Effect of Polytetrafluoroethylene (PTFE) Content on the Properties of Ni-Cu-P-PTFE Composite Coatings
Abstract
1. Introduction
2. Experiment
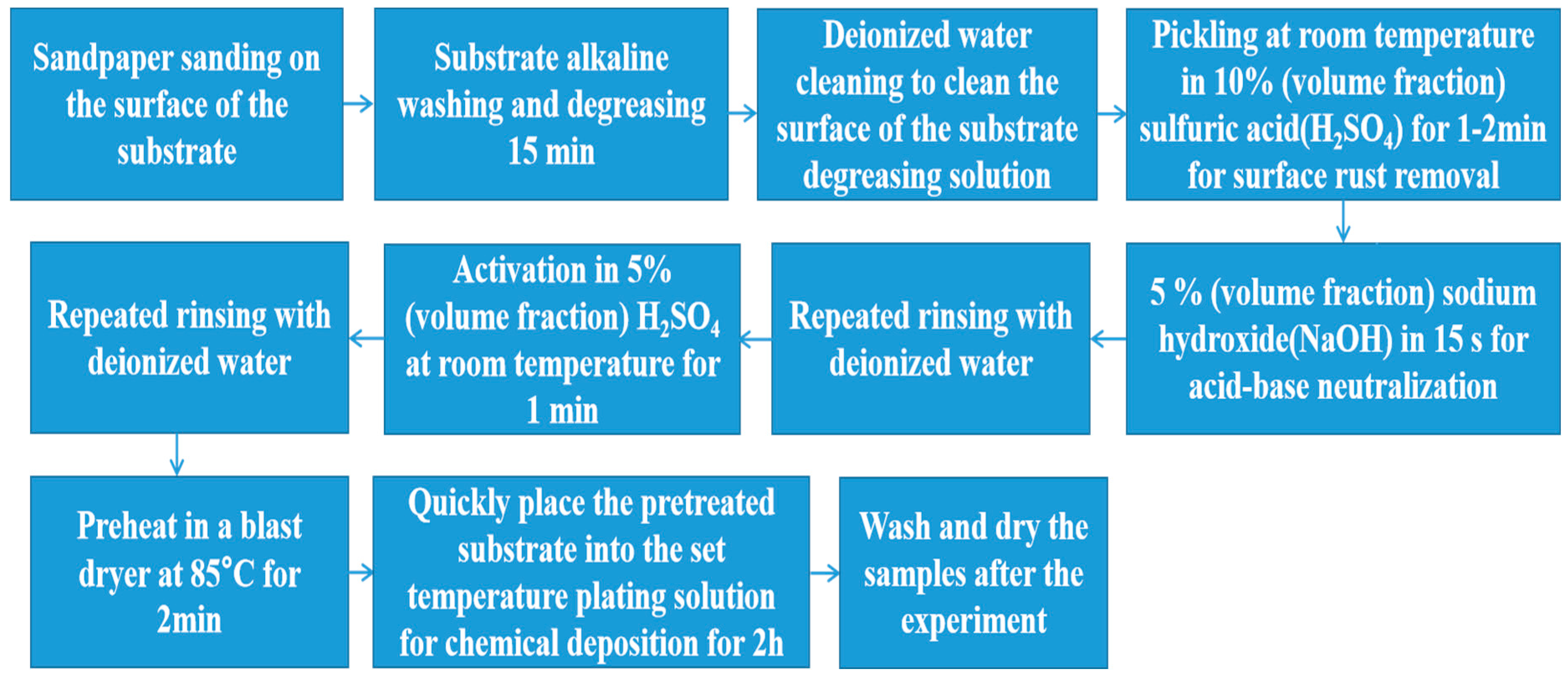
2.1. Preparation and Process of Coating
2.2. Coating Structure Characterization and Phase Analysis
2.3. Characterization of Coating Properties
3. Results Discussion
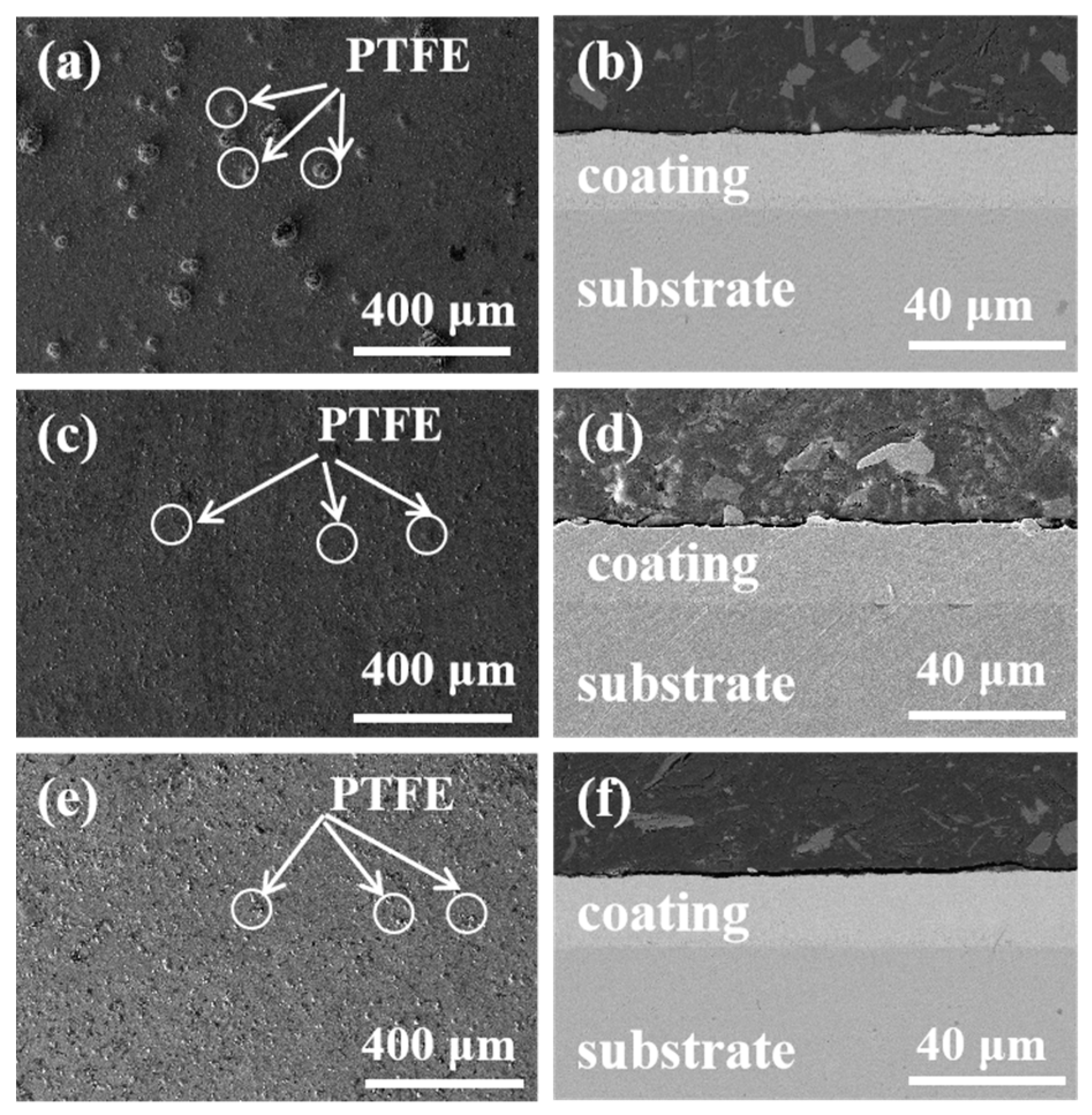
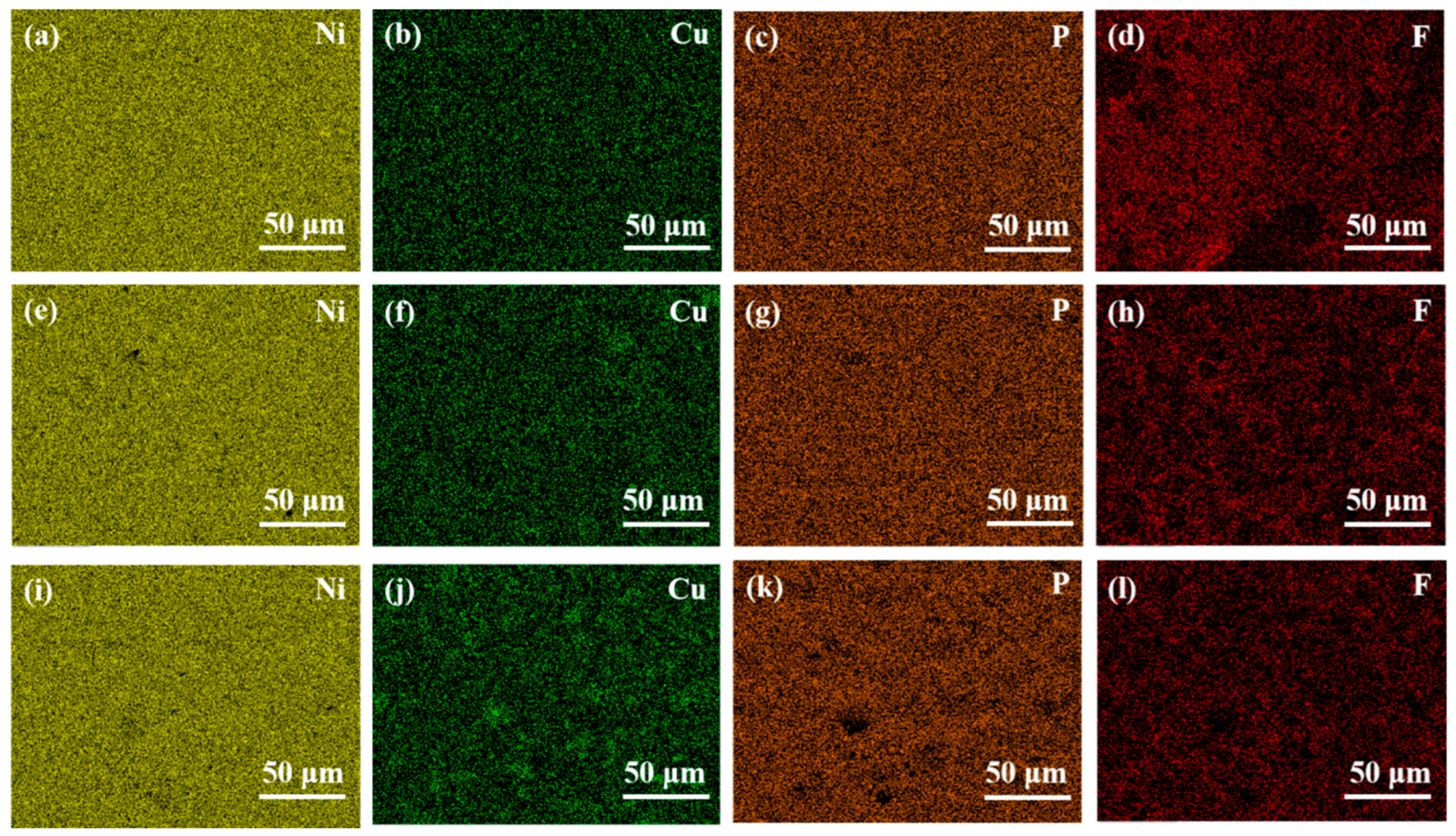
3.1. Surface Morphology and Compositional Analysis of Ni-Cu-P-PTFE Composite Plating
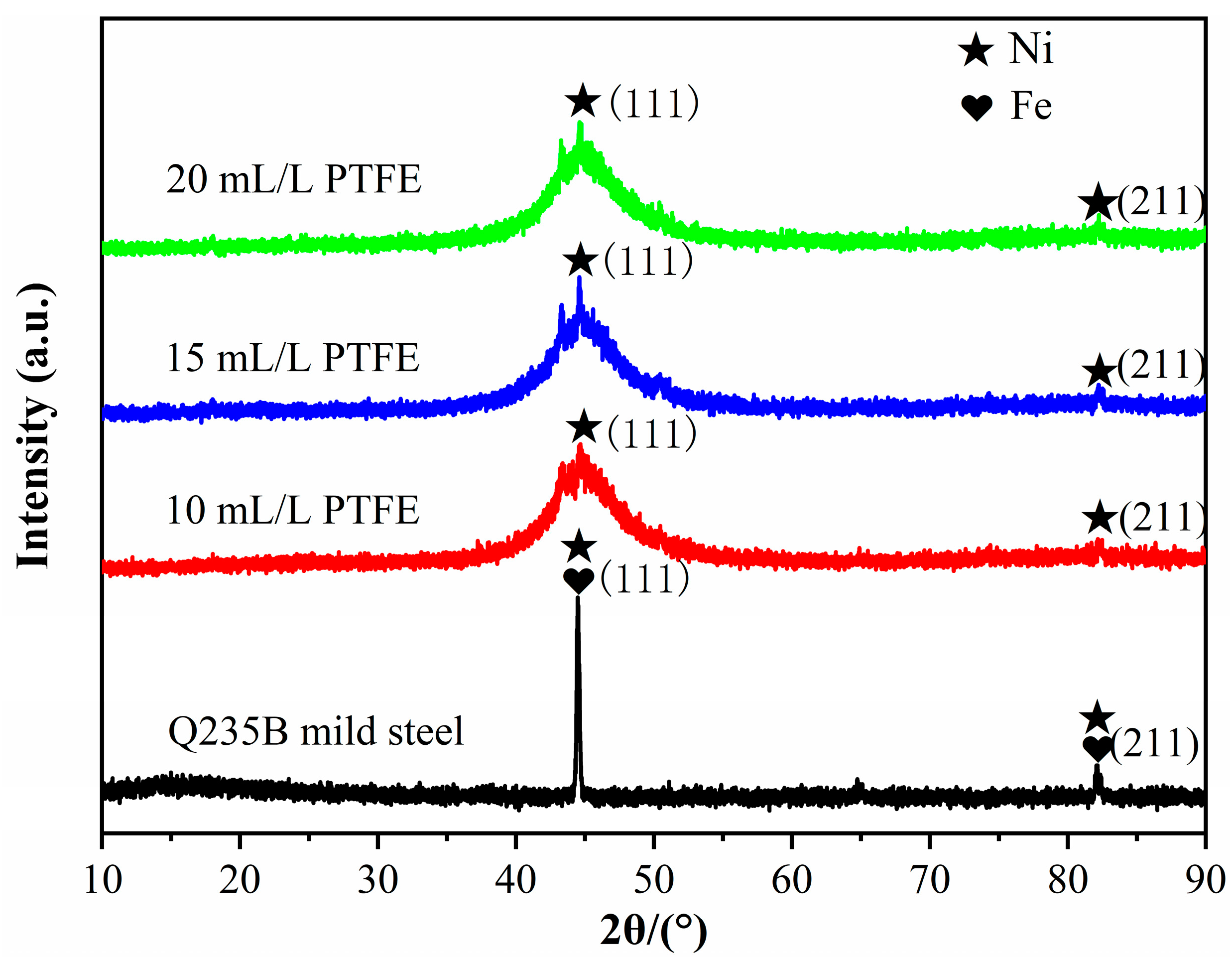
3.2. Physical Phase Analysis of Ni-Cu-P-PTFE Composite Plating
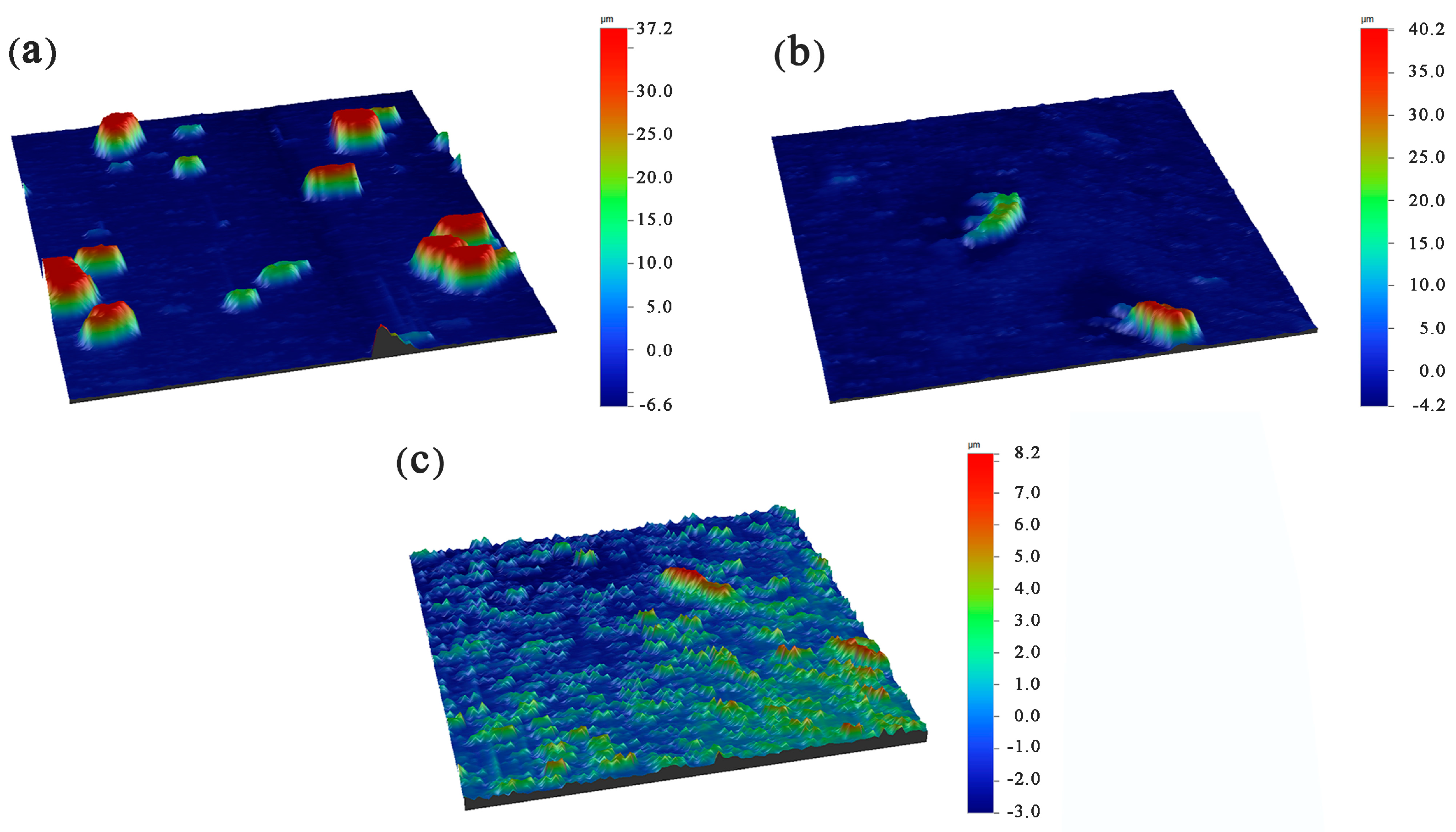
3.3. Surface Roughness of Ni-Cu-P-PTFE Composite Plating
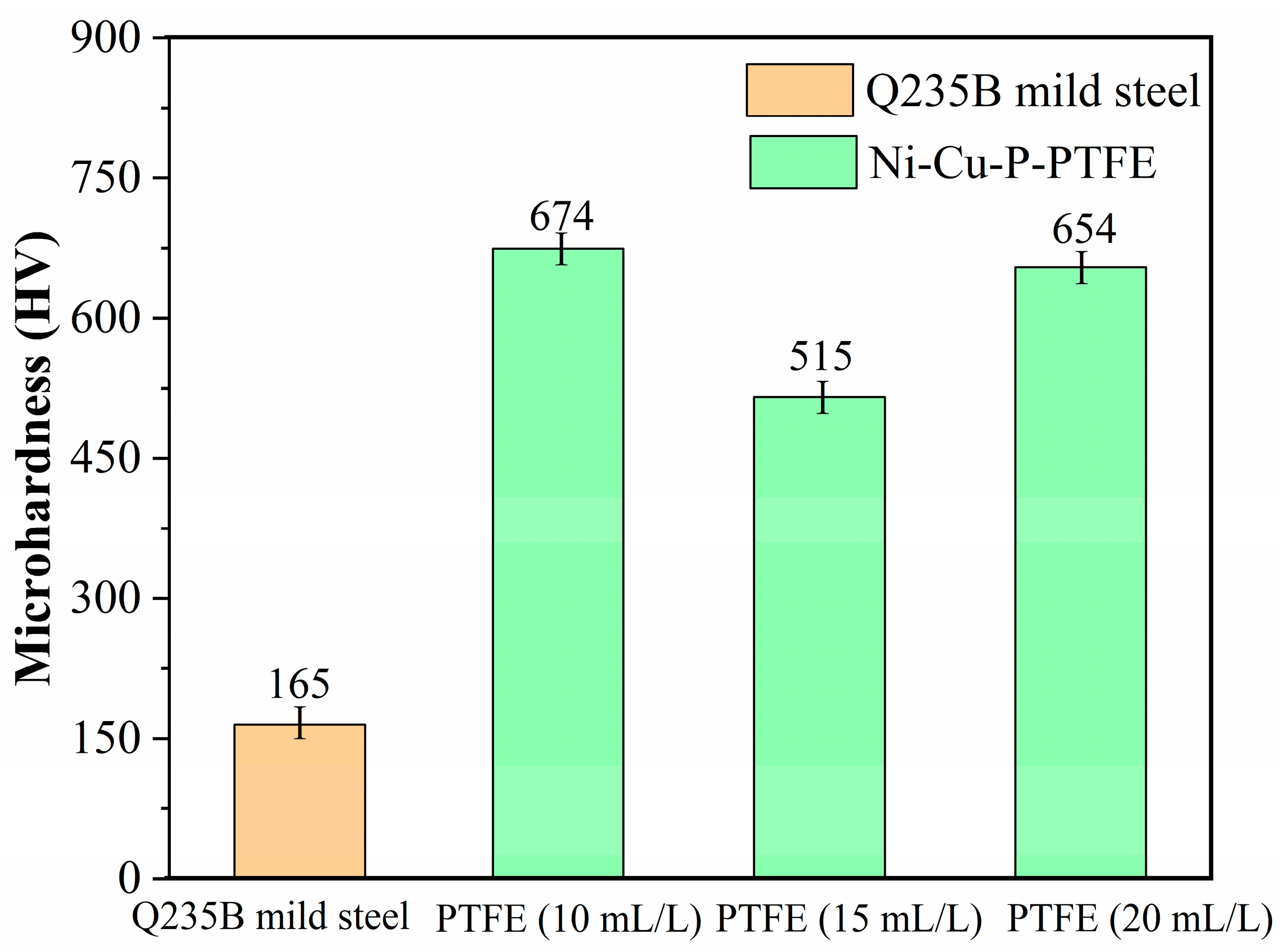
3.4. Microhardness of Ni-Cu-P-PTFE Composite Coatings
3.5. Analysis of the Corrosion Performance of Ni-Cu-P-PTFE Composite Coatings
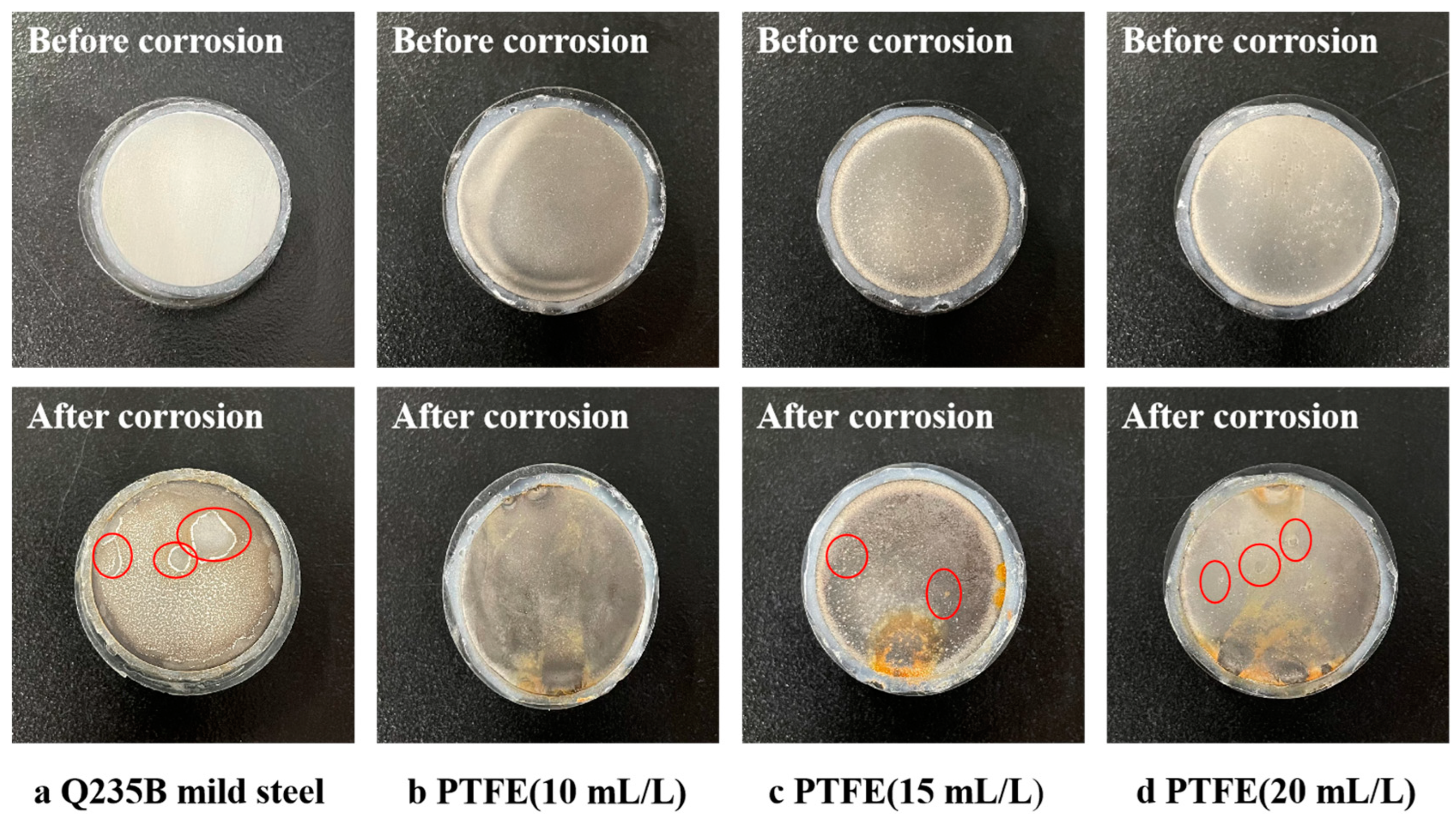
3.5.1. Salt Corrosion Weight Loss Experiments
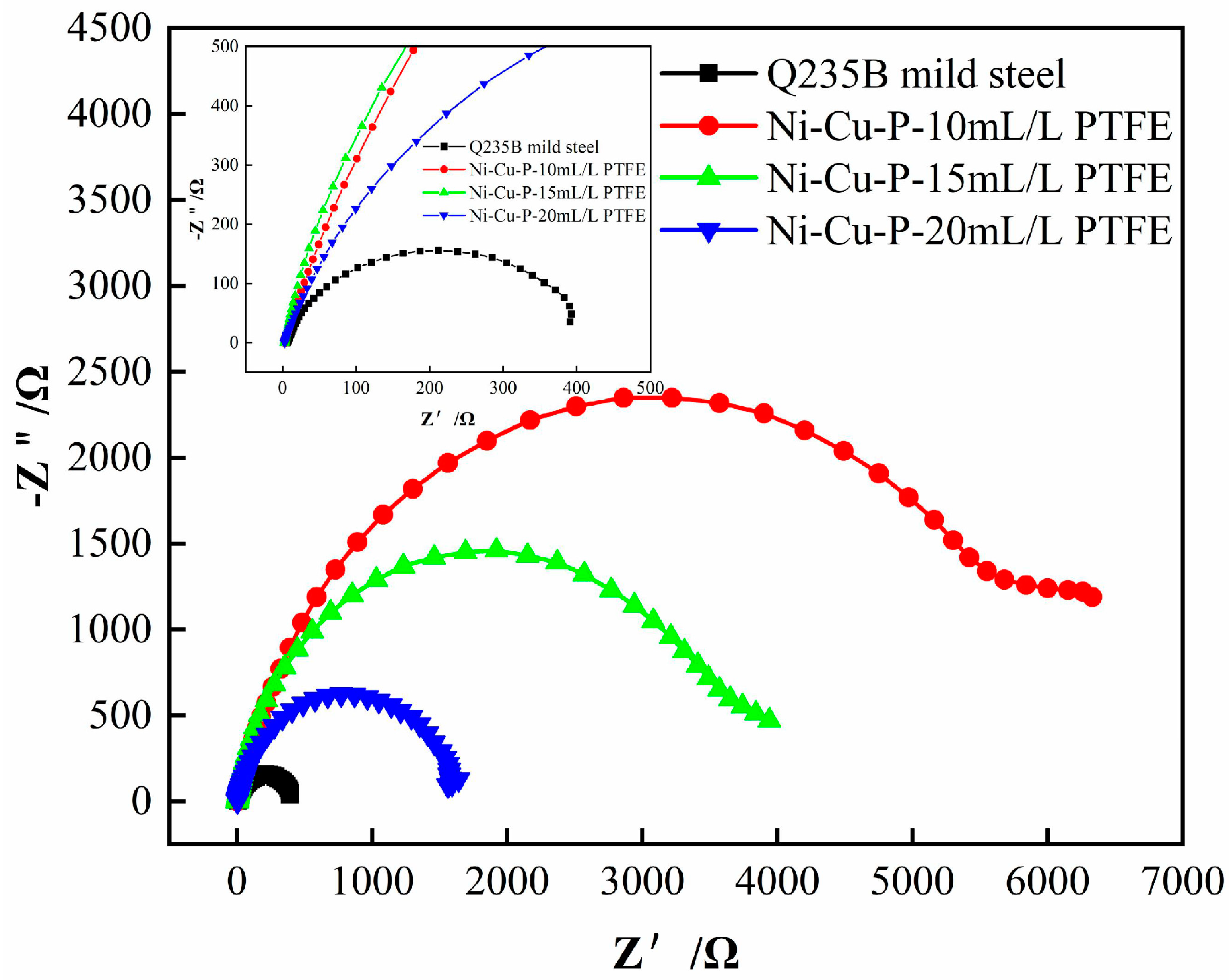
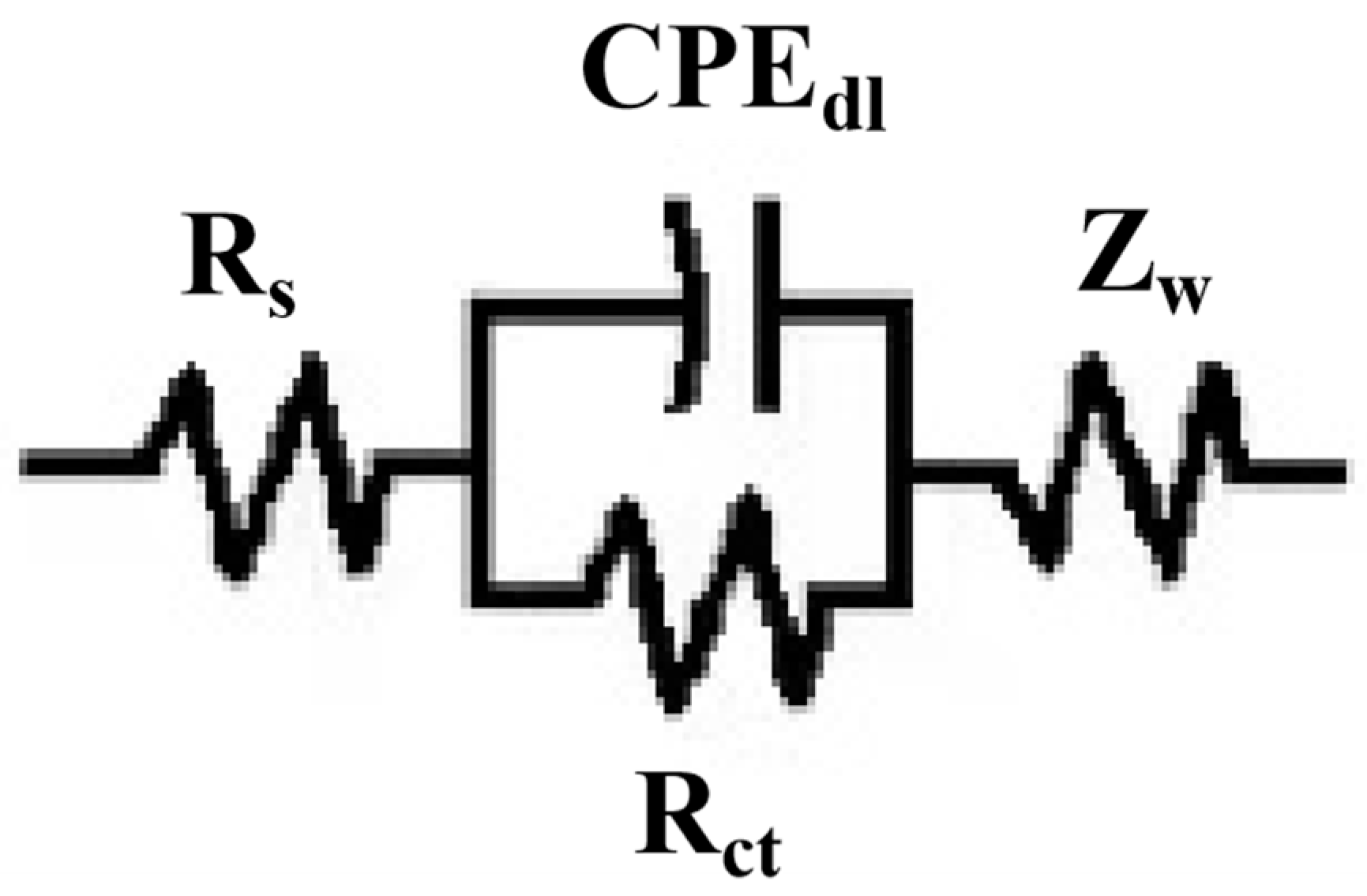
3.5.2. Electrochemical Impedance Spectroscopy Analysis
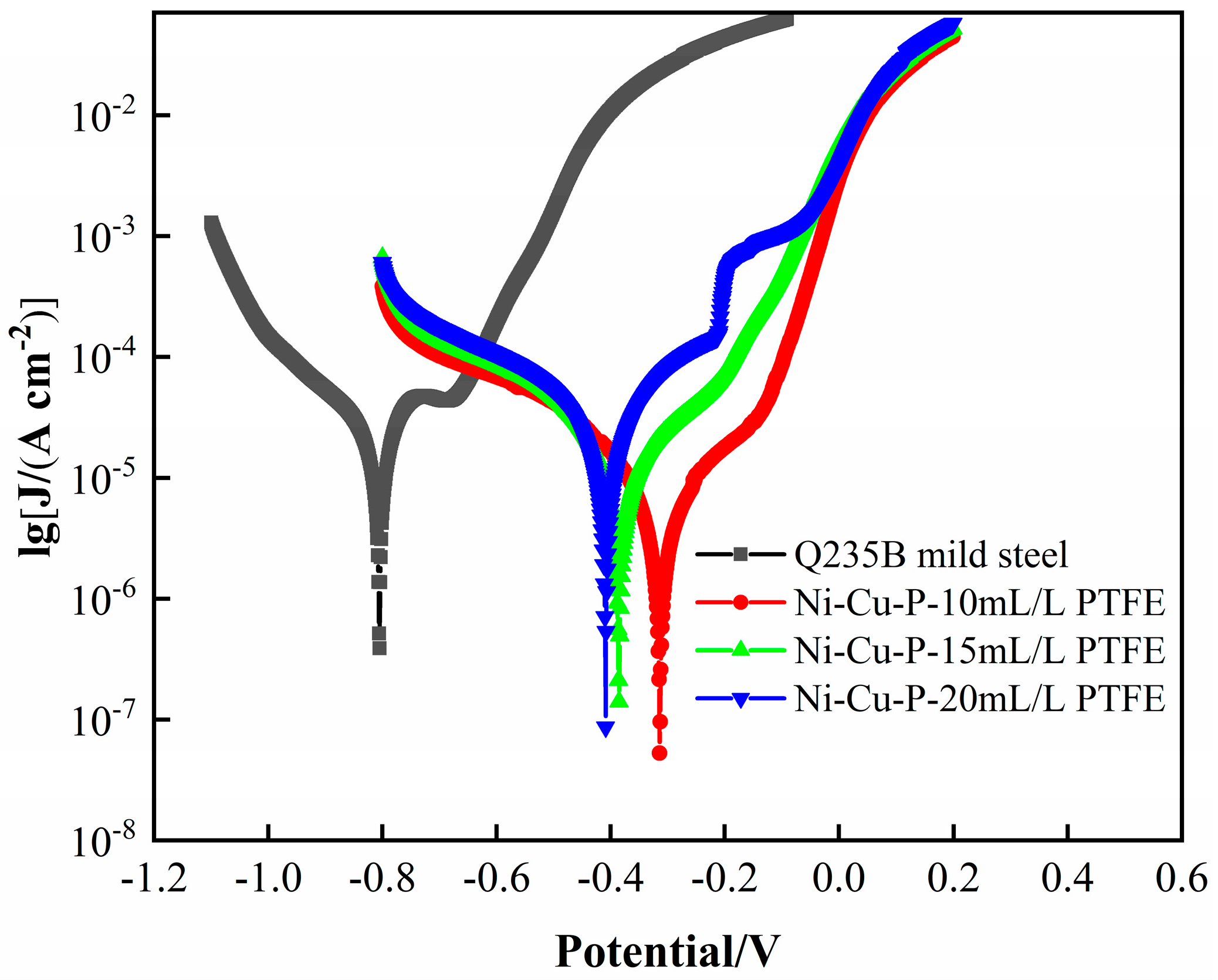
3.5.3. Dynamic Potential Polarization Curve Analysis
4. Conclusions
- Different concentrations of PTFE have almost no effect on the physical phase structure of Ni-Cu-P-PTFE composite plating; Ni-Cu-P-PTFE composite plating has an amorphous structure, the microscopic morphology of Ni-Cu-P-PTFE composite plating surface and cross-section is smooth and dense, and PTFE can be uniformly co-deposited on the substrate surface under the dispersion of CTAB. The three-dimensional contour results showed that the surface roughness of the composite plated layer with a PTFE concentration of 10 mL/L was larger, with a surface roughness value of 5.235. The surface roughness values of the PTFE concentrations of 15 mL/L and 20 mL/L were smaller, with surface roughness values of 1.547 and 1.18, respectively.
- The Ni-Cu-P-PTFE composite coating on the surface of Q235B mild steel can increase the Vickers hardness of its surface. Due to the soft physical properties and loose structure of PTFE, the Vickers hardness of Ni-Cu-P-PTFE composite coating with different concentrations of PTFE varies; the Vickers hardness of Ni-Cu-P-P-PTFE composite coating reached a minimum value of 515 HV when the PTFE concentration was 15 mL/L.
- The results of both the weight loss experiments and electrochemical tests showed that the corrosion resistance of Ni-Cu-P-PTFE composite coating was better than that of Q235B mild steel. The best corrosion resistance was the Ni-Cu-P-PTFE composite coating with a PTFE concentration of 10 mL/L. The weight loss rate was 0.24 mg∙cm−2, the corrosion current density was 7.255 × 10−6 A∙cm−2, and the corrosion voltage was −0.314 V. In addition, the corrosion resistance of the Ni-Cu-P-PTFE composite plating became worse with the increase in PTFE concentration.
- There are few studies on the preparation of Ni-Cu-P-PTFE composite coatings and their properties, and there are almost no studies on the influence of the introduction of PTFE particles on the surface morphology, organization, corrosion resistance, and mechanical properties of Ni-Cu-P-PTFE composite coatings. This also brings difficulties and uncertainties to the application of Ni-Cu-P-PTFE composite coatings. In this paper, three Ni-Cu-P-PTFE composite coatings with different PTFE concentrations were tested and characterized in terms of surface morphology, organization, corrosion resistance, and mechanical properties, which is useful for guiding the performance of Ni-Cu-P-PTFE composite coatings and the deposition mechanism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, E.; Wu, J.; Zhang, D.; Sun, Y.; Chan, J.N. D-phenylalanine inhibits the corrosion of Q235 carbon steel caused by Desulfovibrio sp. Int. Biodeter. Biodegr. 2018, 127, 178–184. [Google Scholar] [CrossRef]
- Usher, K.M.; Kaksonen, A.H.; Cole, I.; Marney, D. Critical review: Microbially influenced corrosion of buried carbon steel pipes. Int. Biodet. Biodegr. 2014, 93, 84–106. [Google Scholar] [CrossRef]
- Hao, Y.S.; Sani, L.A.; Ge, T.J.; Fang, Q.H. Phytic acid doped polyaniline containing epoxy coatings for corrosion protection of Q235 carbon steel. Appl. Surf. Sci. 2017, 419, 826–837. [Google Scholar] [CrossRef]
- Han, Q.H.; Wang, P.P.; Lu, Y. Low-cycle multiaxial fatigue behavior and life prediction of Q235B steel welded material. Int. J. Fatigue 2019, 127, 417–430. [Google Scholar] [CrossRef]
- Feng, J.G.; Chen, Z.G.; Wu, C. Corrosion mechanism and damage characteristic of Q235B steel under the effect of stray current in NS4 simulated soil solution. Process. Safe. Environ. 2022, 164, 38–49. [Google Scholar] [CrossRef]
- Qiu, F.; Bai, Y.Z.; Qu, D.R. Quantitative characterization of Q235B steel electrochemical corrosion by acoustic emission. Int. J. Pres. Ves. Pip. 2022, 104, 76–86. [Google Scholar] [CrossRef]
- Xia, D.H.; Ma, C.; Behnamian, Y.; Ao, S.S.; Song, S.Z.; Xu, L.K. Reliability of the estimation of uniform corrosion rate of Q235B steel under simulated marine atmospheric conditions by electrochemical noise (EN) analyses. Measurement 2019, 148, 65–76. [Google Scholar] [CrossRef]
- Fatima, I.; Fayyaz, O.; Yusuf, M.M. Enhanced electrochemical and mechanical performance of BN reinforced Ni-P based nanocomposite coatings. Diam. Relat. Mater. 2022, 109454, 130. [Google Scholar] [CrossRef]
- He, Y.H.; Zhang, S.H.; He, Y. Strengthening effect of inclusion of Zr Cnano-ceramic particles on the corrosion and wear resistance of Ni-P electroless deposits. Thin Solid Film. 2022, 139364, 756. [Google Scholar]
- Li, Z.Y.; He, Y.; Liu, B. Preparation of super hard nanometer material cBN reinforced Ni-W-P nanocomposite coating and investigation of its mechanical and anti-corrosion properties. Colloid. Surfaces. A 2022, 129600, 651. [Google Scholar]
- Nemane, V.; Chatterjee, S. Evaluation of microstructural, mechanical, and tribological characteristics of Ni-B-W-SiC electroless composite coatings involving multi-pass scratch test. Mater. Charact. 2021, 180, 111414. [Google Scholar] [CrossRef]
- Ram, M.; Kumar, M.J.; Ansari, A. Corrosion Resistance of Electroless Ni-P-SiC/Ni-P-TiO2 -ZrO2 Nano-Coatings in Paper Mill Bleach Plant. Mater. Today Proc. 2021, 21, 1200–1212. [Google Scholar] [CrossRef]
- Guo, F.; Sun, W.C.; Zhang, L. Effect of LaCl3 content on the corrosion resistance of Ni-P coatings. Therm. Process. Technol. 2018, 47, 147–149. [Google Scholar]
- Ying, L.X.; Wu, Y.L.; Nie, C.Y.; Wu, C.X.; Wang, G.X. Improvement of the Tribological Properties and Corrosion Resistance of Epoxy-PTFE Composite Coating by Nanoparticle Modification. Coatings 2021, 36, 53–64. [Google Scholar] [CrossRef]
- Cai, Y.W.; Quan, X.J. Study on the anticorrosion performance of Ni-P-ZrO2 nanocomposite coatings in simulated geothermal water environment. Surf. Technol. 2018, 47, 232–239. [Google Scholar]
- Deng, P.; Qian, B. Study on the performance of hydrophobic Ni-SiO2 coating on the surface of 45# steel for construction. Electroplat. Environ. Prot. 2020, 40, 15–18. [Google Scholar]
- Shi, L.t.; Hu, J.; Lin, X.D.; Fang, L.; Wu, F.; Xie, J.; Meng, F.M. A robust superhydrophobic PPS-PTFE/SiO2 composite coating on AZ31 Mg alloy with excellent wear and corrosion resistance properties. J. Alloy. Compd. 2017, 721, 157–163. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.J.; Geoffrey, M.G.; Zhao, Q. Advanced titanium dioxide-polytetrafluorethylene (TiO2-PTFE) nanocomposite coatings on stainless steel surfaces with antibacterial and anti-corrosion properties. Appl. Surf. Sci. 2019, 490, 231–241. [Google Scholar] [CrossRef]
- Nardi, J.A.; Strauss, J.A.; Fardo, F.M. Wettability and anticorrosion of thin PTFE-like/alumina coatings on carbon steel. Prog. Org. Coat. 2020, 148, 105823. [Google Scholar] [CrossRef]
- Wu, J.X.; Deng, J.X.; Wang, R.; Meng, Y.; Zhang, Z.H.; Lu, Y. The tribological properties and corrosion resistance of PPS/PTFE-bronze coatings deposited by electrohydrodynamic jet deposition. Surf. Coat. Tech. 2022, 436, 128322. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Cao, L. Corrosion and tribological performance of PTFE-coated electroless nickel boron coatings. Surf. Coat. Tech. 2016, 307, 316–323. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Z.F.; Qin, S.Y. Effect of PTFE emulsion on the corrosion resistance of steel surface plating on landscape structures. Electroplat. Finish. 2022, 41, 181–186. [Google Scholar]
- Wang, J.F.; Tian, J.T.; Liu, X.Z.; Yin, Y.S.; Wang, X. Effect of polytetrafluoroethylene content on electrochemical anticorrosion behaviors of electroless deposited Ni-P and Ni-P-polytetrafluoroethylene coatings in seawater. Thin Solid Film. 2011, 519, 5905–5911. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Chen, H.Y.; Zhu, Z.C.; Jen, T.C.; Peng, Y.X. Experimental study on the anti-fouling effects of Ni-Cu-P-PTFE deposit surface of heat exchangers. Appl. Therm. Eng. 2014, 68, 20–25. [Google Scholar] [CrossRef]
- Mafi, I.R.; Dehghanian, C. Comparison of the coating properties and corrosion rates in electroless Ni-P/PTFE composites prepared by different types of surfactants. Appl. Surf. Sci. 2011, 257, 8653–8658. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y. Investigation of graded Ni-Cu-P-PTFE composite coatings with antiscaling properties. Appl. Surf. Sci. 2004, 229, 56–62. [Google Scholar] [CrossRef]
- Walsh, F.C.; Larson, C. Towards improved electroplating of metal-particle composite coatings. Trans. IMF 2020, 98, 288–299. [Google Scholar] [CrossRef]
- Walsh, F.C.; Leon Ponce De, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Mohamed, A.M.A. Electrodeposition of Nanostructured Nickel-Ceramic Composite Coatings: A review. Int. J. Electrochem. Sci. 2014, 9, 1942–1963. [Google Scholar]
- You, Y.H.; Gu, C.D.; Wang, X.L.; Tu, J.P. Electrochemical Preparation and Characterization of Ni–PTFE Composite Coatings from a Non-Aqueous Solution Without Additives. Int. J. Electrochem. Sci. 2012, 7, 12440–12455. [Google Scholar]
- Juniarti, A.; Deana, W.; Sadijah, A.B.B. Synthesis of Dipeptide Benzoylalanylglycine Methyl Ester and Corrosion Inhibitor Evaluation by Tafel Equation. Sains. Malays. 2011, 40, 973–976. [Google Scholar]
- Czagany, C.; Baumli, P. Effect of surfactants on the behavior of the Ni-P bath and on the formation of electroless Ni-P-TiC composite coatings. Appl. Surf. Sci. 2019, 361, 42–49. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Chen, H.Y.; Han, D.T.; Zou, Y.; Zhu, Z.C. Effect of PTFE Addition on the Properties of Electroless Ni-Cu-P-PTFE Deposits. Rare. Metal. Mat. Eng. 2014, 43, 1025–1030. [Google Scholar]
- Chen, H.Y. Preparation and Performance of Ni-Cu-P-PTFE Anti-Scaling Coating; China University of Mining and Technology: Xuzhou, China, 2014. [Google Scholar]
- Ybalaraju, J.N.; Rajam, K.S. Electroless deposition of Ni-Cu-P, Ni-W-Pand Ni-W-Cu-P alloys. Surf. Coat. Tech. 2005, 195, 154–161. [Google Scholar] [CrossRef]
- Molla, H.R.; Modarress, H.; Abdouss, M. Electroless nickel-phosphorus deposition on carbon steel CK-75 and study of the effects of some parameters on properties of the deposits. J. Coat. Technol. Res. 2012, 9, 183–188. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cao, L.; Wang, Y.Y.; Wan, Y. Corrosion resistance and tribological properties of Ni-B coatings on 45 steel surfaces. China Surf. Eng. 2015, 28, 63–69. [Google Scholar]
- Oloyede, O.; Zhu, L.; Hong, L. Deposition and electrochemical corrosion behavior of electroless Ni-P/Ni-W-P duplex plating on mild steel substrate. J. Mater. Sci. Eng. B 2014, 4, 13–19. [Google Scholar]

| Reagent Name | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| NiSO4∙6H2O | 30 g/L | 30 g/L | 30 g/L |
| CuSO4∙5H2O | 0.8 g/L | 0.8 g/L | 0.8 g/L |
| NaH2PO2∙H2O | 30 g/L | 30 g/L | 30 g/L |
| C6H5Na3O7∙2H2O | 6 g/L | 6 g/L | 6 g/L |
| C3H6O3 | 15 mL/L | 15 mL/L | 15 mL/L |
| CH3COONa | 15 g/L | 15 g/L | 15 g/L |
| C2H5NO2 | 5 g/L | 5 g/L | 5 g/L |
| CH4N2S | suitable amount | suitable amount | suitable amount |
| CTAB | 0.5 g/L | 0.5 g/L | 0.5 g/L |
| 60 wt% PTFE | 10 mL/L | 15 mL/L | 20 mL/L |
| Temperature | 85 °C~87 °C | 85 °C~87 °C | 85 °C~87 °C |
| PH | 4.5~4.6 | 4.5~4.6 | 4.5~4.6 |
| Ni-Cu-P-PTFE | |||
|---|---|---|---|
| PTFE (10 mL/L) | PTFE (15 mL/L) | PTFE (20 mL/L) | |
| Element | Weight % | Weight % | Weight % |
| F | 26 | 31 | 28 |
| P | 31 | 27 | 29 |
| Ni | 34 | 36 | 35 |
| Cu | 9 | 6 | 8 |
| Simple | Rs/Ω·cm2 | Rct/Ω·cm2 |
|---|---|---|
| Q235B mild Stell | 1.014 | 387 |
| Ni-Cu-P-10 mL/L PTFE | 2.987 | 5.386 × 103 |
| Ni-Cu-P-15 mL/L PTFE | 2.654 | 3.875 × 103 |
| Ni-Cu-P-20 mL/L PTFE | 2.446 | 1.683 × 103 |
| Simple | Ecorr /V | Icorr /(A∙cm−2) | OCP/V |
|---|---|---|---|
| Q235B mild Stell | −0.805 | 8.499 × 10−5 | −0.549 |
| Ni-Cu-P-10 mL/L PTFE | −0.314 | 7.255 × 10−6 | −0.307 |
| Ni-Cu-P-15 mL/L PTFE | −0.385 | 1.551 × 10−5 | −0.338 |
| Ni-Cu-P-20 mL/L PTFE | −0.409 | 2.508 × 10−5 | −0.377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wu, P.; Lan, L.; Wang, Y.; Ning, Y.; Wang, Y.; Qin, Y. Effect of Polytetrafluoroethylene (PTFE) Content on the Properties of Ni-Cu-P-PTFE Composite Coatings. Materials 2023, 16, 1966. https://doi.org/10.3390/ma16051966
Liang X, Wu P, Lan L, Wang Y, Ning Y, Wang Y, Qin Y. Effect of Polytetrafluoroethylene (PTFE) Content on the Properties of Ni-Cu-P-PTFE Composite Coatings. Materials. 2023; 16(5):1966. https://doi.org/10.3390/ma16051966
Chicago/Turabian StyleLiang, Xinghua, Penggui Wu, Lingxiao Lan, Yujiang Wang, Yujuan Ning, Yu Wang, and Yunmei Qin. 2023. "Effect of Polytetrafluoroethylene (PTFE) Content on the Properties of Ni-Cu-P-PTFE Composite Coatings" Materials 16, no. 5: 1966. https://doi.org/10.3390/ma16051966
APA StyleLiang, X., Wu, P., Lan, L., Wang, Y., Ning, Y., Wang, Y., & Qin, Y. (2023). Effect of Polytetrafluoroethylene (PTFE) Content on the Properties of Ni-Cu-P-PTFE Composite Coatings. Materials, 16(5), 1966. https://doi.org/10.3390/ma16051966





