MWCNTs-TiO2 Incorporated-Mg Composites to Improve the Mechanical, Corrosion and Biological Characteristics for Use in Biomedical Fields
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Preparation
2.2. Microstructure Characterization and Mechanical Property Testing
2.3. Electrochemical and Immersion Tests
2.4. Antibacterial Properties
2.5. In Vitro Biocompatibility
2.6. Statistical Analysis
3. Results and Discussion
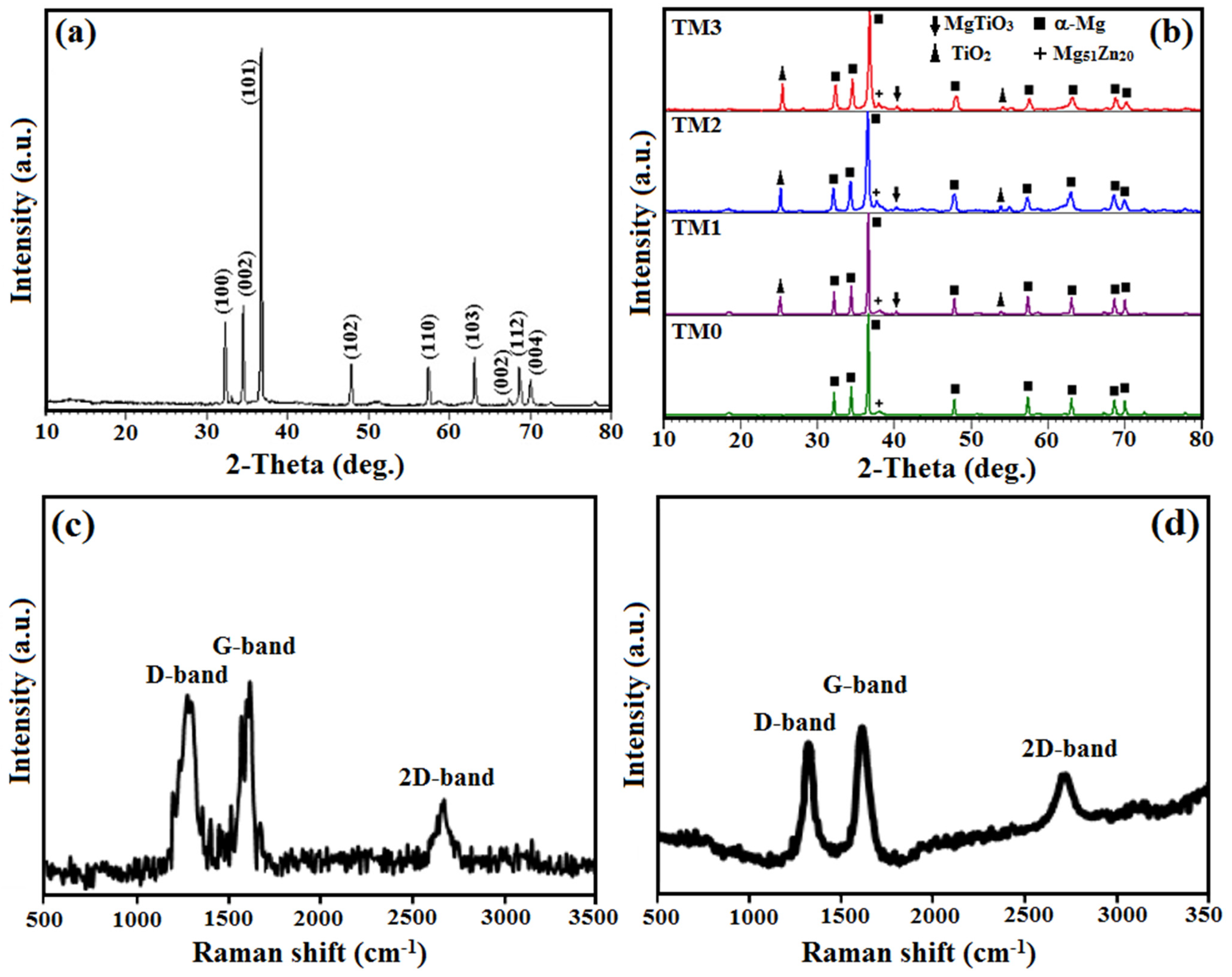
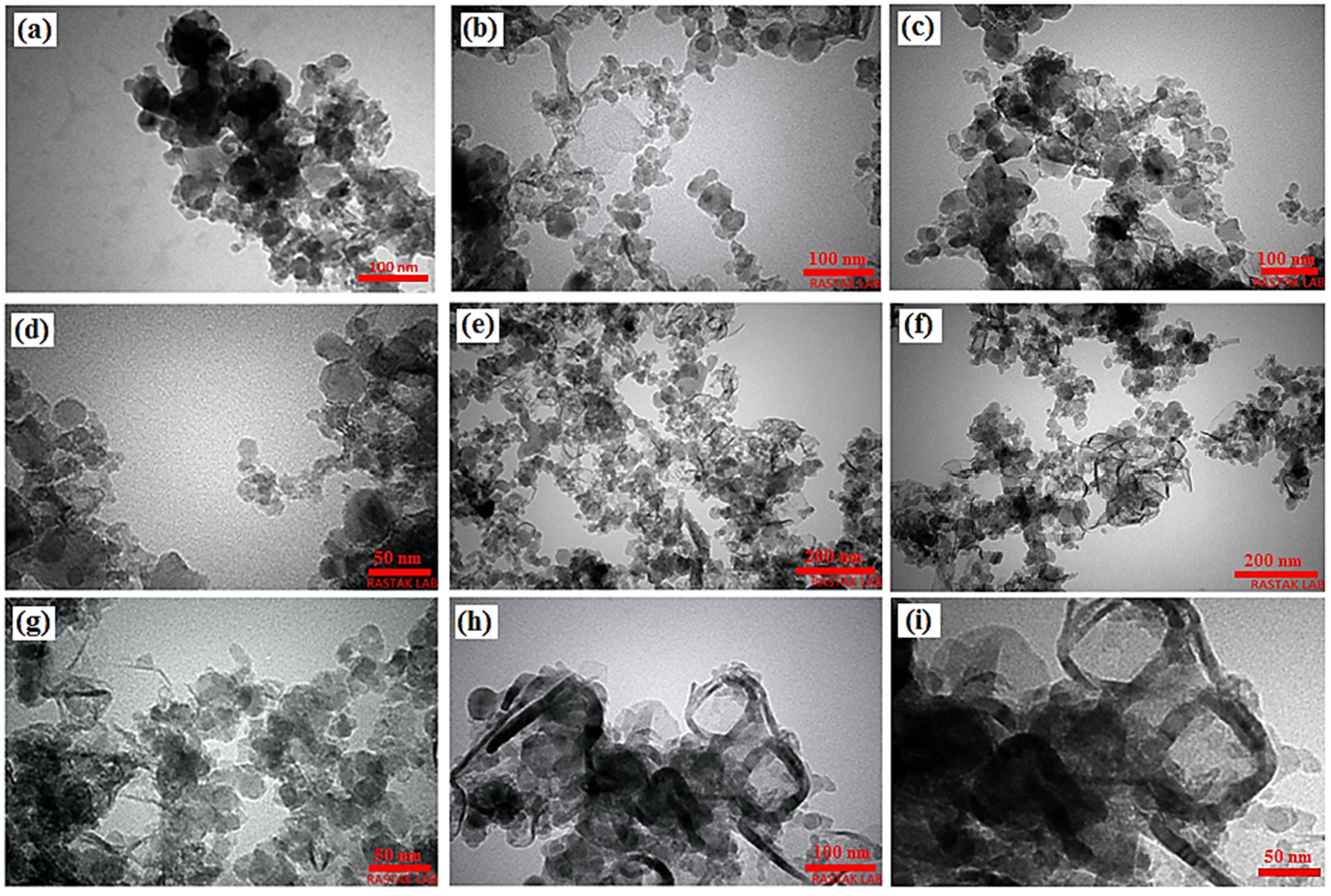
3.1. Microstructure Characterization, XRD and Raman Analysis
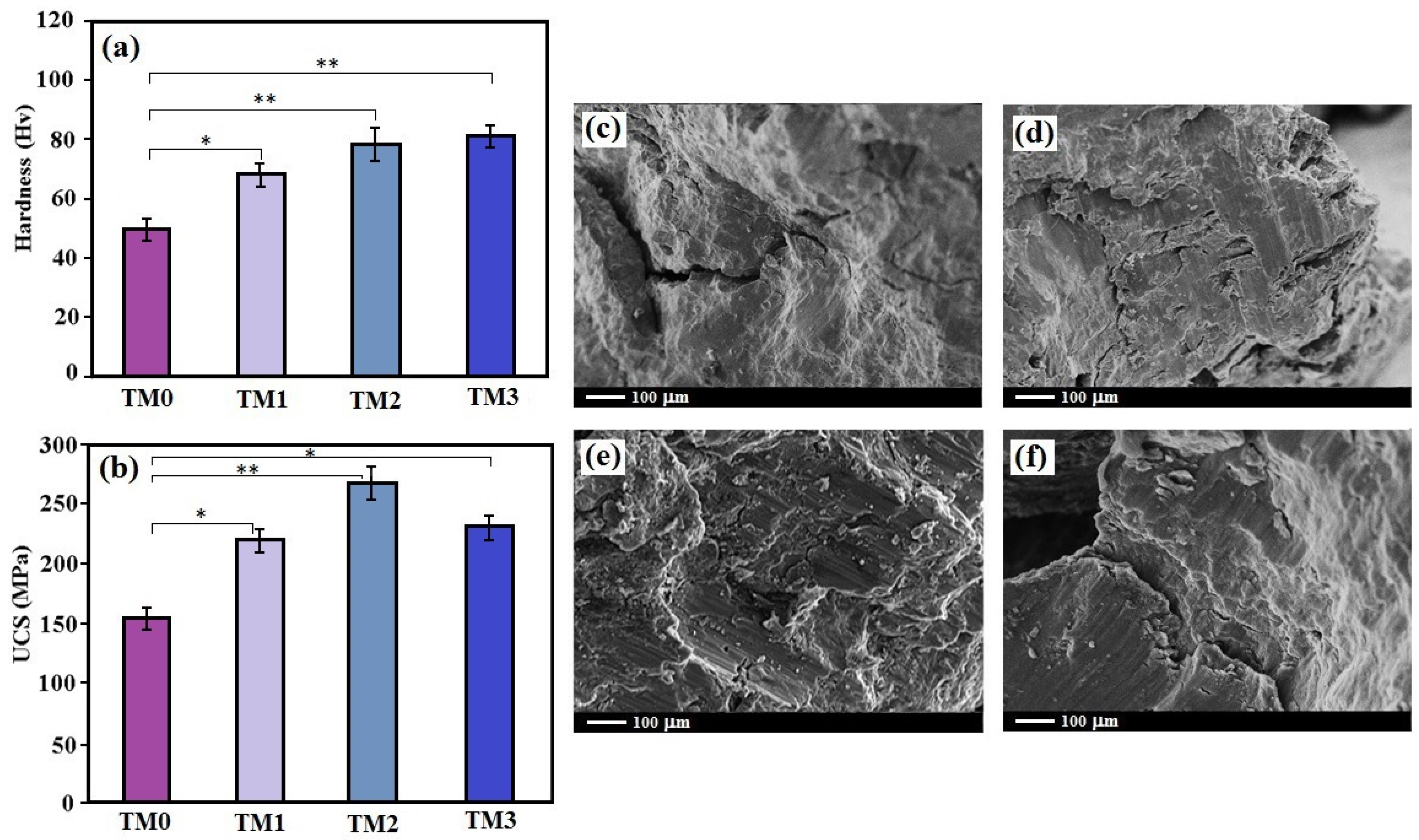
3.2. Mechanical Characteristics
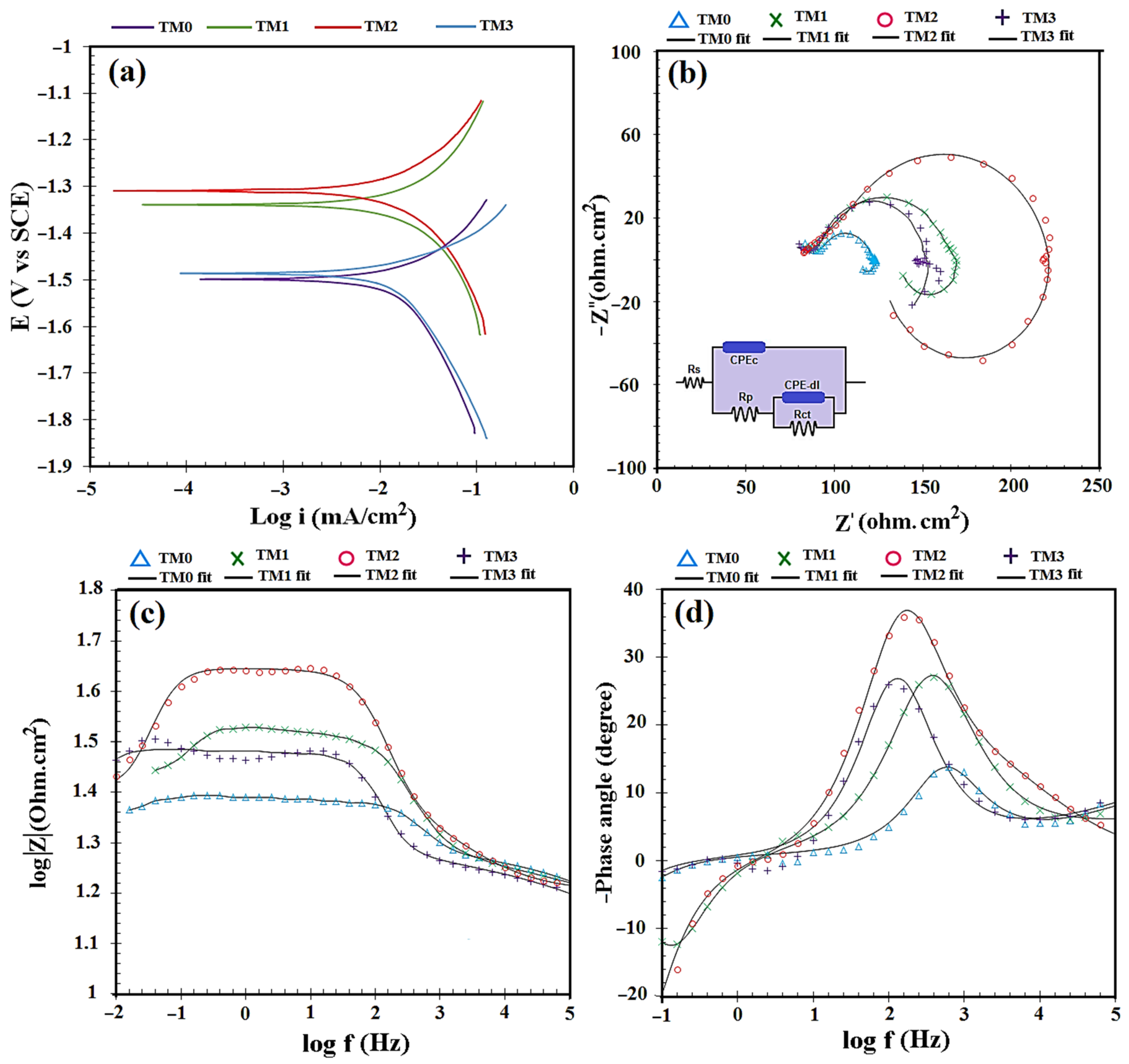
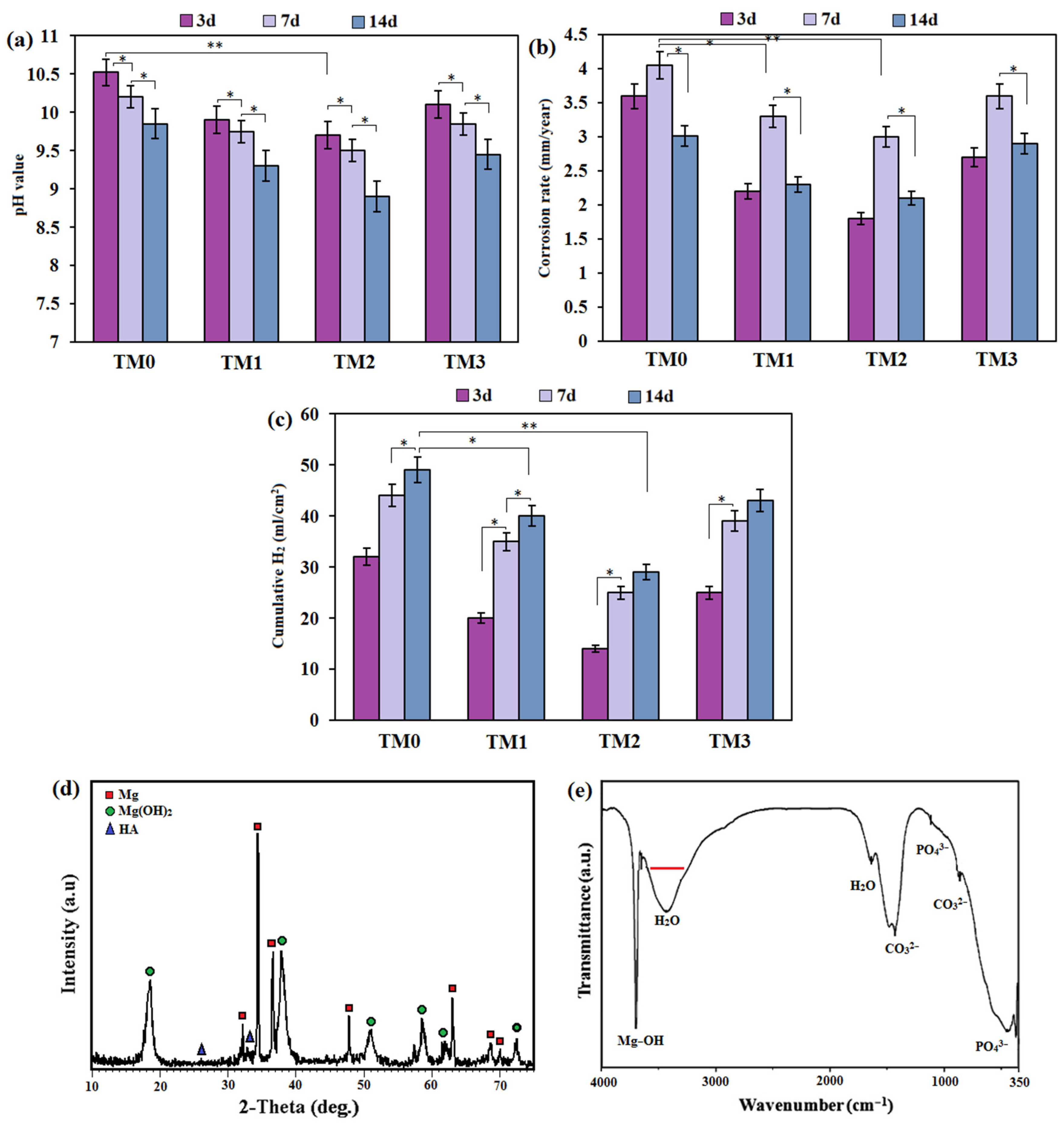
3.3. Corrosion Behavior
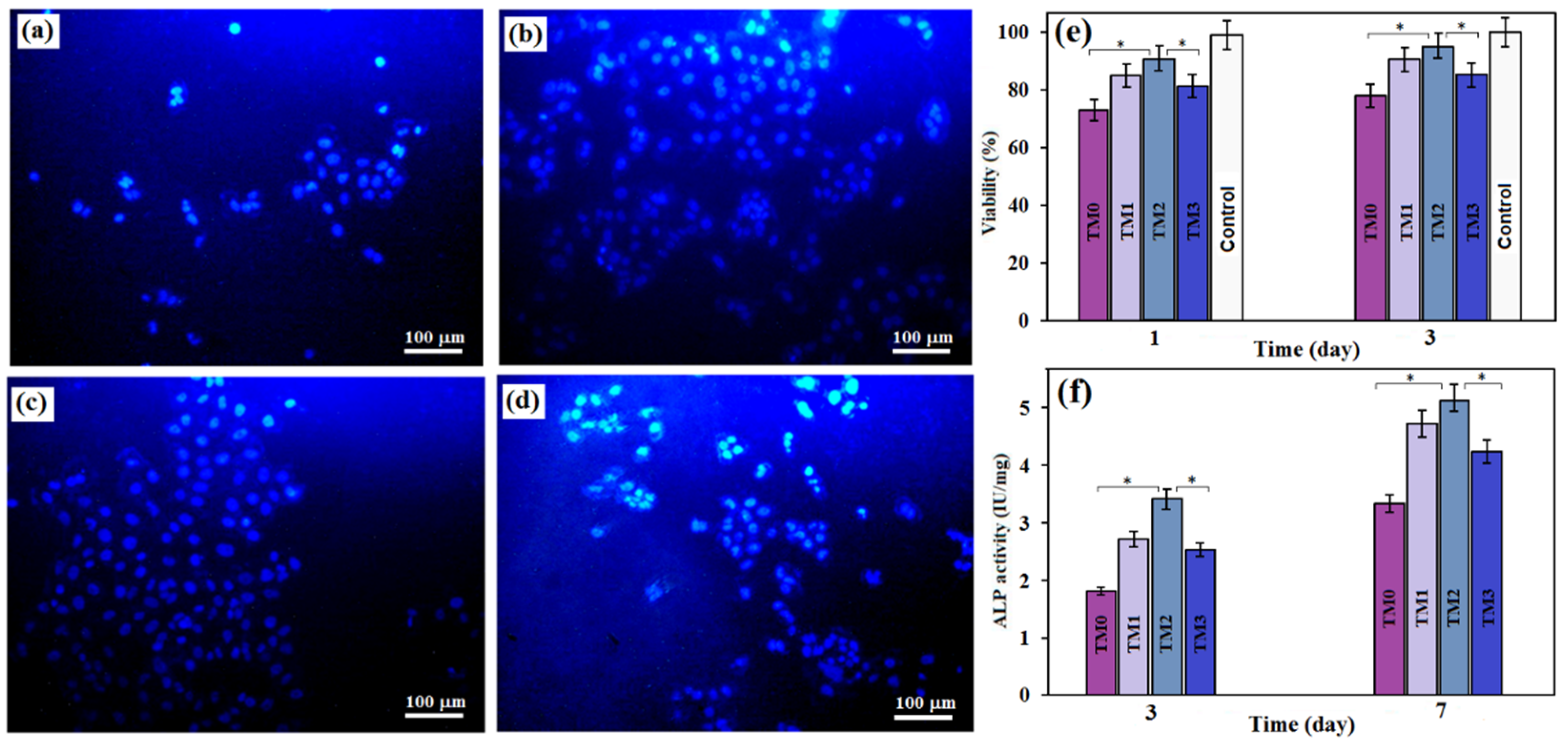
3.4. Evaluation of Biocompatibility
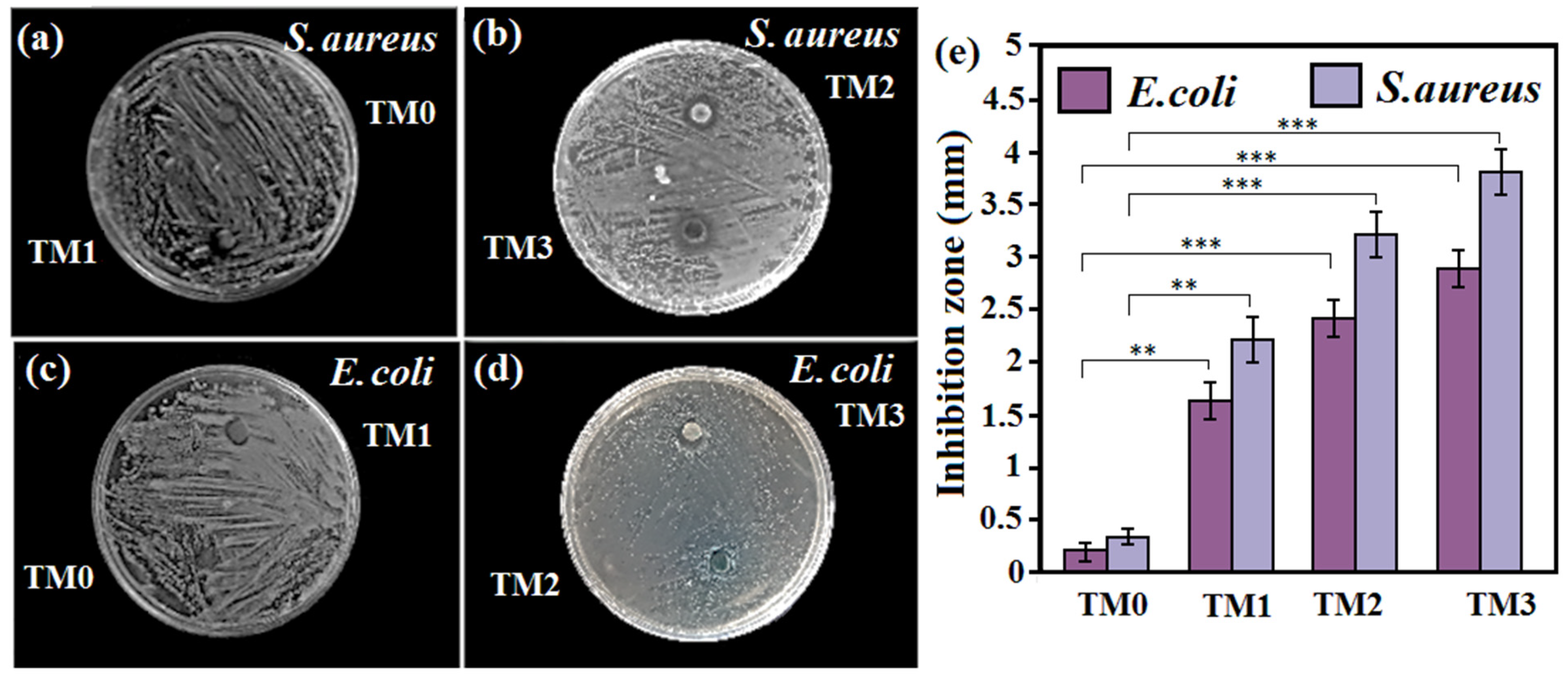
3.5. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, J.; Du, W.; Wang, Z.; Li, S.; Liu, K.; Du, X. Combination of enhanced thermal conductivity and strength of MWCNTs reinforced Mg-6Zn matrix composite. J. Alloys Compd. 2020, 838, 155573. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, L.; Zhao, M.; Feng, P.; Yang, Y.; Guo, W.; Gao, C.; Yuan, F. Microstructure, biodegradation, antibacterial and mechanical properties of ZK60-Cu alloys prepared by selective laser melting technique. J. Mater. Sci. Technol. 2018, 34, 1944–1952. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Lu, Y.; Asif, M.; Hussain, S.; She, J.; Gou, J.; Mao, J. Effect of graphene nanoplatelets (GNPs) addition on strength and ductility of magnesium-titanium alloys. J. Magnes. Alloys 2013, 1, 242–248. [Google Scholar] [CrossRef]
- Mindivan, H.; Efe, A.; Kosatepe, A.H.; Kayali, E. Fabrication and characterization of carbon nanotube reinforced magnesium matrix composites. Appl. Surf. Sci. 2014, 318, 234–243. [Google Scholar] [CrossRef]
- Xiong, P.; Jia, Z.; Li, M.; Zhou, W.; Yan, J.; Wu, Y.; Cheng, Y.; Zheng, Y. Biomimetic Ca, Sr/P-Doped Silk Fibroin Films on Mg-1Ca Alloy with Dramatic Corrosion Resistance and Osteogenic Activities. ACS Biomater. Sci. Eng. 2018, 4, 3163–3176. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Wang, B.; Yang, Y.; Peng, S.; Gao, C. 3D honeycomb nanostructure-encapsulated magnesium alloys with superior corrosion resistance and mechanical properties. Compos. Part B Eng. 2019, 162, 611–620. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Zhang, X. Dynamic degradation behavior of MgZn alloy in circulating m-SBF. Mater. Lett. 2010, 64, 1996–1999. [Google Scholar] [CrossRef]
- Zhou, J.; Zhong, K.; Zhao, C.; Meng, H.; Qi, L. Effect of carbon nanotubes grown temperature on the fracture behavior of carbon fiber reinforced magnesium matrix composites: Interlaminar shear strength and tensile strength. Ceram. Int. 2021, 47, 6597–6607. [Google Scholar] [CrossRef]
- Shen, M.; Han, B.; Ying, T. Micromechanical simulations and experimental characteristics of randomly distributed carbon nanotubes reinforced Mg matrix composites. J. Alloys Compd. 2022, 924, 166589. [Google Scholar] [CrossRef]
- Munir, K.S.; Wen, C.; Li, Y.C. Carbon Nanotubes and Graphene as Nanoreinforcements in Metallic Biomaterials: A Review. Adv. Biosyst. 2019, 3, e1800212. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Hussain, S.; Gou, J.; Mao, J. Improved strength and ductility of magnesium with addition of aluminum and graphene nanoplatelets (Al+GNPs) using semi powder metallurgy method. J. Ind. Eng. Chem. 2015, 23, 243–250. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, X.; Hu, X.; Meng, L.; Song, Z.; Li, X.; Sun, Z.; Zhang, Q.; Wu, K. Achieving ultra-high strengthening and toughening efficiency in carbon nanotubes/magnesium composites via constructing micro-nano layered structure. Compos. Part A Appl. Sci. Manuf. 2019, 119, 225–234. [Google Scholar] [CrossRef]
- Prabhu, D.B.; Gopalakrishnan, P.; Ravi, K. Morphological studies on the development of chemical conversion coating on surface of Mg–4Zn alloy and its corrosion and bio mineralisation behaviour in simulated body fluid. J. Alloys Compd. 2020, 812, 152146. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Koç, M. Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends. J. Magnes. Alloys 2021, 9, 392–415. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Zadpoor, A.A. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Li, S.; Yin, D.; Xie, J.; Rommens, P.M.; Xiang, Z.; Liu, M.; Ritz, U. Recent progress in Mg-based alloys as a novel bioabsorbable biomaterials for orthopedic applications. J. Magnes. Alloys 2022, 10, 1428–1456. [Google Scholar] [CrossRef]
- Hou, J.; Du, W.; Parande, G.; Gupta, M.; Li, S. Significantly enhancing the strength + ductility combination of Mg-9Al alloy using multi-walled carbon nanotubes. J. Alloys Compd. 2019, 790, 974–982. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloys 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Nie, K.; Wang, X.; Deng, K.; Hu, X.; Wu, K. Magnesium matrix composite reinforced by nanoparticles—A review. J. Magnes. Alloys 2021, 9, 57–77. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. In Materials Science and Engineering: R: Reports; Elsevier: Amsterdam, The Netherlands, 2014; Volume 77, pp. 1–34. [Google Scholar]
- Yuan, Q.-H.; Zeng, X.-S.; Liu, Y.; Luo, L.; Wu, J.-B.; Wang, Y.-C.; Zhou, G.-H. Microstructure and mechanical properties of AZ91 alloy reinforced by carbon nanotubes coated with MgO. Carbon 2016, 96, 843–855. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Kaushik, V.; Kumar, B.N.; Kumar, S.S.; Vignesh, M. Magnesium role in additive manufacturing of biomedical implants–challenges and opportunities. Addit. Manuf. 2022, 55, 102802. [Google Scholar]
- Yuan, Q.-H.; Zhou, G.-H.; Liao, L.; Liu, Y.; Luo, L. Interfacial structure in AZ91 alloy composites reinforced by graphene nanosheets. Carbon 2018, 127, 177–186. [Google Scholar] [CrossRef]
- Nai, M.H.; Wei, J.; Gupta, M. Interface tailoring to enhance mechanical properties of carbon nanotube reinforced magnesium composites. Mater. Des. 2014, 60, 490–495. [Google Scholar] [CrossRef]
- Han, G.; Wang, Z.; Liu, K.; Li, S.; Du, X.; Du, W. Synthesis of CNT-reinforced AZ31 magnesium alloy composites with uniformly distributed CNTs. Mater. Sci. Eng. A 2015, 628, 350–357. [Google Scholar] [CrossRef]
- Ali, M.; Hussein, M.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Paramsothy, M.; Chan, J.; Kwok, R.; Gupta, M. Addition of CNTs to enhance tensile/compressive response of magnesium alloy ZK60A. Compos. Part A Appl. Sci. Manuf. 2011, 42, 180–188. [Google Scholar] [CrossRef]
- Shanmugam, B.K.; Rangaraj, S.; Subramani, K.; Srinivasan, S.; Aicher, W.K.; Venkatachalam, R. Biomimetic TiO2-chitosan/sodium alginate blended nanocomposite scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2020, 110, 110710. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.; Awwad, N.S. Antibacterial activity of TiO2 doped ZnO composite synthesized via laser ablation route for antimicrobial application. J. Mater. Res. Technol. 2020, 9, 9434–9441. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Nai, M.H.; Almajid, A.; Gupta, M. Development of high performance Mg–TiO2 nanocomposites targeting for biomedical/structural applications. Mater. Des. 2015, 65, 104–114. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Khoshnava, S.M.; Pagan, E.; Chen, X. Co-incorporation of graphene oxide/silver nanoparticle into poly-L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater. Sci. Eng. C 2020, 111, 110812. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R. Reduced graphene oxide (RGO) reinforced Mg biocomposites for use as orthopedic applications: Mechanical properties, cytocompatibility and antibacterial activity. J. Magnes. Alloys 2021, 10, 3612–3627. [Google Scholar] [CrossRef]
- Shuai, C.; Zan, J.; Qi, F.; Wang, G.; Liu, Z.; Yang, Y.; Peng, S. nMgO-incorporated PLLA bone scaffolds: Enhanced crystallinity and neutralized acidic products. Mater. Des. 2019, 174, 107801. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. Magnesium-graphene nano-platelet composites: Corrosion behavior, mechanical and biological properties. J. Alloys Compd. 2020, 821, 153379. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Xie, L.; Zhang, L.-C.; Wu, L.; Fu, Y.; Wang, L.; Lu, W. Electrochemical and in vitro behavior of the nanosized composites of Ti-6Al-4V and TiO2 fabricated by friction stir process. Appl. Surf. Sci. 2017, 423, 331–339. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir, M.R.A.; Izman, S.; Bakhsheshi-Rad, H.R.; Farahany, S. Effect of mechanical alloying on the phase evolution, microstructure and bio-corrosion properties of a Mg/HA/TiO2/MgO nanocomposite. Ceram. Int. 2014, 40, 16743–16759. [Google Scholar] [CrossRef]
- Sul, Y.-T. Osseoinductive Magnesium-Titanate Implant and Method of Manufacturing the Same. U.S. Patent 7,452,566, 18 November 2008. [Google Scholar]
- Ramezanzade, S.G.R.; Ebrahimi, M. Torabi Parizi and H.R. Ezatpour, Synergetic effect of GNPs and MgOs on the mechanical properties of Mg–Sr–Ca alloy. Mater. Sci. Eng. A 2019, 761, 138025. [Google Scholar] [CrossRef]
- Parizi, M.T.; Ebrahimi, G.; Ezatpour, H.; Paidar, M. The structure effect of carbonaceous reinforcement on the microstructural characterization and mechanical behavior of AZ80 magnesium alloy. J. Alloys Compd. 2019, 809, 151682. [Google Scholar] [CrossRef]
- Cui, Z.; Li, W.; Cheng, L.; Gong, D.; Cheng, W.; Wang, W. Effect of nano-HA content on the mechanical properties, degradation and biocompatible behavior of Mg-Zn/HA composite prepared by spark plasma sintering. Mater. Charact. 2019, 151, 620–631. [Google Scholar] [CrossRef]
- Jaiswal, S.; Kumar, R.M.; Gupta, P.; Kumaraswamy, M.; Roy, P.; Lahiri, D. Mechanical, corrosion and biocompatibility behaviour of Mg-3Zn-HA biodegradable composites for orthopaedic fixture accessories. J. Mech. Behav. Biomed. Mater. 2018, 78, 442–454. [Google Scholar] [CrossRef]
- Shuai, C.; Zeng, Z.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Graphene oxide assists polyvinylidene fluoride scaffold to reconstruct electrical microenvironment of bone tissue. Mater. Des. 2020, 190, 108564. [Google Scholar] [CrossRef]
- Baradaran, S.; Moghaddam, E.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Moghaddam, M.R.N.; Alias, Y. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon 2014, 69, 32–45. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium-based composites reinforced with graphene nanoplatelets as biodegradable implant materials. J. Alloys Compd. 2020, 828, 154461. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, M.; Wen, C.; Li, Y. Microstructure, mechanical and corrosion properties of hot-pressed graphene nanoplatelets-reinforced Mg matrix nanocomposites for biomedical applications. J. Alloys Compd. 2021, 887, 161379. [Google Scholar] [CrossRef]
- Sunil, B.R.; Ganapathy, C.; Kumar, T.S.; Chakkingal, U. Processing and mechanical behavior of lamellar structured degradable magnesium–hydroxyapatite implants. J. Mech. Behav. Biomed. Mater. 2014, 40, 178–189. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Dou, J.; Yu, H.; Chen, C. Layer by layer assembled chitosan (TiO2)-heparin composite coatings on MAO-coated Mg alloys. Mater. Lett. 2020, 281, 128640. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Yue, C.; Zhang, T.; Li, P.; Xing, Z.; Chen, Y. A tough graphene nanosheet/hydroxyapatite composite with improved in vitro biocompatibility. Carbon 2013, 61, 105–115. [Google Scholar] [CrossRef]
- Khazeni, D.; Saremi, M.; Soltani, R. Development of HA-CNTs composite coating on AZ31 Magnesium alloy by cathodic electrodeposition. Part 2: Electrochemical and in-vitro behavior. Ceram. Int. 2019, 45, 11186–11194. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, B.; Bin, S.; Peng, S.; Gao, C. TiO2-Induced In Situ Reaction in Graphene Oxide-Reinforced AZ61 Biocomposites to Enhance the Interfacial Bonding. ACS Appl. Mater. Interfaces 2020, 12, 23464–23473. [Google Scholar] [CrossRef]
- Wei, D.; Du, Q.; Guo, S.; Jia, D.; Wang, Y.; Li, B.; Zhou, Y. Structures, bonding strength and in vitro bioactivity and cytotoxicity of electrochemically deposited bioactive nano-brushite coating/TiO2 nanotubes composited films on titanium. Surf. Coat. Technol. 2018, 340, 93–102. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.M.; Kuntal, K.K.; Gupta, P.; Das, S.; Jayaganthan, R.; Roy, P.; Lahiri, D. Sol–Gel Derived Hydroxyapatite Coating on Mg-3Zn Alloy for Orthopedic Application. Jom 2015, 67, 702–712. [Google Scholar] [CrossRef]
- Willumeit, R.; Möhring, A.; Feyerabend, F. Optimization of Cell Adhesion on Mg Based Implant Materials by Pre-Incubation under Cell Culture Conditions. Int. J. Mol. Sci. 2014, 15, 7639–7650. [Google Scholar] [CrossRef]
- Webster, T.J.; Waid, M.C.; McKenzie, J.L.; Price, R.L.; Ejiofor, J.U. Nano-biotechnology: Carbon nanofibres as improved neural and orthopaedic implants. Nanotechnology 2004, 15, 48–54. [Google Scholar] [CrossRef]
- Abutalib, M.; Rajeh, A. Enhanced structural, electrical, mechanical properties and antibacterial activity of Cs/PEO doped mixed nanoparticles (Ag/TiO2) for food packaging applications. Polym. Test. 2021, 93, 107013. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Wang, Y.; Zhang, C.Y. TiO2-based nanosystem for cancer therapy and antimicrobial treatment: A review. Chem. Eng. J. 2022, 431, 133714. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non-Crystalline Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Ulu, A.; Birhanlı, E.; Köytepe, S.; Ateş, B. Chitosan/polypropylene glycol hydrogel composite film designed with TiO2 nanoparticles: A promising scaffold of biomedical applications. Int. J. Biol. Macromol. 2020, 163, 529–540. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zou, F.; Li, D.; Yao, Y. A high-sensitivity thin-film MWNT@ PDA-AgNP nanocomposite sensor for acquiring microscopic deformations. Compos. Sci. Technol. 2022, 229, 109689. [Google Scholar] [CrossRef]
- Naebe, M.; Lin, T.; Tian, W.; Dai, L.; Wang, X. Effects of MWNT nanofillers on structures and properties of PVA electrospun nanofibres. Nanotechnology 2007, 18, 225605. [Google Scholar] [CrossRef]
- Mehwish, N.; Xu, M.; Zaeem, M.; Lee, B.H. Mussel-Inspired Surface Functionalization of Porous Albumin Cryogels Supporting Synergistic Antibacterial/Antioxidant Activity and Bone-Like Apatite Formation. Gels 2022, 8, 679. [Google Scholar] [CrossRef]
- Kiran, A.S.K.; Kumar, T.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and Bioactive Surface Modifications of Titanium Implants by PCL/TiO2 Nanocomposite Coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, S.; Wang, W.; Liu, J.; Liu, D.; Chen, X.; Li, W.; Mei, B. Microstructure, Interface and Strengthening Mechanism of Ni-CNTs/AZ91 Magnesium Matrix Composites. Materials 2022, 15, 7946. [Google Scholar] [CrossRef]
- Kúdela, S.; Koráb, J.; Štefánik, P. Effect of Temperature on the Complex Modulus of Mg-Based Unidirectionally Aligned Carbon Fiber Composites. Materials 2022, 15, 7812. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, J.; Zhang, Y.; Xue, B.; Wang, S. Mechanism Analysis of Nanosecond Pulse Laser Etching of SiCp/Mg Composites. Materials 2022, 15, 7654. [Google Scholar] [CrossRef] [PubMed]
- Lesz, S.; Karolus, M.; Gabryś, A.; Kremzer, M. Influence of Milling Time on Phase Composition and Product Structure of Mg-Zn-Ca-Ag Alloys Obtained by Mechanical Synthesis. Materials 2022, 15, 7333. [Google Scholar] [CrossRef]
- Chen, J.; Dai, J.; Qian, J.; Li, W.; Li, R.; Pang, D.; Wan, G.; Li, P.; Xu, S. Influence of Surface Roughness on Biodegradability and Cytocompatibility of High-Purity Magnesium. Materials 2022, 15, 3991. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Domantovsky, A.G.; Kaminsky, V.V.; Pytskii, I.S.; Emelyanenko, K.A.; Boinovich, L.B. The Mechanisms of Antibacterial Activity of Magnesium Alloys with Extreme Wettability. Materials 2021, 14, 5454. [Google Scholar] [CrossRef]
- Helmholz, H.; Adejube, B.; Luthringer-Feyerabend, B.; Willumeit-Römer, R. In Vitro Investigation on Degradable Mg-Based Biomaterial under the Impact of the Serum Glycoprotein Fetuin. Materials 2021, 14, 5005. [Google Scholar] [CrossRef]
- Bakhtiari-Zamani, H.; Saebnoori, E.; Bakhsheshi-Rad, H.R.; Berto, F. Corrosion and Wear Behavior of TiO2/TiN Duplex Coatings on Titanium by Plasma Electrolytic Oxidation and Gas Nitriding. Materials 2022, 15, 8300. [Google Scholar] [CrossRef]
- Wang, S.-H.; Lee, S.-P.; Yang, C.-W.; Lo, C.-M. Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. Materials 2021, 14, 441. [Google Scholar] [CrossRef]
- Yang, C.-W.; Wang, G.-K. Effect of Hydrothermal (Sr)-Hydroxyapatite Coatings on the Corrosion Resistance and Mg2+ Ion Release to Enhance Osteoblastic Cell Responses of AZ91D Alloy. Materials 2020, 13, 591. [Google Scholar] [CrossRef]
- Mehwish, N.; Kausar, A.; Siddiq, M. High-performance polyvinylidene fluoride/poly(styrene–butadiene–styrene)/functionalized MWCNTs-SCN-Ag nanocomposite membranes. Iran. Polym. J. 2015, 24, 549–559. [Google Scholar] [CrossRef]
- Mehwish, N.; Kausar, A.; Siddiq, M.; Raheel, M. Design and properties of polyvinylidene fluoride/poly (styrene-butadiene-styrene)/functionalized multi-walled carbon nanotube nanocomposite membranes. J. Plast. Film. Sheeting 2015, 31, 118–143. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Berto, F. Functionalized carbon nanotube-encapsulated magnesium-based nanocomposites with outstanding mechanical and biological properties as load-bearing bone implants. Mater. Des. 2022, 213, 110354. [Google Scholar] [CrossRef]
- Shajahan; Mo, Y.; Kibria, A.F.; Kim, M.; Nahm, K. High growth of SWNTs and MWNTs from C2H2 decomposition over Co–Mo/MgO catalysts. Carbon 2004, 42, 2245–2253. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirzade-Iranaq, M.T.; Omidi, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Abazari, S.; Teymouri, N.; Naeimi, F.; Sergi, C.; Ismail, A.F.; Sharif, S.; et al. MWCNTs-TiO2 Incorporated-Mg Composites to Improve the Mechanical, Corrosion and Biological Characteristics for Use in Biomedical Fields. Materials 2023, 16, 1919. https://doi.org/10.3390/ma16051919
Amirzade-Iranaq MT, Omidi M, Bakhsheshi-Rad HR, Saberi A, Abazari S, Teymouri N, Naeimi F, Sergi C, Ismail AF, Sharif S, et al. MWCNTs-TiO2 Incorporated-Mg Composites to Improve the Mechanical, Corrosion and Biological Characteristics for Use in Biomedical Fields. Materials. 2023; 16(5):1919. https://doi.org/10.3390/ma16051919
Chicago/Turabian StyleAmirzade-Iranaq, Mohammad Taher, Mahdi Omidi, Hamid Reza Bakhsheshi-Rad, Abbas Saberi, Somayeh Abazari, Nadia Teymouri, Farid Naeimi, Claudia Sergi, Ahmad Fauzi Ismail, Safian Sharif, and et al. 2023. "MWCNTs-TiO2 Incorporated-Mg Composites to Improve the Mechanical, Corrosion and Biological Characteristics for Use in Biomedical Fields" Materials 16, no. 5: 1919. https://doi.org/10.3390/ma16051919
APA StyleAmirzade-Iranaq, M. T., Omidi, M., Bakhsheshi-Rad, H. R., Saberi, A., Abazari, S., Teymouri, N., Naeimi, F., Sergi, C., Ismail, A. F., Sharif, S., & Berto, F. (2023). MWCNTs-TiO2 Incorporated-Mg Composites to Improve the Mechanical, Corrosion and Biological Characteristics for Use in Biomedical Fields. Materials, 16(5), 1919. https://doi.org/10.3390/ma16051919










