Bioactive Carbonate Apatite Cement with Enhanced Compressive Strength via Incorporation of Silica Calcium Phosphate Composites and Calcium Hydroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation Specimen Groups
2.2. Compressive Strength Measurement
2.3. Preparation and Calculation of SBF
2.4. Bioactivity Evaluation Procedure
2.5. Scanning Electron Microscope and Energy Dispersive Spectroscopy (SEM-EDS)
2.6. X-ray Diffraction (XRD)
2.7. Fourier-Transform Infrared (FTIR)
2.8. Statistical Analysis
3. Results
3.1. Compressive Strength Evaluation
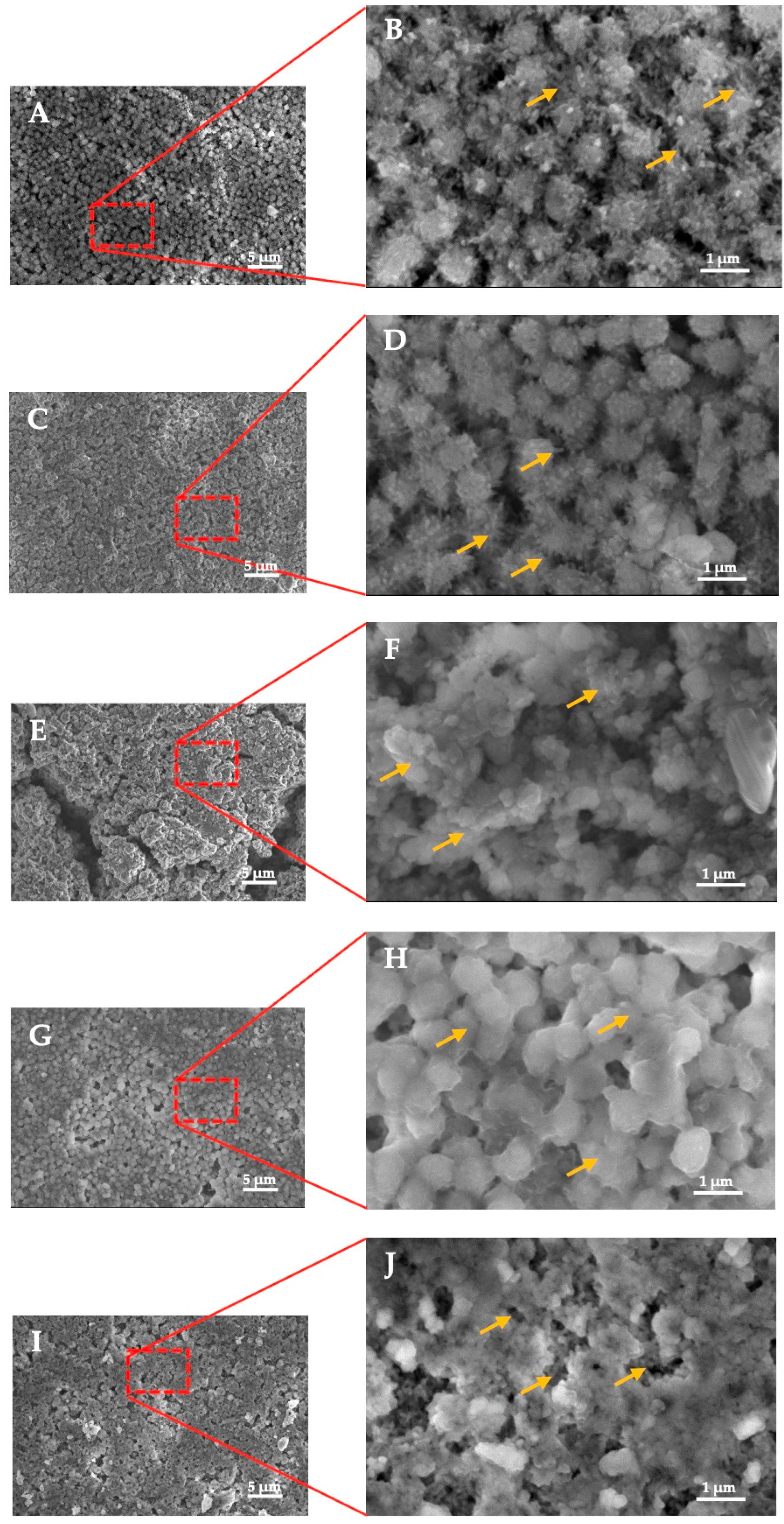
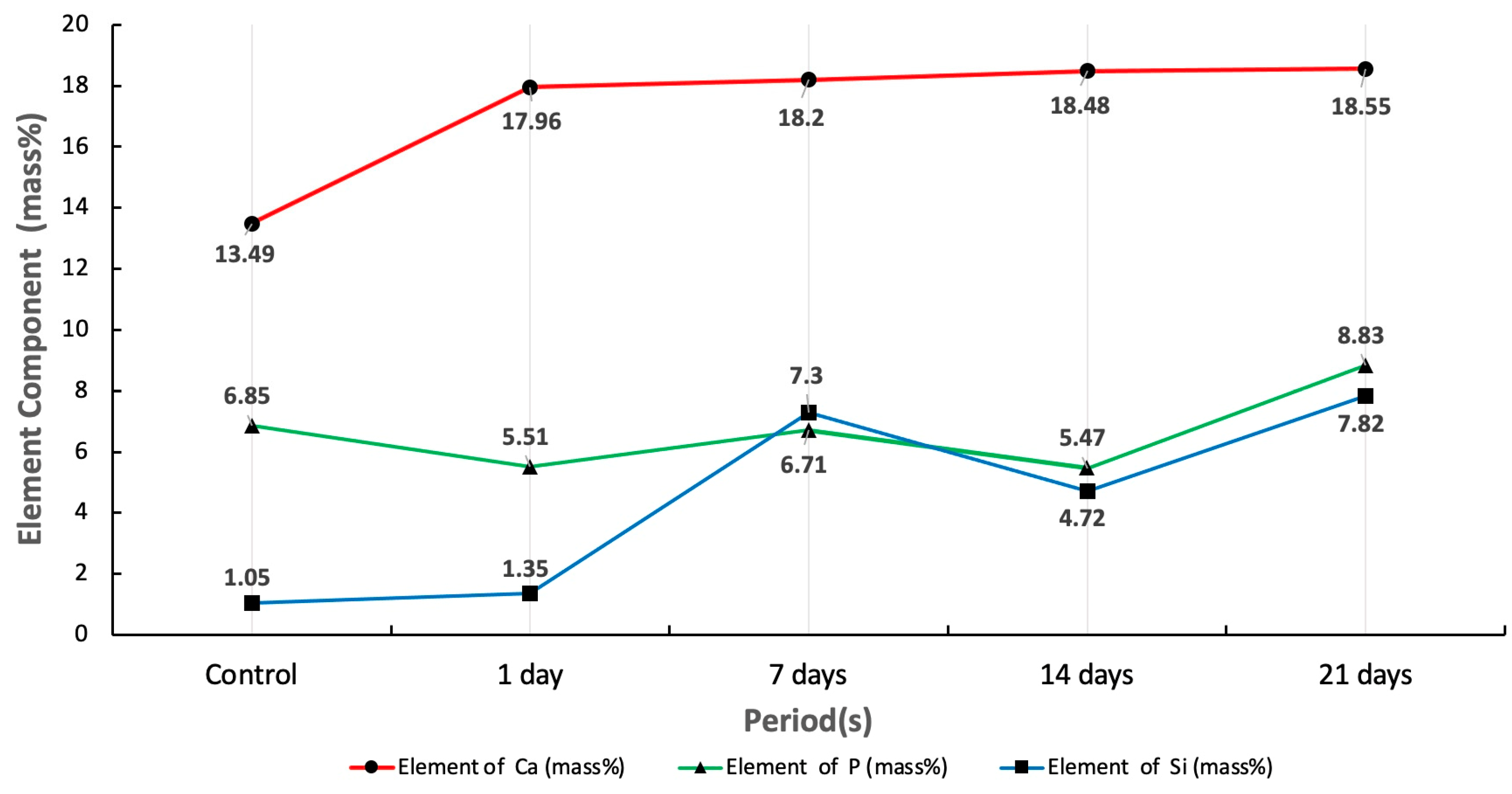
3.2. SEM-EDS Analysis
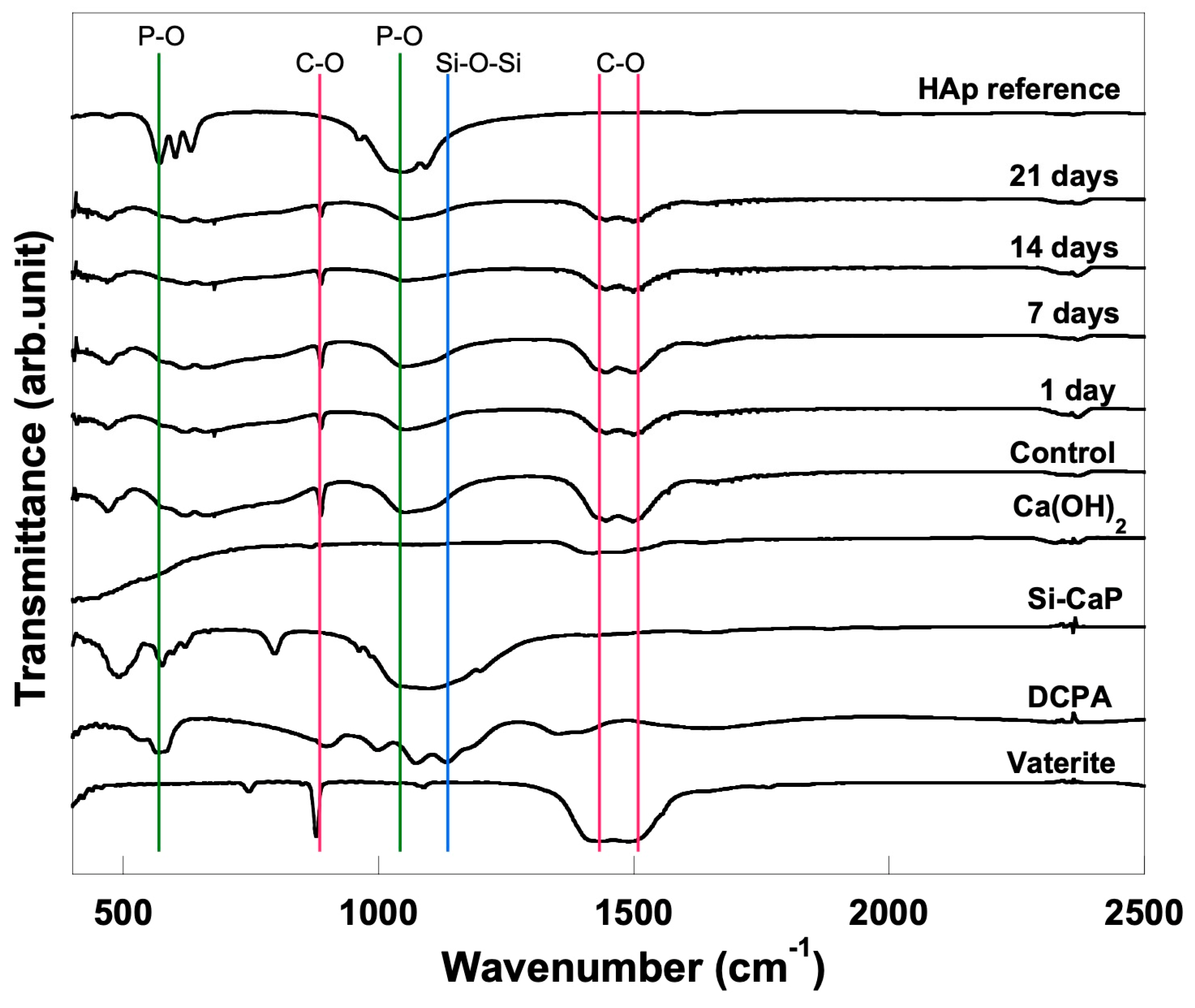
3.3. FTIR Analysis
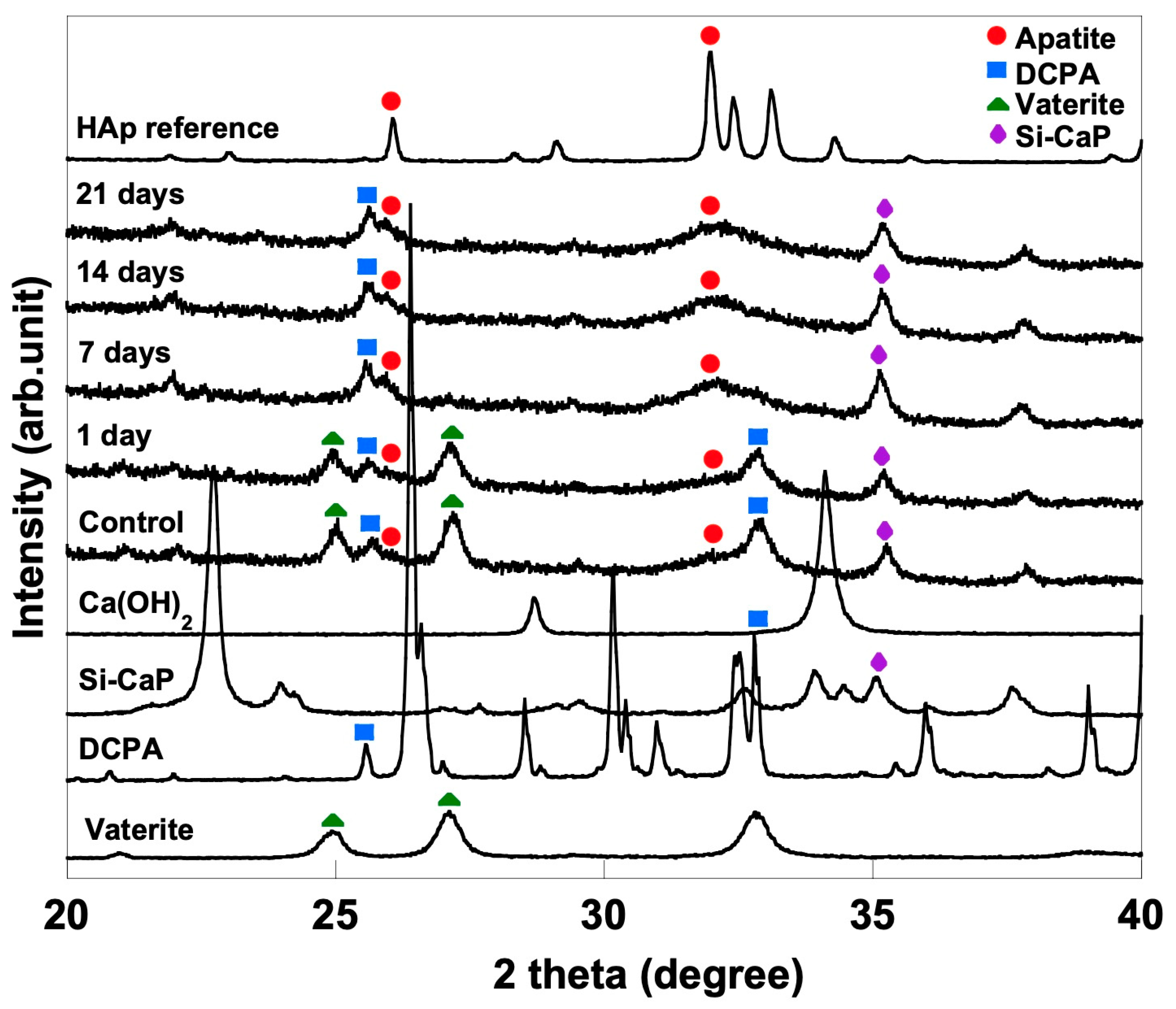
3.4. XRD Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium orthophosphate cements and concretes. Materials 2009, 2, 221–291. [Google Scholar] [CrossRef]
- Zakaria, M.N.; Cahyanto, A.; El-ghannam, A. Calcium release and physical properties of modified carbonate apatite cement as pulp capping agent in dental application. Biomat. Res. 2018, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Al-sanabani, J.S.; Madfa, A.A.; Al-sanabani, F.A. Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 2013, 876132. [Google Scholar] [CrossRef]
- Zakaria, M.N.; Fitriani, N.; Pauziah, N.; Sabirin, I.P.; Cahyanto, A. Evaluation of carbonate apatite cement in inducing formation of reparative dentin in exposed dental pulp. Key Eng. Mater. 2017, 758, 250–254. [Google Scholar] [CrossRef]
- Zakaria, M.N.; Cahyanto, A.; El-Ghannam, A. Basic properties of novel bioactive cement based on silica calcium phosphate composite and carbonate apatite. Key Eng. Mater. 2016, 720, 147–152. [Google Scholar] [CrossRef]
- Huang, J. Biology and Engineering Stem Cell Niches, Chapter 20 Design and Development of Ceramics and Glasses; Elsevier: London, UK, 2017; pp. 315–329. [Google Scholar]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Cahyanto, A.; Restunaesha, M.; Zakaria, M.N.; Rezano, A.; El-Ghannam, A. Compressive strength evaluation and phase analysis of pulp capping materials based on carbonate apatite-SCPC using different concentration of SCPC and calcium hydroxide. Key Eng. Mater. 2018, 782, 15–20. [Google Scholar] [CrossRef]
- Aniket; Young, A.; Marriott, I.; El-Ghannam, A. Promotion of pro-osteogenic responses by a bioactive ceramic coating. J. Biomed. Mater. Res. Part A 2012, 100, 3314–3325. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non. Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Sathorn, C.; Parashos, P.; Messer, H. Antibacterial efficacy of calcium hydroxide intracanal dressing: A systematic review and meta-analysis. Int. Endod. J. 2007, 40, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xiao, Y. Evaluation of the in vitro bioactivity of bioceramics. Bone Tissue Regener. Insights 2009, 2, 25–29. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodríguez-Lozano, F.J.; Llena, C.; Sauro, S.; Forner, L. Bioactivity of bioceramic materials used in the dentin-pulp complex therapy: A systematic review. Materials 2019, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Bahrololoom, M.E.; Shariat, M.H.; Bagheri, R.A. Bioactive glasses in dentistry: A review. J. Dent. Biomater. 2015, 2, 1–9. [Google Scholar]
- Shibata, H.; Yokoi, T.; Goto, T.; Kim, I.Y.; Kawashita, M.; Kikuta, K.; Ohtsuki, C. Behavior of hydroxyapatite crystals in a simulated body fluid: Effects of crystal face. J. Ceram. Soc. Jpn. 2013, 12, 807–812. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Simulated body fluid and the novel bioactive materials derived from it. J. Biomed. Mater. Res. Part A 2019, 107, 968–977. [Google Scholar] [CrossRef]
- El-Ghannam, A. Advanced bioceramic composite for bone tissue engineering: Design principles and structure bioactivity relationship. J. Biomed. Mater. Res. Part A 2004, 69, 490–501. [Google Scholar] [CrossRef]
- Cahyanto, A.; Maruta, M.; Tsuru, K.; Matsuya, S.; Ishikawa, K. Fabrication of bone cement that fully transforms to carbonate apatite. Dent. Mater. J. 2015, 34, 394–401. [Google Scholar] [CrossRef]
- BS ISO 23317:2014; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. BSI Standards Publication: London, UK, 2014; pp. 1–24.
- Cahyanto, A.; Tsuru, K.; Ishikawa, K. Carbonate apatite formation during the setting reaction of apatite cement. Adv. Bioceram. Porous Ceram. V 2010, 3, 147–164. [Google Scholar]
- Gupta, G.; Kirakodu, S.; White, D.; El-Ghannam, A. Dissolution kinetics of a Si-rich nanocomposite and its effect on osteoblast gene expression. J. Biomed. Mater. Res. Part A 2006, 80, 486–496. [Google Scholar] [CrossRef]
- Najdanović, J.; Rajković, J.; Najman, S. Bioactive biomaterials: Potential for application in bone regenerative medicine. In Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices, 1st ed.; Zivic, F., Affatato, S., Trajanovic, M., Schnabelrauch, M., Grujovic, N., Choy, K., Eds.; Springer International Publisher: New York, NY, USA, 2017; pp. 333–360. [Google Scholar]
- Smith, A.J.; Duncan, H.F.; Diogenes, A.; Simon, S.; Cooper, P.R. Exploiting the bioactive properties of the dentin-pulp complex in regenerative endodontics. J. Endod. 2016, 42, 47–56. [Google Scholar] [CrossRef]
- Daitou, F.; Maruta, M.; Kawachi, G.; Tsuru, K.; Matsuya, S.; Terada, Y.; Ishikawa, K. Fabrication of carbonate apatite block based on internal dissolution precipitation reaction of dicalcium phosphate and calcium carbonate. Dent. Mater. J. 2010, 29, 303–308. [Google Scholar] [CrossRef]
- Tsuru, K.; Yoshimoto, A.; Kanazawa, M.; Sugiura, Y.; Nakashima, Y.; Ishikawa, K. Fabrication of carbonate apatite block through a dissolution-precipitation reaction using calcium hydrogen phosphate dihydrate block as a precursor. Materials 2017, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.S.K.; Tseng, Y.H.; Mou, Y.; Hsu, Y.-C.; Yang, C.-M.; Chan, J.C.C. Mechanistic study of apatite formation on bioactive glass surface using 31P solid-state NMR spectroscopy. Chem. Mater. 2005, 17, 4493–4501. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef] [PubMed]
- Grandfield, K.; Palmquist, A.; Engqvist, H.; Thomsen, P. Resolving the CaP-bone interface: A review of discoveries with light and electron microscopy. Biomatter 2012, 2, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.M. Hydroxyapatite-based materials: Synthesis and Characterization. In Biomedical Engineering-Frontiers and Challenges, 1st ed.; Fazel-Rezai, R., Ed.; Intech: Rijeka, Croatia, 2011; pp. 75–96. [Google Scholar]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef]

| Groups | Powder Compositions | Additional Powder |
|---|---|---|
| Control | 60% DCPA and 40% vaterite | |
| 1 | 60% DCPA and 30% vaterite | 0% Si-CaP and 10% Ca(OH)2 |
| 2 | 3% Si-CaP and 7% Ca(OH)2 | |
| 3 | 5% Si-CaP and 5% Ca(OH)2 | |
| 4 | 7% Si-CaP and 3% Ca(OH)2 | |
| 5 | 10% Si-CaP and 0% Ca(OH)2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cahyanto, A.; Liemidia, M.; Karlina, E.; Zakaria, M.N.; Shariff, K.A.; Sukotjo, C.; El-Ghannam, A. Bioactive Carbonate Apatite Cement with Enhanced Compressive Strength via Incorporation of Silica Calcium Phosphate Composites and Calcium Hydroxide. Materials 2023, 16, 2071. https://doi.org/10.3390/ma16052071
Cahyanto A, Liemidia M, Karlina E, Zakaria MN, Shariff KA, Sukotjo C, El-Ghannam A. Bioactive Carbonate Apatite Cement with Enhanced Compressive Strength via Incorporation of Silica Calcium Phosphate Composites and Calcium Hydroxide. Materials. 2023; 16(5):2071. https://doi.org/10.3390/ma16052071
Chicago/Turabian StyleCahyanto, Arief, Michella Liemidia, Elin Karlina, Myrna Nurlatifah Zakaria, Khairul Anuar Shariff, Cortino Sukotjo, and Ahmed El-Ghannam. 2023. "Bioactive Carbonate Apatite Cement with Enhanced Compressive Strength via Incorporation of Silica Calcium Phosphate Composites and Calcium Hydroxide" Materials 16, no. 5: 2071. https://doi.org/10.3390/ma16052071
APA StyleCahyanto, A., Liemidia, M., Karlina, E., Zakaria, M. N., Shariff, K. A., Sukotjo, C., & El-Ghannam, A. (2023). Bioactive Carbonate Apatite Cement with Enhanced Compressive Strength via Incorporation of Silica Calcium Phosphate Composites and Calcium Hydroxide. Materials, 16(5), 2071. https://doi.org/10.3390/ma16052071









