Abstract
In this work, the facile fabrication of Co3O4 particles/reduced graphene oxide (Co3O4/rGO) composites on Indium tin oxide (ITO) slide was achieved by an electrophoretic deposition and annealing process. The deposition time and ratio of the precursors were optimized. Structural characterization and chemical composition investigation indicated successful loading of Co3O4 particles on graphene sheets. When applied as a non-enzymatic H2O2 sensor, Co3O4/rGO showed significant electrocatalytic activity, with a wide linear range (0.1–19.5 mM) and high sensitivity (0.2247 mA mM−1 cm−2). The good anti-interference ability, reproducibility, and long-term stability of the constructed sensor were also presented. The application of Co3O4/rGO in real sample analysis was evaluated in human urine sample with satisfactory results, indicating the feasibility of the sensor in physiological and medical applications.
1. Introduction
Hydrogen peroxide (H2O2) is a molecule that plays important roles in industrial, pharmacological, and clinical processes [1,2]. On account of its strong oxidizing ability, H2O2 is often used for sterilization and food production [3]. As a by-product of biochemical reactions during metabolism, the amount of H2O2 should be regulated, as over-production of H2O2 in the body can induce diseases such as cancer, Alzheimer’s disease, and cardiovascular disorders [4,5]. The quantitative determination of H2O2 is important for industrial and medical purposes. Electrochemical H2O2 sensors immobilized with enzymes were reported with high specificity and sensitivity [6]. However, enzymatic sensors suffer from issues such as high cost, poor recycling ability, complicated procedures, etc. Therefore, increasing attention has focused on non-enzymatic H2O2 sensing.
Transition metal nanoparticles (Cu, Co, Au, Ag, etc.) and metal oxides (CuO, Cu2O, Co3O4) are attractive materials for non-enzymatic H2O2 sensing [7].Among these, Co3O4 seems to be a promising candidate with low-cost, high abundance, minimal surface fouling, and improved selectivity [8], which are advantageous in H2O2 detection. However, Co3O4-based catalysts usually exhibit unsatisfactory performance due to the poor conductivity, few active sites or agglomeration problems [9]. In order to enhance the catalytic ability of Co3O4, researchers have combined Co3O4 with carbon-based materials such as graphene and carbon nanotubes to form hybrids [10,11]. Graphene, a 2D carbon nanosheets, displays properties such as a high surface area, good electrical conductivity, and chemical stability. In electrochemical applications, reduced graphene oxide (rGO), one derivative of graphene, has drawn much attention, as its functional groups and defect sites can act as active sites for the bonding of nanomaterials and can facilitate electron transfer. Reports have shown that outstanding electrochemical sensing performance was achieved by incorporating Co3O4 with rGO [12,13]. Various methods have been explored for the preparation of Co3O4 and rGO composites such as the solvothermal method [14], hydrothermal method [15], or laser irradiation method [16]. Indeed, hydrothermal/solvothermal methods are widely used synthesis strategies for uniform morphology of the material by adjusting experimental conditions and precursors. For instance, Co3O4 nanowires on 3D graphene were prepared by a hydrothermal procedure at 120 °C for 16 h [17]. Hollow and mesoporous Co3O4 spheres were synthesized by the surfactant-assisted solvothermal method, with autoclaving at 200 °C for 4 h in absolute methanol [18]. Although the process is simple without expensive experimental facilities such as the irradiation method, the long reaction time and inhomogeneous thermal distribution in the autoclave could affect the evenness and repeatability of materials [19].
Electrophoretic deposition (EPD) has emerged as a cost-effective and facile strategy to fabricate electrochemical catalysts at room temperature [20]. In an EPD-coating process, particles in the colloidal suspensions migrate to the electrode with opposite charge under applied voltage, yielding uniform coatings on various substrates with a controllable deposition process by simply adjusting the deposition parameters such as the applied voltage and deposition time [21]. In addition, the conductivity and the degree of packing density of deposited layers can be controlled well by EPD, which could enhance the efficiency of the electrocatalyst layers [22]. The EPD process takes a relatively shorter time, such as a few seconds or minutes. Prompt EPD also makes the scaling up to large dimensions possible [23]. In previous work, we showed the use of the EPD technique to fabricate carbon-based nanocomposites for non-enzymatic glucose sensing [24]. Herein, we report the facile fabrication of a Co3O4 particle/reduced graphene oxide (Co3O4/rGO)-modified ITO interface by one-step EPD and a subsequent annealing process. The Co3O4 particles/rGO composites were applied to non-enzymatic H2O2 detection with a wide linear range and feasibility in real sample analysis.
2. Materials and Methods
2.1. Chemicals
Cobalt (II) nitrate hexahydrate (Co(NO3)2·6H2O), α-D-glucose, uric acid (UA), ascorbic acid (AA), dopamine hydrochloride (DA), ethanol, and sodium hydroxide were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China) and used as received. Then, 0.1 M phosphate buffer (PBS, pH = 7.4) was prepared using Na2HPO4 and NaH2PO4 by adjusting the pH with H2SO4 or NaOH. All chemicals were of analytical grade and were used as received without any further purification. Graphene (reduced graphene oxide, purity: >98 wt %) was purchased from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Sciences (Chengdu, China). Indium tin oxide (ITO) slides (10 Ω/sq) were obtained from Zhuhai Kaivo Optoelectronic Technology Co., Ltd, Zhuhai City, China. A urine sample was collected from a healthy colleague in our group. The water used in all experiments was purified with an ultrafiltration system from Ulupure Co.(Chengdu, China, resistivity = 18.25 MΩ.cm).
2.2. Preparation of the Co3O4/rGO/ITO Interfaces
The Co3O4/rGO-modified ITO interfaces were obtained through electrophoretic deposition. Prior to modification, the ITO slides were cleaned thoroughly by successive sonication in acetone, ethanol, and deionized water, followed by blow drying. In the typical EPD process, platinum foil (1 × 1.5cm2) and an ITO slide (1 × 1.5cm2) function as the anode and cathode, respectively, with a parallel distance of 1 cm in the cell. After sonication for 1 h, a suspension of graphene (0.25 mg ml−1) and Co(NO3)2·6H2O (0.5mg ml−1) in ethanol was transferred to the cell, and a DC voltage of 50 V was applied for 1–3 min. Then, the interfaces were washed with deionized water and dried in the air, followed by annealing at 400 °C for 1 h under the protection of an argon atmosphere. The interfaces coated with Co3O4 particles/rGO with different ratios of Co2+ vs. graphene (3:1, 1:1, 1:2, 1:3) were deposited under the same conditions unless mentioned otherwise. The electrode modified with rGO or Co3O4 alone was obtained using the same method in the absence of Co2+ salts or graphene.
2.3. Instrumentation
A Rigaku (Tokyo, Japan) DMAX-2500PC X-ray diffractometer (XRD) with Cu Kα radiation was employed to verify the crystalline phases of the samples. The microstructure structure and morphology of the interfaces were obtained by using a thermal field scanning electron microscope (SEM, SU-70, Hitachi, Tokyo, Japan) equipped with an energy dispersive spectrometer. The surface composition and element properties were characterized by X-ray photoelectron spectroscopy (XPS, AXIS SUPRA, Shimadzu, Kyoto, Japan). Raman spectroscopy measurements were performed by a Raman microscope (Renishaw inVia, UK) with a laser excitation of 532 nm.
Electrochemical measurements were carried out using a CHI 660E electrochemical workstation (Chenhua Instrument, Shanghai, China) using the 3-electrode system, in which the Co3O4/rGO/ITO interface worked as the working electrode, Ag/AgCl as the reference electrode, and the platinum plate as the counter electrode. Cyclic voltammetry (CV) curves were recorded using aqueous solutions of 0.1 M NaOH/0.1 M phosphate buffer (PBS, pH = 7.4) as the electrolyte. The chronoamperometric performances of the Co3O4/rGO/ITO electrode were performed in stirred electrolyte solutions under N2-saturated PBS, with the successive addition of H2O2. In real sample analysis, urine was diluted by PBS and spiked with a fixed concentration of H2O2. The response of the proposed sensor towards H2O2 in the urine sample was recorded.
3. Results and Discussion
3.1. Characterization of the Co3O4/rGO Composites
The Co3O4/rGO composites were successfully deposited on the ITO interface via a facile electrophoretic method and subsequent thermal annealing process. As illustrated in Figure 1, positively charged Co2+ with graphene moved towards the cathode with the applied voltage of 50 V as the driving force. At the surface of the ITO, the reduction of NO3− ions occurred and the generated OH− reacted with absorbed Co2+, forming Co(OH)2 on the graphene sheets. The coating scheme is as follows [25,26]:
NO3− + H2O + 2e− → NO2− + 2OH−
2H2O + 2e− → H2 + 2OH−
Co2+ + 2OH− → Co(OH)2
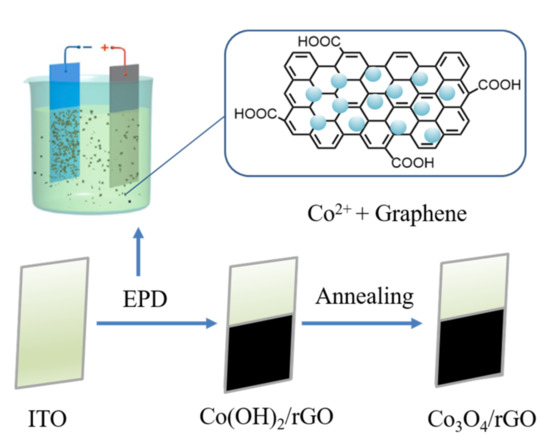
Figure 1.
Schematic illustration of the fabrication of the Co3O4/rGO composites.
After the EPD, the coated interfaces were annealed at 400 °C for 1 h in an Ar atmosphere to convert Co(OH)2/rGO to Co3O4/rGO. In order to verify the crystalline structure of the synthesized Co3O4/rGO composite, XRD analysis was conducted. As shown in Figure 2, the XRD pattern of graphene displayed a peak at 26.4°, corresponding to the (002) plane of graphene. After integration with Co3O4, diffraction peaks at 20°, 31.2°, 37°, 45.1°, and 60° appeared, which can be ascribed to the respective (111), (220), (311), (400), and (511) planes of Co3O4 (JCPDS no. 42-1467) [8,25]. The peak of (002) for graphene became broader, indicating that the structure of graphene was restored after EPD with Co [27]. The morphology of the as-prepared Co3O4/rGO composites was characterized by SEM. EDX spectroscopy analysis was used to examine the chemical composition. In Figure 3a, rGO presents thin layer-by-layer assembly of several nanosheets. After Co(NO3)2·6H2O (0.5 mg ml−1) addition, the rGO nanosheets were decorated with Co3O4 particles in the size of 0.27 ± 0.06 µm (estimated from 100 particles) (Figure 3b–d). The effect of the deposition time on the morphology of the Co3O4 particles/rGO was evaluated by SEM. Figure 3b–d presents the images of Co3O4 particles/rGO with varied deposition times of 1 min, 2 min, and 3 min, respectively. It can be seen that after 1 min deposition, several Co3O4 particles were anchored on the graphene sheets (Figure 3b). The Co3O4 particles were homogenously distributed with a much higher density when the deposition time was 2 min (Figure 3c). However, the Co3O4 particles agglomerated as clusters after 3 min deposition, as depicted in Figure 3d. Similar results were found during the EPD process of the Ag nanoparticles [28], the mechanism of which could be explained by Ostwald ripening [29,30]. The EDX spectrum (Figure 3e) performed on Co3O4/rGO-modified ITO (2 min deposition) comprised signals mainly due to Co, O, C, Si, In, and Sn, consistent with the chemical composition of the material-coated substrate. Signals of Ca, Na, Mg, Al, and Au could be impurities of ITO. The distributions of Co, C, and O were illustrated by EDX mapping in Figure 3f, g, h, respectively, presenting the uniform distribution of Co3O4 and graphene. The Co atomic concentration was estimated to be 6.04 at%, suggesting the successful deposition of Co.
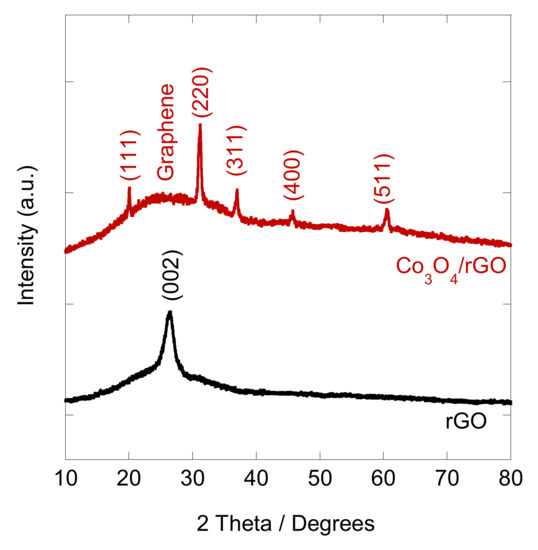
Figure 2.
XRD patterns of rGO and Co3O4/rGO composites.
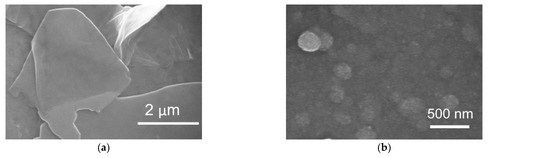
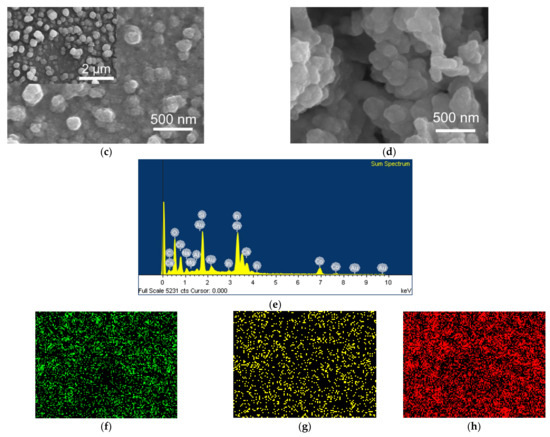
Figure 3.
SEM images of (a) rGO; Co3O4/rGO with a deposition time of (b) 1 min; (c) 2 min (Inset: low magnification image); (d) 3 min; EDX results of Co3O4/rGO (2 min deposition): (e)EDX spectrum and EDX elemental mapping (f) Co; (g) C; (h) O.
The chemical composition of the Co3O4/rGO composite was further investigated by X-ray photoelectron spectroscopy (XPS). As shown in Figure 4a, the full survey of XPS spectra for Co3O4/rGO clearly demonstrated a series of signals corresponding to the characteristic peaks of Co 3p, Co 3s, C 1s, O 1s, and Co 2p, indicating the existence of Co, O, and C [31]. Figure 4b shows the high resolution of the C 1s spectrum of the Co3O4/rGO composites. It could be deconvoluted into three peaks at 284.86 eV, 286.11 eV, and 288.40 eV, which are assigned to sp2 carbon, C-O, and C=O, respectively, with sp2 carbon being dominant [32,33]. The Co 2p core spectrum was deconvoluted and displayed in Figure 4c. The nonsymmetric spin-orbit doublets with shake-up satellites indicate the existence of Co2+ and Co3+ [34]. The peaks at 779.6 and 794.6 eV with a satellite signal at 789.7 eV were characteristic of Co3+, and peaks at 781.1 and 796.2 eV with satellite at 804.4 were attributed to Co2+ [35], indicating the formation of Co3O4.
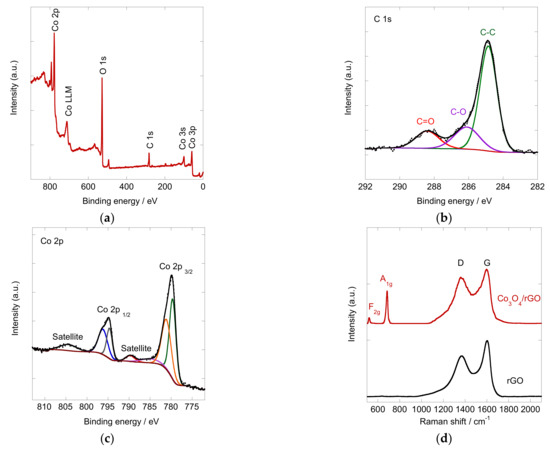
Figure 4.
High-resolution XPS of the Co3O4/rGO composite: (a) full spectrum; (b) C1s and (c) Co 2p spectrum and (d) Raman spectra of Co3O4/rGO (red) and rGO (black).
To gain more insight into the composition of the deposited composites, Raman analysis was conducted. Figure 4d shows the Raman spectra of rGO and Co3O4/rGO, which displayed the main features of graphene-based materials with D (defects and disorders in the graphitic lattice) and G band (crystalline part of graphene) [36]. The Raman spectrum of rGO exhibited a D band at ~1366 cm−1 and a G band at ~1603 cm−1. In comparison, the D and G bands of Co3O4/rGO slightly shifted towards a lower wavenumber and were located at 1361 and 1597 cm−1. The red shifts of the D and G bands of Co3O4/rGO indicated the charge transfer between rGO and Co3O4 [37]. The ID/IG intensity ratio in Raman spectra could provide information on the disorder caused by the defects related to vacancies. The value of ID/IG of rGO was 0.73. After integration with Co3O4, the number increased to 0.86, indicating that the co-deposition of Co3O4 particles with rGO caused more defects [38], which will serve as active sites for electrocatalytic reactions. Characteristic intensity peaks at 520 and 680 cm−1 were observed on the Co3O4/rGO spectrum, which are attributed to the respective F2g and A1g vibrational modes of Co3O4 [39,40]. The Raman results showed good accordance with the XPS spectra, indicating the successful incorporation of Co3O4 on the rGO sheets.
3.2. Electrocatalytic Performance of Co3O4/rGO in H2O2 Reduction
Prior to the investigation of the electrocatalytic performance of the Co3O4/rGO interface, cyclic voltammograms (CV) over a potential range of −0.1-0.7 V at various scan rates were recorded in 0.1 M NaOH aqueous solution (Figure 5a). Two reversible redox peaks of Co3O/CoOOH, CoOOH/CoO2 were observed under alkaline conditions. The reactions can be explained by the following Equations (4) and (5) [17,41]. With the increase in scan rates, the redox peak current increased with positively shifted oxidative peaks and negatively shifted reductive peaks, manifesting a surface-controlled electrochemical process. The typical redox peaks also confirmed the fabrication of Co3O4/rGO composites.
Co3O4 + OH− + H2O ⇌ 3CoOOH + e−
CoOOH + OH− ⇌ CoO2+ H2O + e−
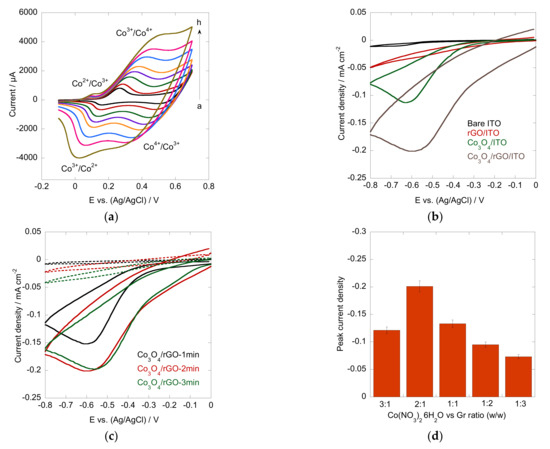
Figure 5.
(a) CV scans of Co3O4/rGO in 0.1 M NaOH at different scan rates (a-h: 10–300 mV s−1); (b) CV of bare ITO (black), rGO/ITO (red), Co3O4/ITO (green), and Co3O4/rGO (grey) in the presence of 1 mM H2O2 in N2-saturated 0.1 M PBS buffer (pH = 7.4) at a scan rate of 50 mV s−1; (c) CV of Co3O4/rGO-1min (black), Co3O4/rGO-2min (red), and Co3O4/rGO-3min (green) in the absence (dashed line) and presence (solid line) of 1 mM H2O2 in N2-saturated 0.1 M PBS buffer (pH = 7.4) at a scan rate of 50 mV s−1; (d) effect of varied ratios of Co2+ vs. graphene.
The electrocatalytic activity of the Co3O4/Co composites toward H2O2 detection was evaluated by CV in N2-saturated 0.1 M PBS buffer (pH = 7.4) at a scan rate of 50 mV s−1. Figure 5b shows the CV recorded from bare ITO, rGO, Co3O4, and Co3O4/rGO composites in the presence of 1 mM H2O2. Bare ITO and the rGO-modified ITO interface did not show any response to H2O2 addition under the identified conditions, whereas ITO modified with Co3O4 displayed a reduction peak at −0.63 V, suggesting that Co3O4 exhibited notable electrocatalytic activity toward H2O2 reduction. It is worth noting that a distinct cathodic peak occurred at −0.59 V with higher current density for the Co3O4/rGO-modified ITO electrode. The highly enhanced electrocatalytic activity and fast electron transfer show the great advantage of Co3O4/rGO composites for H2O2 reduction. The improved performance may be attributed to the synergistic effect of the Co3O4 particles and graphene. The reaction mechanism can be depicted by the following equations [42,43,44]:
Co3O4/rGO + H2O2→Co3O4/rGO-OHads + OH−
Co3O4/rGO-OHads + e−→Co3O4/rGO + OH−
2OH− + 2H+→2H2O
3.3. Optimization of Deposition Conditions
In electrophoretic deposition, the deposition time is an important parameter. Therefore, the effect of the Co3O4/rGO composites with different deposition times toward H2O2 detection was evaluated by CV in the absence and presence of 1 mM H2O2 under otherwise identical conditions (Figure 5c). A higher reduction peak current density was observed at the interfaces after deposition for 2 min (Co:graphene ratio = 2:1 (w/w)) compared to the interface produced with a deposition time of 1 min, indicating its superior electrocatalytic effect. Although the material deposited in 3 min showed a comparable cathodic peak with a film as that observed at 2 min, the background current decreased. These results well matched the SEM observations, in which 2 min deposition exhibited an appropriate density of Co3O4 particles without agglomeration. Furthermore, since Co3O4 particles play an important role in the catalytic reactions, the effect of different ratios of Co(NO3)2·6H2O to graphene (3:1, 2:1, 1:1, 1:2, 1:3, w/w) with a deposition time of 2 min was evaluated (Figure 5d). A higher peak current was observed from the interface modified with a Co(NO3)2·6H2O to graphene ratio of 2:1. Therefore, further electrochemical sensing was carried out on the Co3O4/rGO-modified electrode with a Co2+ to graphene ratio of 2:1 with 2 min deposition.
3.4. Amperometric Detection of H2O2
The amperometric current–time response of the Co3O4/rGO-modified electrode was further investigated with continuous injections of different concentrations of H2O2 into stirred PBS buffer at an applied potential of −0.60 V. As presented in Figure 6a, a staircase response was achieved with increasing concentration of added H2O2. The current density increased sharply and reached a stable value with a quick response within 6 s. Responses to lower additions are zoomed in as the inset in Figure 6a. In addition, the corresponding calibration curve was acquired by plotting the obtained peak current density against the H2O2 concentration in Figure 6b. A good linear relationship was obtained from 0.1 to 19.5 mM, with a regression equation as follows: j (mA cm−2) =0.0293–0.2247[H2O2] (mM), R2 = 0.997. An estimated sensitivity of 0.2247 mA mM−1 cm−2 and a detection limit of 10 µM (signal-to-noise ratio (S/N) = 3) were achieved using the Co3O4/rGO composite-modified electrode. The sensing performance of the proposed electrode showed a comparable wide linear range and high sensitivity to the previously reported H2O2 sensors based on Co or the carbon-related nanostructures listed in Table 1.
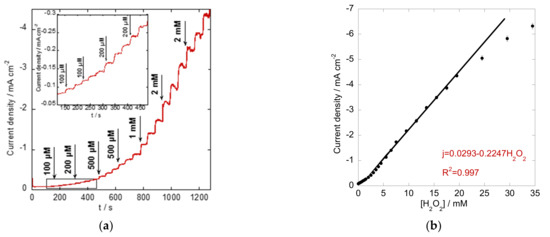
Figure 6.
(a) Amperometric response of the Co3O4/rGO-modified electrode polarized at −0.60 V in N2-saturated 0.1 M PBS solution with the subsequent addition of H2O2 (Inset: responses to lower additions of H2O2); (b) the corresponding calibration curve.

Table 1.
Comparison of the analytical performance of Co3O4/rGO-modified electrodes with previously reported non-enzymatic H2O2 sensors.
3.5. Selectivity, Reproducibility, and Long-Term Stability
The selectivity of the constructed sensor was evaluated by chronoamperometry in the presence of interfering biomolecules. Figure 7a displays the amperometric response of the electrode upon successive additions of 1 mM H2O2, 100 µM ascorbic acid (AA), 100 µM uric acid (UA), 100 µM dopamine (AA), and 500 µM glucose (Glu) in N2-saturated PBS with a biased potential of −0.60 V. No obvious current increase was observed with the injection of the interferences mentioned above, suggesting the outstanding anti-interference ability of the sensor, which is quite important in real sample analysis. Moreover, the reproducibility was examined for four individual Co3O4/rGO-modified electrodes in 0.1 M PBS (pH = 7.4) in the presence of 1 mM H2O2 (Figure 7b). A relative standard deviation (RSD) of 2.69% was found, indicating high reproducibility of the modified electrodes. The long-term stability of the Co3O4/rGO-modified electrode was evaluated by measuring its response to 1 mM H2O2 before and after storage of 15 days. The current retained 95.3% of its initial response after 15 days, suggesting good storage stability of the sensor.

Figure 7.
(a) The amperometric response of the Co3O4/rGO-modified electrode exposed to H2O2 (1 mM), ascorbic acid (AA, 100 µM), uric acid (UA, 100 µM), dopamine (DA, 100 µM), glucose (500 µM), and H2O2 (1 mM) in N2-saturated 0.1 M PBS buffer with an applied potential of −0.60 V; (b) Reproducibility analysis based on four electrodes modified with Co3O4/rGO composites.
3.6. Real Sample Analysis
In order to test the practical applicability of the Co3O4/rGO-modified electrode, the sensor was used for the determination of H2O2 in urine based on the standard addition method. First, 1 mL urine samples were diluted to 10 mL by PBS and spiked with known H2O2 concentrations. The recoveries of the H2O2 in Table 2 were determined by recording the current density at −0.6 V. The recovery value of the spiked H2O2 was in the range of 98–101.5%, indicating feasibility in real sample analysis of the proposed Co3O4/rGO sensor.

Table 2.
Determination of H2O2 in urine samples.
4. Conclusions
In summary, we have demonstrated the easy fabrication of Co3O4 particles/rGO through electrophoretic deposition and subsequent annealing. The electrocatalytic activity of Co3O4/rGO-modified electrodes toward enzyme-free H2O2 sensing was investigated with optimized deposition conditions. The sensor showed a wide linear range from 0.1 to 19.5 mM and a high sensitivity of 0.2247 mA mM−1 cm−2. Moreover, good selectivity, reproducibility, and time stability were achieved for H2O2 detection. The possibility of practical applications was also successfully applied by measuring the H2O2 concentration in human urine samples. Significantly, the synergetic effect of the graphene and cobalt oxide structures facilitated the hybrids, with potential in other electrochemical applications.
Author Contributions
Methodology, investigation, data curation, writing: original draft, fund acquisition, Q.W.; software, validation, Y.W.; resources, formal analysis, G.X.; formal analysis, writing—review & editing, fund acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Q.W. gratefully acknowledges financial support from the National Natural Science Foundation of China (No. 51702187), China Postdoctoral Science Foundation (No. 2017M622193), and the fundamental research funds of Shandong University (No.2017HW003). X.Z. thanks the Key Research and Development Program of Shandong Province (No. 2021CXGC010310).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are presented in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burek, B.; Bormann, S.; Hollmann, F.; Bloh, J.; Holtmann, D. Hydrogen peroxide driven biocatalysis. Green Chem. 2019, 21, 3232–3249. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Hallaj, R.; Soltanian, S.; Mamkhezri, H. Nanomolar detection of hydrogen peroxide on glassy carbon electrode modified with electrodeposited cobalt oxide nanoparticles. Anal. Chim. Acta 2007, 594, 24–31. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.-M.; Barathi, P. Highly stable biomolecule supported by gold nanoparticles/graphene nanocomposite as a sensing platform for H2O2 biosensor application. J. Mater. Chem. B 2016, 4, 6335–6343. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cai, Z.; Gao, Y.; Zhang, H.; Cai, C. Enhancing the electrochemical reduction of hydrogen peroxide based on nitrogen-doped graphene for measurement of its releasing process from living cells. Chem. Commun. 2011, 47, 11327–11329. [Google Scholar] [CrossRef]
- Das, P.; Das, M.; Chinnadayyala, S.R.; Singha, I.M.; Goswami, P. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens. Bioelectron. 2016, 79, 386–397. [Google Scholar] [CrossRef]
- Thatikayala, D.; Ponnamma, D.; Sadasivuni, K.K.; Cabibihan, J.-J.; Al-Ali, A.K.; Malik, R.A.; Min, B. Progress of advanced nanomaterials in the non-enzymatic electrochemical sensing of glucose and H2O2. Biosensors 2020, 10, 151. [Google Scholar] [CrossRef]
- Kogularasu, S.; Govindasamy, M.; Chen, S.-M.; Akilarasan, M.; Mani, V. 3D graphene oxide-cobalt oxide polyhedrons for highly sensitive non-enzymatic electrochemical determination of hydrogen peroxide. Sens. Actuators B Chem. 2017, 253, 773–783. [Google Scholar] [CrossRef]
- Zhang, T.; He, C.; Sun, F.; Ding, Y.; Wang, M.; Peng, L.; Wang, J.; Lin, Y. Co3O4 nanoparticles anchored on nitrogen-doped reduced graphene oxide as a multifunctional catalyst for H2O2 reduction, oxygen reduction and evolution reaction. Sci. Rep. 2017, 7, 43638. [Google Scholar] [CrossRef]
- Hoa, L.T.; Chung, J.S.; Hur, S.H. A highly sensitive enzyme-free glucose sensor based on Co3O4 nanoflowers and 3D graphene oxide hydrogel fabricated via hydrothermal synthesis. Sens. Actuators B Chem. 2016, 223, 76–82. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Chun, D.-M. Fabrication of efficient nanostructured Co3O4-graphene bifunctional catalysts: Oxygen evolution, hydrogen evolution, and H2O2 sensing. Ceram. Int. 2020, 46, 23479–23498. [Google Scholar] [CrossRef]
- Wu, Q.; Sheng, Q.; Zheng, J. Non enzymatic amperometric sensing of hydrogen peroxide using a glassy carbon electrode modified with a sandwich-structured nanocomposite consisting of silver nanoparticles, Co3O4 and reduced graphene oxide. Microchim. Acta 2016, 183, 1943–1951. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, Z.; Zhao, Y.; Wu, Y.; Liu, C.; Wang, X. Highly sensitive electrochemical sensing platform: Carbon cloth enhanced performance of Co3O4/rGO nanocomposite for detection of H2O2. J. Mater. Sci. 2020, 55, 5445–5457. [Google Scholar] [CrossRef]
- Zhang, D.; Zou, W. Decorating reduced graphene oxide with Co3O4 hollow spheres and their application in supercapacitor materials. Curr. Appl. Phys. 2013, 13, 1796–1800. [Google Scholar] [CrossRef]
- Huang, S.; Jin, Y.; Jia, M. Preparation of graphene/Co3O4 composites by hydrothermal method and their electrochemical properties. Electrochim. Acta 2013, 95, 139–145. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Hao, Y.; Yang, X.; Cao, B. Oxygen-vacancy abundant ultrafine Co3O4/graphene composites for high-rate supercapacitor electrodes. Adv. Sci. 2018, 5, 1700659. [Google Scholar] [CrossRef]
- Dong, X.C.; Xu, H.; Wang, X.W.; Huang, Y.X.; Chan Park, M.B.; Zhang, H.; Wang, L.H.; Huang, W.; Chen, P. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- Sun, H.; Sun, X.; Hu, T.; Yu, M.; Lu, F.; Lian, J. Graphene-wrapped mesoporous cobalt oxide hollow spheres anode for high-rate and long-life lithium ion batteries. J. Phys. Chem. C 2014, 118, 2263–2272. [Google Scholar] [CrossRef]
- Shen, K.; Zhai, S.; Wang, S.; Ru, Q.; Hou, X.; San Hui, K.; Nam Hui, K.; Chen, F. Recent progress in binder-free electrodes synthesis for electrochemical energy storage application. Batter. Supercaps 2021, 4, 860–880. [Google Scholar] [CrossRef]
- Daryakenari, A.A.; Mosallanejad, B.; Zare, E.; Ahmadidaryakenari, M.; Delaunay, J.J. Highly efficient electrocatalysts fabricated via electrophoretic deposition for alcohol oxidation, oxygen reduction, hydrogen evolution, and oxygen evolution reactions. Int. J. Hydrog. Energy 2020, 46, 7263–7283. [Google Scholar] [CrossRef]
- Oakes, L.; Westover, A.; Mahjouri-Samani, M.; Chatterjee, S.; Puretzky, A.A.; Rouleau, C.; Geohegan, D.B.; Pint, C.L. Uniform, homogenous coatings of carbon nanohorns on arbitrary substrates from common solvents. ACS Appl. Mater. Interfaces 2013, 5, 13153–13160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhou, X.; Cao, H.; Wang, G.; Liu, Z. Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material. J. Power Sources 2014, 258, 290–296. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Li, M.; Szunerits, S.; Boukherroub, R. Preparation of reduced graphene oxide/Cu nanoparticle composites through electrophoretic deposition: Application for nonenzymatic glucose sensing. RSC Adv. 2015, 5, 15861–15869. [Google Scholar] [CrossRef]
- Wu, X.; Wang, B.; Li, S.; Liu, J.; Yu, M. Electrophoretic deposition of hierarchical Co3O4@ graphene hybrid films as binder-free anodes for high-performance lithium-ion batteries. RSC Adv. 2015, 5, 33438–33444. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Jiang, X.; Yang, N.; Coffinier, Y.; Belkhalfa, H.; Dokhane, N.; Li, M.; Boukherroub, R.; Szunerits, S. Electrophoretic deposition of carbon nanofibers/Co(OH)2 nanocomposites: Application for non-enzymatic glucose sensing. Electroanalysis 2016, 28, 119–125. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, B.; Du, J.; Liu, G.; Guo, Y.; Xiao, D. Electrophoresis deposition of Ag nanoparticles on TiO2 nanotube arrays electrode for hydrogen peroxide sensing. Talanta 2013, 112, 129–135. [Google Scholar] [CrossRef]
- Redmond, P.L.; Hallock, A.J.; Brus, L.E. Electrochemical ostwald ripening of colloidal ag particles on conductive substrates. Nano Lett. 2005, 5, 131–135. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, X.; Wang, X. Microwave-assisted synthesis of spheroidal vaterite CaCO3 in ethylene glycol–water mixed solvents without surfactants. J. Cryst. Growth 2010, 312, 3191–3197. [Google Scholar] [CrossRef]
- Choi, B.G.; Chang, S.-J.; Lee, Y.B.; Bae, J.S.; Kim, H.J.; Huh, Y.S. 3D heterostructured architectures of Co3O4 nanoparticles deposited on porous graphene surfaces for high performance of lithium ion batteries. Nanoscale 2012, 4, 5924–5930. [Google Scholar] [CrossRef]
- Liu, M.; He, S.; Chen, W. Co3O4 nanowires supported on 3D N-doped carbon foam as an electrochemical sensing platform for efficient H2O2 detection. Nanoscale 2014, 6, 11769–11776. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Qiao, W.; Shao, B.; Zhao, Q.; Zhang, L.; Fan, Z. Rapid microwave-assisted synthesis of graphene nanosheet/Co3O4 composite for supercapacitors. Electrochim. Acta 2010, 55, 6973–6978. [Google Scholar] [CrossRef]
- Gao, P.; Zeng, Y.; Tang, P.; Wang, Z.; Yang, J.; Hu, A.; Liu, J. Understanding the synergistic effects and structural evolution of Co(OH)2 and Co3O4 toward boosting electrochemical charge storage. Adv. Funct. Mater. 2022, 32, 2108644. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Jamil, M.F.; Biçer, E.; Kaplan, B.Y.; Gürsel, S.A. One-step fabrication of new generation graphene-based electrodes for polymer electrolyte membrane fuel cells by a novel electrophoretic deposition. Int. J. Hydrog. Energy 2021, 46, 5653–5663. [Google Scholar] [CrossRef]
- Pourzare, K.; Farhadi, S.; Mansourpanah, Y. Graphene oxide/Co3O4 nanocomposite: Synthesis, characterization, and its adsorption capacity for removal of organic dye pollutants from water. Acta Chim. Slov. 2017, 64, 945–958. [Google Scholar] [CrossRef]
- Abarca, G.; Ríos, P.L.; Povea, P.; Cerda-Cavieres, C.; Morales-Verdejo, C.; Arroyo, J.L.; Camarada, M.B. Nanohybrids of reduced graphene oxide and cobalt hydroxide (Co(OH)2|rGO) for the thermal decomposition of ammonium perchlorate. RSC Adv. 2020, 10, 23165–23172. [Google Scholar] [CrossRef]
- Xiong, S.; Yuan, C.; Zhang, X.; Xi, B.; Qian, Y. Controllable synthesis of mesoporous Co3O4 nanostructures with tunable morphology for application in supercapacitors. Chem. A Eur. J. 2009, 15, 5320–5326. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Zeng, J.; Xiong, J.; Zhao, J. Direct electrophoretic deposition of binder-free Co3O4/graphene sandwich-like hybrid electrode as remarkable lithium ion battery anode. ACS Appl. Mater. Interfaces 2017, 9, 32801–32811. [Google Scholar] [CrossRef]
- Yang, J.; Gunasekaran, S. A low-potential, H2O2-assisted electrodeposition of cobalt oxide/hydroxide nanostructures onto vertically-aligned multi-walled carbon nanotube arrays for glucose sensing. Electrochim. Acta 2011, 56, 5538–5544. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Thirumalraj, B.; Chen, S.-M.; Karuppiah, C. Electrochemical co-preparation of cobalt sulfide/reduced graphene oxide composite for electrocatalytic activity and determination of H2O2 in biological samples. J. Colloid Interface Sci. 2018, 509, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.; Thi, M.; Son, N.; Bui, Q.; Nhac-Vu, H.-T.; Ai-Le, P. Mesoporous gold nanoparticles supported cobalt nanorods as a free-standing electrochemical sensor for sensitive hydrogen peroxide detection. J. Electroanal. Chem. 2019, 848, 113359. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.P.; Zhou, Z.; Li, Q.; Huo, L.H.; Zhao, H. Efficient nonenzymatic H2O2 biosensor based on zif-67 MOF derived co nanoparticles embedded n-doped mesoporous carbon composites. Sens. Actuators B Chem. 2018, 276, 142–149. [Google Scholar] [CrossRef]
- Ullah, R.; Rasheed, M.A.; Abbas, S.; Rehman, K.-u.; Shah, A.; Ullah, K.; Khan, Y.; Bibi, M.; Ahmad, M.; Ali, G. Electrochemical sensing of H2O2 using cobalt oxide modified TiO2 nanotubes. Curr. Appl. Phys. 2022, 38, 40–48. [Google Scholar] [CrossRef]
- Liu, D.; Chen, T.; Zhu, W.; Cui, L.; Asiri, A.M.; Lu, Q.; Sun, X. Cobalt phosphide nanowires: An efficient electrocatalyst for enzymeless hydrogen peroxide detection. Nanotechnology 2016, 27, 33LT01. [Google Scholar] [CrossRef]
- Lee, K.K.; Loh, P.Y.; Sow, C.H.; Chin, W.S. CoOOH nanosheet electrodes: Simple fabrication for sensitive electrochemical sensing of hydrogen peroxide and hydrazine. Biosens. Bioelectron. 2013, 39, 255–260. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Novel porous gold-palladium nanoalloy network-supported graphene as an advanced catalyst for non-enzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 2016, 85, 669–678. [Google Scholar] [CrossRef]
- Han, L.; Yang, D.-P.; Liu, A. Leaf-templated synthesis of 3D hierarchical porous cobalt oxide nanostructure as direct electrochemical biosensing interface with enhanced electrocatalysis. Biosens. Bioelectron. 2015, 63, 145–152. [Google Scholar] [CrossRef]
- Han, L.; Wang, Q.; Tricard, S.; Liu, J.; Fang, J.; Zhao, J.; Shen, W. Amperometric detection of hydrogen peroxide utilizing synergistic action of cobalt hexacyanoferrate and carbon nanotubes chemically modified with platinum nanoparticles. RSC Adv. 2013, 3, 281–287. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Li, M.; Szunerits, S.; Boukherroub, R. One-step synthesis of Au nanoparticle–graphene composites using tyrosine: Electrocatalytic and catalytic properties. New J. Chem. 2016, 40, 5473–5482. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).