Estimation of 226Ra and 228Ra Content Using Various Types of Sorbents and Their Distribution in the Surface Layer of the Black Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Sorbents
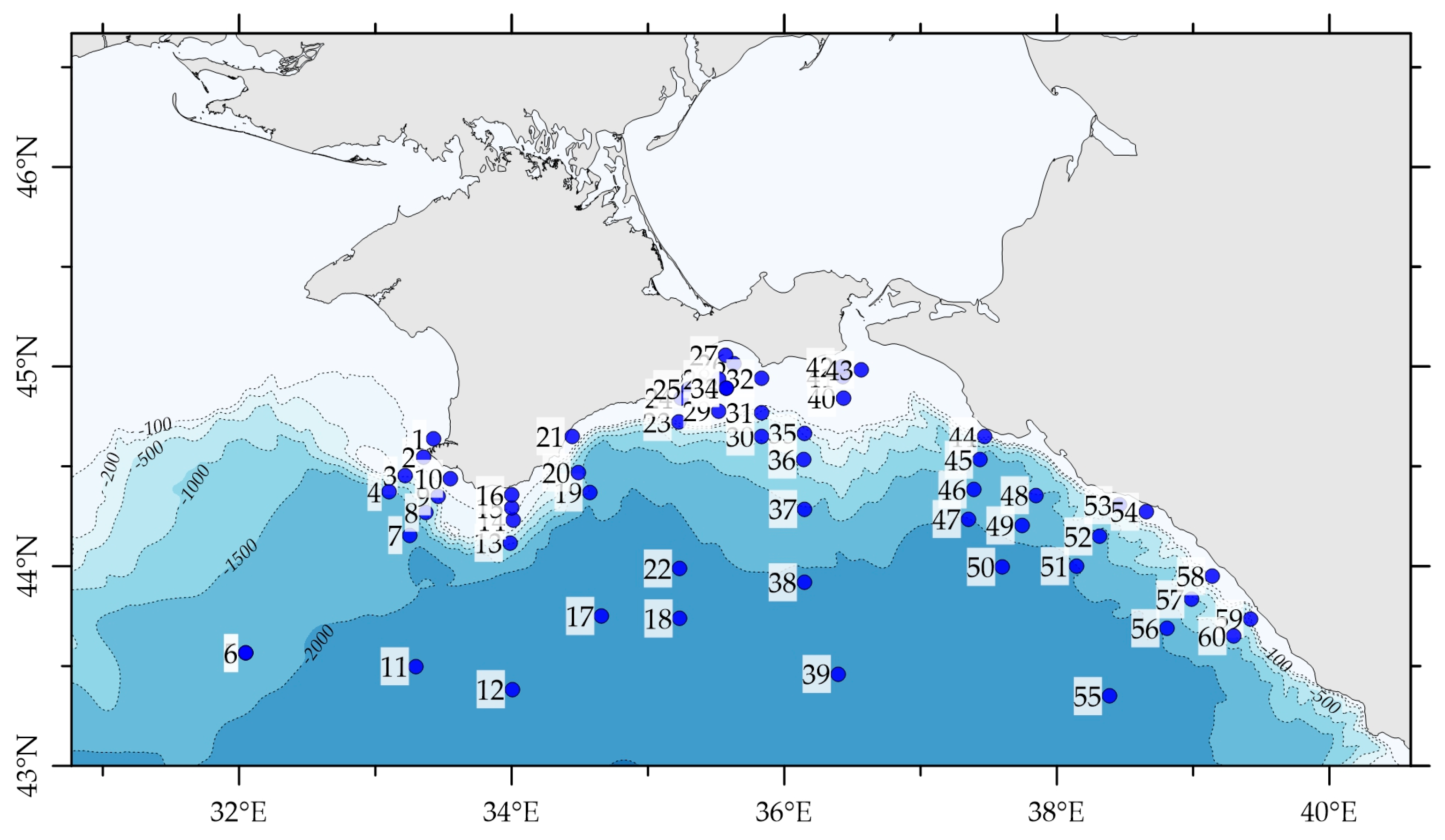
2.2. Hydrological Survey
2.3. Sampling and Recovery of 226Ra and 228Ra
2.4. Determination of the Activity of 226Ra and 228Ra
2.5. Main Biogenic Elements’ Content Determination
3. Results and Discussion
3.1. Assessment of 226Ra and 228Ra Recovery Efficiency by Various Sorbents
- Sorbents based on manganese oxide (PAN-MnO2, Modix, MDM, DMM) [8]:
- Sorbents based on barium silicate (CRM-Sr) [32]:
- Sorbents based on carbonate-containing phosphorus oxide (PD) [27]:
- A 200–250 L volume of seawater is pumped into a container on the vessel board, simultaneously filtering seawater through a polypropylene filter with a pore diameter of 0.5–1 μm.
- Two columns are filled with 25 mL of the Modix, DMM, PAN-MnO2, or CRM-Sr sorbent.
- A 200–250 L volume of seawater is transmitted through columns with sorbents at a rate of 4–8 CV min−1.
- After sorption, the sorbent is dried in an oven at a temperature of 70–80 °C.
- When using granular sorbents, after drying, the sorbent is placed in Petri dishes and sealed. When using the PAN-MnO2 fibrous sorbent, the sorbent after drying is ashed in a muffle furnace at 700 °C for 8 h. The ash is placed in Petri dishes and sealed.
- Measurement of the activity of 226Ra and 228Ra is carried out 3 weeks after sealing (to achieve equilibrium between 226Ra and 214Pb) on a semiconductor gamma spectrometer with an exposure of at least 12 h to achieve a measurement error of not more than 10%.
- The sorption efficiency of 226Ra and 228Ra is calculated by Equation (1) for two absorbers.
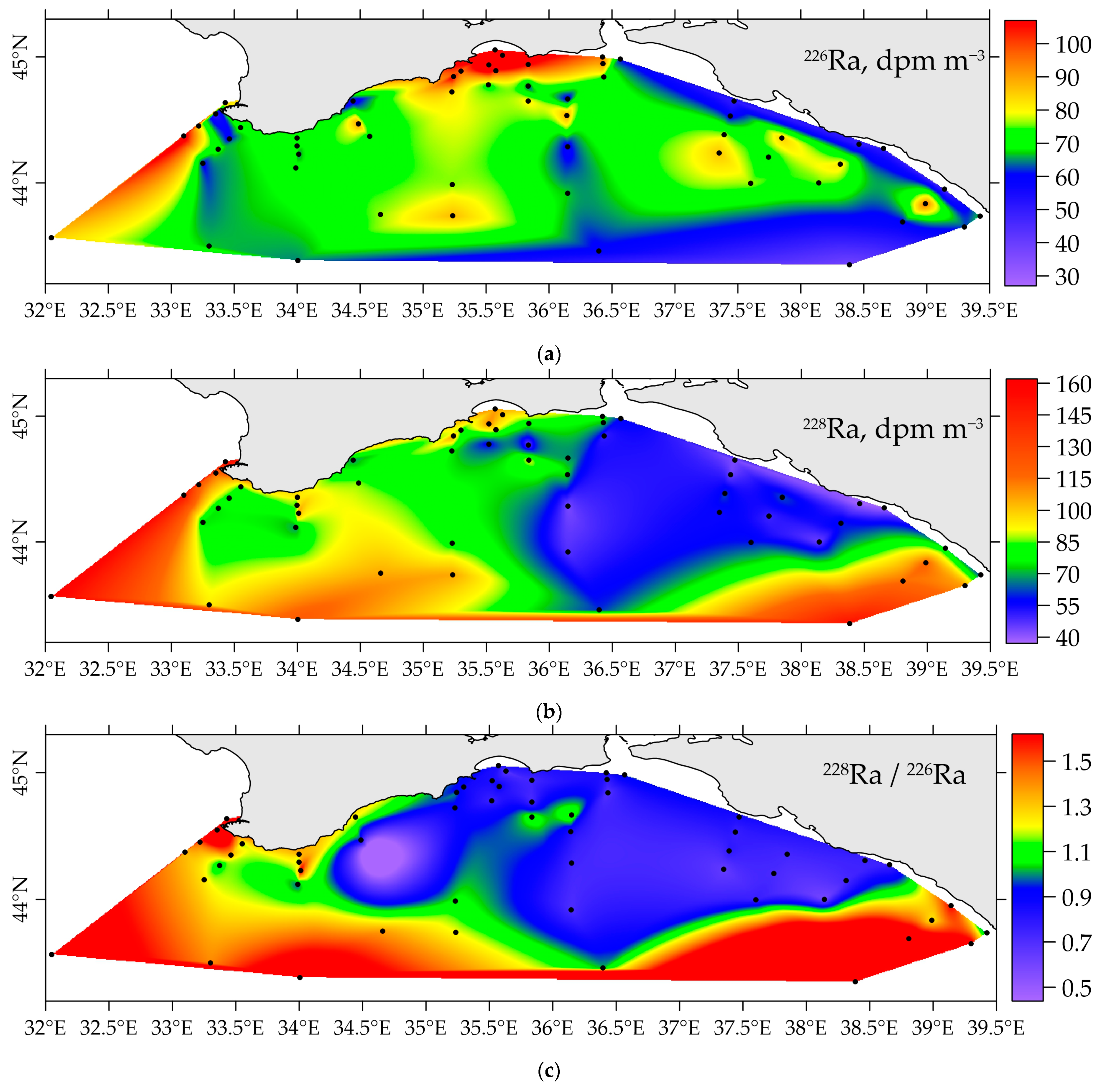
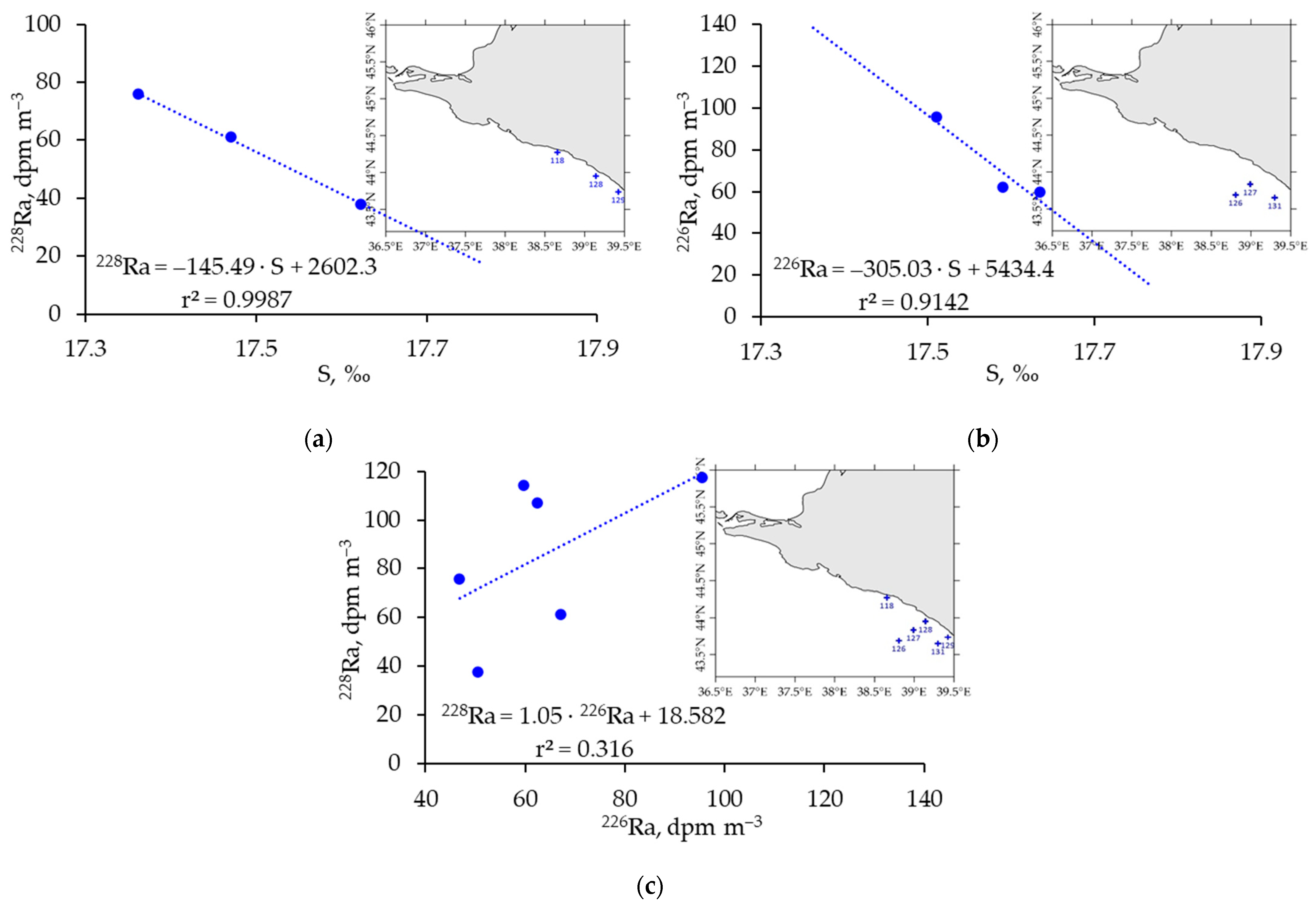
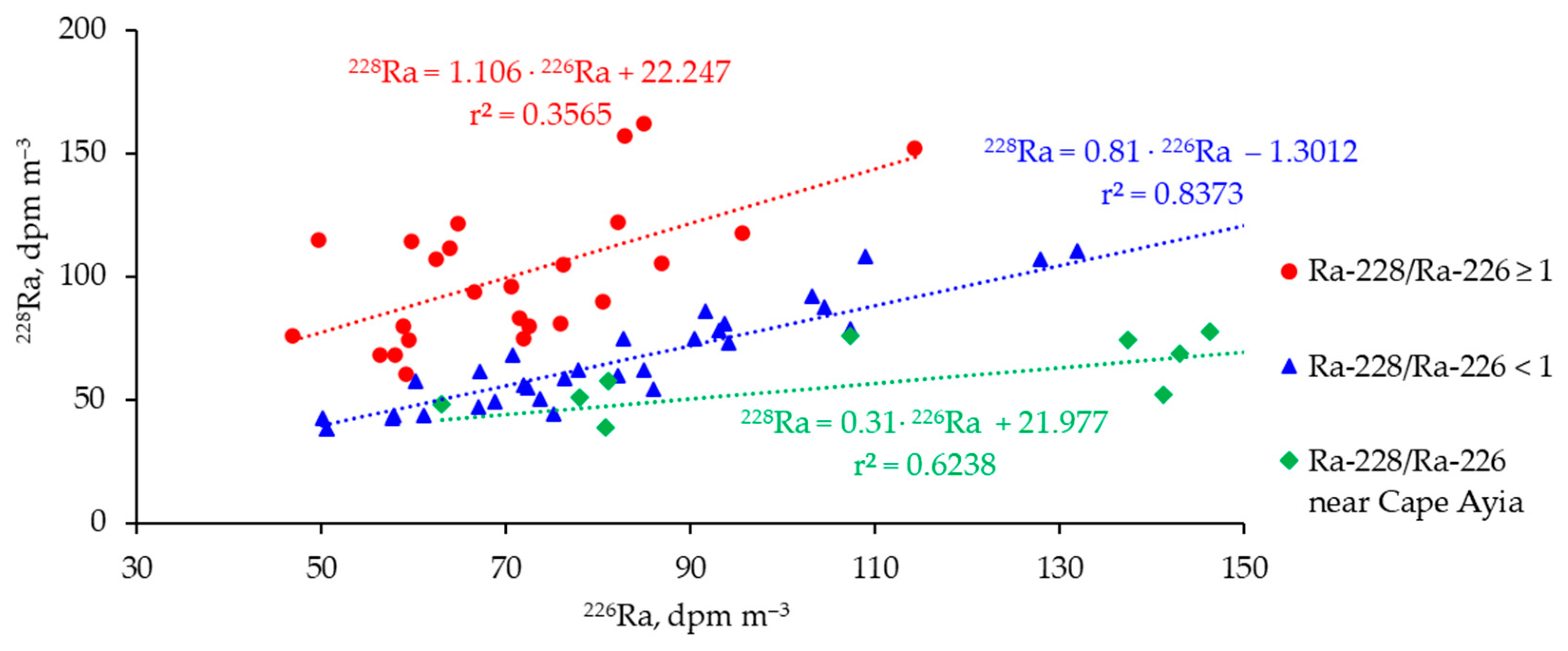
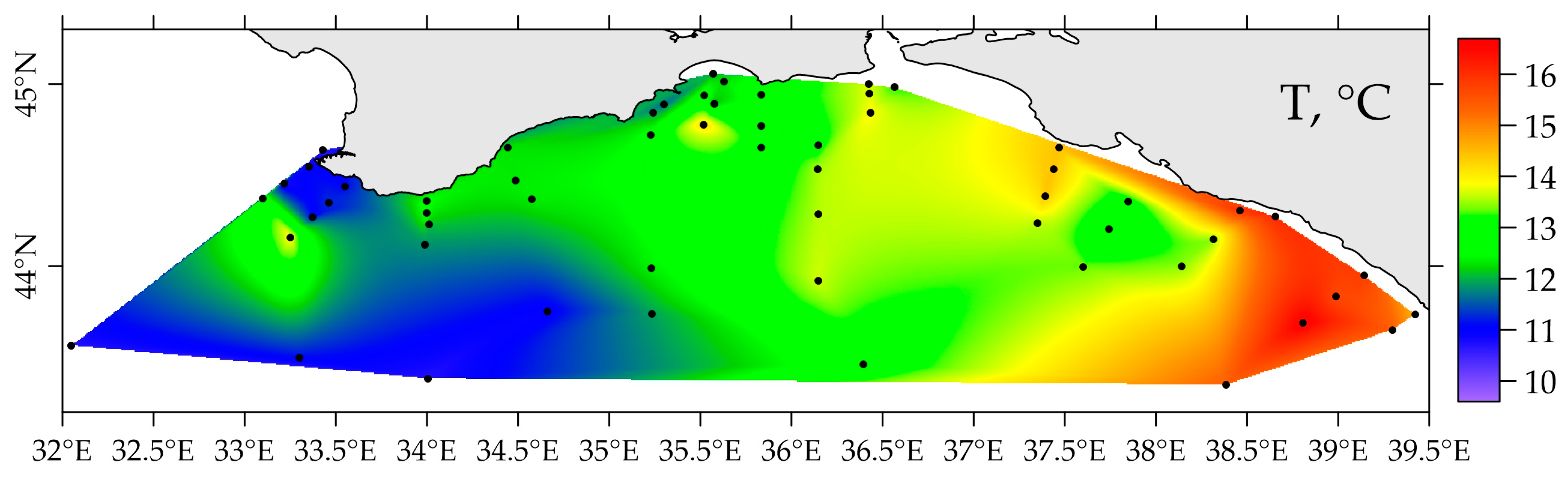
3.2. Distribution of 226Ra and 228Ra Radionuclides in the Surface Layer of the Black Sea in Spring 2021
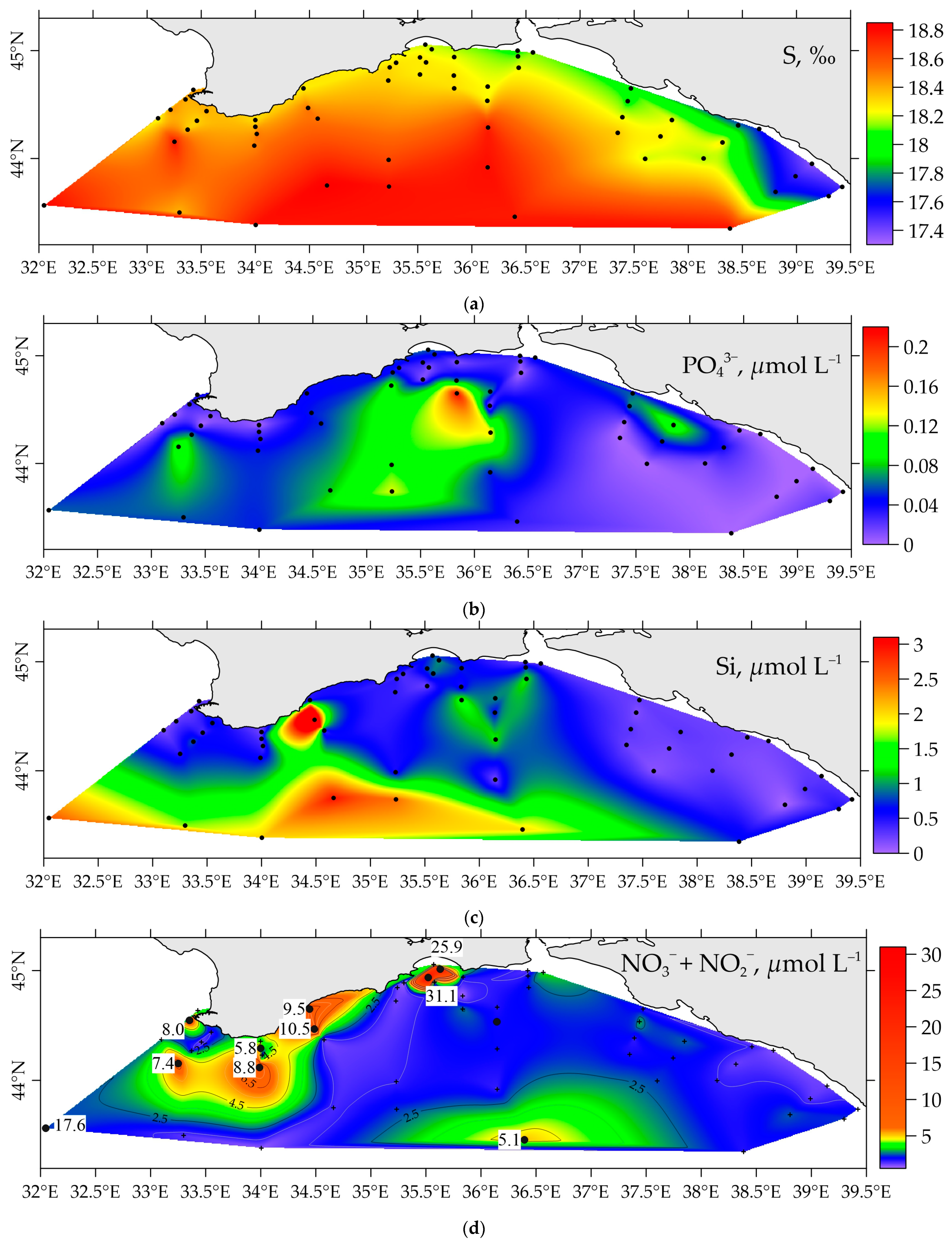
3.3. Distribution of Nutrients in the Surface Water Layer of the Black Sea in Spring 2021
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuclear and Isotopic Techniques for the Characterization of Submarine Groundwater Discharge in Coastal Zones. In Results of a Coordinated Research Project, 2001–2006; IAEA: Vienna, Austria, 2008.
- Hwang, D.-W.; Lee, I.-S.; Choi, M.; Kim, T.-H. Estimating the input of submarine groundwater discharge (SGD) and SGD-derived nutrients in Geoje Bay, Korea using 222Rn-Si mass balance model. Mar. Pollut. Bull. 2016, 110, 119–126. [Google Scholar] [CrossRef]
- Kondrat’ev, S.I.; Shchetinin, Y.T.; Dolotov, N.N.; Androsovich, A.I. Hydrological and chemical characteristics of the submarine freshwater source near Cape Aiya. Phys. Oceanogr. 1998, 9, 217–224. [Google Scholar] [CrossRef]
- Yarusova, S.B.; Shichalin, O.O.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Golub, A.V.; Mayorov, V.Y.; Gerasimenko, A.V.; Papynov, E.K.; Ivanets, A.I.; et al. Synthesis of amorphous KAlSi3O8 for cesium radionuclide immobilization into solid matrices using spark plasma sintering technique. Ceram. Int. 2021, 48, 3808–3817. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Papynov, E.K.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Panasenko, A.E.; Zadorozhny, P.A.; Mayorov, V.Y.; et al. Synthesis and spark plasma sintering of solid-state matrices based on calcium silicate for 60Co immobilization. J. Alloy. Compd. 2022, 912, 165233. [Google Scholar] [CrossRef]
- Dran’kov, A.; Shichalin, O.; Papynov, E.; Nomerovskii, A.; Mayorov, V.; Pechnikov, V.; Ivanets, A.; Buravlev, I.; Yarusova, S.; Zavjalov, A.; et al. Hydrothermal synthesis, structure and sorption performance to cesium and strontium ions of nanostructured magnetic zeolite composites. Nucl. Eng. Technol. 2022, 54, 1991–2003. [Google Scholar] [CrossRef]
- Dovhyi, I.I.; Kremenchutskii, D.A.; Bezhin, N.A.; Tovarchii, Y.Y.; Shibetskaya, Y.G.; Egorin, A.M.; Tokar, E.A.; Tananaev, I.G. MnO2 fiber as a sorbent for radionuclides in oceanographic investigations. J. Radioanal. Nucl. Chem. 2020, 323, 539–547. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Prozorovich, V.G.; Kouznetsova, T.F.; Radkevich, A.V.; Krivoshapkin, P.V.; Krivoshapkina, E.F.; Sillanpää, M. Sorption behavior of 85Sr onto manganese oxides with tunnel structure. J. Radioanal. Nucl. Chem. 2018, 316, 673–683. [Google Scholar] [CrossRef]
- Moore, W.S. Sampling 228Ra in the deep ocean. Deep Sea Res. Oceanogr. Abstr. 1976, 23, 647–651. [Google Scholar] [CrossRef]
- Henderson, P.B.; Morris, P.J.; Moore, W.S.; Charette, M.A. Methodological advances for measuring low-level radium isotopes in seawater. J. Radioanal. Nucl. Chem. 2013, 296, 357–362. [Google Scholar] [CrossRef]
- Surbeck, H. Alpha spectrometry sample preparation using selectively adsorbing thin films. Appl. Radiat. Isot. 2000, 53, 97–100. [Google Scholar] [CrossRef]
- Hartman, M.C.; Buesseler, K.O. Adsorbers for In-Situ Collection and At-Sea Gamma Analysis of Dissolved Thorium-234 in Seawater. Technical Report; Woods Hole Oceanographic Institution: Woods Hole, MA, USA, 1994. [Google Scholar]
- Moon, D.S.; Burnett, W.C.; Nour, S.; Horwitz, P.; Bond, A. Preconcentration of radium isotopes from natural waters using MnO2 resin. Appl. Radiat. Isot. 2003, 59, 255–262. [Google Scholar] [CrossRef]
- Charette, M.A.; Dulaiova, H.; Gonneea, M.E.; Henderson, P.B.; Moore, W.S.; Scholten, J.C.; Pham, M.K. GEOTRACES radium isotopes interlaboratory comparison experiment. Limnol. Oceanogr. Methods 2012, 10, 451–463. [Google Scholar] [CrossRef]
- Rodellas, V.; Garcia-Orellana, J.; Trezzi, G.; Masqué, P.; Stieglitz, T.C.; Bokuniewicz, H.; Cochran, J.K.; Berdalet, E. Using the radium quartet to quantify submarine groundwater discharge and porewater exchange. Geochim. Cosmochim. Acta 2017, 196, 58–73. [Google Scholar] [CrossRef]
- Eikenberg, J.; Tricca, A.; Vezzu, G.; Bajo, S.; Ruethi, M.; Surbeck, H. Determination of 228Ra, 226Ra and 224Ra in natural water via adsorption on MnO2-coated discs. J. Environ. Radioact. 2001, 54, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Ward, A.L.; González-Delgado, A.M. Optimal methods for preparation, separation, and determination of radium isotopes in environmental and biological samples. J. Environ. Radioact. 2021, 228, 106522. [Google Scholar] [CrossRef]
- Dovhyi, I.I.; Bezhin, N.A.; Tananaev, I.G. Sorption methods in marine radiochemistry. Russ. Chem. Rev. 2021, 90, 1544–1565. [Google Scholar] [CrossRef]
- Rodellas, V.; Garcia-Orellana, J.; Masqué, P.; Feldman, M.; Weinstein, Y. Submarine groundwater discharge as a major source of nutrients to the Mediterranean Sea. Proc. Natl. Acad. Sci. USA 2015, 112, 3926–3930. [Google Scholar] [CrossRef]
- Moore, W.S. Oceanic concentrations of 228Radium. Earth Planet. Sci. Lett. 1969, 6, 437–446. [Google Scholar] [CrossRef]
- Grashchenko, S.M.; Nikolaev, D.S.; Kolyadin, L.B.; Kuznetsov, Y.V.; Lazarev, K.F. Radium concentration in the waters of the Black Sea. Trans. (Dokl.) USSR Acad. Sci. 1960, 132, 1171–1172. (In Russian) [Google Scholar]
- Falkner, K.K.; O’Neill, D.J.; Todd, J.F.; Moore, W.S.; Edmond, J.M. Depletion of barium and radium-226 in Black Sea surface waters over the past thirty years. Nature 1991, 350, 491–494. [Google Scholar] [CrossRef]
- O’Neill, D.J.; Todd, J.F.; Moore, W.S. 226Ra in the Black Sea and Sea of Marmara. Earth Planet. Sci. Lett. 1992, 110, 7–21. [Google Scholar] [CrossRef]
- Moore, W.S.; Falkner, K.K. Cycling of radium and barium in the Black Sea. J. Environ. Radioact. 1999, 43, 247–254. [Google Scholar] [CrossRef]
- Tazoe, H.; Obata, H.; Hara, T.; Inoue, M.; Tanaka, T.; Nishioka, J. Vertical profiles of 226Ra and 228Ra activity concentrations in the western subarctic gyre of the Pacific Ocean. Front. Mar. Sci. 2022, 9, 824862. [Google Scholar] [CrossRef]
- Inoue, M.; Hanaki, S.; Kameyama, H.; Kumamoto, Y.; Nagao, S. Unique current connecting Southern and Indian Oceans identified from radium distributions. Sci. Rep. 2022, 12, 1781. [Google Scholar] [CrossRef]
- Bezhin, N.A.; Dovhyi, I.I.; Kapranov, S.V.; Bobko, N.I.; Milyutin, V.V.; Kaptakov, V.O.; Kozlitin, E.A.; Tananaev, I.G. Separation of radiostrontium from seawater using various types of sorbents. J. Radioanal. Nucl. Chem. 2021, 328, 1199–1209. [Google Scholar] [CrossRef]
- Bezhin, N.A.; Dovhyi, I.I.; Tokar, E.A.; Tananaev, I.G. Physical and chemical regularities of cesium and strontium recovery from the seawater by sorbents of various types. J. Radioanal. Nucl. Chem. 2021, 330, 1101–1111. [Google Scholar] [CrossRef]
- Methodology for Measuring the Volumetric Activity of Radium Isotopes (226Ra, 228Ra) in Samples of Natural Waters by Alpha–Beta Radiometric Method with Radiochemical Preparation; Federal State Budgetary Institution “All-Russian Scientific-Research Institute of Mineral Resources Named after N.M. Fedorovsky” (FSBI VIMS): Moscow, Russia, 2021. (In Russian)
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. (Eds.) Methods of Seawater Analysis, 3rd ed.; WILEY-VCH Verlag: Weinheim, Germany, 2007. [Google Scholar]
- Wurl, O. (Ed.) Practical Guidelines for the Analysis Of Seawater; CRC Press, Taylor & Francis Group: New York, NY, USA, 2009. [Google Scholar]
- Avramenko, V.A.; Egorin, A.M.; Maiorov, V.Y.; Sokolnitskaya, T.A.; Sergienko, V.I. Processing of liquid radioactive waste containing seawater. Issues Radiat. Saf. 2015, 3, 42–51. (In Russian) [Google Scholar]
- Pechenyuk, S.I. Present state of research on the sorption of inorganic compounds by oxide hydroxides from aqueous solutions. Russ. Chem. Rev. 1992, 61, 385–398. [Google Scholar] [CrossRef]
- Voronina, A.V.; Belokonova, N.V.; Suetina, A.K.; Semenishchev, V.S. Sorption of 90Sr by a T-3K carbonate-containing zirconium dioxide. J. Radioanal. Nucl. Chem. 2022, 331, 4021–4030. [Google Scholar] [CrossRef]
- Garcia-Orellana, J.; Rodellas, V.; Tamborski, J.; Diego-Feliu, M.; van Beek, P.; Weinstein, Y.; Charette, M.; Alorda-Kleinglass, A.; Michael, H.A.; Stieglitz, T.; et al. Radium isotopes as submarine groundwater discharge (SGD) tracers: Review and recommendations. Earth-Sci. Rev. 2021, 220, 103681. [Google Scholar] [CrossRef]
- Moore, W.S. Sources and fluxes of submarine groundwater discharge delineated by radium isotopes. Biogeochemistry 2003, 66, 5–93. [Google Scholar] [CrossRef]
- Porcelli, D. Chapter 4 Investigating groundwater processes using U- and Th-series nuclides. In Radioactivity in the Environment; Krishnaswami, S., Kirk Cochran, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 13, pp. 105–153. [Google Scholar] [CrossRef]
- Moore, W.S. Determining coastal mixing rates using radium isotopes. Cont. Shelf Res. 2000, 20, 1993–2007. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Geibert, W.; Woodward, E.M.S.; Wyatt, N.J.; Lohan, M.C.; Achterberg, E.P.; Henderson., G.M. Radium-228-derived ocean mixing and trace element inputs in the South Atlantic. Biogeosciences 2021, 18, 1645–1671. [Google Scholar] [CrossRef]
- Sanial, V.; Kipp, L.E.; Henderson, P.B.; van Beek, P.; Reyss, J.-L.; Hammond, D.E.; Hawco, N.J.; Saito, M.A.; Resing, J.A.; Sedwick, P.; et al. Radium-228 as a tracer of dissolved trace element inputs from the Peruvian continental margin. Mar. Chem. 2018, 201, 20–34. [Google Scholar] [CrossRef]
- Key, R.M.; Stallard, R.F.; Moore, W.S.; Sarmiento, J.L. Distribution and flux of 226Ra and 228Ra in the Amazon River estuary. J. Geophys. Res. 1985, 90, 6995–7004. [Google Scholar] [CrossRef]
- Li, Y.-H.; Mathieu, G.; Biscaye, P.; Simpson, H.J. The flux of 226Ra from estuarine and continental shelf sediments. Earth Planet. Sci. Lett. 1977, 37, 237–241. [Google Scholar] [CrossRef]
- Moore, W.S. Radium isotopes as tracers of submarine groundwater discharge in Sicily. Cont. Shelf Res. 2006, 26, 852–861. [Google Scholar] [CrossRef]
- Dovhyi, I.I.; Bezhin, N.A.; Kremenchutskii, D.A.; Kozlovskaia, O.N.; Chepyzhenko, A.I.; Verterich, A.V.; Tovarchiy, Y.Y.; Shibetskaya, Y.G.; Chaykin, D.Y. Studying Submarine Groundwater Discharge at Cape Ayia: A Multi-Tracer Approach. Phys. Oceanogr. 2021, 28, 52–66. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlovskaia, O.N.; Shibetskaia, I.G.; Bezhin, N.A.; Tananaev, I.G. Estimation of 226Ra and 228Ra Content Using Various Types of Sorbents and Their Distribution in the Surface Layer of the Black Sea. Materials 2023, 16, 1935. https://doi.org/10.3390/ma16051935
Kozlovskaia ON, Shibetskaia IG, Bezhin NA, Tananaev IG. Estimation of 226Ra and 228Ra Content Using Various Types of Sorbents and Their Distribution in the Surface Layer of the Black Sea. Materials. 2023; 16(5):1935. https://doi.org/10.3390/ma16051935
Chicago/Turabian StyleKozlovskaia, Ol’ga N., Iuliia G. Shibetskaia, Nikolay A. Bezhin, and Ivan G. Tananaev. 2023. "Estimation of 226Ra and 228Ra Content Using Various Types of Sorbents and Their Distribution in the Surface Layer of the Black Sea" Materials 16, no. 5: 1935. https://doi.org/10.3390/ma16051935
APA StyleKozlovskaia, O. N., Shibetskaia, I. G., Bezhin, N. A., & Tananaev, I. G. (2023). Estimation of 226Ra and 228Ra Content Using Various Types of Sorbents and Their Distribution in the Surface Layer of the Black Sea. Materials, 16(5), 1935. https://doi.org/10.3390/ma16051935








