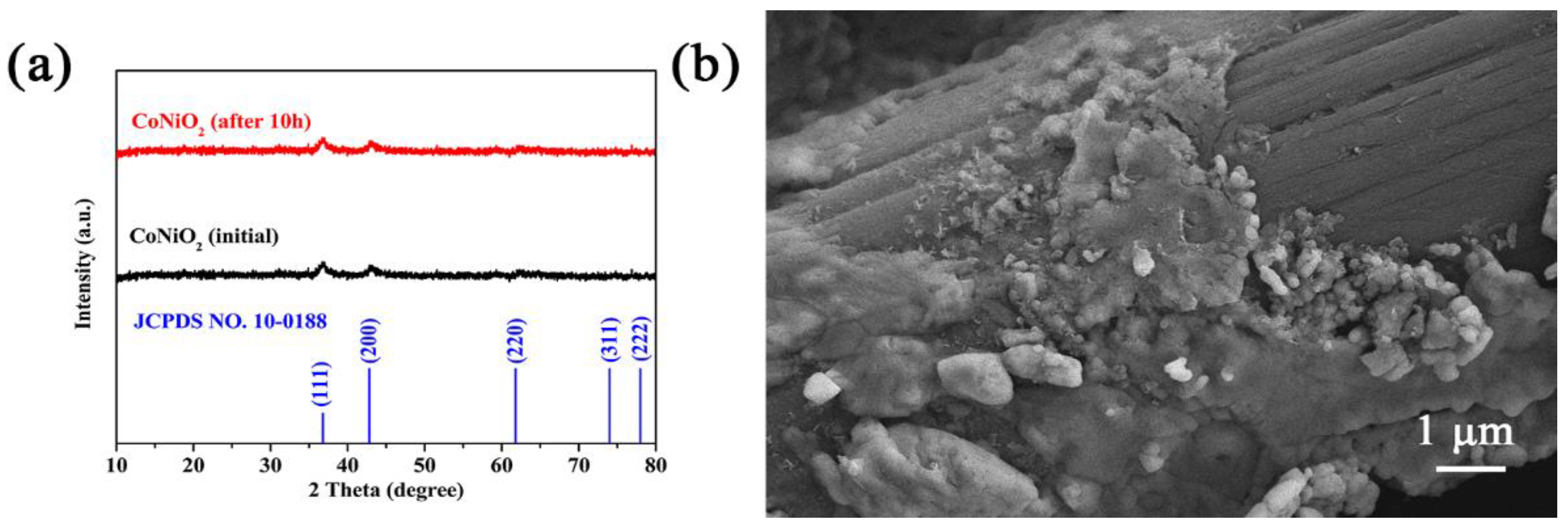
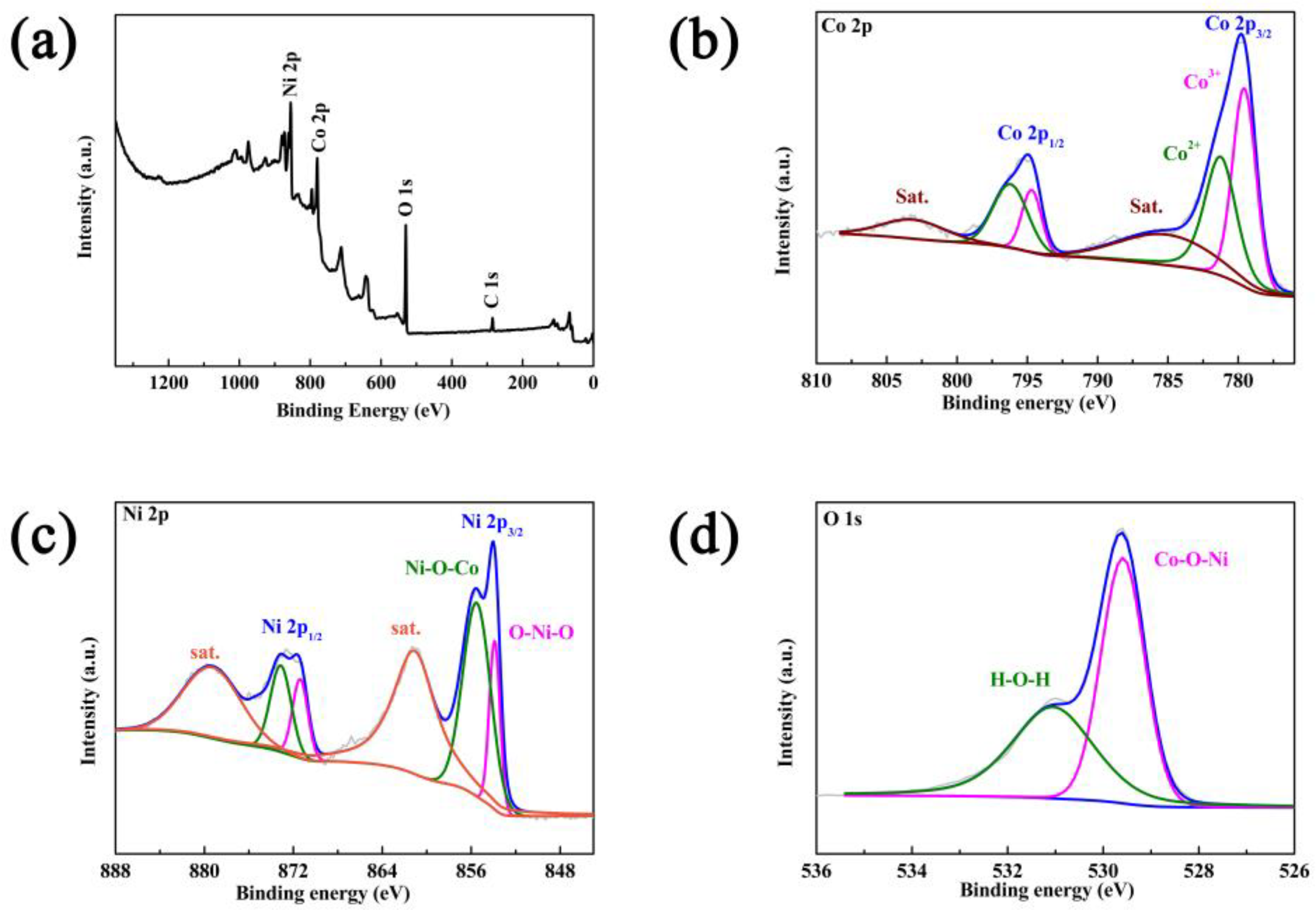
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L. Technology: Hydrogen-fuelled vehicles. Nature 2009, 460, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, Z.; Bai, X.; Zhang, X.; Gao, R.; Yuan, W.; Chen, Z.; Li, Z.; Li, Y. In situ formed edge-rich Ni3S2-NiOOH heterojunctions for oxygen evolution reaction. J. Electrochem. Soc. 2022, 169, 054532. [Google Scholar] [CrossRef]
- Battiato, S.; Bruno, L.; Pellegrino, A.L.; Terrasi, A.; Mirabella, S. ×Optimized electroless deposition of NiCoP electrocalysts for enhanced water splitting. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Ledendecker, M.; Mondschein, J.S.; Kasian, O.; Geiger, S.; Gohl, D.; Schalenbach, M.; Zeradjanin, A.; Cherevko, S.; Schaak, R.E.; Mayrhofer, K. Stability and activity of non-noble-metal-based catalysts toward the hydrogen evolution reaction. Angew Chem. Int. Ed. Engl. 2017, 56, 9767–9771. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, H.; Zhang, Y.; Luo, P.; Liu, L.; Wang, Y. Striking hierarchical urchin-like peapoded NiCo2O4@C as advanced bifunctional electrocatalyst for overall water splitting. J. Power Source 2017, 372, 46–53. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, Z.; Xu, H.; Liu, L.; Guan, Y.; Gu, X.; Wang, Y. Crystal-plane engineering of NiCo2O4 electrocatalysts towards efficient overall water splitting. J. Catal. 2018, 357, 238–246. [Google Scholar] [CrossRef]
- Wang, L.Y.; Gu, C.D.; Ge, X.; Zhang, J.L.; Zhu, H.Y.; Tu, J.P. A NiCo2O4 shell on a hollow Ni nanorod array core for water splitting with enhanced electrocatalytic performance. Chemnanomat 2018, 4, 124–131. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, E.; Sun, G. Layered transition-metal hydroxides for alkaline hydrogen evolution reaction. Chin. J. Catal. 2020, 41, 574–591. [Google Scholar] [CrossRef]
- Hu, C.; Lv, C.; Liu, S.; Shi, Y.; Song, J.; Zhang, Z.; Cai, J.; Watanabe, A. Nickel phosphide electrocatalysts for hydrogen evolution reaction. Catalysts 2020, 10, 88. [Google Scholar] [CrossRef]
- Wei, Y.; Soomro, R.A.; Xie, X.; Xu, B. Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D MXenes. J. Energy Chem. 2021, 55, 244–255. [Google Scholar] [CrossRef]
- Shahroudi, A.; Esfandiari, M.; Habibzadeh, S. Nickel sulfide and phosphide electrocatalysts for hydrogen evolution reaction: Challenges and future perspectives. RSC Adv. 2022, 12, 29440–29468. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Kundu, S.; Noda, S. Progess in nickel chalcogenide electrocatalyzed hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 4174–4192. [Google Scholar] [CrossRef]
- Ge, Z.; Fu, B.; Zhao, J.; Li, X.; Ma, B.; Chen, Y. A review of the electrocatalysts on hydrogen evolution reaction with an emphasis on Fe, Co and Ni-based phosphides. J Mater. Sci. 2020, 55, 14081–14104. [Google Scholar] [CrossRef]
- Bai, S.; Yang, M.; Jiang, J.; He, X.; Zou, J.; Xiong, Z.; Liao, G.; Liu, S. Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. NPJ 2D Mater. Appl. 2021, 5, 78. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Paudel, D.R.; Dhakal, P.P.; Kim, N.H.; Lee, J.H. Hybridized bimetallic phosphides of Ni–Mo, Co–Mo, and Co–Ni in a single ultrathin-3D-nanosheets for efficient HER and OER in alkaline media. Compos. Part B Eng. 2022, 239, 109992. [Google Scholar] [CrossRef]
- Battiato, S.; Pellegrino, A.L.; Pollicino, A.; Terrasi, A.; Mirabella, S. Composition-controlled chemical bath deposition of Fe-doped NiO microflowers for boosting oxygen evolution reaction. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.J.; Jang, Y.H. Electrodeposition of Stable Noble-Metal-Free Co-P Electrocatalysts for Hydrogen Evolution Reaction. Materials 2023, 16, 593. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, Y.D.; Yan, Q.; Yin, J.L.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; Cao, D.X.; Wang, G.L. In situ growth of Ni0.85Se on graphene as a robust electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 10486–10493. [Google Scholar] [CrossRef]
- Singh, K.P.; Shin, C.H.; Lee, H.Y.; Razmjooei, F.; Sinhamahapatra, A.; Kang, J.; Yu, J.S. TiO2/ZrO2 nanoparticle composites for electrochemical hydrogen evolution. ACS Appl. Nano Mater. 2020, 3, 3634–3645. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Feng, L.; Jin, C.; Ren, M.; Xing, H.; Shi, J. Fabrication of the flower-like NiCo2O4 @ Ni(OH)2/NiOOH composites supported on nickel foam by the green solvent dimethyl sulfoxide with high performance in supercapacitor and hydrogen evolution reaction. J. Phys. Chem. Solids 2021, 159, 110257. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Wang, J.; Geng, Y.; Zhang, B.; He, J.; Xue, J.; Frauenheim, T.; Li, M. Ni/Mo Bimetallic-Oxide-Derived Heterointerface-Rich Sulfide Nanosheets with Co-Doping for Efficient Alkaline Hydrogen Evolution by Boosting Volmer Reaction. Small 2021, 17, e2006730. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Yu, Y.; Ahmad, M.; Sun, H. Facile synthesis of single-crystal mesoporous CoNiO2 nanosheets assembled flowers as anode materials for lithium-ion batteries. Electrochim. Acta 2014, 132, 404–409. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Yu, Y.; Li, J.; Ahmad, M.; Sun, H. Hierarchical CoNiO2 structures assembled from mesoporous nanosheets with tunable porosity and their application as lithium-ion battery electrodes. New J. Chem. 2014, 38, 3084–3091. [Google Scholar] [CrossRef]
- Yeom, H.C.; Moon, D.J.; Lee, K.Y.; Kim, S.W. Formation and characterization of Ni nanofiber catalysts on nickel metallic foam by electrospinning process. J. Nanosci. Nanotechnol. 2015, 15, 5167–5170. [Google Scholar] [CrossRef]
- Wang, D.; Guo, J.; Hu, D.; Xu, Q.; Zhang, L.G.; Wang, J.D. Co@Co3O4 Prepared in situ from metallic Co as an efficient semiconductor catalyst for photocatalytic water oxidation. ACS Sustain. Chem. Eng. 2018, 6, 8300–8307. [Google Scholar] [CrossRef]
- Yan, X.; Li, K.; Lyu, L.; Song, F.; He, J.; Niu, D.; Liu, L.; Hu, X.; Chen, X. From water oxidation to reduction: Transformation from Ni(x)Co(3-x)O4 nanowires to NiCo/NiCoO(x) heterostructures. ACS Appl. Mater. Interfaces 2016, 8, 3208–3214. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, P.; Zhang, X.; Dai, X.; Ma, Y.; Wang, Y.; Jiang, Y.; Liu, M.; Wang, Y. Bimetallic thin film NiCo-NiCoO2@NC as a superior bifunctional electrocatalyst for overall water splitting in alkaline media. J. Mater. Chem. A 2017, 5, 15901–15912. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, S.; Liu, W.; Wang, X.; Li, Q.; Zhao, Z.; Geng, F. Coupling molecularly ultrathin sheets of NiFe-layered double hydroxide on NiCo2O4 nanowire arrays for highly efficient overall water-splitting activity. ACS Appl. Mater. Interfaces 2017, 9, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim. Acta 2020, 361, 137040. [Google Scholar] [CrossRef]
- Wei, B.B.; Wu, J.; Mei, G.; Qi, Z.B.; Hu, W.S.; Wang, Z.C. NiCo2O4 nanowire arrays rich in oxygen deficiencies for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 6612–6617. [Google Scholar] [CrossRef]
- Kong, Q.; Feng, W.; Ma, S.; Sun, F.; Xie, X.; Sun, C. Hydrothermal synthesis of nanoporous NiO rods self-supported on Ni foam as efficient electrocatalysts for hydrogen evolution reaction. JOM 2018, 71, 621–625. [Google Scholar] [CrossRef]
- Hao, S.; Cao, Q.; Yang, L.; Che, R. Morphology-optimized interconnected Ni3S2 nanosheets coupled with Ni(OH)2 nanoparticles for enhanced hydrogen evolution reaction. J. Alloys Compd. 2020, 827, 154163. [Google Scholar] [CrossRef]
- Sung, M.C.; Lee, G.H.; Kim, D.W. CeO2/Co(OH)2 hybrid electrocatalysts for efficient hydrogen and oxygen evolution reaction. J. Alloys Compd. 2019, 800, 450–455. [Google Scholar] [CrossRef]
- Ullah, N.; Xie, M.; Chen, L.; Yaseen, W.; Zhao, W.; Yang, S.; Xu, Y.; Xie, J. Novel 3D graphene ornamented with CoO nanoparticles as an efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Mater. Chem. Phys. 2021, 261, 124237. [Google Scholar] [CrossRef]
- Mohan Kumar, G.; Ilanchezhiyan, P.; Siva, C.; Madhankumar, A.; Kang, T.W.; Kim, D.Y. Co-Ni based hybrid transition metal oxide nanostructures for cost-effective bi-functional electrocatalytic oxygen and hydrogen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 391–400. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Tahira, A.; Tang, P.; Liu, X.; Morante, J.R.; Fahlman, M.; Arbiol, J.; Vagin, M.; Vomiero, A. MoSx@NiO composite nanostructures: An advanced nonprecious catalyst for hydrogen evolution reaction in alkaline media. Adv. Funct. Mater. 2019, 29, 1807562. [Google Scholar] [CrossRef]

| Catalyst | Current Density (mA·cm−2) | Potential (mV) | Reference |
|---|---|---|---|
| NiFe-LDH/NiCo2O4 | 10 | 192 | [31] |
| NiCo2O4 nanowies | 10 | 294 | [31] |
| Ni/NiO | 10 | 226 | [32] |
| Air- NiCo2O4 | 10 | 226 | [33] |
| Ni@NiO | 10 | 192 | [34] |
| Ni3S2@Ni(OH)2 | 10 | 237 | [35] |
| CeO2/Co(OH)2 | 10 | 317 | [36] |
| CoO-3D graphen | 10 | 360 | [37] |
| Co-Ni hybrid oxides | 10 | 378 | [38] |
| MoSx@NiO | 10 | 406 | [39] |
| CoNiO2 | 10 | 170 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Yao, J.; Yin, J.; Wang, G.; Zhu, K.; Yan, J.; Cao, D.; Zhu, M. Hierarchical CoNiO2 Microflowers Assembled by Mesoporous Nanosheets as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Materials 2023, 16, 2204. https://doi.org/10.3390/ma16062204
Zhang D, Yao J, Yin J, Wang G, Zhu K, Yan J, Cao D, Zhu M. Hierarchical CoNiO2 Microflowers Assembled by Mesoporous Nanosheets as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Materials. 2023; 16(6):2204. https://doi.org/10.3390/ma16062204
Chicago/Turabian StyleZhang, Dingfu, Jiaxin Yao, Jinling Yin, Guiling Wang, Kai Zhu, Jun Yan, Dianxue Cao, and Min Zhu. 2023. "Hierarchical CoNiO2 Microflowers Assembled by Mesoporous Nanosheets as Efficient Electrocatalysts for Hydrogen Evolution Reaction" Materials 16, no. 6: 2204. https://doi.org/10.3390/ma16062204
APA StyleZhang, D., Yao, J., Yin, J., Wang, G., Zhu, K., Yan, J., Cao, D., & Zhu, M. (2023). Hierarchical CoNiO2 Microflowers Assembled by Mesoporous Nanosheets as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Materials, 16(6), 2204. https://doi.org/10.3390/ma16062204









