A Randomized Clinical Trial Comparing Implants Placed in Two Different Biomaterials Used for Maxillary Sinus Augmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment Procedures
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
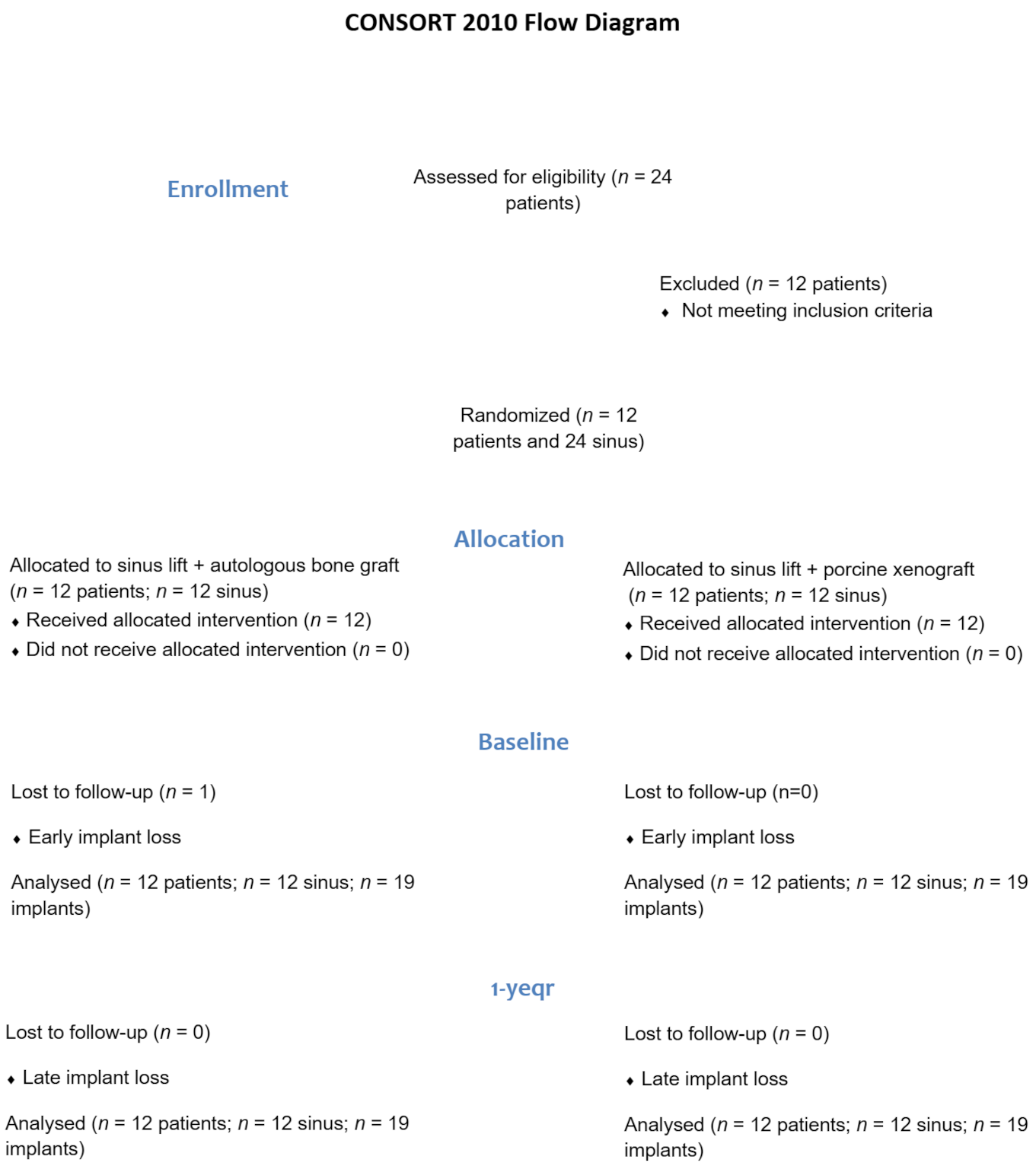
3.1. Patient and Intervention Characteristics
3.2. Implant Survival
3.3. Survival of the Prosthesis and Hardware Complications
3.4. Periimplant Soft Tissue Condition and Biological Complication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RCT | randomised clinical trial |
| CI | confidence interval |
| SD | standard deviation |
| M | mesial |
| D | distal |
| A | autologous bone graft |
| X | porcine xenograft |
| t0 | prosthesis delivery |
| t1 | one year follow-up |
| mm | millimetres |
| DBB | demineralized bovine bone |
| PRP | platelet-rich plasma |
References
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Donos, N.; Alcoforado, G.; Balmer, M.; Gurzawska, K.; Mardas, N.; Milinkovic, I.; Nisand, D.; Rocchietta, I.; Stavropoulos, A.; et al. Therapeutic concepts and methods for improving dental implant outcomes. Summary and consensus statements. The 4th EAO Consensus Conference 2015. Clin. Oral Implants Res. 2015, 26 (Suppl. S11), 202–206. [Google Scholar] [CrossRef] [PubMed]
- Rapani, M.; Rapani, C.; Ricci, L. Schneider membrane thickness classification evaluated by cone-beam computed tomography and its importance in the predictability of perforation. Retrospective analysis of 200 patients. Br. J. Oral Maxillofac. Surg. 2016, 54, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Seong, W.J.; Barczak, M.; Jung, J.; Basu, S.; Olin, P.S.; Conrad, H.J. Prevalence of sinus augmentation associated with maxillary posterior implants. J. Oral Implantol. 2013, 39, 680–688. [Google Scholar] [CrossRef]
- Stacchi, C.; Spinato, S.; Lombardi, T.; Bernardello, F.; Bertoldi, C.; Zaffe, D.; Nevins, M. Minimally Invasive Management of Implant-Supported Rehabilitation in the Posterior Maxilla, Part, I. Sinus Floor Elevation: Biologic Principles and Materials. Int. J. Periodontics Restor. Dent. 2020, 40, e85–e93. [Google Scholar] [CrossRef]
- Salgar, N. Osseodensified Crestal Sinus Window Augmentation: An Alternative Procedure to the Lateral Window Technique. J. Oral Implantol. 2021, 47, 45–55. [Google Scholar] [CrossRef]
- Cruz, R.S.; Lemos, C.A.A.; Batista, V.E.S.; Oliveira, H.; Gomes, J.M.L.; Pellizzer, E.P.; Verri, F.R. Short implants versus longer implants with maxillary sinus lift. A systematic review and meta-analysis. Braz. Oral Res. 2018, 32, e86. [Google Scholar] [CrossRef]
- Correia, F.; Gouveia, S.; Pozza, D.H.; Felino, A.C.; Faria-Almeida, R. Lateral window technique: A focus review. Oral Surg. 2021, 15, 421–430. [Google Scholar] [CrossRef]
- Yin, L.; Yu, Z.; Chen, Z.; Huang, B.; Zhang, K.; Zhou, A.; Li, X. Analysis of Bone Height Changes after Maxillary Sinus Augmentation with Simultaneous and Delayed Placement of Dental Implants: A Clinical and Radiographic Study. J. Prosthodont. 2016, 25, 440–445. [Google Scholar] [CrossRef]
- Van den Bergh, J.P.; ten Bruggenkate, C.M.; Disch, F.J.; Tuinzing, D.B. Anatomical aspects of sinus floor elevations. Clin. Oral Implant. Res. 2000, 11, 256–265. [Google Scholar] [CrossRef]
- Delilbasi, C.; Gurler, G. Comparison of piezosurgery and conventional rotative instruments in direct sinus lifting. Implant. Dent. 2013, 22, 662–665. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implant. Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Valente, N.A.; Iezzi, G.; Petrini, M.; Derchi, G.; Barone, A. Maxillary sinus augmentation with three different biomaterials: Histological, histomorphometric, clinical, and patient-reported outcomes from a randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 86–95. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; de Buitrago, J.G.; Padial-Molina, M.; Fernandez-Barbero, J.E.; Ata-Ali, J.; O’Valle, F. Histopathological comparison of healing after maxillary sinus augmentation using xenograft mixed with autogenous bone versus allograft mixed with autogenous bone. Clin. Oral Implant. Res. 2018, 29, 192–201. [Google Scholar] [CrossRef]
- Baena, R.R.Y.; Pastorino, R.; Gherlone, E.F.; Perillo, L.; Lupi, S.M.; Lucchese, A. Histomorphometric Evaluation of Two Different Bone Substitutes in Sinus Augmentation Procedures: A Randomized Controlled Trial in Humans. Int. J. Oral Maxillofac. Implant. 2017, 32, 188–194. [Google Scholar] [CrossRef]
- Kolerman, R.; Nissan, J.; Rahmanov, M.; Vered, H.; Cohen, O.; Tal, H. Comparison between mineralized cancellous bone allograft and an alloplast material for sinus augmentation: A split mouth histomorphometric study. Clin. Implant Dent. Relat. Res. 2017, 19, 812–820. [Google Scholar] [CrossRef]
- Monje, A.; O'Valle, F.; Monje-Gil, F.; Ortega-Oller, I.; Mesa, F.; Wang, H.L.; Galindo-Moreno, P. Cellular, Vascular, and Histomorphometric Outcomes of Solvent-Dehydrated vs Freeze-Dried Allogeneic Graft for Maxillary Sinus Augmentation: A Randomized Case Series. Int. J. Oral Maxillofac. Implant. 2017, 32, 121–127. [Google Scholar] [CrossRef]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.; Faria e Almeida, R. The applications of regenerative medicine in sinus lift procedures: A systematic review. Clin. Implant. Dent. Relat. Res. 2018, 20, 229–242. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Husler, J.; Hammerle, C.H.; Jung, R.E. EAO Supplement Working Group 4—EAO CC 2015 Short implants versus sinus lifting with longer implants to restore the posterior maxilla: A systematic review. Clin. Oral Implant. Res. 2015, 26 (Suppl. S11), 154–169. [Google Scholar] [CrossRef]
- Al-Moraissi, E.; Elsharkawy, A.; Abotaleb, B.; Alkebsi, K.; Al-Motwakel, H. Does intraoperative perforation of Schneiderian membrane during sinus lift surgery causes an increased the risk of implants failure?: A systematic review and meta regression analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 882–889. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, e1000251. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.C.; Faria-Almeida, R. Advantages of Porcine Xenograft over Autograft in Sinus Lift: A Randomised Clinical Trial. Materials 2021, 14, 3439. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, M.; Martegani, P.; D'Avenia, F.; Farneti, M.; Capri, D.; Paolantoni, G.; Landi, L. Simultaneous sinus augmentation with implant placement: Histomorphometric comparison of two different grafting materials. A multicenter double-blind prospective randomized controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2013, 28, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Pagliani, L.; Andersson, P.; Lanza, M.; Nappo, A.; Verrocchi, D.; Volpe, S.; Sennerby, L. A collagenated porcine bone substitute for augmentation at Neoss implant sites: A prospective 1-year multicenter case series study with histology. Clin. Implant Dent. Relat. Res. 2012, 14, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Barausse, C.; Barone, A.; Zucchelli, G.; Piattelli, M.; Pistilli, R.; Ippolito, D.R.; Simion, M. Interpositional Augmentation Technique in the Treatment of Posterior Mandibular Atrophies: A Retrospective Study Comparing 129 Autogenous and Heterologous Bone Blocks with 2 to 7 Years Follow-Up. Int. J. Periodontics Restor. Dent. 2017, 37, 469–480. [Google Scholar] [CrossRef]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef]
- Esposito, M.; Barausse, C.; Pistilli, R.; Sammartino, G.; Grandi, G.; Felice, P. Short implants versus bone augmentation for placing longer implants in atrophic maxillae: One-year post-loading results of a pilot randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 257–268. [Google Scholar]
- Al-Dajani, M. Incidence, Risk Factors, and Complications of Schneiderian Membrane Perforation in Sinus Lift Surgery: A Meta-Analysis. Implant Dent. 2016, 25, 409–415. [Google Scholar] [CrossRef]
- Danesh-Sani, S.A.; Loomer, P.M.; Wallace, S.S. A comprehensive clinical review of maxillary sinus floor elevation: Anatomy, techniques, biomaterials and complications. Br. J. Oral Maxillofac. Surg. 2016, 54, 724–730. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Mordenfeld, A.; Becktor, J.P.; Jensen, S.S. Maxillary Sinus Floor Augmentation With Synthetic Bone Substitutes Compared With Other Grafting Materials: A Systematic Review and Meta-analysis. Implant Dent 2018, 27, 363–374. [Google Scholar] [CrossRef]
- Bennardo, F.; Barone, S.; Buffone, C.; Colangeli, W.; Antonelli, A.; Giudice, A. Removal of dental implants displaced into the maxillary sinus: A retrospective single-center study. Head Face Med. 2022, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Meloni, S.M.; Jovanovic, S.A.; Lolli, F.M.; Cassisa, C.; De Riu, G.; Pisano, M.; Lumbau, A.; Luglie, P.F.; Tullio, A. Grafting after sinus lift with anorganic bovine bone alone compared with 50:50 anorganic bovine bone and autologous bone: Results of a pilot randomised trial at one year. Br. J. Oral Maxillofac. Surg. 2015, 53, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.K.; Shaik, M.; Nadella, K.R.; Chintapalli, B.M. Comparative study of alveolar bone height and implant survival rate between autogenous bone mixed with platelet rich plasma versus venous blood for maxillary sinus lift augmentation procedure. J. Maxillofac. Oral Surg. 2015, 14, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Sierra-Baztan, A.; Jimenez-Guerra, A.; Espana-Lopez, A.; Ortiz-Garcia, I.; Nunez-Marquez, E.; Moreno-Munoz, J.; Rondon-Romero, J.L.; Lopez-Lopez, J.; Monsalve-Guil, L. Long-Term Clinical Study of Implants Placed in Maxillary Sinus Floor Augmentation Using Beta-Tricalcium Phosphate. Int. J. Environ. Res. Public Health 2021, 18, 9975. [Google Scholar] [CrossRef]
- Rickert, D.; Vissink, A.; Slot, W.J.; Sauerbier, S.; Meijer, H.J.; Raghoebar, G.M. Maxillary sinus floor elevation surgery with BioOss(R) mixed with a bone marrow concentrate or autogenous bone: Test of principle on implant survival and clinical performance. Int. J. Oral Maxillofac. Surg. 2014, 43, 243–247. [Google Scholar] [CrossRef]
- Tomasi, C.; Derks, J. Etiology, occurrence, and consequences of implant loss. Periodontol. 2000 2022, 88, 13–35. [Google Scholar] [CrossRef]
- Sousa, V.; Mardas, N.; Farias, B.; Petrie, A.; Needleman, I.; Spratt, D.; Donos, N. A systematic review of implant outcomes in treated periodontitis patients. Clin. Oral Implant. Res. 2016, 27, 787–844. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Smoking and the risk of peri-implantitis. A systematic review and meta-analysis. Clin. Oral Implant. Res. 2015, 26, e62–e67. [Google Scholar] [CrossRef]
- Sayardoust, S.; Grondahl, K.; Johansson, E.; Thomsen, P.; Slotte, C. Implant survival and marginal bone loss at turned and oxidized implants in periodontitis-susceptible smokers and never-smokers: A retrospective, clinical, radiographic case-control study. J. Periodontol. 2013, 84, 1775–1782. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef]
- Correia, F.; Gouveia, S.; Felino, A.C.; Costa, A.L.; Almeida, R.F. Survival Rate of Dental Implants in Patients with History of Periodontal Disease: A Retrospective Cohort Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 927–934. [Google Scholar] [CrossRef]
- Stacchi, C.; Troiano, G.; Montaruli, G.; Mozzati, M.; Lamazza, L.; Antonelli, A.; Giudice, A.; Lombardi, T. Changes in implant stability using different site preparation techniques: Osseodensification drills versus piezoelectric surgery. A multi-center prospective randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Lindgren, C.; Mordenfeld, A.; Johansson, C.B.; Hallman, M. A 3-year clinical follow-up of implants placed in two different biomaterials used for sinus augmentation. Int. J. Oral Maxillofac. Implant. 2012, 27, 1151–1162. [Google Scholar]
- Pohl, V.; Thoma, D.S.; Sporniak-Tutak, K.; Garcia-Garcia, A.; Taylor, T.D.; Haas, R.; Hammerle, C.H. Short dental implants (6 mm) versus long dental implants (11–15 mm) in combination with sinus floor elevation procedures: 3-year results from a multicentre, randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 438–445. [Google Scholar] [CrossRef]
- Felice, P.; Pistilli, R.; Piattelli, M.; Soardi, E.; Barausse, C.; Esposito, M. 1-stage versus 2-stage lateral sinus lift procedures: 1-year post-loading results of a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 65–75. [Google Scholar]
- Mordenfeld, A.; Lindgren, C.; Hallman, M. Sinus Floor Augmentation Using Straumann(R) BoneCeramic and Bio-Oss(R) in a Split Mouth Design and Later Placement of Implants: A 5-Year Report from a Longitudinal Study. Clin. Implant Dent. Relat. Res. 2016, 18, 926–936. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Abril-Garcia, D.; Carrillo-Galvez, A.B.; Zurita, F.; Martin-Morales, N.; O'Valle, F.; Padial-Molina, M. Maxillary sinus floor augmentation comparing bovine versus porcine bone xenografts mixed with autogenous bone graft. A split-mouth randomized controlled trial. Clin. Oral Implant. Res. 2022, 33, 524–536. [Google Scholar] [CrossRef]
- Younes, F.; Eghbali, A.; Goemaere, T.; De Bruyckere, T.; Cosyn, J. Patient-Reported Outcomes After Lateral Wall Sinus Floor Elevation: A Systematic Review. Implant Dent. 2018, 27, 236–245. [Google Scholar] [CrossRef]
- Meloni, S.M.; Jovanovic, S.A.; Pisano, M.; Xhanari, E.; De Riu, G.; Tullio, A.; Tallarico, M. Sinus lift grafting with anorganic bovine bone vs 50% autologous bone mixed with 50% anorganic bovine bone: 2 years after loading results from a randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 425–432. [Google Scholar]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Bruxism and dental implant failures: A multilevel mixed effects parametric survival analysis approach. J. Oral Rehabil. 2016, 43, 813–823. [Google Scholar] [CrossRef]
- Esposito, M.; Zucchelli, G.; Barausse, C.; Pistilli, R.; Trullenque-Eriksson, A.; Felice, P. Four mm-long versus longer implants in augmented bone in atrophic posterior jaws: 4-month post-loading results from a multicentre randomised controlled trial. Eur. J. Oral Implantol. 2016, 9, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Cannizzaro, G.; Barausse, C.; Cosci, F.; Soardi, E.; Felice, P. Cosci versus Summers technique for crestal sinus lift: 3-year results from a randomised controlled trial. Eur. J. Oral Implantol. 2014, 7, 129–137. [Google Scholar] [PubMed]
- Esposito, M.; Piattelli, M.; Pistilli, R.; Pellegrino, G.; Felice, P. Sinus lift with guided bone regeneration or anorganic bovine bone: 1-year post-loading results of a pilot randomised clinical trial. Eur. J. Oral Implantol. 2010, 3, 297–305. [Google Scholar] [PubMed]
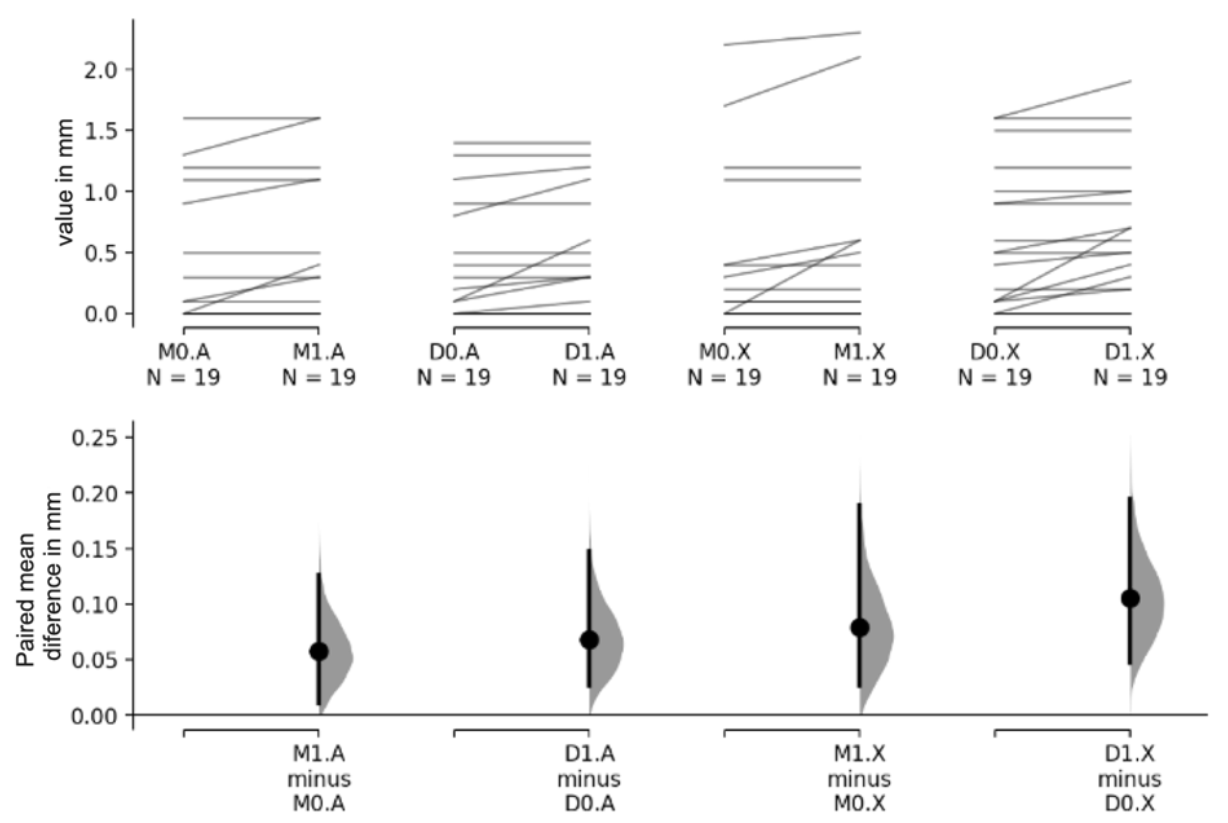
| (M + D)0 | (M + D)1 | (M + D)1 − (M + D)0 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI Lower | 95% CI Upper | Mean | SD | 95% CI Lower | 95% CI Upper | Mean | SD | 95% CI Lower | 95% CI Upper | p-Value | ||
| Material | A | 0.374 | 0.510 | 0.437 | 0.532 | 0.063 | 0.126 | 0.022 | 0.105 | 0.004 | ||||
| X | 0.497 | 0.614 | 0.589 | 0.640 | 0.092 | 0.163 | 0.038 | 0.146 | 0.001 | |||||
| Total | 0.436 | 0.564 | 0.513 | 0.590 | 0.078 | 0.146 | ||||||||
| Difference (X − A) | 0.124 | −0.382 | 0.134 | 0.153 | −0.422 | 0.117 | 0.029 | −0.096 | 0.038 | |||||
| p-value | 0.343 | 0.262 | 0.390 | |||||||||||
| M0 | M1 | M1 − M0 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI Lower | 95% CI Upper | Mean | SD | 95% CI Lower | 95% CI Upper | Mean | SD | 95% CI Lower | 95% CI Upper | p-Value | ||
| Material | A | 0.374 | 0.549 | 0.432 | 0.579 | 0.058 | 0.122 | 0.011 | 0.126 | 0.129 | ||||
| X | 0.405 | 0.655 | 0.484 | 0.710 | 0.079 | 0.165 | 0.026 | 0.189 | <0.001 | |||||
| Total | 0.389 | 0.596 | 0.458 | 0.640 | 0.068 | 0.144 | ||||||||
| Difference (X − A) | 0.032 | −0.316 | 0.421 | 0.053 | −0.316 | 0.468 | 0.021 | −0.063 | 0.117 | |||||
| p-value | 0.868 | 0.804 | 0.625 | |||||||||||
| D0 | D1 | D1 − D0 | ||||||||||||
| Mean | SD | 95% CI Lower | 95% CI Upper | Mean | SD | 95% IC Lower | 95% IC Upper | Mean | SD | 95% CI Lower | 95% CI Upper | p-Value | ||
| Material | A | 0.374 | 0.484 | 0.442 | 0.497 | 0.068 | 0.134 | 0.026 | 0.147 | <0.001 | ||||
| X | 0.589 | 0.573 | 0.695 | 0.561 | 0.105 | 0.165 | 0.047 | 0.195 | 0.008 | |||||
| Total | 0.482 | 0.535 | 0.568 | 0.538 | 0.087 | 0.149 | ||||||||
| Difference (X − A) | 0.216 | −0.105 | 0.547 | 0.253 | −0.073 | 0.574 | 0.037 | −0.058 | 0.132 | |||||
| p-value | 0.222 | 0.150 | 0.503 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, F.; Gouveia, S.A.; Pozza, D.H.; Felino, A.C.; Faria-Almeida, R. A Randomized Clinical Trial Comparing Implants Placed in Two Different Biomaterials Used for Maxillary Sinus Augmentation. Materials 2023, 16, 1220. https://doi.org/10.3390/ma16031220
Correia F, Gouveia SA, Pozza DH, Felino AC, Faria-Almeida R. A Randomized Clinical Trial Comparing Implants Placed in Two Different Biomaterials Used for Maxillary Sinus Augmentation. Materials. 2023; 16(3):1220. https://doi.org/10.3390/ma16031220
Chicago/Turabian StyleCorreia, Francisco, Sónia Alexandre Gouveia, Daniel Humberto Pozza, António Campos Felino, and Ricardo Faria-Almeida. 2023. "A Randomized Clinical Trial Comparing Implants Placed in Two Different Biomaterials Used for Maxillary Sinus Augmentation" Materials 16, no. 3: 1220. https://doi.org/10.3390/ma16031220
APA StyleCorreia, F., Gouveia, S. A., Pozza, D. H., Felino, A. C., & Faria-Almeida, R. (2023). A Randomized Clinical Trial Comparing Implants Placed in Two Different Biomaterials Used for Maxillary Sinus Augmentation. Materials, 16(3), 1220. https://doi.org/10.3390/ma16031220







