Influence of the Addition of Rare Earth Elements on the Energy Storage and Optical Properties of Bi0.5Na0.5TiO3–0.06BaTiO3 Polycrystalline Thin Films
Abstract
1. Introduction
2. Materials and Methods
3. Results
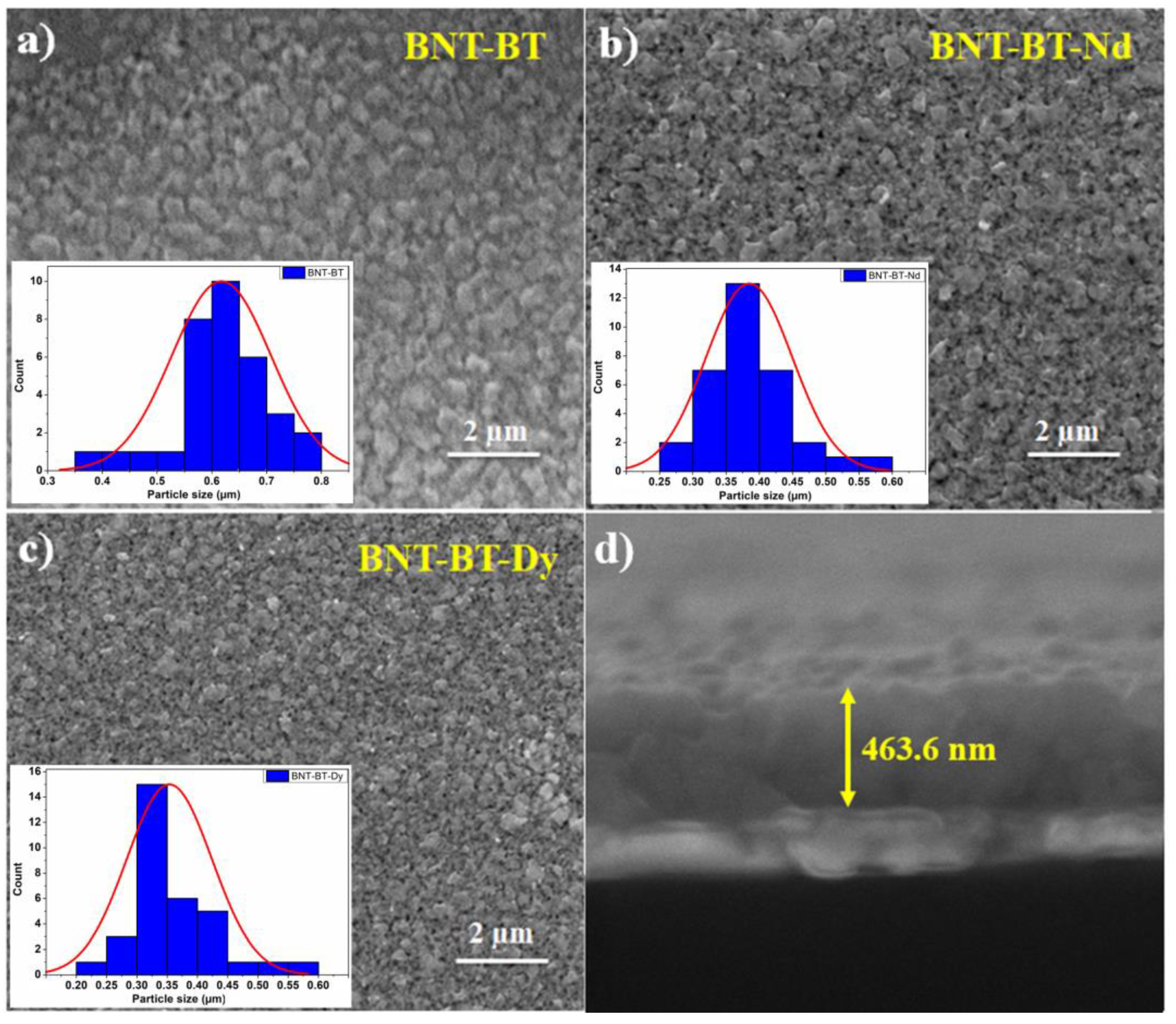
3.1. Microstructural and Structural Investigations
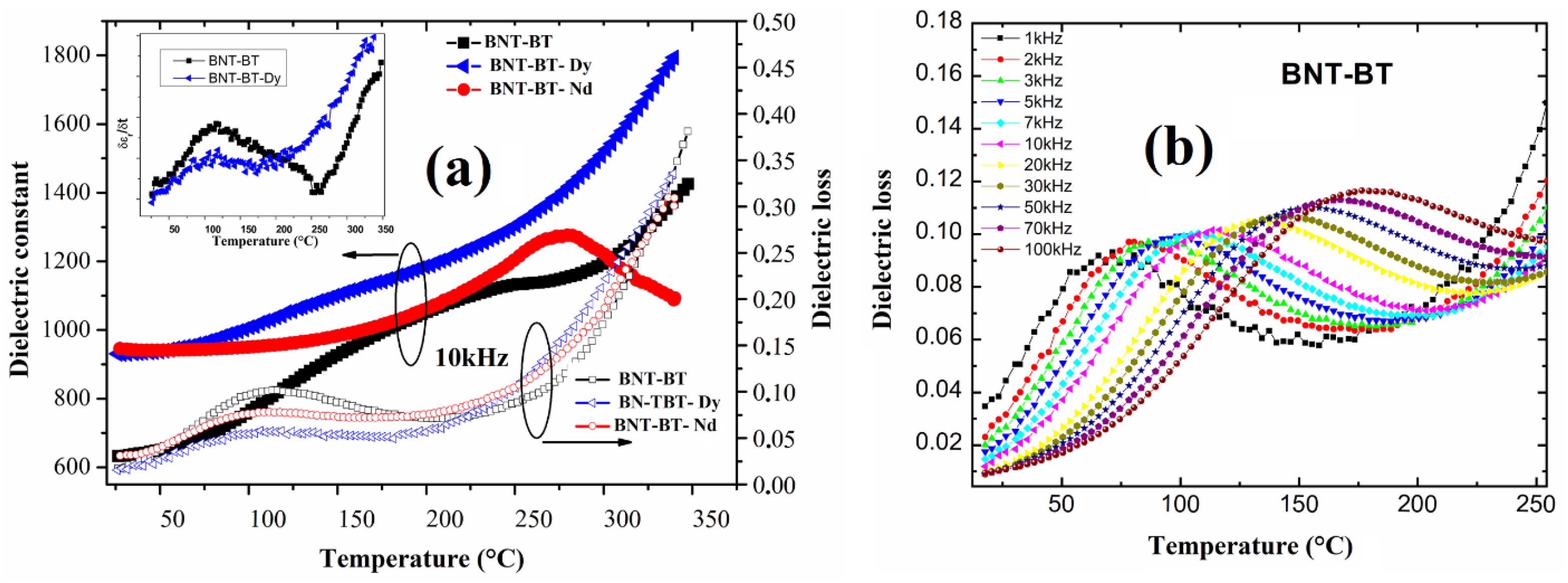
3.2. Dielectric Investigations
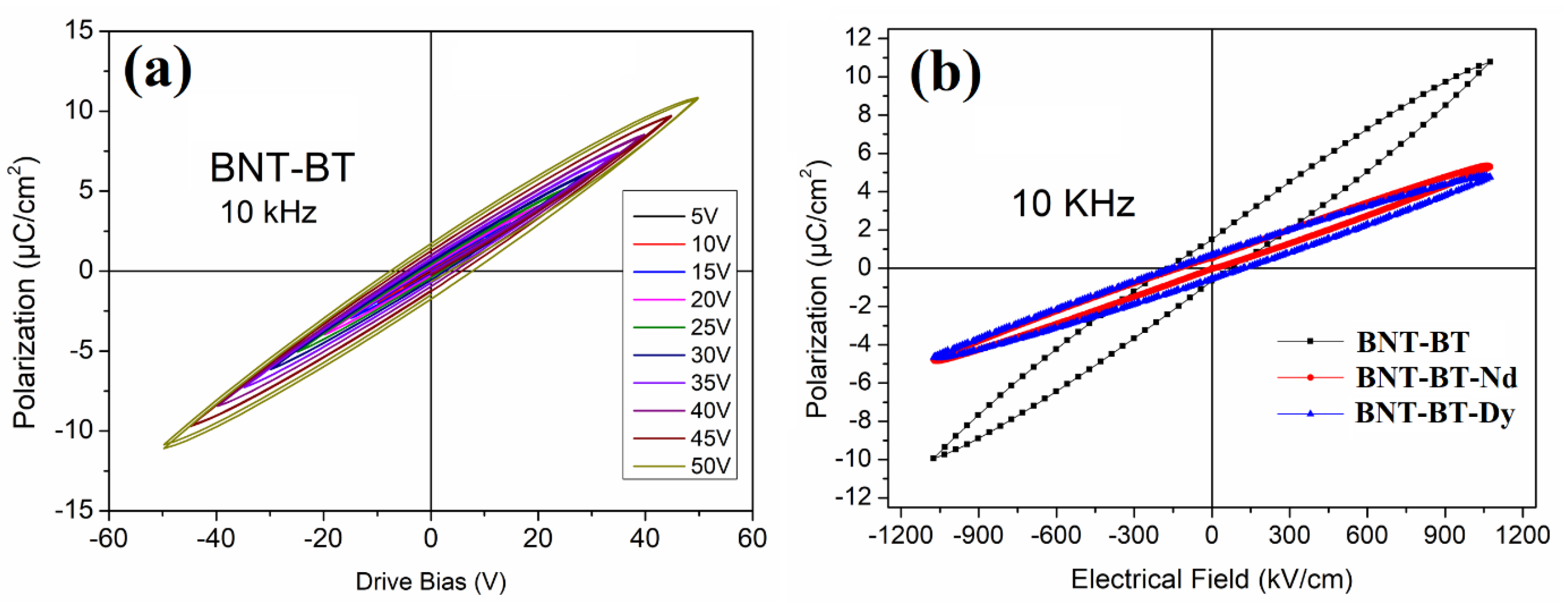
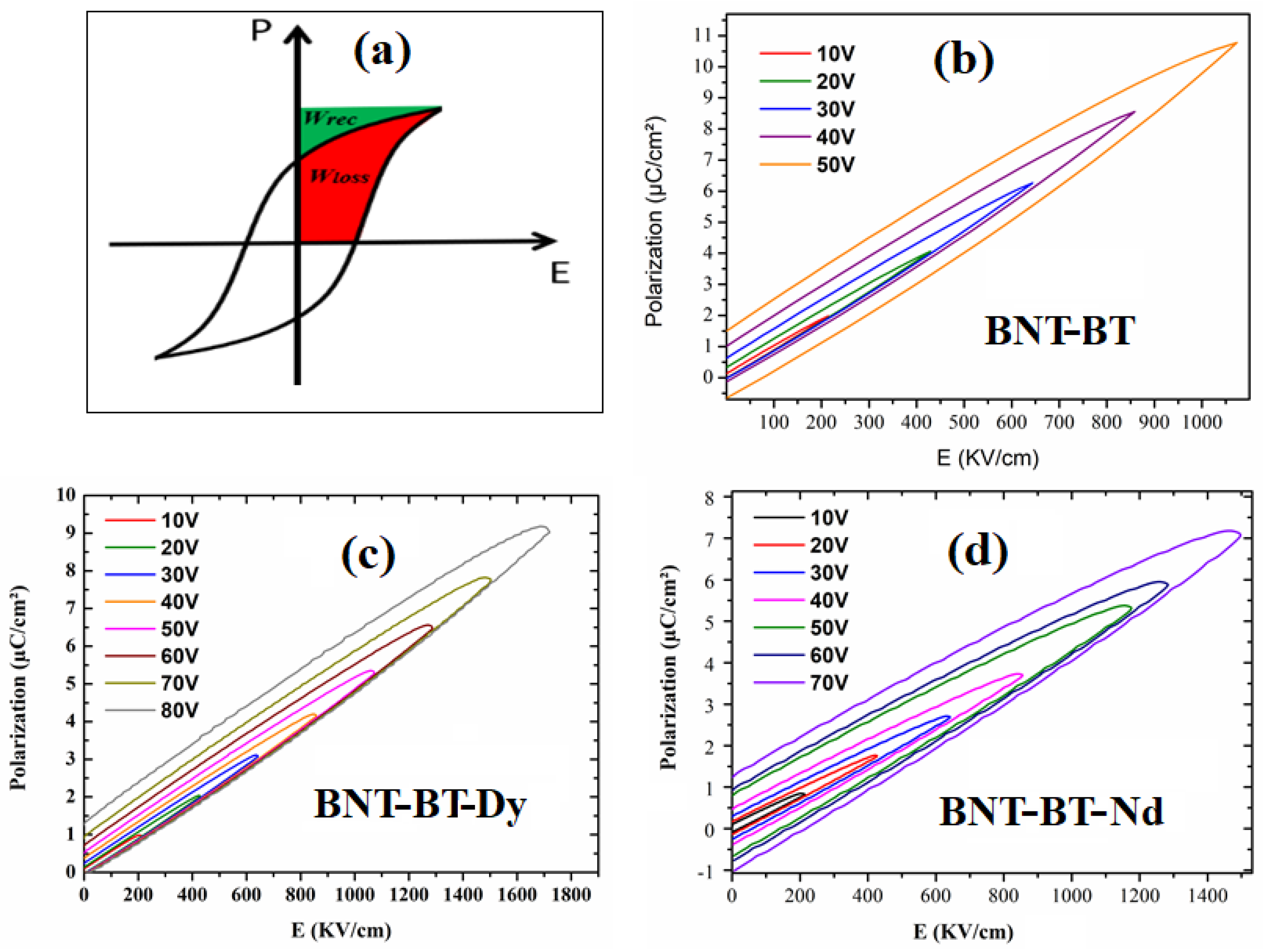
3.3. Ferroelectric and Energy Storage Investigations
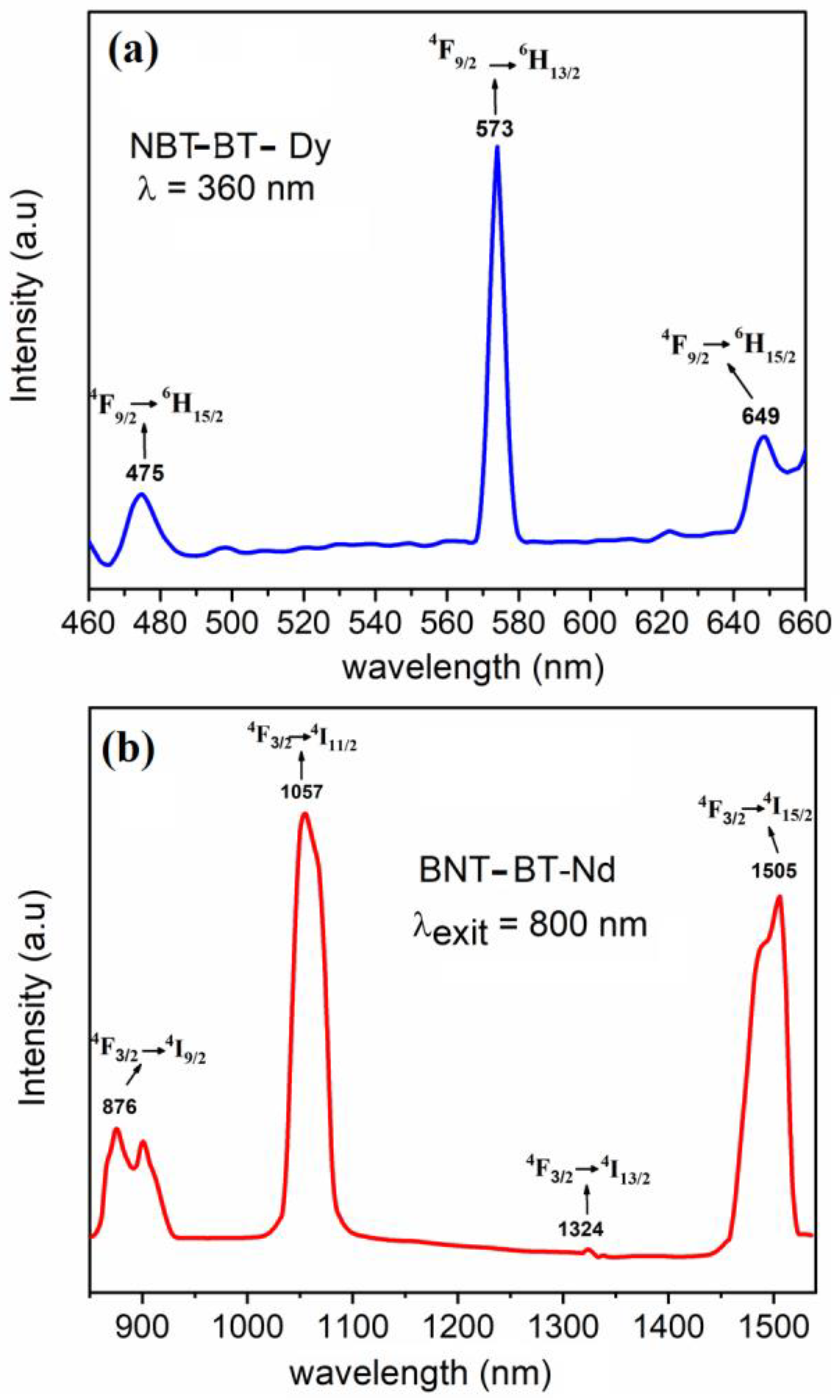
3.4. Optical Investigations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nihal, K. Energy Storage Devices for Electronic Systems: Rechargeable Batteries and Supercapacitors; School of Engineering, The University of Waikato: Hamilton, New Zealand, 2015. [Google Scholar]
- Zannen, M.; Belhadi, J.; Benyoussef, M.; Khemakhem, H.; Zaidat, K.; El Marssi, M.; Lahmar, A. Electrostatic energy storage in antiferroelectric like perovskite. Superlattices Microstruct. 2019, 127, 43–48. [Google Scholar] [CrossRef]
- Benyoussef, M.; Belhadi, J.; Lahmar, A.; El Marssi, M. Tailoring the dielectric and energy storage properties in BaTiO3/BaZrO3 superlattices. Mater. Lett. 2019, 234, 279–282. [Google Scholar] [CrossRef]
- Benyoussef, M.; Zannen, M.; Belhadi, J.; Manoun, B.; Kutnjak, Z.; Vengust, D.; Spreitzer, M.; El Marssi, M.; Lahmar, A. Structural, dielectric, and ferroelectric properties of Na0.5(Bi1-xNdx)0.5TiO3 ceramics for energy storage and electrocaloric applications. Ceram. Int. 2021, 47, 26539–26551. [Google Scholar] [CrossRef]
- Benyoussef, M.; Zannen, M.; Belhadi, J.; Manoun, B.; Dellis, J.L.; El Marssi, M.; Lahmar, A. Dielectric, ferroelectric, and energy storage properties in dysprosium doped sodium bismuth titanate ceramics. Ceram. Int. 2018, 44, 19451–19460. [Google Scholar] [CrossRef]
- Mezzourh, H.; Belkhadir, S.; Mezzane, D.; Amjoud, M.; Choukri, E.; Lahmar, A.; Gagou, Y.; Kutnjak, Z.; El Marssi, M. Enhancing the dielectric, electrocaloric and energy storage properties of lead-free Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics prepared via sol-gel process. Phys. B Condens. Matter 2021, 603, 412760. [Google Scholar] [CrossRef]
- Xie, Y.; Hao, H.; Xie, J.; Zhang, S.; Cao, M.; Yao, Z.; Liu, H. Ultra-high energy storage density and enhanced dielectric properties in BNT-BT based thin film. Ceram. Int. 2021, 47, 23259–23266. [Google Scholar] [CrossRef]
- Chandrasekhar, M.; Khatua, D.K.; Pattanayak, R.; Kumar, P. Dielectric relaxation and conduction mechanism studies of BNT-BT-BKT ceramics. J. Phys. Chem. Solids 2017, 111, 160–166. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Sun, N.; Du, J.; Li, X.; Zhang, L.; Zhang, Q.; Hao, X. High energy-storage performance of BNT-BT-NN ferroelectric thin films prepared by RF magnetron sputtering. J. Alloys Compd. 2018, 750, 228–234. [Google Scholar] [CrossRef]
- Kim, M.-K.; Ji, S.-Y.; Lim, J.-H.; Kim, S.-W.; Jeong, D.-Y. Energy storage performance and thermal stability of BNT-SBT with artificially modulated nano-grains via aerosol deposition method. J. Asian Ceram. Soc. 2022, 10, 196–202. [Google Scholar] [CrossRef]
- Belarbi, M.; Tamraoui, Y.; Manoun, B.; Cantaluppi, A.; Gagou, Y.; Taibi, K.; El Marssi, M.; Lahmar, A. Structural, dielectric and energy storage properties of Neodymium niobate with tetragonal tungsten bronze structure. Phys. B Condens. Matter 2021, 618, 413185. [Google Scholar] [CrossRef]
- Daniel, W.; Margieh, U. Energy Storage Capacitor Technology Comparison and Selection. In Proceedings of the 3rd PCNS, KYOCERA AVX, Milano, Italy, 7–10 September 2021; pp. 1–12. Available online: https://epci.eu/energy-storage-capacitor-technology-comparison-and-selection/ (accessed on 6 February 2023).
- Belhadi, J.; Hanani, Z.; Trstenjak, U.; Shepelin, N.A.; Bobnar, V.; Koster, G.; Hlinka, J.; Pergolesi, D.; Lippert, T.; El Marssi, M.; et al. Large imprint in epitaxial 0.67Pb(Mg1/3Nb2/3)O3-0.33PbTiO3 thin films for piezoelectric energy harvesting applications. Appl. Phys. Lett. 2022, 121, 182903. [Google Scholar] [CrossRef]
- Ait Tamerd, M.; Abraime, B.; El Rhazouani, O.; Lahmar, A.; El Marssi, M.; Hamedoun, M.; Benyoussef, A.; El Kenz, A. Modelling of the ferroelectric and energy storage properties of PbZr1−xTixO3 thin films using Monte Carlo simulation. Mater. Res. Express 2019, 6, 126429. [Google Scholar] [CrossRef]
- Radley-Gardner, O.; Beale, H.; Zimmermann, R. Directive 2011/83/EU of the European Parliament and of the Council. In Fundamental Texts On European Private Law; Hart Publishing: London, UK, 2020; pp. 629–665. [Google Scholar] [CrossRef]
- Gao, F.; Dong, X.; Mao, C.; Liu, W.; Zhang, H.; Yang, L.; Cao, F.; Wang, G. Energy-Storage Properties of 0.89Bi0.5Na0.5TiO3–0.06BaTiO3–0.05K0.5Na0.5NbO3 Lead-Free Anti-ferroelectric Ceramics. J. Am. Ceram. Soc. 2011, 94, 4382–4386. [Google Scholar] [CrossRef]
- Chandrasekhar, M.; Kumar, P. Synthesis and characterizations of BNT–BT and BNT–BT–KNN ceramics for actuator and energy storage applications. Ceram. Int. 2015, 41, 5574–5580. [Google Scholar] [CrossRef]
- Yang, H.; Yan, F.; Lin, Y.; Wang, T.; Wang, F. High energy storage density over a broad temperature range in sodium bismuth titanate-based lead-free ceramics. Sci. Rep. 2017, 7, 8726. [Google Scholar] [CrossRef]
- Wendari, T.P.; Arief, S.; Mufti, N.; Blake, G.R.; Baas, J.; Suendo, V.; Prasetyo, A.; Insani, A.; Zulhadjri, Z. Lead-Free Aurivillius Phase Bi2LaNb1.5Mn0.5O9: Structure, Ferroelectric, Magnetic, and Magnetodielectric Effects. Inorg. Chem. 2022, 61, 8644–8652. [Google Scholar] [CrossRef]
- McCabe, E.E.; Bousquet, E.; Stockdale, C.P.J.; Deacon, C.A.; Tran, T.T.; Halasyamani, P.S.; Stennett, M.C.; Hyatt, N.C. Proper Ferroelectricity in the Dion–Jacobson Material CsBi2Ti2NbO10: Experiment and Theory. Chem. Mater. 2015, 27, 8298–8309. [Google Scholar] [CrossRef]
- Wang, H.; Gou, G.; Li, J. Ruddlesden–Popper perovskite sulfides A3B2S7: A new family of ferroelectric photovoltaic materials for the visible spectrum. Nano Energy 2016, 22, 507–513. [Google Scholar] [CrossRef]
- Davies, P.K.; Wu, H.; Borisevich, A.Y.; Molodetsky, I.E.; Farber, L. Crystal Chemistry of Complex Perovskites: New Cation-Ordered Dielectric Oxides. Annu. Rev. Mater. Res. 2008, 38, 369–401. [Google Scholar] [CrossRef]
- Jo, W.; Schaab, S.; Sapper, E.; Schmitt, L.A.; Kleebe, H.J.; Bell, A.J.; Rödel, J. On the phase identity and its thermal evolution of lead free (Bi1/2Na1/2)TiO3-6 mol BaTiO3. J. Appl. Phys. 2011, 110, 074106. [Google Scholar] [CrossRef]
- Viola, G.; Ning, H.; Wei, X.; Deluca, M.; Adomkevicius, A.; Khaliq, J.; Reece, J.; Yan, H. Dielectric relaxation, lattice dynamics and polarization mechanisms in Bi0.5Na0.5TiO3-based lead-free ceramics. J. Appl. Phys. 2013, 114, 014107. [Google Scholar] [CrossRef]
- Chu, B.J.; Chen, D.; Li, G.; Yin, Q. Electrical properties of Na1/2Bi1/2TiO3–BaTiO3 ceramics. J. Eur. Ceram. Soc. 2002, 22, 2115–2121. [Google Scholar] [CrossRef]
- Pan, H.; Zeng, Y.; Shen, Y.; Lin, Y.; Ma, J.; Li, L.; Nan, C. BiFeO3–SrTiO3 thin film as a new lead-free relaxor-ferroelectric capacitor with ultrahigh energy storage performance. J. Mater. Chem. 2017, 5, 5920–5926. [Google Scholar] [CrossRef]
- Lahmar, A.; Belhadi, J.; El Marssi, M.; Zannen, M.; Khemakhem, H.; Al-Dahoudi, N. Energy storage property of Lead-free Na0.5Bi0.5TiO3 ceramic and thin film. In Proceedings of the 2017 International Conference in Energy and Sustainability in Small Developing Economies (ES2DE), Funchal, Portugal, 10–12 July 2017; pp. 1–4. [Google Scholar]
- Cho, S.; Yun, C.; Kim, Y.S.; Wang, H.; Jian, J.; Zhang, W.; Huang, J.; Wang, X.; Wang, H.; MacManus-Driscoll, J.L. Strongly enhanced dielectric and energy storage properties in lead-free perovskite titanate thin films by alloying. Nano Energy 2018, 45, 398–406. [Google Scholar] [CrossRef]
- Gallegos-Melgar, A.; Espinosa-Arbelaez, D.G.; Flores-Ruiz, F.J.; Lahmar, A.; Dellis, J.L.; Lemée, N.; Espinoza-Beltran, F.J.; Muñoz-Saldaña, J. Ferroelectric properties of manganese doped (Bi1/2 Na1/2)TiO3 and (Bi1/2 Na1/2 )TiO3-BaTiO3 epitaxial thin films. Appl. Surf. Sci. 2015, 359, 923–930. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, W.L.; Cao, W.P.; Zhang, T.D.; Bai, T.R.G.L.; Yu, Y.; Hou, Y.F.; Feng, Y.; Fei, W.D. Enhanced energy-storage performance of 0.94NBT-0.06BT thin films induced by a Pb0.8La0.1Ca0.1Ti0.975O3 seed layer. Ceram. Int. 2016, 42, 14788–14792. [Google Scholar] [CrossRef]
- Alonso-Sanjosé, D.; Jiménez, R.; Bretos, I.; Calzada, M.L. Lead-free ferroelectric (Na1/2Bi1/2)TiO3-BaTiO3 thin films in the morphotropic phase boundary composition: Solution processing and properties. J. Am. Ceram. Soc. 2009, 92, 2218–2225. [Google Scholar] [CrossRef]
- Badapanda, T.; Sahoo, S.; Nayak, P. Dielectric, Ferroelectric and Piezoelectric study of BNT-BT solid solutions around the MPB region. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Orissa, India, 9–10 December 2017; Volume 178, p. 12032. [Google Scholar] [CrossRef]
- Aissa, M.; Zannen, M.; Alzahrani, H.A.H.; Belhadi, J.; Hadouch, Y.; Mezzane, D.; El Marssi, M.; Majdoub, M.; Lahmar, A. Multifunctionality of rare earth doped 0.925Na0.5Bi0.5TiO3-0.075K0.5Na0.5NbO3 ferroelectric ceramics. J. Alloys Compd. 2022, 921, 166188. [Google Scholar] [CrossRef]
- Lahmar, A.; Ehrenberg, H.; Antic-Fidancev, E.; Ganschow, S.; Zriouil, M.; Elouadi, B. Single crystal structure determination and infrared fluorescence of the system (K3Sr1−xNdx) (Nd1−xSr1+x) Nb10O30. Mater. Res. Bull. 2012, 47, 2566–2572. [Google Scholar] [CrossRef]
- Chahal, R.; Starecki, F.; Doualan, J.-L.; Němec, P.; Trapananti, A.; Prestipino, C.; Tricot, G.; Boussard-Pledel, C.; Michel, K.; Braud, A.; et al. Nd3+: Ga-Ge-Sb-S glasses and fibers for luminescence in mid-IR: Synthesis, structural characterization and rare earth spectroscopy. Opt. Mater. Express 2018, 8, 1650. [Google Scholar] [CrossRef]




| Samples | Emax (kV/cm) | Wrec (J/cm3) | η (%) | Ref. |
|---|---|---|---|---|
| BNT-BT | 1080 (real) | 4.5 | 45 | This work |
| BNT-BT-xNd (x = 0.01) | 1080 (real) 1780 (real) | 2 5.3 | 68 59 | This work |
| 2500 (effective) | 12 | 58 | ||
| BNT-BT-xDy (x = 0.01) | 1080 (real) 1780 (real) | 2.5 6.6 | 79 72 | This work |
| 2500 (effective) | 16 | 64 | ||
| BNT-BT-xMn (x = 0.2) | 2000 | 25 | 82 | [7] |
| BNT-BT-xMn (x = 0.6) | 2500 | 54 | 59 | [7] |
| BNT-xHo (=0.02) | 500 | 9.4 | 55 | [2] |
| BNT-xEr (=0.02) | 500 | 9.2 | 57 | [2] |
| BNT-BT-NaNbO3 (=0.1) | 3170 | 32 | 90 | [9] |
| 6BNT-4ST | 10.4 | 64.5 | 64.5 | [10] |
| 0.94BNT-0.06BT with PLCT seed layers | 17.2 | 74.3 | 74.3 | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaoui, I.H.; Moussa, M.; Lemée, N.; Le Marrec, F.; Cantaluppi, A.; Favry, D.; Lahmar, A. Influence of the Addition of Rare Earth Elements on the Energy Storage and Optical Properties of Bi0.5Na0.5TiO3–0.06BaTiO3 Polycrystalline Thin Films. Materials 2023, 16, 2197. https://doi.org/10.3390/ma16062197
Alaoui IH, Moussa M, Lemée N, Le Marrec F, Cantaluppi A, Favry D, Lahmar A. Influence of the Addition of Rare Earth Elements on the Energy Storage and Optical Properties of Bi0.5Na0.5TiO3–0.06BaTiO3 Polycrystalline Thin Films. Materials. 2023; 16(6):2197. https://doi.org/10.3390/ma16062197
Chicago/Turabian StyleAlaoui, Ilham Hamdi, Mebarki Moussa, Nathalie Lemée, Françoise Le Marrec, Anna Cantaluppi, Delphine Favry, and Abdelilah Lahmar. 2023. "Influence of the Addition of Rare Earth Elements on the Energy Storage and Optical Properties of Bi0.5Na0.5TiO3–0.06BaTiO3 Polycrystalline Thin Films" Materials 16, no. 6: 2197. https://doi.org/10.3390/ma16062197
APA StyleAlaoui, I. H., Moussa, M., Lemée, N., Le Marrec, F., Cantaluppi, A., Favry, D., & Lahmar, A. (2023). Influence of the Addition of Rare Earth Elements on the Energy Storage and Optical Properties of Bi0.5Na0.5TiO3–0.06BaTiO3 Polycrystalline Thin Films. Materials, 16(6), 2197. https://doi.org/10.3390/ma16062197








