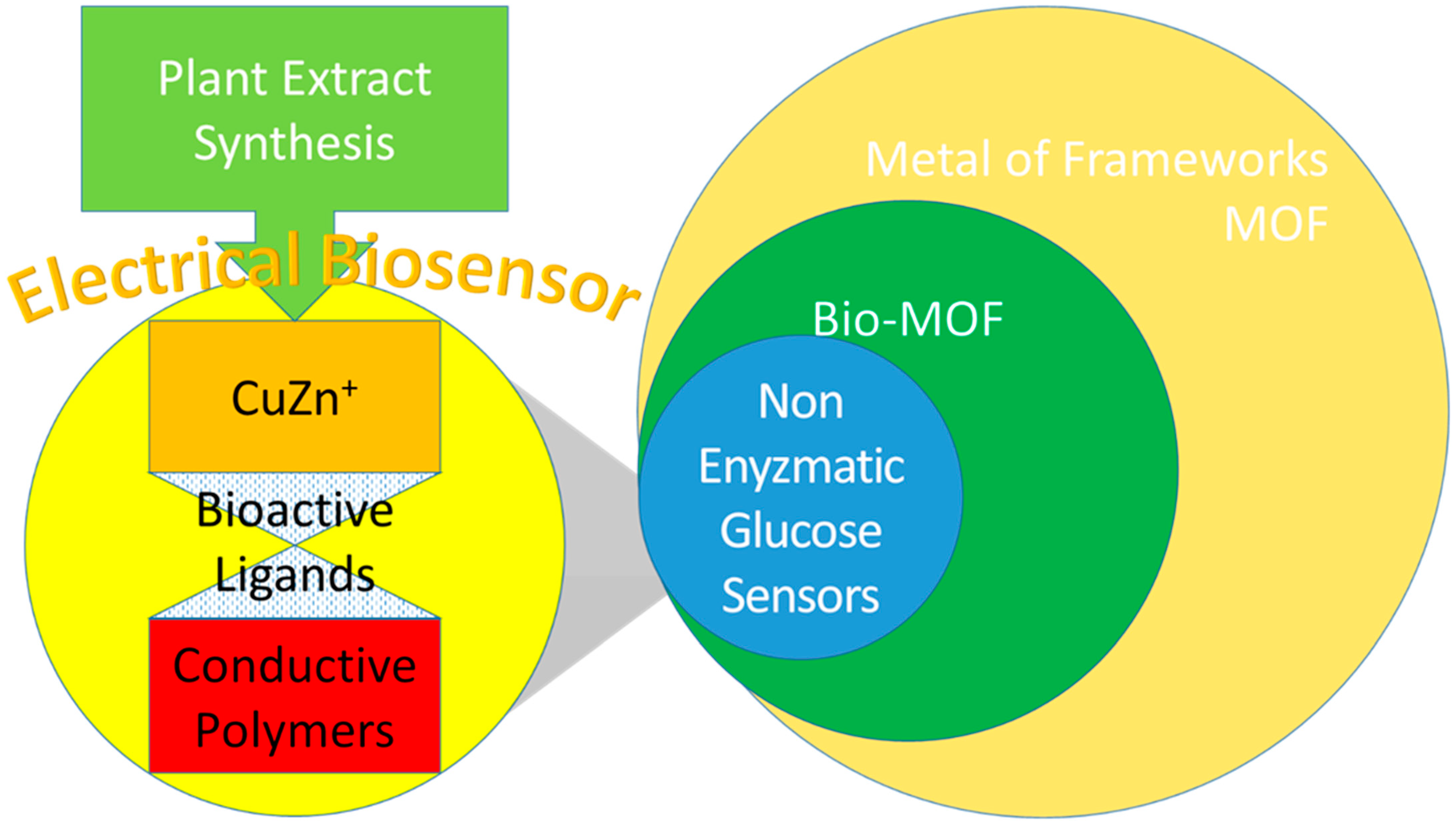
CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems
Abstract
1. Introduction
2. Electrical Biosensors for Electrical Trigger in Drug Delivery Systems
Additional Features Enhancements of Electrical Biosensors for Electrical Triggers
3. Cu and Zn as the Main Composites in Electrical Biosensors for Electrical Triggers
3.1. Cu in Non-Enzymatic Glucose and Electrical Biosensors
3.2. Cu in MOF Compositions for Electrical Biosensors
3.3. Zn in MOF Compositions for Electrical Biosensors
4. Other Stimuli and Chemotherapies
4.1. Cu and Zn for Redox and pH Stimuli in Electrical Biosensors
4.2. Cu and Zn as API in Chemotherapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eveness, J.; Cao, L.; Kiely, J.; Luxton, R. Equivalent circuit model of a non-faradaic impedimetric ZnO nano-crystal biosensor. J. Electroanal. Chem. 2022, 906, 116003. [Google Scholar] [CrossRef]
- Khan, A.N.; Ermakov, A.; Saunders, T.; Giddens, H.; Gould, D.; Sukhorukov, G.; Hao, Y. Electrical characterization of micron sized chambers used as a depot for drug delivery. IEEE Sens. J. 2022, 22, 18162–18169. [Google Scholar] [CrossRef]
- Lo, J.-I.; Peng, Y.-C.; Lu, H.-C.; Tseng, T.-R.; Cheng, B.-M. Monitoring the Temperature of a Mo/Si Mirror with Photoluminescence in Extreme-Ultraviolet Lithography. ACS Appl. Electron. Mater. 2022, 4, 3435–3439. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Su, W.; Zhang, W.; Xu, Z.; Wang, J.; Chen, J. Fabrication of a Free-Standing MWCNT Electrode by Electric Field Force for an Ultra-Sensitive MicroRNA-21 Nano-Genosensor. Small 2022, 18, 2201791. [Google Scholar] [CrossRef] [PubMed]
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.-T.; Suresh, S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS Bull. 2010, 35, 382–388. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Huey-Jen Hsu, S.; Chou, D.-A.; Wang, G.-J.; Chang, C.-C. Surface plasmon-enhanced fluorescence and surface-enhanced Raman scattering dual-readout chip constructed with silver nanowires: Label-free clinical detection of direct-bilirubin. Biosens. Bioelectron. 2022, 213, 114440. [Google Scholar] [CrossRef]
- Adhikari, J.; Rizwan, M.; Ahmed, M.U. Development of a label-free electrochemiluminescence biosensor for the sensitive detection of porcine gelatin using carbon nanostructured materials. Sens. Diagn. 2022, 1, 968–976. [Google Scholar] [CrossRef]
- Hong, S.P.; Mohd-Naim, N.F.; Keasberry, N.A.; Ahmed, M.U. Electrochemical Detection of β-Lactoglobulin Allergen Using Titanium Dioxide/Carbon Nanochips/Gold Nanocomposite-based Biosensor. Electroanalysis 2022, 34, 684–691. [Google Scholar] [CrossRef]
- Beg, S.; Handa, M.; Shukla, R.; Rahman, M.; Almalki, W.H.; Afzal, O.; Altamimi, A.S.A. Wearable smart devices in cancer diagnosis and remote clinical trial monitoring: Transforming the healthcare applications. Drug Discov. Today 2022, 27, 103314. [Google Scholar] [CrossRef]
- Nocerino, V.; Miranda, B.; Tramontano, C.; Chianese, G.; Dardano, P.; Rea, I.; De Stefano, L. Plasmonic Nanosensors: Design, Fabrication, and Applications in Biomedicine. Chemosensors 2022, 10, 150. [Google Scholar] [CrossRef]
- Zhou, F.; Pan, W.; Chang, Y.; Su, X.; Duan, X.; Xue, Q. A Supported Lipid Bilayer-Based Lab-on-a-Chip Biosensor for the Rapid Electrical Screening of Coronavirus Drugs. ACS Sens. 2022, 7, 2084–2092. [Google Scholar] [CrossRef]
- Gao, D.; Lv, J.; Lee, P.S. Natural Polymer in Soft Electronics: Opportunities, Challenges, and Future Prospects. Adv. Mater. 2022, 34, 2105020. [Google Scholar] [CrossRef]
- Han, W.B.; Yang, S.M.; Rajaram, K.; Hwang, S. Materials and Fabrication Strategies for Biocompatible and Biodegradable Conductive Polymer Composites toward Bio-Integrated Electronic Systems. Adv. Sustain. Syst. 2022, 6, 2100075. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent advancements in synthesis, properties, and applications of conductive polymers for electrochemical energy storage devices: A review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Jang, H.-K.; Kim, J.; Park, J.-S.; Moon, J.B.; Oh, J.; Lee, W.-K.; Kang, M.-G. Synthesis and Characterization of a Conductive Polymer Blend Based on PEDOT:PSS and Its Electromagnetic Applications. Polymers 2022, 14, 393. [Google Scholar] [CrossRef]
- Dědek, I.; Kupka, V.; Jakubec, P.; Šedajová, V.; Jayaramulu, K.; Otyepka, M. Metal-organic framework/conductive polymer hybrid materials for supercapacitors. Appl. Mater. Today 2022, 26, 101387. [Google Scholar] [CrossRef]
- Moharramnejad, M.; Ehsani, A.; Salmani, S.; Shahi, M.; Malekshah, R.E.; Robatjazi, Z.S.; Parsimehr, H. Zinc-based metal-organic frameworks: Synthesis and recent progress in biomedical application. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3339–3354. [Google Scholar] [CrossRef]
- Cun, J.-E.; Fan, X.; Pan, Q.; Gao, W.; Luo, K.; He, B.; Pu, Y. Copper-based metal–organic frameworks for biomedical applications. Adv. Colloid Interface Sci. 2022, 305, 102686. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shuang, E.; Liu, J.; Sheng, K.; Zhang, X. Endogenous calcium enriched hydrochar catalyst derived from water hyacinth for glucose isomerization. Sci. Total Environ. 2022, 807, 150660. [Google Scholar] [CrossRef]
- Sun, W.; Lu, Z.; Zuo, K.; Xu, S.; Shi, B.; Wang, H. High efficiency electrochemical disinfection of Pseudomons putida using electrode of orange peel biochar with endogenous metals. Chemosphere 2022, 289, 133138. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.-L.; Chao, Q.; Li, Q.; Kong, R.; Fan, G.-C.; Luo, X. A DNAzyme-based normalized fluorescence strategy for direct quantification of endogenous zinc in living cells. Chem. Commun. 2022, 58, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Danilova, E.D.; Litvinovskaya, R.P.; Zlobin, I.E.; Kolomeichuk, L.V.; Murgan, O.K.; Sauchuk, A.L.; Khripach, V.A.; Kuznetsov, V.V.; Efimova, M.V. Polymetallic Stress Changes the Endogenous Status of Brassinosteroids and Reduces the Effectiveness of Photochemical Reactions Photosystem II in Barley Plants. Dokl. Biochem. Biophys. 2022, 504, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S855–S872. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, B.; Li, M.; Huang, Y.; Li, L. Heterostructures Made of Upconversion Nanoparticles and Metal–Organic Frameworks for Biomedical Applications. Adv. Sci. 2022, 9, 2103911. [Google Scholar] [CrossRef]
- Ge, X.; Wong, R.; Anisa, A.; Ma, S. Recent development of metal-organic framework nanocomposites for biomedical applications. Biomaterials 2022, 281, 121322. [Google Scholar] [CrossRef]
- Bahrani, S.; Hashemi, S.A.; Mousavi, S.M.; Arjmand, M.; Ghalamfarsa, F.; Ghaedi, M. Conductive polymers in green analytical chemistry. In Conductive Polymers in Analytical Chemistry; American Chemical Society: Washington, DC, USA, 2022; pp. 1–37. [Google Scholar] [CrossRef]
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2022, 34, 2105063. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion-conducting polyelectrolytes for energy devices. Trends Chem. 2022, 4, 236–249. [Google Scholar] [CrossRef]
- Delvart, A.; Moreau, C.; Cathala, B. Dextrans and dextran derivatives as polyelectrolytes in layer-by-layer processing materials—A review. Carbohydr. Polym. 2022, 293, 119700. [Google Scholar] [CrossRef]
- Zhao, Z.; Bourne, P.E. Harnessing systematic protein–ligand interaction fingerprints for drug discovery. Drug Discov. Today 2022, 27, 103319. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Wang, X.; Liang, S.; Li, N.; An, H. Progress in research on natural cellulosic fibre modifications by polyelectrolytes. Carbohydr. Polym. 2022, 278, 118966. [Google Scholar] [CrossRef]
- Le Goas, M.; Saber, J.; Bolívar, S.G.; Rabanel, J.-M.; Awogni, J.-M.; Boffito, D.C.; Banquy, X. (In)stability of ligands at the surface of inorganic nanoparticles: A forgotten question in nanomedicine? Nano Today 2022, 45, 101516. [Google Scholar] [CrossRef]
- Yan, Y.; Wan, B.; Mansor, M.; Wang, X.; Zhang, Q.; Kappler, A.; Feng, X. Co-sorption of metal ions and inorganic anions/organic ligands on environmental minerals: A review. Sci. Total Environ. 2022, 803, 149918. [Google Scholar] [CrossRef] [PubMed]
- Al Sharabati, M.; Sabouni, R.; Husseini, G.A. Biomedical Applications of Metal−Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials 2022, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, H.; Jiang, H.; Yang, P.; Luo, L.; Niu, Q.; You, T. Photoactivities regulating of inorganic semiconductors and their applications in photoelectrochemical sensors for antibiotics analysis: A systematic review. Biosens. Bioelectron. 2022, 216, 114634. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Mao, J.; Sun, D.; Zhang, Q.; Cheng, L.; Yang, X.; Li, P. Strategies to control mycotoxins and toxigenic fungi contamination by nano-semiconductor in food and agro-food: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–25. [Google Scholar] [CrossRef]
- Adesoye, S.; Dellinger, K. ZnO and TiO2 nanostructures for surface-enhanced Raman scattering-based bio-sensing: A review. Sens. Bio-Sensing Res. 2022, 37, 100499. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, H.; Wang, Y.; Zhang, Y.-H.P. Protein engineering for electrochemical biosensors. Curr. Opin. Biotechnol. 2022, 76, 102751. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules. Enzyme Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef]
- Saira, F.; Yaqub, A.; Razzaq, H.; Sohail, M.G.; Saleemi, S.; Mumtaz, M.; Rafiq, M.A.; Qaisar, S. Hollow nanocages for electrochemical glucose sensing: A comprehensive review. J. Mol. Struct. 2022, 1268, 133646. [Google Scholar] [CrossRef]
- Borzehandani, M.Y.; Sathar, M.H.A.; Abdulmalek, E.; Rahman, M.B.A.; Latif, M.A.M. In Silico identification of the mechanism of fluorouracil adsorption inside MIL-101(Mg) metal-organic framework. AIP Conf. Proc. 2022, 2506, 060003. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.-X. Cu-based nanoparticle toxicity to zebrafish cells regulated by cellular discharges. Environ. Pollut. 2022, 292, 118296. [Google Scholar] [CrossRef] [PubMed]
- Tricase, A.; Imbriano, A.; Macchia, E.; Sarcina, L.; Scandurra, C.; Torricelli, F.; Cioffi, N.; Torsi, L.; Bollella, P. Enzyme based amperometric wide field biosensors: Is single-molecule detection possible? Electrochem. Sci. Adv. 2022. [Google Scholar] [CrossRef]
- Osuna, V.; Vega-Rios, A.; Zaragoza-Contreras, E.A.; Estrada-Moreno, I.A.; Dominguez, R.B. Progress of Polyaniline Glucose Sensors for Diabetes Mellitus Management Utilizing Enzymatic and Non-Enzymatic Detection. Biosensors 2022, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Gu, L.; Duan, Z.; Pan, D.; Xu, Z.; Gong, Q.; Li, Y.; Zhu, H.; Luo, K. Anticancer nanomedicines harnessing tumor microenvironmental components. Expert Opin. Drug Deliv. 2022, 19, 337–354. [Google Scholar] [CrossRef]
- Zou, R.; Wang, X.; Li, S.; Chan, H.C.S.; Vogel, H.; Yuan, S. The role of metal ions in G protein-coupled receptor signalling and drug discovery. WIREs Comput. Mol. Sci. 2022, 12, e1565. [Google Scholar] [CrossRef]
- Avan, A.; Członkowska, A.; Gaskin, S.; Granzotto, A.; Sensi, S.L.; Hoogenraad, T.U. The Role of Zinc in the Treatment of Wilson’s Disease. Int. J. Mol. Sci. 2022, 23, 9316. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Behbudi, G.; Gholami, A.; Hashemi, S.A.; Nejad, Z.M.; Bahrani, S.; Chiang, W.-H.; Wei, L.C.; Omidifar, N. Shape-controlled synthesis of zinc nanostructures mediating macromolecules for biomedical applications. Biomater. Res. 2022, 26, 4. [Google Scholar] [CrossRef]
- Quiñones Vélez, G.; Carmona-Sarabia, L.; Rivera Raíces, A.A.; Hu, T.; Peterson-Peguero, E.A.; López-Mejías, V. High affinity zoledronate-based metal complex nanocrystals to potentially treat osteolytic metastases. Mater. Adv. 2022, 3, 3251–3266. [Google Scholar] [CrossRef]
- Atoufi, Z.; Zarrintaj, P.; Motlagh, G.H.; Amiri, A.; Bagher, Z.; Kamrava, S.K. A novel bio electro active alginate-aniline tetramer/agarose scaffold for tissue engineering: Synthesis, characterization, drug release and cell culture study. J. Biomater. Sci. Polym. Ed. 2017, 28, 1617–1638. [Google Scholar] [CrossRef]
- Lestari, W.W.; Arvinawati, M.; Martien, R.; Kusumaningsih, T. Green and facile synthesis of MOF and nano MOF containing zinc(II) and benzen 1,3,5-tri carboxylate and its study in ibuprofen slow-release. Mater. Chem. Phys. 2018, 204, 141–146. [Google Scholar] [CrossRef]
- Wang, H.; Jian, Y.; Kong, Q.; Liu, H.; Lan, F.; Liang, L.; Ge, S.; Yu, J. Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sens. Actuators B Chem. 2018, 257, 561–569. [Google Scholar] [CrossRef]
- Forero, J.; Roa, E.; Reyes, J.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications. Materials 2022, 15, 1421. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, W.; Kwak, K.; Choi, H.; Kim, D.-H. Electric Pulse Responsive Magnetic Nanoclusters Loaded with Indoleamine 2,3-Dioxygenase Inhibitor for Synergistic Immuno-Ablation Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 54415–54425. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Wells, C.; Jennings, A.; Ghimire, M.; Mishra, S.R.; Morshed, B.I. Electric Stimulus-Responsive Chitosan/MNP Composite Microbeads for a Drug Delivery System. IEEE Trans. Biomed. Eng. 2020, 67, 226–233. [Google Scholar] [CrossRef]
- Gharehdaghi, Z.; Rahimi, R.; Naghib, S.M.; Molaabasi, F. Fabrication and application of copper metal–organic frameworks as nanocarriers for pH-responsive anticancer drug delivery. J. Iran. Chem. Soc. 2022, 19, 2727–2737. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Behbudi, G.; Hashemi, S.A.; Babapoor, A.; Chiang, W.-H.; Ramakrishna, S.; Rahman, M.M.; Lai, C.W.; Gholami, A.; Omidifar, N.; et al. Recent Progress in Electrochemical Detection of Human Papillomavirus (HPV) via Graphene-Based Nanosensors. J. Sens. 2021, 2021, 6673483. [Google Scholar] [CrossRef]
- Patil, S.B.; Inamdar, S.Z.; Das, K.K.; Akamanchi, K.G.; Patil, A.V.; Inamadar, A.C.; Reddy, K.R.; Raghu, A.V.; Kulkarni, R.V. Tailor-made electrically-responsive poly(acrylamide)-graft-pullulan copolymer based transdermal drug delivery systems: Synthesis, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101525. [Google Scholar] [CrossRef]
- Sabater, B. Entropy Perspectives of Molecular and Evolutionary Biology. Int. J. Mol. Sci. 2022, 23, 4098. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Y.; Yang, Z. A novel electrochemical sensor based on a glassy carbon electrode modified with Cu–MWCNT nanocomposites for determination of hydroquinone. Anal. Methods 2016, 8, 2568–2575. [Google Scholar] [CrossRef]
- Zheng, W.; Hu, L.; Lee, L.Y.S.; Wong, K.-Y. Copper nanoparticles/polyaniline/graphene composite as a highly sensitive electrochemical glucose sensor. J. Electroanal. Chem. 2016, 781, 155–160. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Lü, H.; Hui, N. Phytic acid doped poly(3,4-ethylenedioxythiophene) modified with copper nanoparticles for enzymeless amperometric sensing of glucose. Microchim. Acta 2020, 187, 49. [Google Scholar] [CrossRef] [PubMed]
- Sattar, T.; Athar, M.; Najamul Haq, M. Hydrothermal Synthesis, Characterization, and In Vitro Drug Adsorption Studies of Some Nano-BioMOFs. J. Nanomater. 2016, 2016, 7803480. [Google Scholar] [CrossRef]
- Sattar, T.; Athar, M. Some Nano Bio-Mofs Evaluated For Storage Purpose of Drugs. Biomed. J. Sci. Tech. Res. 2018, 2, 2348–2359. [Google Scholar] [CrossRef]
- Mohammadhassan, Z.; Mohammadkhani, R.; Mohammadi, A.; Zaboli, K.A.; Kaboli, S.; Rahimi, H.; Nosrati, H.; Danafar, H. Preparation of copper oxide nanoparticles coated with bovine serum albumin for delivery of methotrexate. J. Drug Deliv. Sci. Technol. 2022, 67, 103015. [Google Scholar] [CrossRef]
- Azizi Vahed, T.; Naimi-Jamal, M.R.; Panahi, L. Alginate-coated ZIF-8 metal-organic framework as a green and bioactive platform for controlled drug release. J. Drug Deliv. Sci. Technol. 2019, 49, 570–576. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Huang, L.; Zhang, W.; Wang, R.; Yue, T.; Sun, J.; Li, G.; Wang, J. The highly efficient elimination of intracellular bacteria via a metal organic framework (MOF)-based three-in-one delivery system. Nanoscale 2019, 11, 9468–9477. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Z.; Deng, T.; Chen, J.; Wen, Y.; Fu, X.; Zhou, L.; Zhu, Z.; Yu, C. A natural polysaccharide mediated MOF-based Ce6 delivery system with improved biological properties for photodynamic therapy. J. Mater. Chem. B 2020, 8, 1481–1488. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Li, Z.; Govindan, R.; Jayakumar, R.; Wang, C.; Long Gu, F. Zinc oxide-quercetin nanocomposite as a smart nano-drug delivery system: Molecular-level interaction studies. Appl. Surf. Sci. 2021, 536, 147741. [Google Scholar] [CrossRef]
- Sanati, A.; Esmaeili, Y.; Bidram, E.; Shariati, L.; Rafienia, M.; Mahshid, S.; Parlak, O. Recent advancement in electrode materials and fabrication, microfluidic designs, and self-powered systems for wearable non-invasive electrochemical glucose monitoring. Appl. Mater. Today 2022, 26, 101350. [Google Scholar] [CrossRef]
- de Vries, F.; Otten, E. Reversible On/Off Switching of Lactide Cyclopolymerization with a Redox-Active Formazanate Ligand. ACS Catal. 2022, 12, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, Q.; Tang, Z.; Liu, C.; Li, Z.; Wu, A.; Lin, H. Tumor Microenvironment Stimuli-Responsive Fluorescence Imaging and Synergistic Cancer Therapy by Carbon-Dot–Cu2+ Nanoassemblies. Angew. Chem. Int. Ed. 2020, 59, 21041–21048. [Google Scholar] [CrossRef] [PubMed]
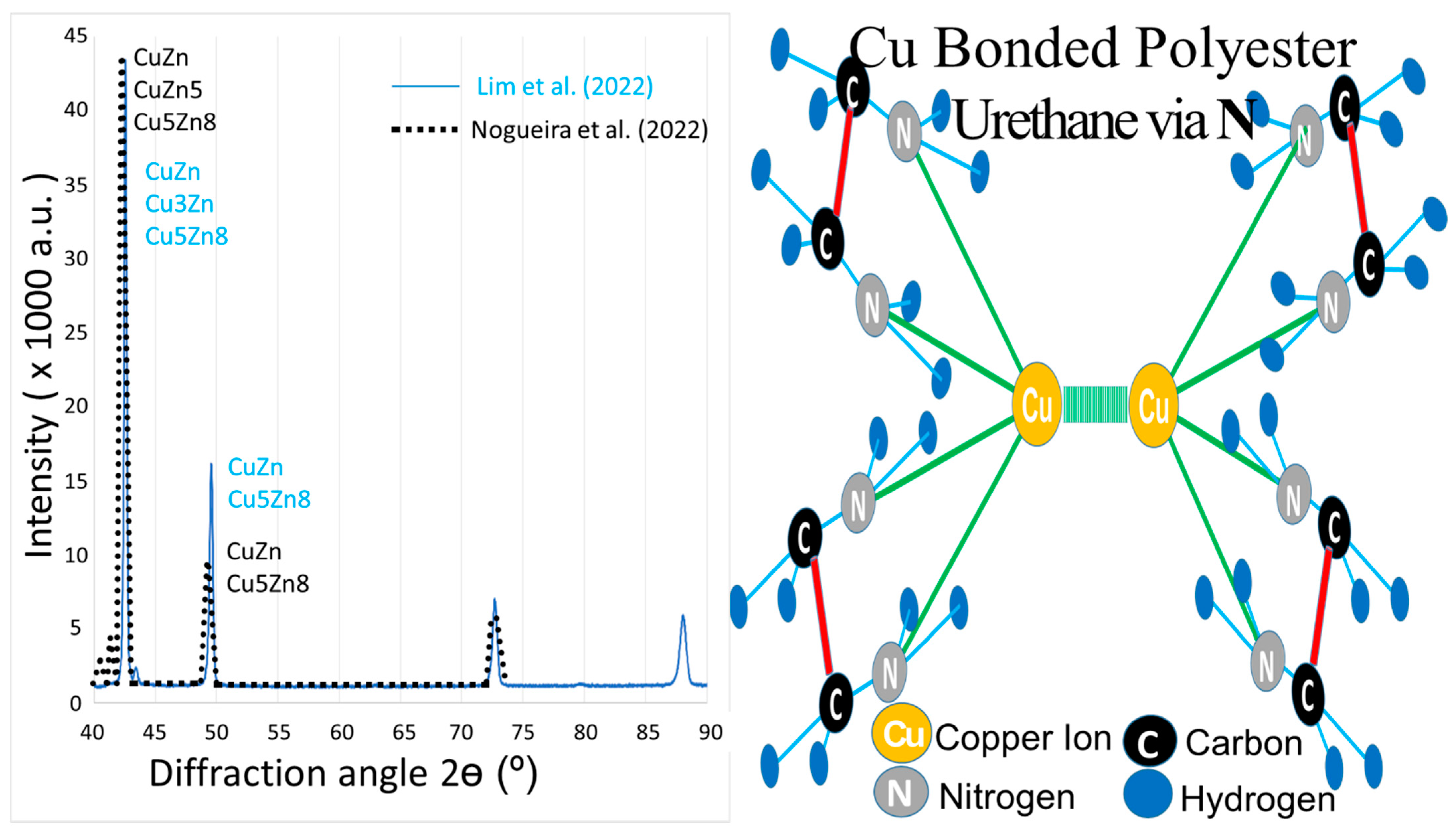
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. Structural Strength Analyses for Low Brass Filler Biomaterial with Anti-Trauma Effects in Articular Cartilage Scaffold Design. Materials 2022, 15, 4446. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, I.D.; Maçoas, E.M.; Montemor, M.F.; Alves, M.M. Biomedical potential of 3D Zn and ZnCu foams produced by dynamic hydrogen bubble template. Appl. Surf. Sci. 2022, 580, 152207. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Numerical Simulation Study on Relationship between the Fracture Mechanisms and Residual Membrane Stresses of Metallic Material. J. Funct. Biomater. 2022, 13, 20. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Yang, Q.; Gao, Y.; Duan, X.; Fu, Q.; Chu, C.; Pan, X.; Cui, X.; Sun, Y. Cuprous oxide nanoparticles trigger ER stress-induced apoptosis by regulating copper trafficking and overcoming resistance to sunitinib therapy in renal cancer. Biomaterials 2017, 146, 72–85. [Google Scholar] [CrossRef]
- Rabiee, N.; Atarod, M.; Tavakolizadeh, M.; Asgari, S.; Rezaei, M.; Akhavan, O.; Pourjavadi, A.; Jouyandeh, M.; Lima, E.C.; Mashhadzadeh, A.H.; et al. Green metal-organic frameworks (MOFs) for biomedical applications. Microporous Mesoporous Mater. 2022, 335, 111670. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Ion, D.; Niculescu, A.-G.; Mușat, F.; Andronic, O.; Grumezescu, A.M.; Bolocan, A. Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications. Pharmaceutics 2022, 14, 435. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Qiu, L.; Li, Y.; Marraiki, N.; Elgorban, A.M.; Xue, L. Green synthesized zinc oxide nanoparticles regulates the apoptotic expression in bone cancer cells MG-63 cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111644. [Google Scholar] [CrossRef]
- Cassano, R.; Curcio, F.; Di Gioia, M.L.; Trombino, S. Copper nanoparticles-based stimuli-responsive approaches. In Stimuli-Responsive Nanocarriers; Academic Press: Cambridge, MA, USA, 2022; pp. 413–428. [Google Scholar] [CrossRef]
| Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|
| AT | Dex | Alg/agarose/AT | Conductivity and suitable for neuroregenerative medicine. | [50] |
| Zn | Ibuprofen | BTC2/Zn3 | Electro-sonochemitry MOF at 15 V, 40 KHz with a slower release function. | [51] |
| Cu | miR-155 | Au@Cu | DPV detection limit of 0.35 fM, so highly sensitive detection of miR-155. | [52] |
| CuZn | HAp | CuZn/gelatin/Chi | Increased antibacterial activity and non-toxicity for osteoprogenitor cells. | [53] |
| Biosensor | API | Drug Carrier | Remarks | Ref. |
|---|---|---|---|---|
| IONPs | IDOi | IONP/IDOi | Synergistic electric pulse and local magnetic field effects on immuno-ablation cancer therapy | [55] |
| PEGDMA | Vcm | IONP/Chi/PEGDMA | Stimulated control release and targeted delivery | [56] |
| Cu | Dox | IONP/Cu3(BTC)2 | API released 85.5% at pH 5 and adsorbed API with 40.5 wt.%. | [57] |
| Cu | Dox | GO/Cu-TCPP | API released 98.9% at pH 5 and adsorbed API with 45.7 wt.%. | [57] |
| Plt/PVA | RT | polyAAm/Plt/PVA | Efficient reservoir for transdermal DDS | [59] |
| Cu Biosensor | Findings | Ref. |
|---|---|---|
| Cu-MWCNT | Increased electrochemical signals with a detection limit of 0.04 μM. | [61] |
| Cu/PANI/GO | A sensitivity of 0.15 Acm−2M−1 with a detection limit of 0.27 µM. | [62] |
| Cu/PEDOT/PA | A sensitivity of 79.27 Acm−2M−1 with a detection limit of 0.28 µM. | [63] |
| Drug Carrier | API | Findings with Slow Release | Ref. |
|---|---|---|---|
| CS, CP, and CT | Rsv | Corresponding carriers with DAs of 0.25, 0.15, and 0.25 g/g. | [64] |
| TH, Tms and Glp | Corresponding carriers with DAs of 0.095, 0.200, and 0.316 g/g TH; 0.083, 0.160, and 0.085 g/g Tms; and 0.041, 0.138, and 0.138 g/g Glp. | [65] | |
| CuO/BSA | Mtx | Drug loading efficiency of 8.70% and 75% release in proteinase K enzyme at pH 7.4. | [66] |
| Drug Carrier | API | Findings | Ref. |
|---|---|---|---|
| ZIF-8/Alg | Mfm | 11.6 Å pore size, 83.5% loading efficiency, and 6.68 wt.% payload. | [67] |
| ZIF-8/HA | Tet | 98% Tet clearance rate under acidic conditions and pH-responsive. | [68] |
| ZIF-8/HA | Ce6 | 88.4% of HepG2 cell death was by ROS. | [69] |
| ZnO/Qct | Qct | High biocompatibility with 3T3-L1 cells and effective MCF-7 growth inhibition. | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A. CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems. Materials 2022, 15, 7672. https://doi.org/10.3390/ma15217672
Lim YY, Miskon A, Zaidi AMA. CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems. Materials. 2022; 15(21):7672. https://doi.org/10.3390/ma15217672
Chicago/Turabian StyleLim, Yan Yik, Azizi Miskon, and Ahmad Mujahid Ahmad Zaidi. 2022. "CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems" Materials 15, no. 21: 7672. https://doi.org/10.3390/ma15217672
APA StyleLim, Y. Y., Miskon, A., & Zaidi, A. M. A. (2022). CuZn Complex Used in Electrical Biosensors for Drug Delivery Systems. Materials, 15(21), 7672. https://doi.org/10.3390/ma15217672






