Investigation of Gamma-Ray Shielding Properties of Bismuth Oxide Nanoparticles with a Bentonite–Gypsum Matrix
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Samples Preparation
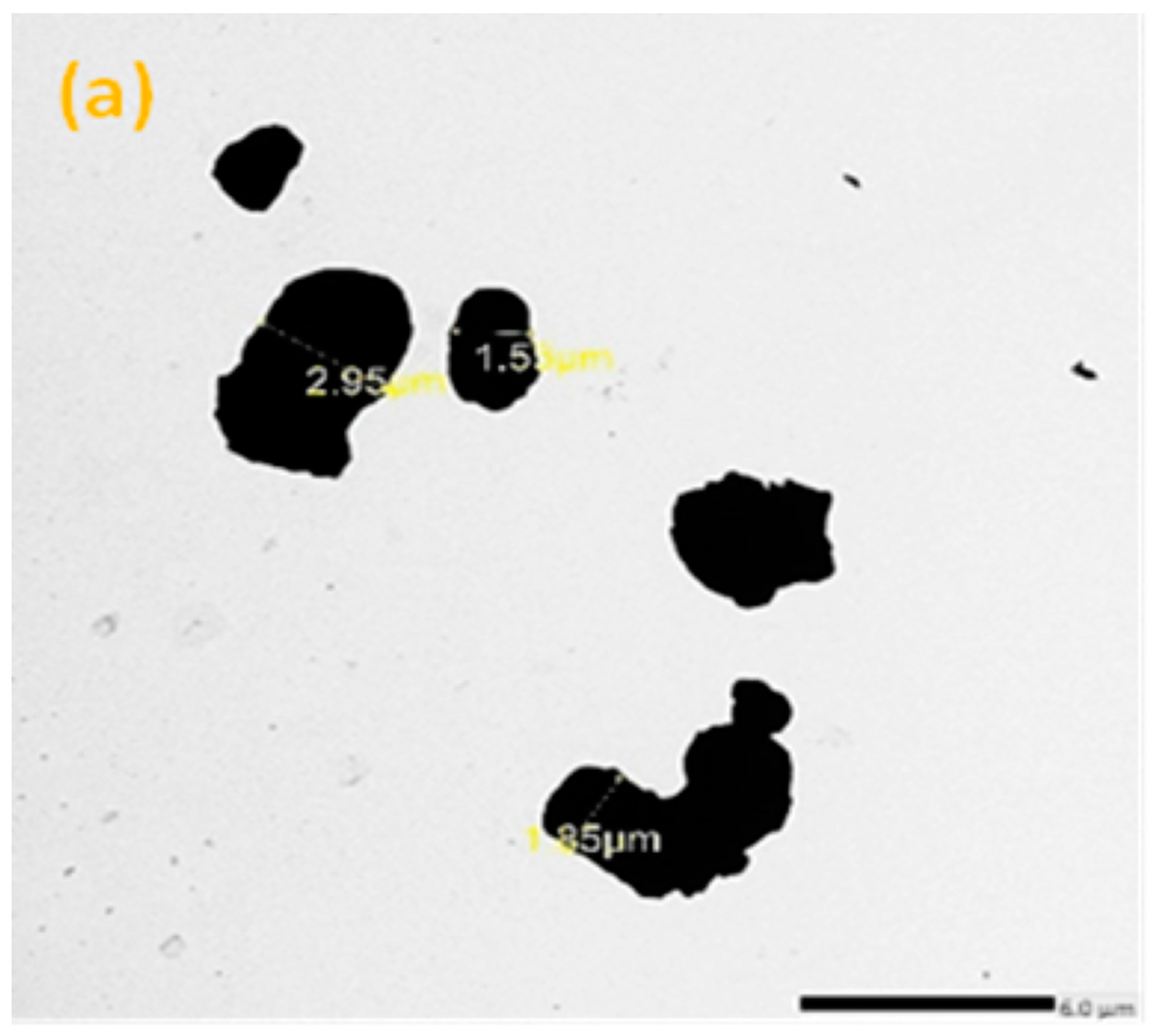
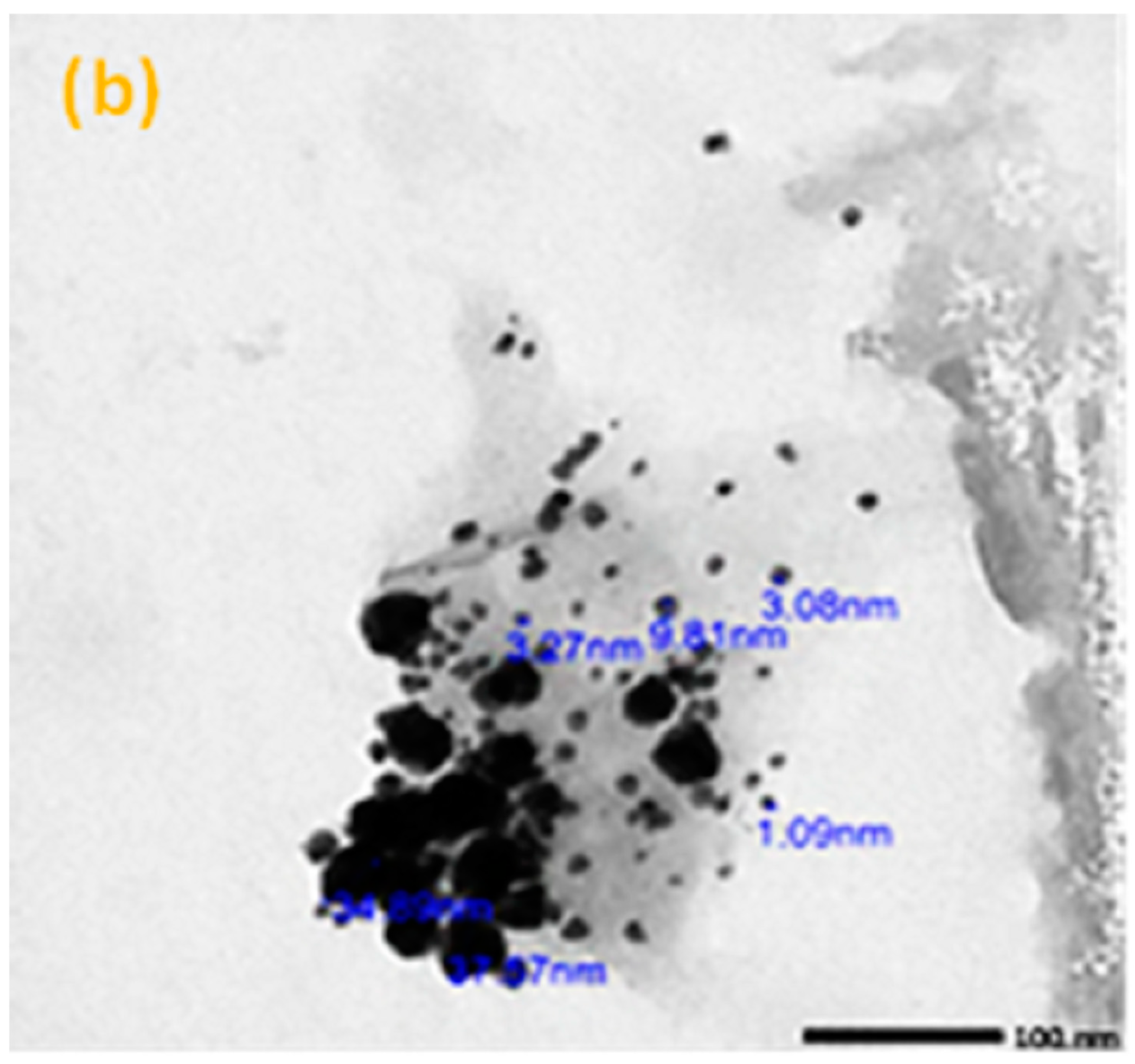
2.3. Morphology Test
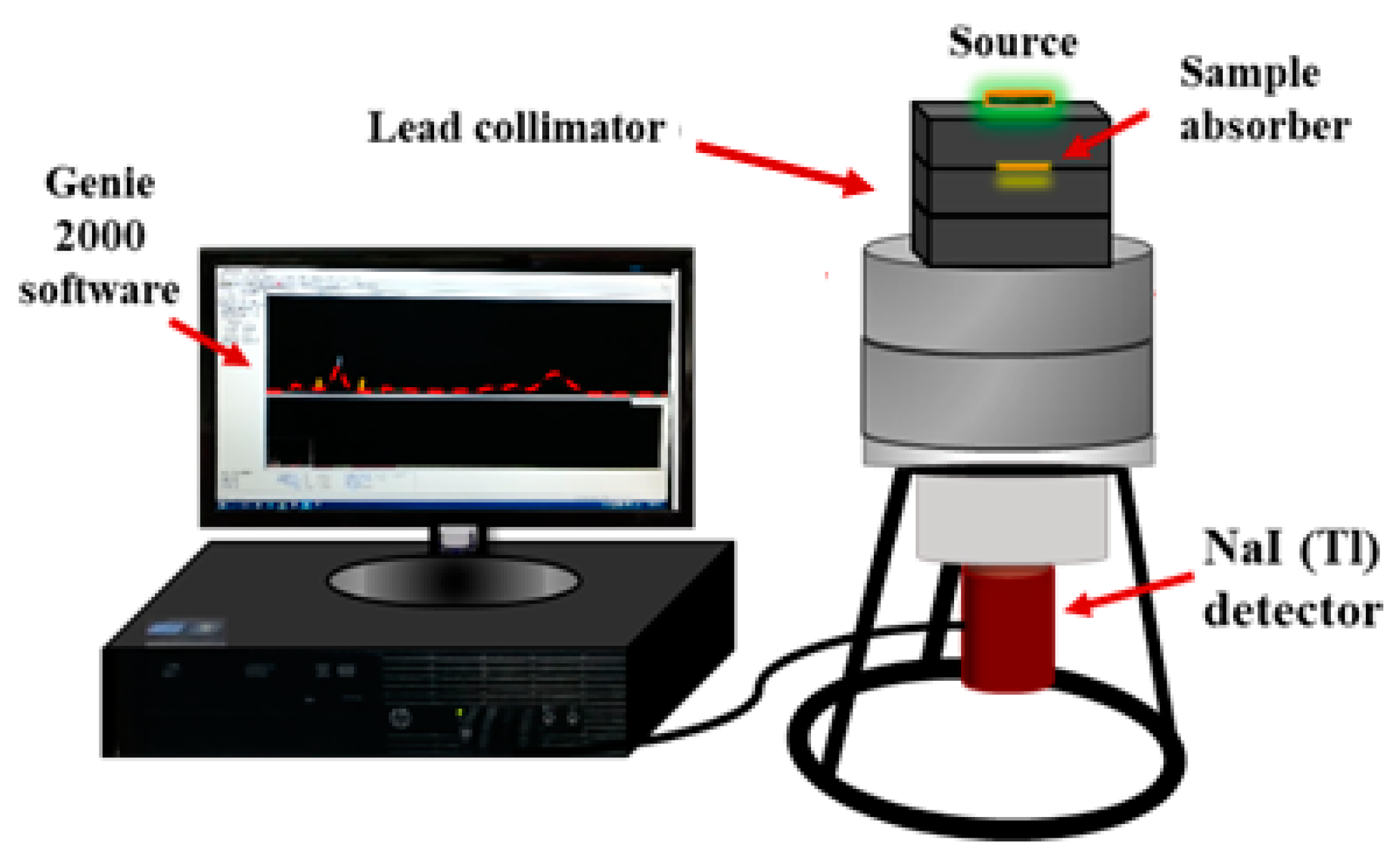
2.4. Radiation Measurements
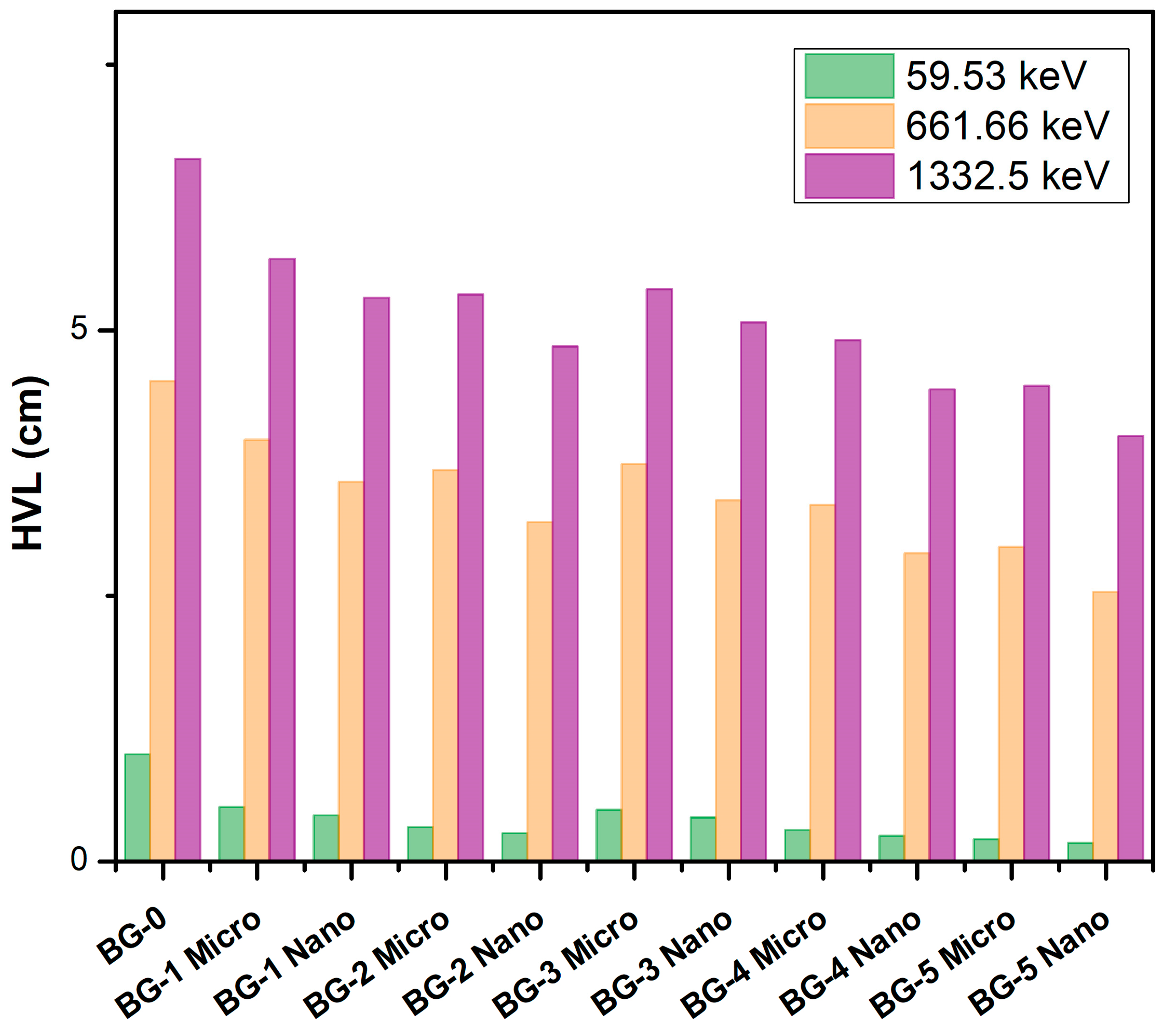
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavaz, E.; Ghanim, E.H.; Abouhaswa, A.S. Optical, structural and nuclear radiation security properties of newly fabricated V2O5-SrO-PbO glass system. J. Non Cryst. Solids 2020, 538, 120045. [Google Scholar] [CrossRef]
- Agar, O.; Khattari, Z.Y.; Sayyed, M.I.; Tekin, H.O.; Al-Omari, S.; Maghrabi, M.; Zaid, M.H.M.; Kityk, I.V. Evaluation of the shielding parameters of alkaline earth based phosphate glasses using MCNPX code. Result. Phys. 2019, 12, 101–106. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Almuqrin, A.H.; Kurtulus, R.; Javier-Hila, A.V.; Kaky, K.; Kavas, T. X-ray shielding characteristics of P2O5–Nb2O5 glass doped with Bi2O3 by using EPICS2017 and Phy-X/PSD. Appl. Phys. A 2021, 127, 243. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Al-Hadeethi, Y.; AlShammari, M.M.; Ahmed, S.H.; Rammah, Y.S. Physical, optical and gamma radiation shielding competence of newly borotellurite based glasses: TeO2–B2O3–ZnO–Li2O3–Bi2O3. Ceram. Int. 2021, 47, 611–618. [Google Scholar] [CrossRef]
- Ozdogan, H.; Kilicoglu, O.; Akman, F.; Agar, O. Comparison of Monte Carlo simulations and theoretical calculations of nuclear shielding characteristics of various borate glasses including Bi, V, Fe, and Cd. Appl. Radiat. Isot. 2022, 189, 110454. [Google Scholar] [CrossRef] [PubMed]
- Çakıroğlu, M.; Kaplan, A.; Süzen, A. Experimental and DBN-Based neural network extraction of radiation attenuation coefficient of dry mixture shotcrete produced using different additives. Radiat. Phys. Chem. 2021, 188, 109636. [Google Scholar] [CrossRef]
- Akman, F.; Khattari, Z.Y.; Kaçal, M.R.; Sayyed, M.I.; Afaneh, F. The radiation shielding features for some silicide, boride and oxide types ceramics. Radiat. Phys. Chem. 2019, 160, 9–14. [Google Scholar] [CrossRef]
- Tiamduangtawan, P.; Kamkaew, C.; Kuntonwatchara, S.; Wimolmala, E.; Saenboonruang, K. Comparative mechanical, self-healing, and gamma attenuation properties of PVA hydrogels containing either nano- or micro-sized Bi2O3 for use as gamma-shielding materials. Radiat. Phys. Chem. 2020, 177, 109164. [Google Scholar] [CrossRef]
- Intom, S.; Kalkornsurapranee, E.; Johns, J.; Kaewjaeng, S.; Kothan, S.; Hongtong, W.; Chaiphaksa, W.; Kaewkhao, J. Mechanical and radiation shielding properties of flexible material based on natural rubber/Bi2O3 composites. Radiat. Phys. Chem. 2020, 172, 108772. [Google Scholar] [CrossRef]
- Kurtuluş, R.; Buriahi, M.S.; Issa, S.A.M.; Tekin, H.O.; Kavas, T.; Kavaz, E. Physical, structural, mechanical and radiation shielding features of waste pharmaceutical glasses doped with Bi2O3. Optik 2022, 261, 169108. [Google Scholar] [CrossRef]
- Luchechko, A.; Vasyltsiv, V.; Kostyk, L.; Tsvetkova, O.; Popov, A.I. Shallow and deep trap levels in X-ray irradiated β-Ga2O3: Mg. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 441, 12–17. [Google Scholar] [CrossRef]
- Averback, R.S.; Ehrhart, P.; Popov, A.I.; Sambeek, A.V. Defects in ion implanted and electron irradiated MgO and Al2O3. Radiat. Eff. Defects Solids 1995, 136, 169–173. [Google Scholar] [CrossRef]
- Kiatwattanacharoen, S.; Srimaroeng, P.; Kothan, S.; Jumpee, C.; Kim, H.J.; Kaewjaeng, S. A study of X-ray Radiation Shielding Properties of Bricks Contained Barium Sulfate. AIP Conf. Proc. 2020, 2279, 060004. [Google Scholar]
- Gouda, M.M.; Badawi, M.S.; El-Khatib, A.M.; Hussien, N.S.; Abbas, M.I. Calculation of NaI(Tl) Detector Full-Energy Peak Efficiency Using the Efficiency Transfer Method For Small Radioactive Cylindrical Sources. Nucl. Technol. Radiat. Prot. J. 2016, 31, 150–158. [Google Scholar] [CrossRef]
- Badawi, M.S.; Ruskov, I.; Gouda, M.M.; El-Khatib, A.M.; Alotiby, M.F.; Mohamed, M.M.; Thabet, A.A.; Abbas, M.I. A numerical approach to calculate the full-energy peak efficiency of HPGe well-type detectors using the effective solid angle ratio. J. Instrum. 2014, 9, P07030. [Google Scholar] [CrossRef]
- Abd-Elzaher, M.; Badawi, M.S.; El-Khatib, A.M.; Thabet, A.A. Determination of full energy peak efficiency of NaI (Tl) detector depending on efficiency transfer principle for conversion form experimental values. World J. Nucl. Sci. Technol. 2012, 2, 3. [Google Scholar] [CrossRef]
- Gouda, M.M. Calibration of NaI (Tl) Cylindrical Detector Using Axially Shifted Radioactive Cylindrical Sources. Nucl. Technol. Radiat. Prot. 2019, 34, 353–360. [Google Scholar] [CrossRef]
- Badawi, M.S.; Gouda, M.M.; Nafee, S.S.; El-Khatib, A.M.; El-Mallah, E.A. New algorithm for studying the effect of self attenuation factor on the efficiency of γ-rays detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. 2012, 696, 164–170. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Abbas, M.I.; Sayyed, M.I.; Khandaker, U.; Abd-Elzaher, M.; Khalil, M.M.; Elsafi, M.; Gouda, M.M. Assessment of γ-radiation shielding behavior of some mixed nature clays. Radiat. Phys. Chem 2022, 200, 110236. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Abbas, A.M.; Hammoury, S.I.; Gouda, M.M.; Zard, K.; Elsafi, M. Effect of PbO-Nanoparticles on Dimethyl Polysiloxane for use in Radiation Shielding Applications. Sci. Rep. 2022, 12, 15722. [Google Scholar] [CrossRef]
- Elsafi, M.; Sayyed, M.I.; Almuqrin, A.H.; Gouda, M.M.; El-Khatib, A.M. Analysis of particle size on mass dependent attenuation capability of bulk and nanoparticle PbO radiation shields. Results Phys. 2021, 26, 104458. [Google Scholar] [CrossRef]
- Elsafi, M.; Shehata, A.G.; El-Khatib, A.M.; Abbas, A.M.; Sayyed, M.I.; Khandaker, M.U.; Gouda, M.M. Effect of iron and ferrosilicon materials to enhance the radiation shielding ability of bentonite clay. Radiat. Phys. Chem. 2022, 110235. [Google Scholar] [CrossRef]
- Gouda, M.M.; Abbas, A.M.; Hammoury, S.I.; Zard, K.; El-Khatib, A.M. Nano tin oxide/dimethyl polysiloxane reinforced composite as a flexible radiation protecting material. Sci. Rep. 2023, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today: Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Doma, A.S.; Badawi, M.S.; Abu-Rayan, A.E.; Aly, N.S.; Alzahrani, J.S.; Abbas, A.M. Conductive natural and waste rubbers composites-loaded with lead powder as environmental flexible gamma radiation shielding material. Mater. Res. Express 2020, 7, 105309. [Google Scholar] [CrossRef]
- Kurudirek, M. Effective atomic numbers and electron densities of some human tissues and dosimetric materials for mean energies of various radiation sources relevant to radiotherapy and medical applications. Radiat. Phys. Chem. 2014, 102, 139–146. [Google Scholar] [CrossRef]
- Sharaf, J.M.; Saleh, H. Gamma-ray energy buildup factor calculations and shielding effects of some Jordanian building structures. Radiat. Phys. Chem 2015, 110, 87–95. [Google Scholar] [CrossRef]
- Alabsy, M.T.; Alzahrani, J.S.; Sayyed, M.I.; Abbas, M.I.; Tishkevich, D.I.; El-Khatib, A.M.; Elsafi, M. Gamma-Ray Attenuation and Exposure Buildup Factor of Novel Polymers in Shielding Using Geant4 Simulation. Materials 2021, 14, 5051. [Google Scholar] [CrossRef]
- Berger, M. XCOM: Photon cross Section Database (Version 1.3). 2005. Available online: https://physics.nist.gov/xcom (accessed on 28 September 1998).
- Alsayed, Z.; Badawi, M.S.; Awad, R.; Thabet, A.A.; El-Khatib, A.M. Study of some γ-ray attenuation parameters for new shielding materials composed of nano ZnO blended with high density polyethylene. Nucl. Technol. Radiat. Prot. 2019, 34, 342–352. [Google Scholar] [CrossRef]
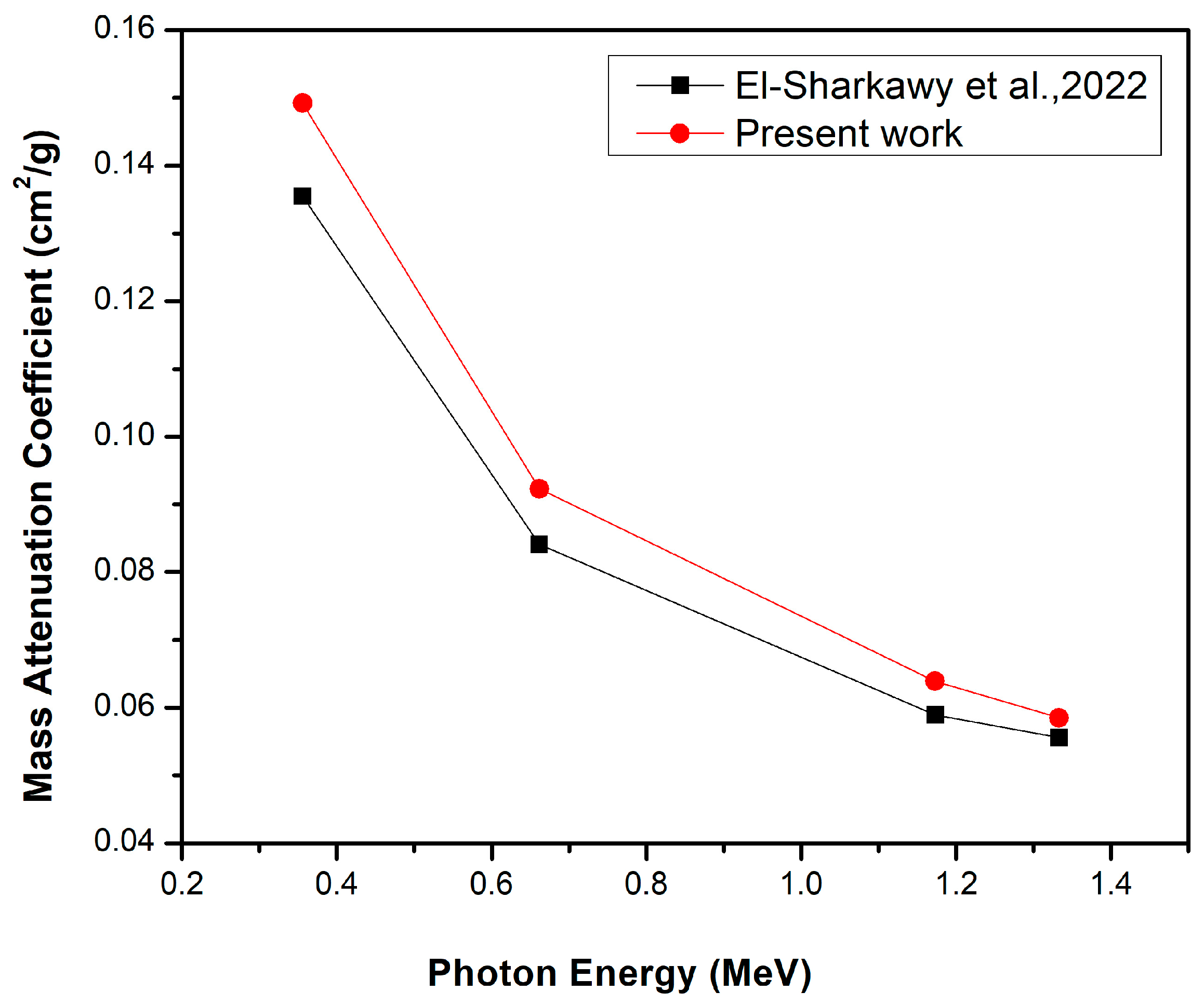
- El-Sharkawy, R.M.; Allam, E.A.; El-Taher, A.; Elsaman, R.; Massoud, E.E.; Mahmoud, M.E. Synergistic effects on gamma-ray shielding by novel light-weight nanocomposite materials of bentonite containing nano Bi2O3 additive. Ceram. Int. 2022, 48, 7291–7303. [Google Scholar] [CrossRef]


| Metal Oxide | Weight Fraction (%) | |
|---|---|---|
| Bentonite | Gypsum | |
| Na2O | 1.30 | 0 |
| MgO | 1.18 | 0 |
| Al2O3 | 20.35 | 0 |
| SiO2 | 49.65 | 0 |
| SO3 | 1.96 | 41.27 |
| K2O | 1.28 | 0 |
| CaO | 10.92 | 58.73 |
| TiO2 | 2.74 | 0 |
| FeO | 10.62 | 0 |
| Sample Codes | Compositions (wt %) | ||
|---|---|---|---|
| Main Matrix | Bismuth Oxide (Bi2O3) | ||
| Bentonite | Gypsum | ||
| BG-0 | 67 | 33 | 0 |
| BG-1 | 27 | 6 | |
| BG-2 | 20 | 13 | |
| BG-3 | 61 | 33 | 6 |
| BG-4 | 54 | 13 | |
| BG-5 | 56.7 | 23.3 | 20 |
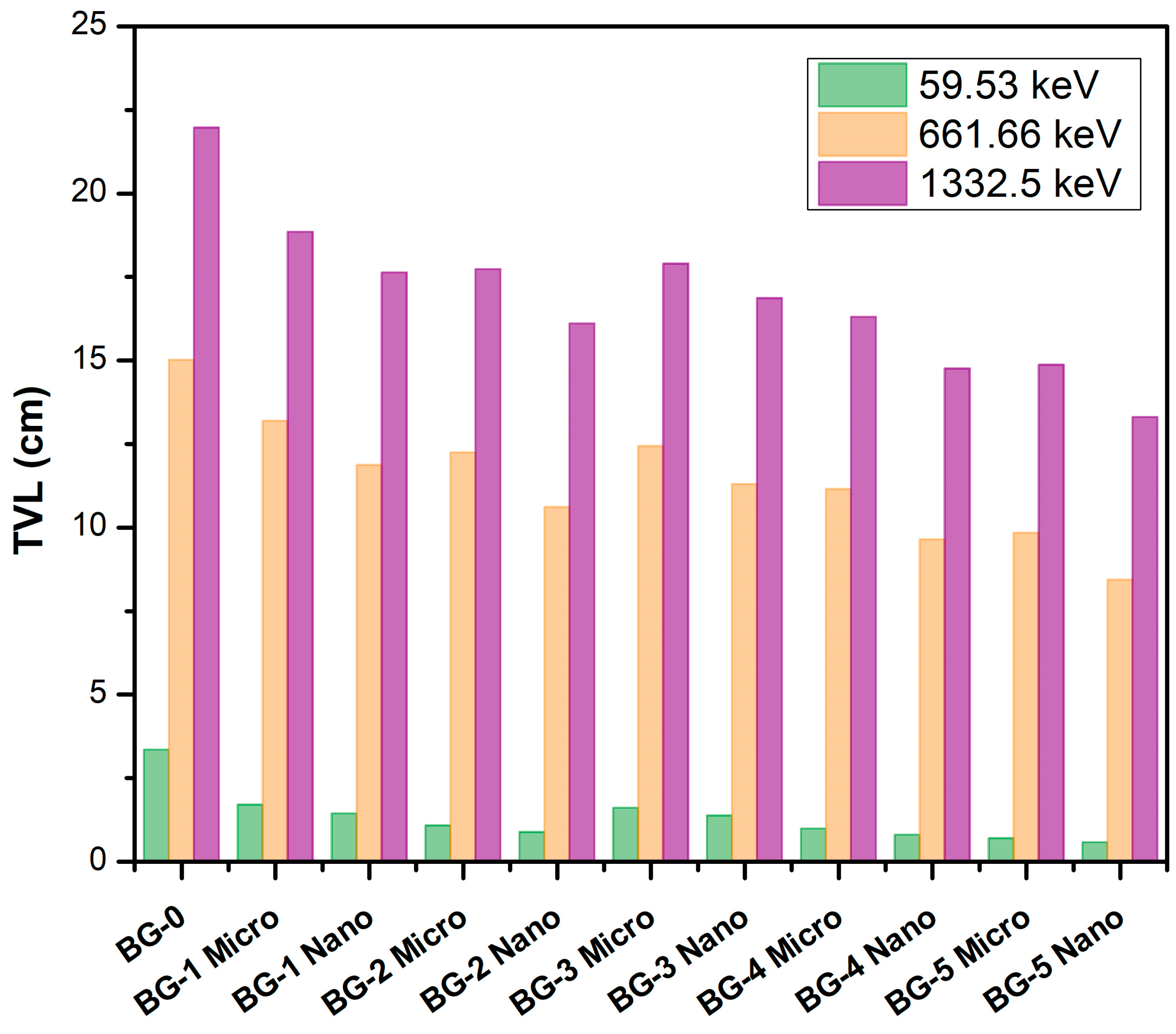
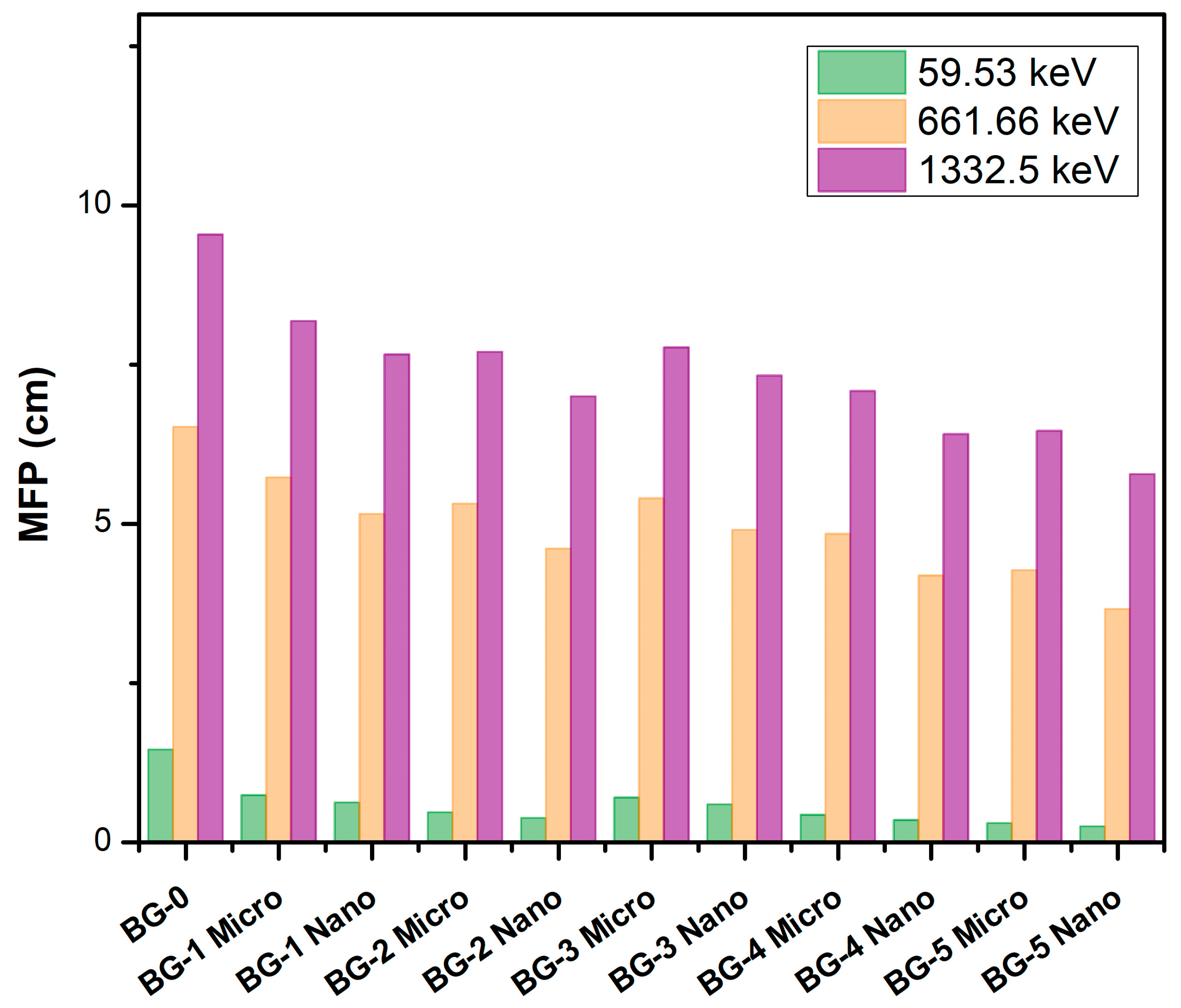
| Sample Code/wt% | Energy (keV) | Mass Attenuation Coefficient (cm2 g−1) | Linear Attenuation Coefficient (cm−1) | Density (g cm−3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (MAC)XCOM | (MAC)Micro | ∆1 % | (MAC)Nano | ∆2 % | (LAC)Micro | (LAC)Nano | Micro | Nano | ||
| BG-0 0 wt % | 59.53 | 0.3580 | 0.3522 | 1.647 | 0.6861 | 1.948 ± 0.01 | ||||
| 80.99 | 0.2238 | 0.2215 | 1.038 | 0.4315 | ||||||
| 356.01 | 0.0993 | 0.0979 | 1.430 | 0.1907 | ||||||
| 661.66 | 0.0766 | 0.0787 | −2.668 | 0.1533 | ||||||
| 1173.23 | 0.0583 | 0.0586 | −0.512 | 0.1142 | ||||||
| 1332.5 | 0.0547 | 0.0538 | 1.673 | 0.1048 | ||||||
| BG-1 6 wt % | 59.53 | 0.6018 | 0.5858 | 2.731 | 0.6772 | 15.603 | 1.3473 | 1.5901 | 2.300 ± 0.02 | 2.348 ± 0.01 |
| 80.99 | 0.3256 | 0.3277 | −0.641 | 0.3631 | 10.803 | 0.7537 | 0.8526 | |||
| 356.01 | 0.1087 | 0.1104 | −1.540 | 0.1183 | 7.156 | 0.2539 | 0.2778 | |||
| 661.66 | 0.0781 | 0.0759 | 2.899 | 0.0826 | 8.827 | 0.1746 | 0.1939 | |||
| 1173.23 | 0.0584 | 0.0571 | 2.277 | 0.0607 | 6.305 | 0.1313 | 0.1425 | |||
| 1332.5 | 0.0547 | 0.0531 | 3.013 | 0.0556 | 4.708 | 0.1221 | 0.1305 | |||
| BG-2 13 wt % | 59.53 | 0.8863 | 0.8646 | 2.510 | 1.0051 | 16.250 | 2.1139 | 2.5831 | 2.445 ± 0.03 | 2.570 ± 0.02 |
| 80.99 | 0.4442 | 0.4329 | 2.610 | 0.4932 | 13.929 | 1.0584 | 1.2675 | |||
| 356.01 | 0.1196 | 0.1170 | 2.222 | 0.1301 | 11.197 | 0.2861 | 0.3344 | |||
| 661.66 | 0.0799 | 0.0769 | 3.901 | 0.0844 | 9.753 | 0.1880 | 0.2169 | |||
| 1173.23 | 0.0585 | 0.0572 | 2.273 | 0.0613 | 7.168 | 0.1399 | 0.1575 | |||
| 1332.5 | 0.0547 | 0.0531 | 3.013 | 0.0556 | 4.708 | 0.1298 | 0.1429 | |||
| BG-3 6 wt % | 59.53 | 0.6049 | 0.6008 | 0.682 | 0.6954 | 15.746 | 1.4209 | 1.6655 | 2.365 ± 0.02 | 2.395 ± 0.02 |
| 80.99 | 0.3267 | 0.3209 | 1.807 | 0.3704 | 15.425 | 0.7589 | 0.8871 | |||
| 356.01 | 0.1088 | 0.1068 | 1.873 | 0.1304 | 22.097 | 0.2526 | 0.3123 | |||
| 661.66 | 0.0782 | 0.0783 | −0.128 | 0.0851 | 8.685 | 0.1852 | 0.2038 | |||
| 1173.23 | 0.0584 | 0.0595 | −1.849 | 0.0636 | 6.891 | 0.1407 | 0.1523 | |||
| 1332.5 | 0.0547 | 0.0544 | 0.551 | 0.0570 | 4.779 | 0.1287 | 0.1365 | |||
| BG-4 13 wt % | 59.53 | 0.8930 | 0.9128 | −2.169 | 1.0609 | 16.225 | 2.3094 | 2.8294 | 2.530 ± 0.02 | 2.667 ± 0.03 |
| 80.99 | 0.4467 | 0.4512 | −0.997 | 0.5137 | 13.852 | 1.1415 | 1.3700 | |||
| 356.01 | 0.1198 | 0.1213 | −1.237 | 0.1348 | 11.129 | 0.3069 | 0.3595 | |||
| 661.66 | 0.0800 | 0.0816 | −1.961 | 0.0895 | 9.681 | 0.2064 | 0.2387 | |||
| 1173.23 | 0.0586 | 0.0572 | 2.448 | 0.0616 | 7.692 | 0.1447 | 0.1643 | |||
| 1332.5 | 0.0548 | 0.0558 | −1.792 | 0.0585 | 4.839 | 0.1412 | 0.1560 | |||
| BG-5 20 wt % | 59.53 | 1.1760 | 1.1632 | 1.100 | 1.3286 | 14.219 | 3.2744 | 3.9300 | 2.815 ± 0.03 | 2.958 ± 0.01 |
| 80.99 | 0.5649 | 0.5483 | 3.028 | 0.6204 | 13.150 | 1.5435 | 1.8351 | |||
| 356.01 | 0.1306 | 0.1324 | −1.360 | 0.1492 | 12.689 | 0.3727 | 0.4413 | |||
| 661.66 | 0.0818 | 0.0831 | −1.564 | 0.0923 | 11.071 | 0.2339 | 0.2730 | |||
| 1173.23 | 0.0587 | 0.0581 | 1.033 | 0.0639 | 9.983 | 0.1636 | 0.1890 | |||
| 1332.5 | 0.0547 | 0.0550 | −0.545 | 0.0585 | 6.364 | 0.1548 | 0.1730 | |||
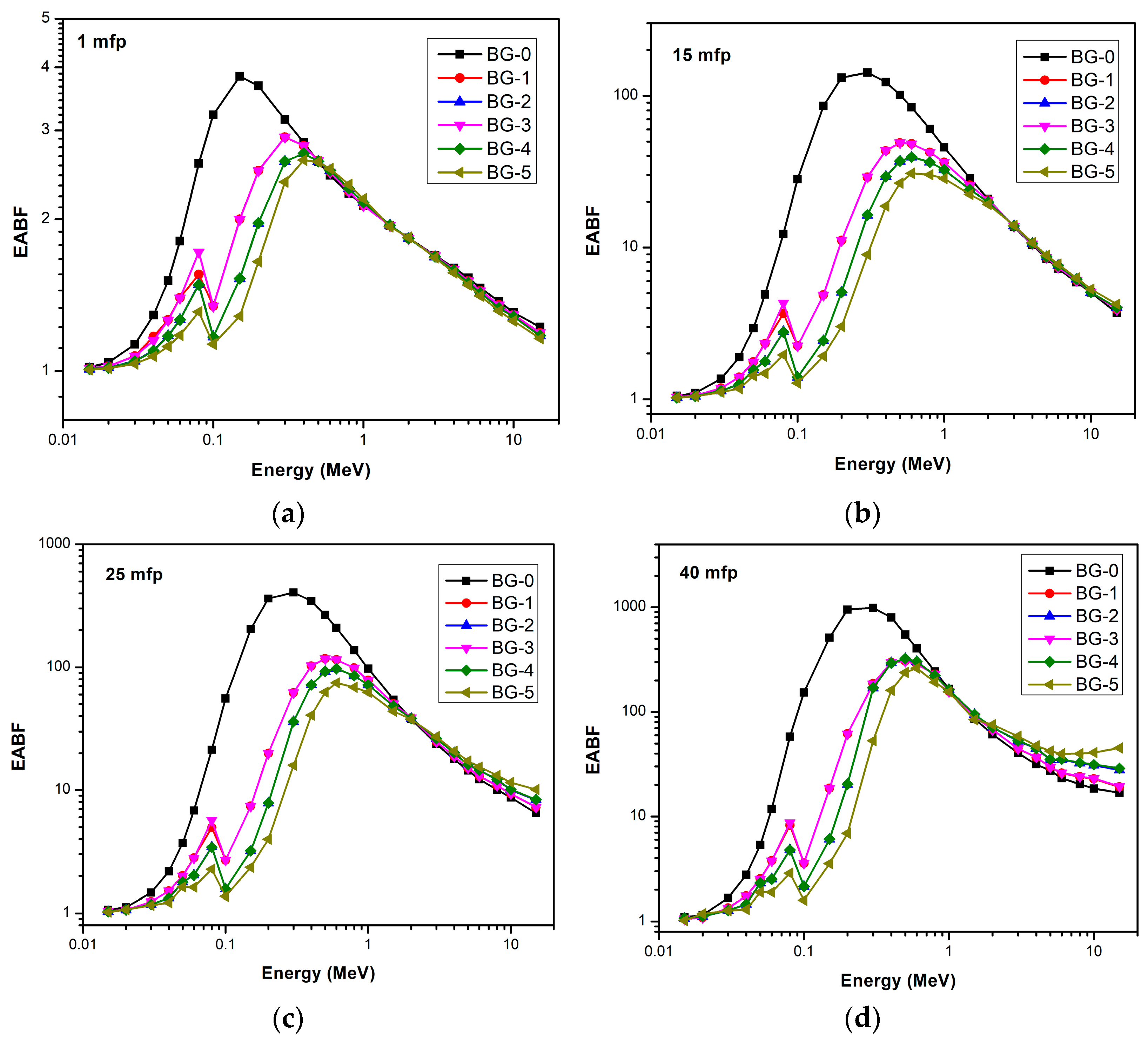
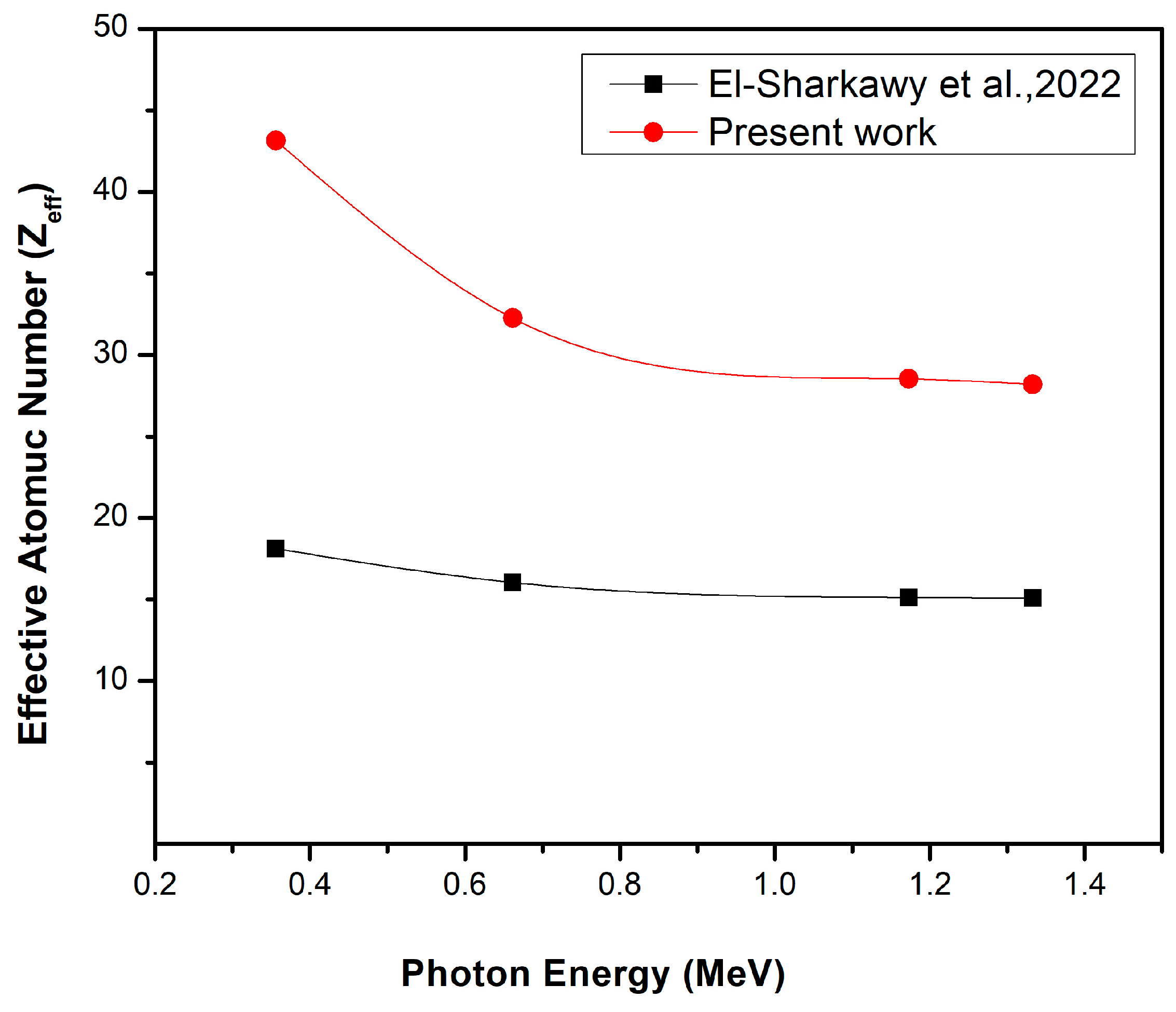
| Energy (keV) | BG-0 | BG-1 | BG-2 | BG-3 | BG-4 | BG-5 |
|---|---|---|---|---|---|---|
| 59.53 | 17.1943 | 49.5183 | 62.7007 | 49.6420 | 62.9440 | 69.1968 |
| 80.99 | 15.6597 | 42.7528 | 56.7242 | 42.7966 | 56.8427 | 64.3237 |
| 356.01 | 13.3757 | 24.6872 | 34.9620 | 24.7671 | 35.1137 | 43.1547 |
| 661.66 | 13.3390 | 19.4663 | 26.0590 | 19.5548 | 26.2427 | 32.2615 |
| 1173.23 | 13.3323 | 18.0070 | 23.2816 | 18.0978 | 23.4748 | 28.5231 |
| 1332.50 | 13.3346 | 17.8930 | 23.0561 | 17.9839 | 23.2498 | 28.2105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.I.; El-Khatib, A.M.; Elsafi, M.; El-Shimy, S.N.; Dib, M.F.; Abdellatif, H.M.; Baharoon, R.; Gouda, M.M. Investigation of Gamma-Ray Shielding Properties of Bismuth Oxide Nanoparticles with a Bentonite–Gypsum Matrix. Materials 2023, 16, 2056. https://doi.org/10.3390/ma16052056
Abbas MI, El-Khatib AM, Elsafi M, El-Shimy SN, Dib MF, Abdellatif HM, Baharoon R, Gouda MM. Investigation of Gamma-Ray Shielding Properties of Bismuth Oxide Nanoparticles with a Bentonite–Gypsum Matrix. Materials. 2023; 16(5):2056. https://doi.org/10.3390/ma16052056
Chicago/Turabian StyleAbbas, Mahmoud I., Ahmed M. El-Khatib, Mohamed Elsafi, Sarah N. El-Shimy, Mirvat F. Dib, Hala M. Abdellatif, Raqwana Baharoon, and Mona M. Gouda. 2023. "Investigation of Gamma-Ray Shielding Properties of Bismuth Oxide Nanoparticles with a Bentonite–Gypsum Matrix" Materials 16, no. 5: 2056. https://doi.org/10.3390/ma16052056
APA StyleAbbas, M. I., El-Khatib, A. M., Elsafi, M., El-Shimy, S. N., Dib, M. F., Abdellatif, H. M., Baharoon, R., & Gouda, M. M. (2023). Investigation of Gamma-Ray Shielding Properties of Bismuth Oxide Nanoparticles with a Bentonite–Gypsum Matrix. Materials, 16(5), 2056. https://doi.org/10.3390/ma16052056








