Impact of Natural-Based Viscosity Modifiers of Inhalation Drugs on the Dynamic Surface Properties of the Pulmonary Surfactant
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Surface Tension Variations during Area Alterations
3.2. Characterization of Surface Rheological Properties in the Presence of MPS
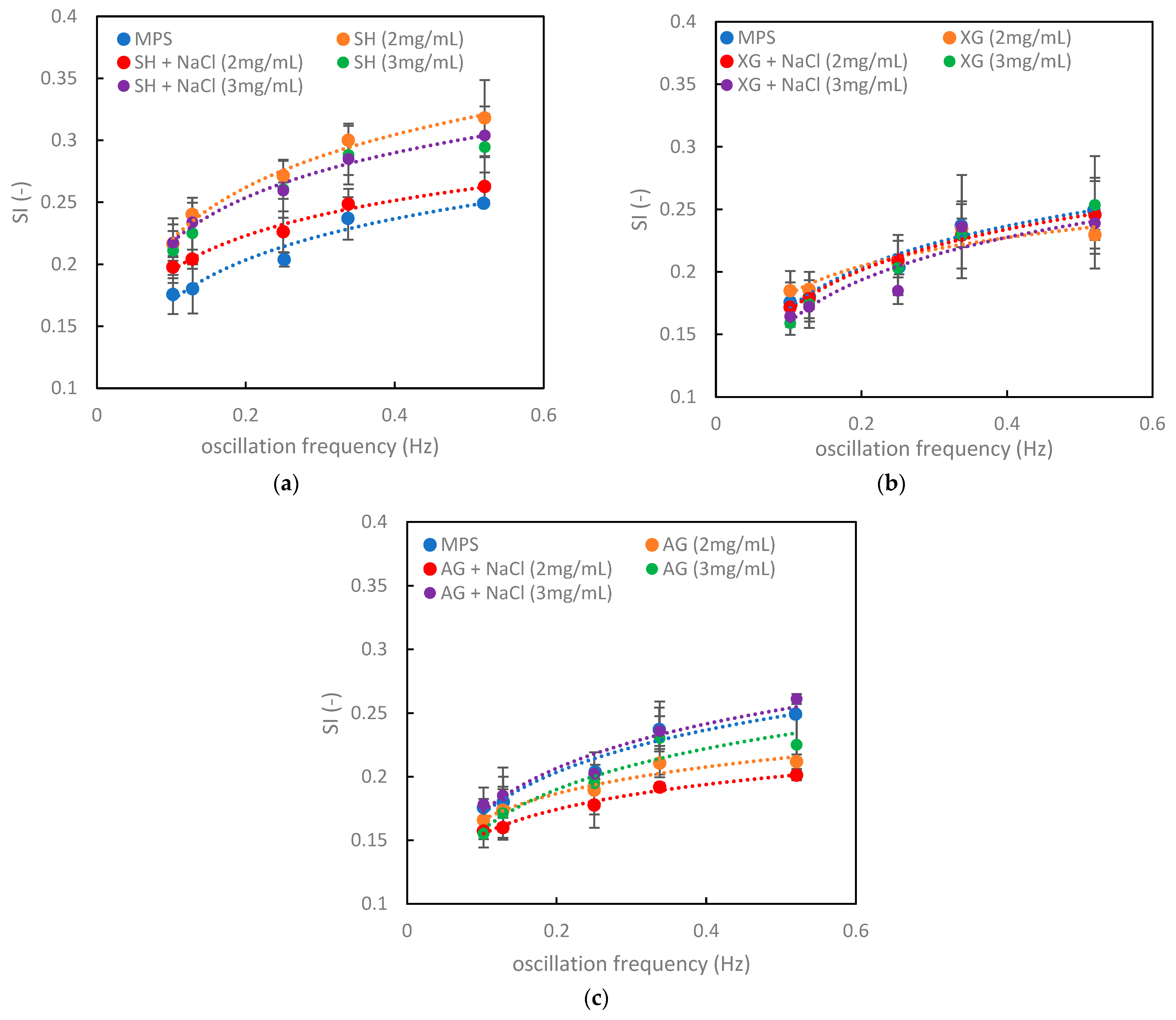
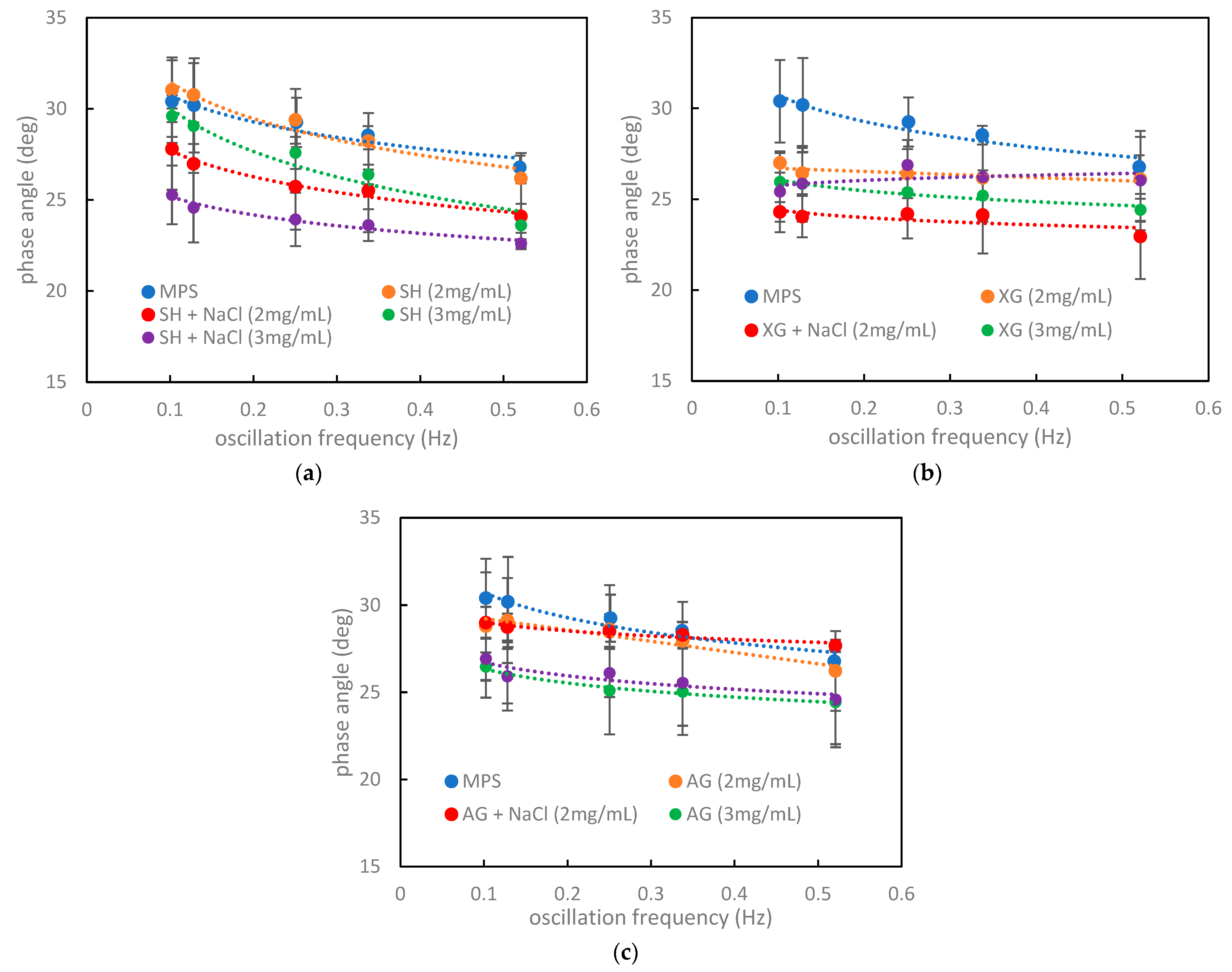
3.3. Relations between the Numerical Criteria, Rheological Parameters, and Hysteresis Shape in the Context of the Impact of Inhaled VMs on the Pulmonary Surfactant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, H.C.; Schum, G.M. Models of the human lung airways and their application to inhaled particle deposition. Bull. Math. Biol. 1980, 42, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; Peters, J.I.; Williams III, R.O. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fernández, T.A.; Casan, C.P. Deposition of inhaled particles in the lungs. Arch. Bronconeumol. 2012, 48, 240–246. [Google Scholar] [CrossRef]
- Kuempel, E.D.; Sweeney, L.M.; Morris, J.B.; Jarabek, A.M. Advances in inhalation dosimetry models and methods for occupational risk assessment and exposure limit derivation. J. Occup. Environ. Hyg. 2015, 12, S18–S40. [Google Scholar] [CrossRef] [PubMed]
- McCallion, O.N.; Taylor, K.M.; Thomas, M.; Taylor, A.J. Nebulization of fluids of different physicochemical properties with air-jet and ultrasonic nebulizers. Pharm. Res. 1995, 12, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Oesterheld, N.; Knuedeler, M.C.; Seeger, W. On the correlation of output rate and aerodynamic characteristics in vibrating-mesh-based aqueous aerosol delivery. Int. J. Pharm. 2014, 461, 34–37. [Google Scholar]
- Broniarz-Press, L.; Sosnowski, T.R.; Matuszak, M.; Ochowiak, M.; Jabłczyńska, K. The effect of shear and extensional viscosities on atomization of Newtonian and non-Newtonian fluids in ultrasonic inhaler. Int. J. Pharm. 2015, 485, 41–49. [Google Scholar] [CrossRef]
- Ghazanfari, T.; Elhissi, A.M.A.; Ding, Z.; Taylor, K.M.G. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int. J. Pharm. 2007, 339, 103–111. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Sosnowski, T.R. Sodium hyaluronate as a potential viscosity modifier of nebulization drugs—Preliminary studies. In Chemical and Process Engineering for the Environment and Health; Sosnowski, T.R., Szwast, M., Eds.; Sieć Badawcza Łukasiewicz—Instytut Technologii Eksploatacji: Radom, Poland, 2020; Volume 1, pp. 166–174. [Google Scholar]
- Dobrowolska, K.; Kinowska, M.; Sosnowski, T.R. Nebulization of solutions containing guar gum as a viscosity modifier of natural origin. In Respiratory Drug Delivery 2022; RDD-Online: Orlando, FL, USA, 2022. [Google Scholar]
- Mazzone Sr, M.; Peri, O.; Moschetti, V. Evaluation of Mucoadhesive Properties of Xanthan Gum Hydrogels versus Marketed Ophthalmic Gel Formulations Using a Tensile Strength Method. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1966. [Google Scholar]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchi, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Rocha, C.M.R.; Gonçalves, M.P. Agar (Chapter 24). In Woodhead Publishing Series in Food Science, Technology and Nutrition, Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 731–765. [Google Scholar]
- Parra, E.; Pérez-Gil, J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem. Phys. Lipids 2015, 185, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Seifart, C.; Barth, P.J. Effect of air pollutants on the pulmonary surfactant system. Eur. J. Clin. Investig. 1998, 28, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska, K.; Jabłczyńska, K.; Kondej, D.; Sosnowski, T.R. Interactions of insoluble micro-and nanoparticles with the air-liquid interface of the model pulmonary fluids. Physicochem. Probl. 2018, 54, 151–162. [Google Scholar]
- Wang, F.; Liu, J.; Zeng, H. Interactions of particulate matter and pulmonary surfactant: Implications for human health. Adv. Colloid Interface Sci. 2020, 284, 102244. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Leach, A.G.; Riley, F. An investigation into drug partitioning behaviour in simulated pulmonary surfactant monolayers with associated molecular modelling. Surf. Interface Anal. 2018, 50, 369–377. [Google Scholar] [CrossRef]
- Hidalgo, A.; Garcia-Mouton, C.; Autilio, C.; Carravilla, P.; Orellana, G.; Islam, M.N.; Bhattacharya, J.; Bhattacharya, S.; Cruz, A.; Pérez-Gil, J. Pulmonary surfactant and drug delivery: Vehiculization, release and targeting of surfactant/tacrolimus formulations. J. Control. Release 2021, 329, 205–222. [Google Scholar] [CrossRef]
- Gradoń, L.; Podgórski, A. Hydrodynamical model of pulmonary clearance. Chem. Eng. Sci. 1989, 44, 741–749. [Google Scholar] [CrossRef]
- Escolar, J.D.; Escolar, A. Lung hysteresis: A morphological view. Histol. Histopathol. 2004, 19, 159–166. [Google Scholar]
- Wüstneck, R.; Perez-Gil, J.; Wüstneck, N.; Cruz, A.; Fainerman, V.B.; Pison, U. Interfacial properties of pulmonary surfactant layers. Adv. Colloid Interface Sci. 2005, 117, 33–58. [Google Scholar] [CrossRef]
- Gradoń, L.; Podgórski, A.; Sosnowski, T.R. Experimental and theoretical investigations of transport properties of DPPC monolayer. J. Aerosol. Med. 1996, 9, 357–367. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Gradoń, L.; Skoczek, M.; Droździel, H. Experimental evaluation of the importance of the pulmonary surfactant for oxygen transfer rate in human lungs. Int. J. Occup. Saf. Ergon. 1998, 4, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R. Particles on the lung surface—Physicochemical and hydrodynamic effects. Curr. Opin. Colloid Interface Sci. 2018, 36, 1–9. [Google Scholar] [CrossRef]
- Korchowiec, B.; Stachowicz-Kuśnierz, A.; Korchowiec, J. The role of DPPG in lung surfactant exposed to benzo[a] pyrene. Environ. Sci. Process. Impacts 2019, 21, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Piosik, E.; Zaryczniak, A.; Mylkie, K.; Ziegler-Borowska, M. Probing of Interactions of Magnetite Nanoparticles Coated with Native and Aminated Starch with a DPPC Model Membrane. Int. J. Mol. Sci. 2021, 22, 5939. [Google Scholar] [CrossRef]
- SCOGS Database. Available online: https://www.fda.gov (accessed on 14 July 2022).
- Kondej, D.; Sosnowski, T.R. Interfacial rheology for the assessment of potential health effects of inhaled carbon nanomaterials at variable breathing conditions. Sci. Rep. 2020, 10, 14044. [Google Scholar] [CrossRef]
- Clements, J.A.; Hustead, R.F.; Johnson, R.P. Pulmonary surface tension and alveolar stability. J. Appl. Physiol. 1961, 16, 444–450. [Google Scholar] [CrossRef]
- Notter, R.H.; Taubold, R.; Mavis, R.D. Hysteresis in saturated phospholipid films and its potential relevance for lung surfactant function in vivo. Exp. Lung Res. 1982, 3, 109–127. [Google Scholar] [CrossRef]
- Lyklema, J. Fundamentals of Interface and Colloid Science; Academic Press: London, UK; San Diego, CA, USA, 2000. [Google Scholar]
- Ravera, F.; Ferrari, M.; Santini, E.; Liggieri, L. Influence of surface processes on the dilational visco-elasticity of surfactant solutions. Adv. Colloid Interface Sci. 2005, 117, 75–100. [Google Scholar] [CrossRef]
- Bykov, G.; Loglio, G.; Miller, R.; Milyaeva, O.Y.; Michailov, A.V.; Noskov, B.A. Dynamic properties and relaxation processes in surface layer of pulmonary surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 14–21. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; García, A.E. Concentration dependence of NaCl ion distributions around DPPC lipid bilayers. Interdiscip Sci. 2011, 3, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Musilová, L.; Kašpárková, V.; Mráček, A.; Minařík, A.; Minařík, M. The behaviour of hyaluronan solutions in the presence of Hofmeister ions: A light scattering, viscometry and surface tension study. Carbohydr. Polym. 2019, 212, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kutálkováa, E.; Hrnčiříka, J.; Witaseka, R.; Ingra, M. Effect of solvent and ions on the structure and dynamics of a hyaluronan molecule. Carbohydr. Polym. 2020, 234, 115919. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, A.; Pittsley, J.; Senti, F.R. Polysaccharide B-1459: A new hydrocolloid polyelectrolyte produced from glucose by bacterial fermentation. J. Appl. Polym. Sci. 1961, 5, 519. [Google Scholar] [CrossRef]
- Bezemer, L.; Ubbink, J.; De Kooker, J.; Kuil, M.; Leyte, J. On the conformational transitions of native xanthan. Macromolecules 1993, 26, 6436. [Google Scholar] [CrossRef]
- Holzwarth, G. Conformation of the extracellular polysaccharide of Xanthomonas campestris. Biochemistry 1976, 15, 4333. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Liberatore, M.W. Rheology and viscosity scaling of the polyelectrolyte xanthan gum. J. Appl. Polym. Sci. 2009, 114, 4076–4084. [Google Scholar] [CrossRef]
- Wüstneck, R.; Wüstneck, N.; Moser, B.; Karageorgieva, V.; Pison, U. Surface Dilatational Behavior of Pulmonary Surfactant Components Spread on the Surface of a Pendant Drop. 1. Dipalmitoyl Phosphatidylcholine and Surfactant Protein C. Langmuir 2002, 18, 1119–1124. [Google Scholar] [CrossRef]
- Jabłczyńska, K.; Sosnowski, T.R. Adsorption and co-adsorption of polyaldehydedextran nanoparticles and nonionic surfactantat an air–water interface: Potential implications for pulmonary drug delivery. Chem. Process Eng. 2017, 38, 67–77. [Google Scholar] [CrossRef]
- Podgórski, A.; Sosnowski, T.R.; Gradoń, L. Deactivation of the pulmonary surfactant dynamics by toxic aerosols and gases. J. Aerosol. Med. 2001, 14, 455–466. [Google Scholar] [CrossRef]
- Schleh, C.; Hohlfeld, J.M. Interaction of nanoparticles with the pulmonary surfactant system. Inhal. Toxicol. 2009, 21, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Santini, E. Lung surfactant-particles at fluid interfaces for toxicity assessments. Curr. Opin. Colloid Interface Sci. 2019, 39, 24–39. [Google Scholar] [CrossRef]
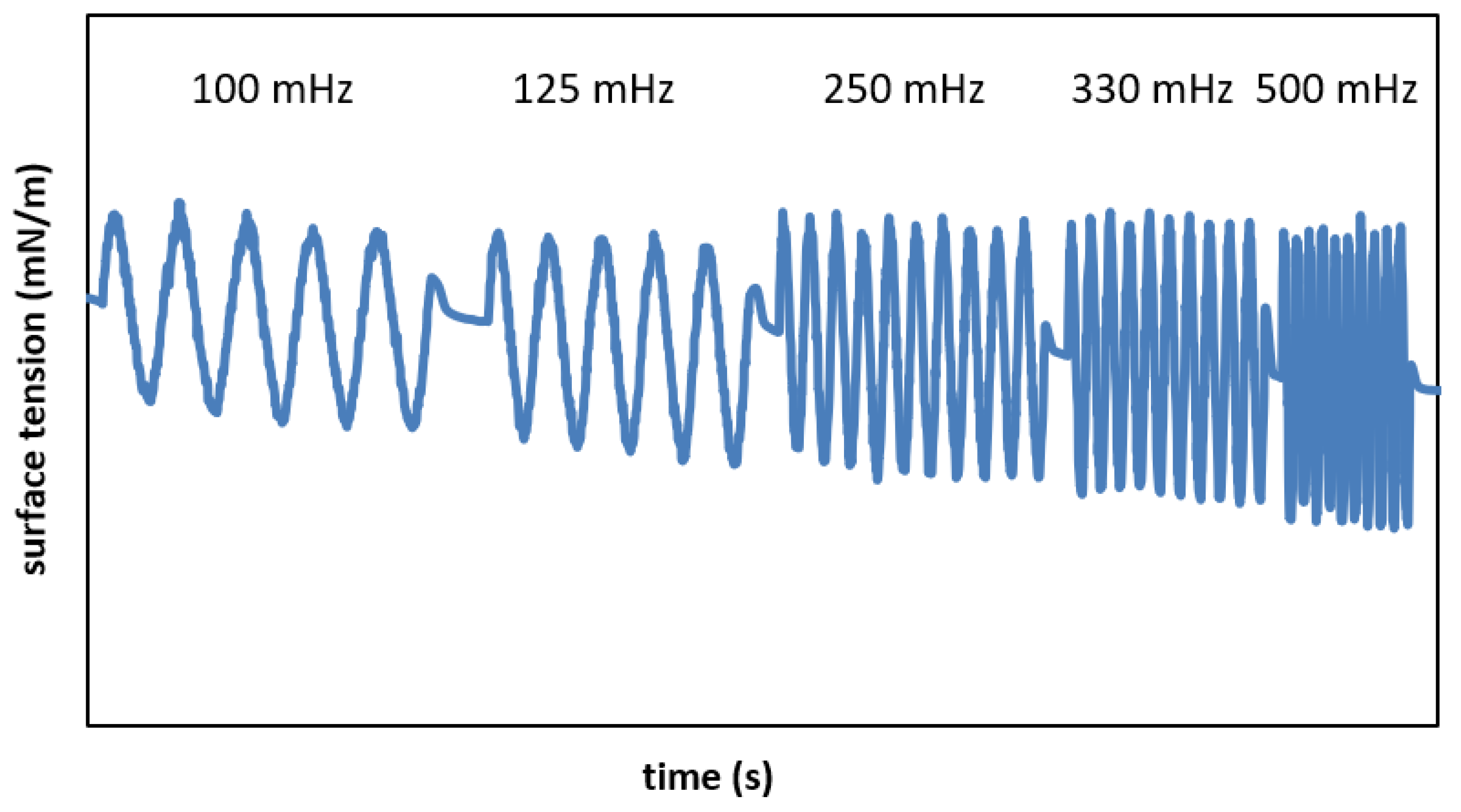


| f = 100 mHz | f = 125 mHz | f = 250 mHz | f = 330 mHz | f = 500 mHz | |
|---|---|---|---|---|---|
| σmin (mN/m) | 29.62 0.45 | 29.35 1.14 | 28.67 0.21 | 28.42 1.72 | 27.92 1.42 |
| SI (-) | 0.18 0.02 | 0.18 0.02 | 0.20 0.01 | 0.24 0.02 | 0.25 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrowolska, K.; Miros, M.; Sosnowski, T.R. Impact of Natural-Based Viscosity Modifiers of Inhalation Drugs on the Dynamic Surface Properties of the Pulmonary Surfactant. Materials 2023, 16, 1975. https://doi.org/10.3390/ma16051975
Dobrowolska K, Miros M, Sosnowski TR. Impact of Natural-Based Viscosity Modifiers of Inhalation Drugs on the Dynamic Surface Properties of the Pulmonary Surfactant. Materials. 2023; 16(5):1975. https://doi.org/10.3390/ma16051975
Chicago/Turabian StyleDobrowolska, Katarzyna, Małgorzata Miros, and Tomasz R. Sosnowski. 2023. "Impact of Natural-Based Viscosity Modifiers of Inhalation Drugs on the Dynamic Surface Properties of the Pulmonary Surfactant" Materials 16, no. 5: 1975. https://doi.org/10.3390/ma16051975
APA StyleDobrowolska, K., Miros, M., & Sosnowski, T. R. (2023). Impact of Natural-Based Viscosity Modifiers of Inhalation Drugs on the Dynamic Surface Properties of the Pulmonary Surfactant. Materials, 16(5), 1975. https://doi.org/10.3390/ma16051975






