Effect of Adding Gadolinium Oxide Promoter on Nickel Catalyst over Yttrium-Zirconium Oxide Support for Dry Reforming of Methane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Catalysts
2.3. Catalyst Activity
2.4. Catalyst Characterization
3. Results and Discussion
3.1. Nitrogen Physisorption Analysis
3.2. Hydrogen Temperature-Programmed Reduction (H2-TPR)
3.3. Carbon Dioxide Temperature-Programmed Desorption (CO2-TPD)
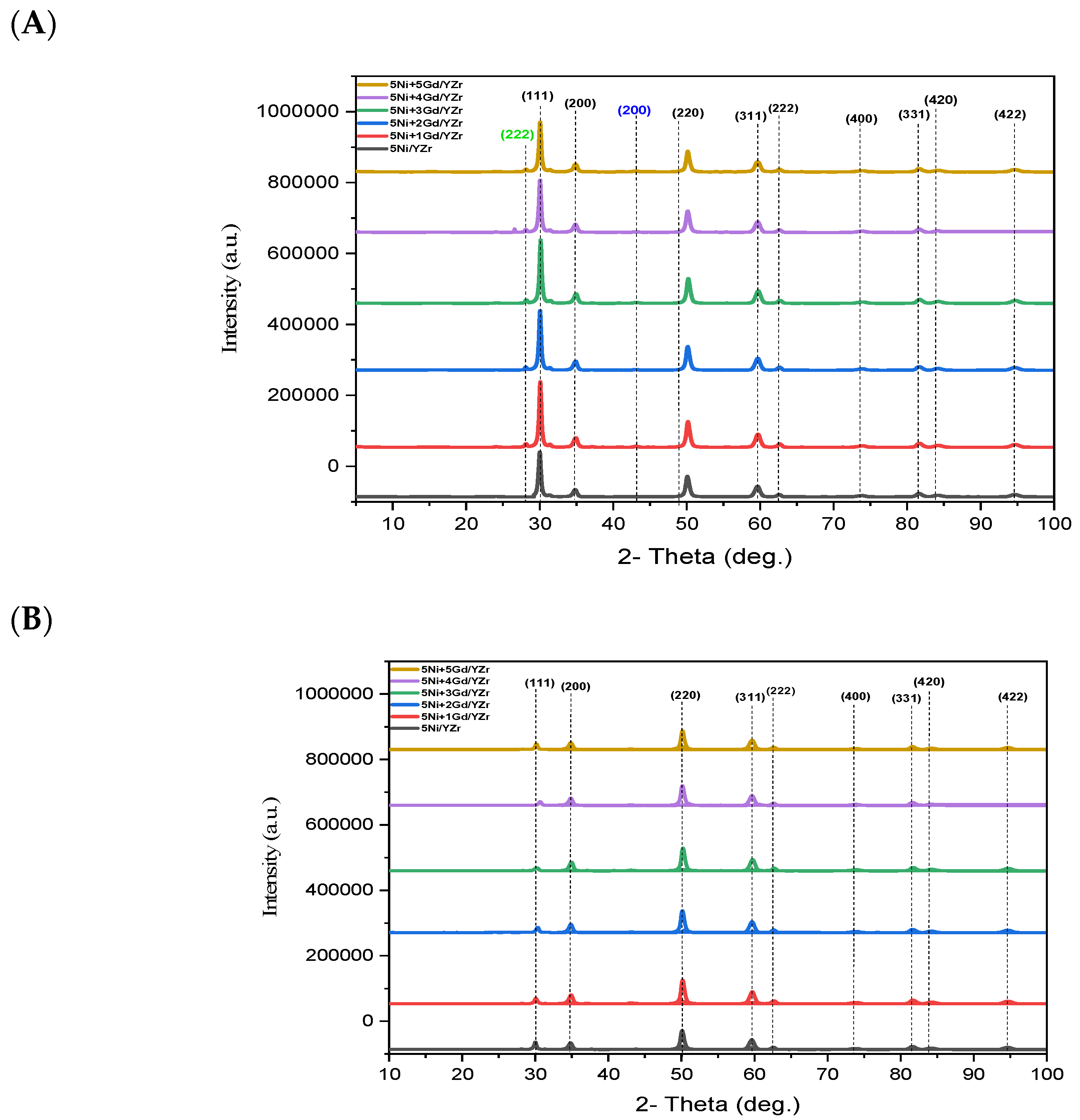
3.4. XRD Analysis
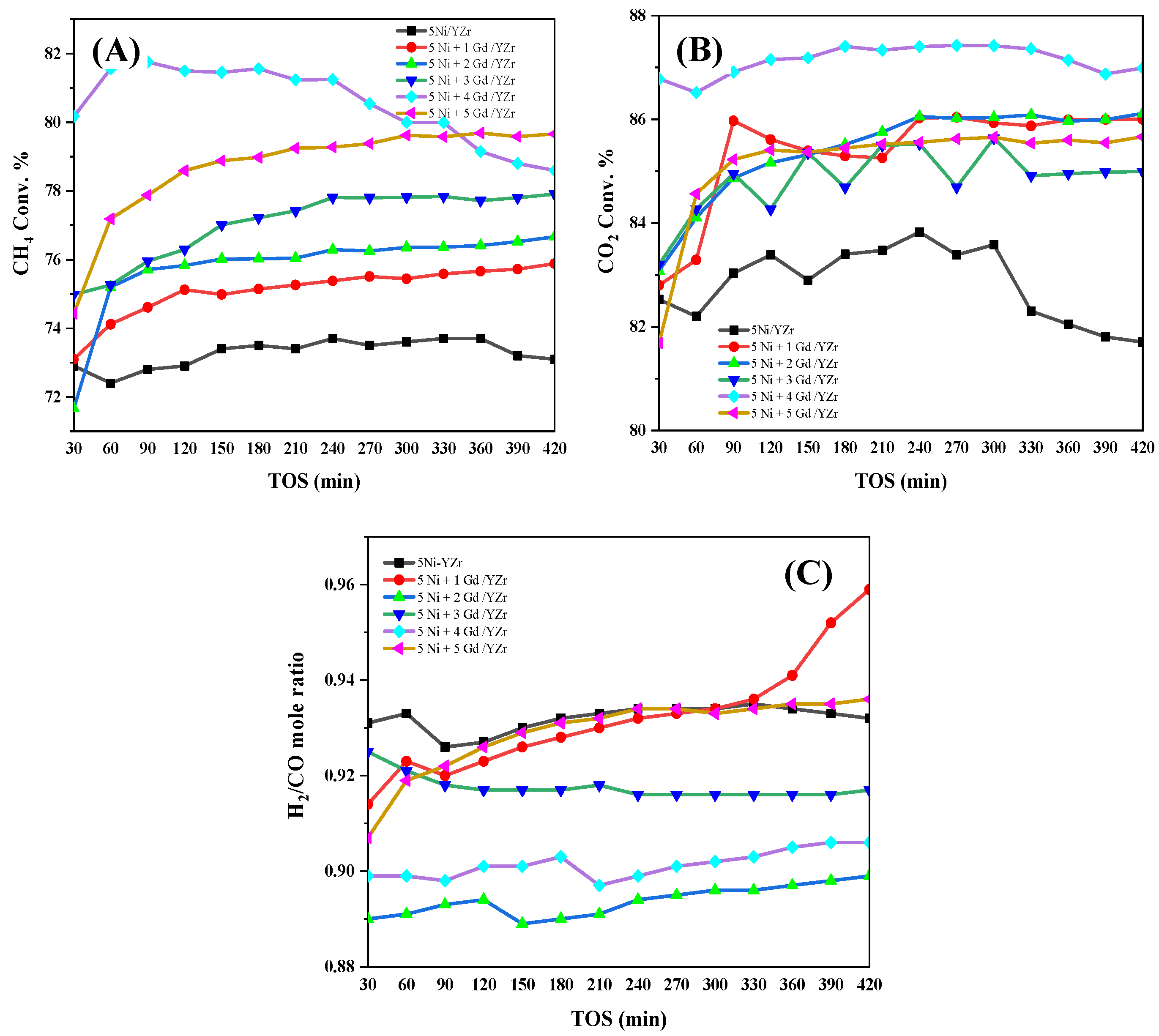
3.5. Catalytic Activity
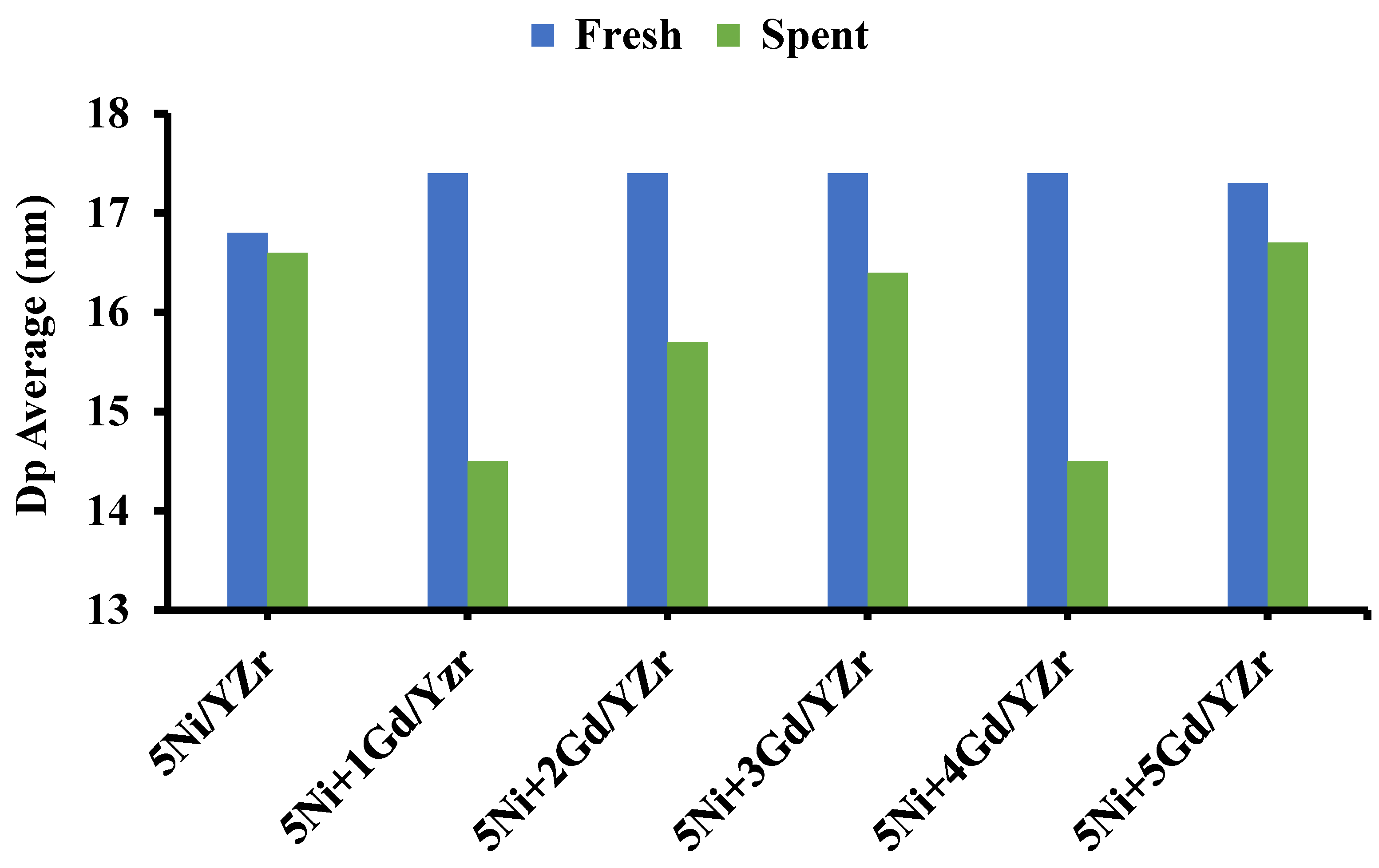
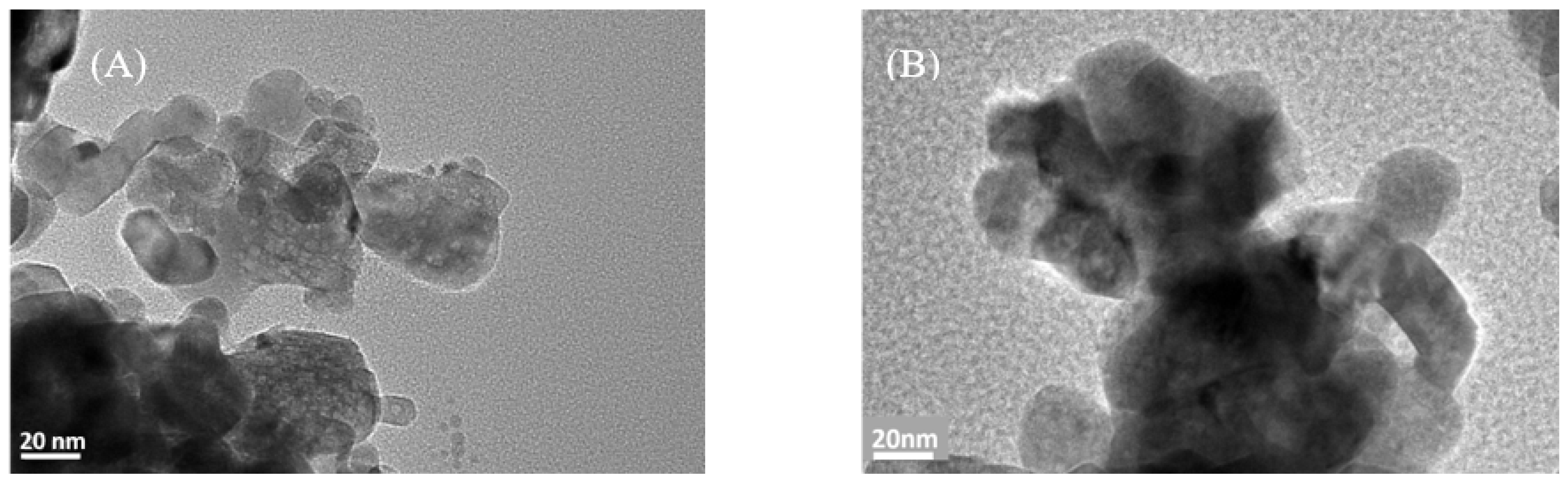
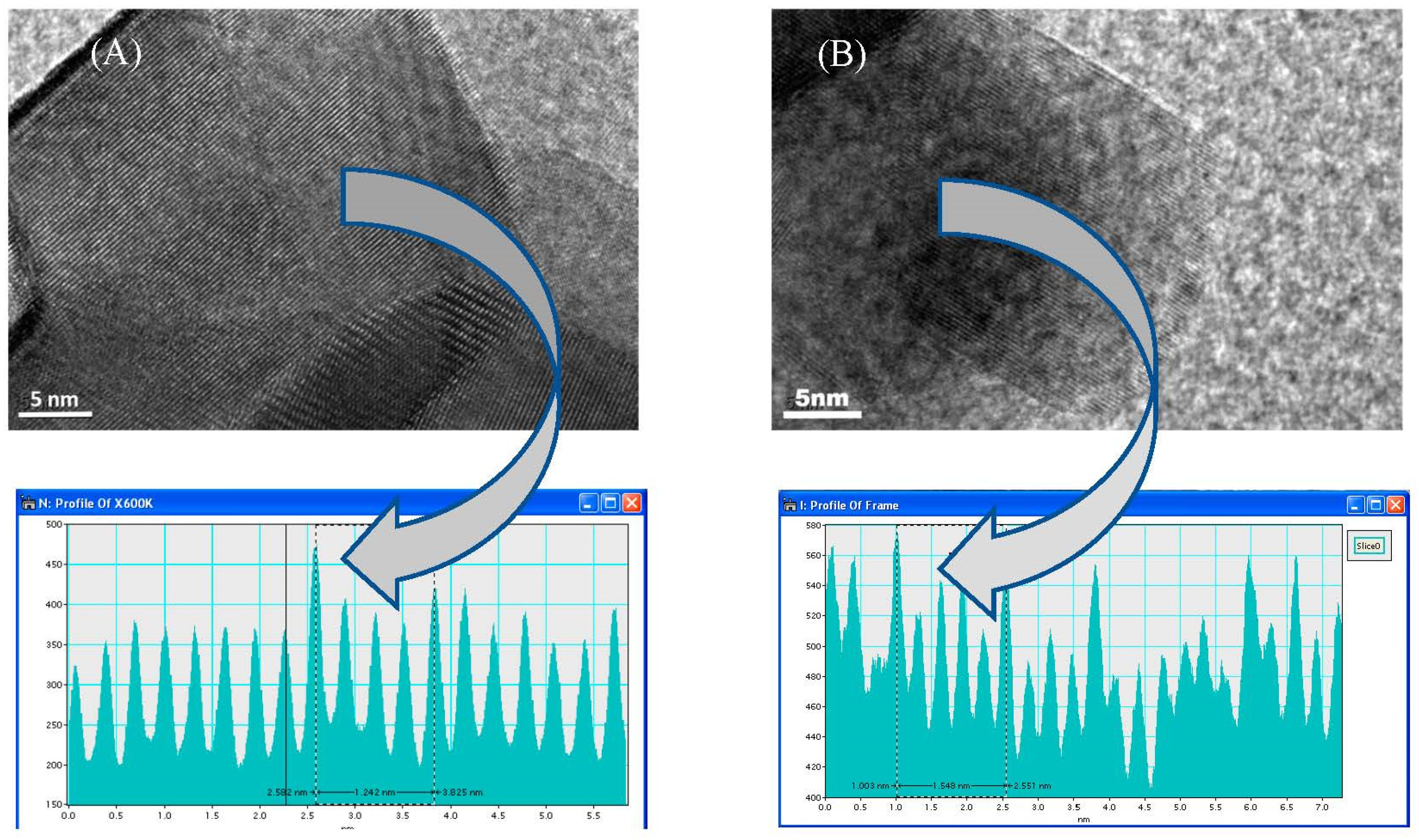
3.6. Transmission Electron Microscope (TEM)
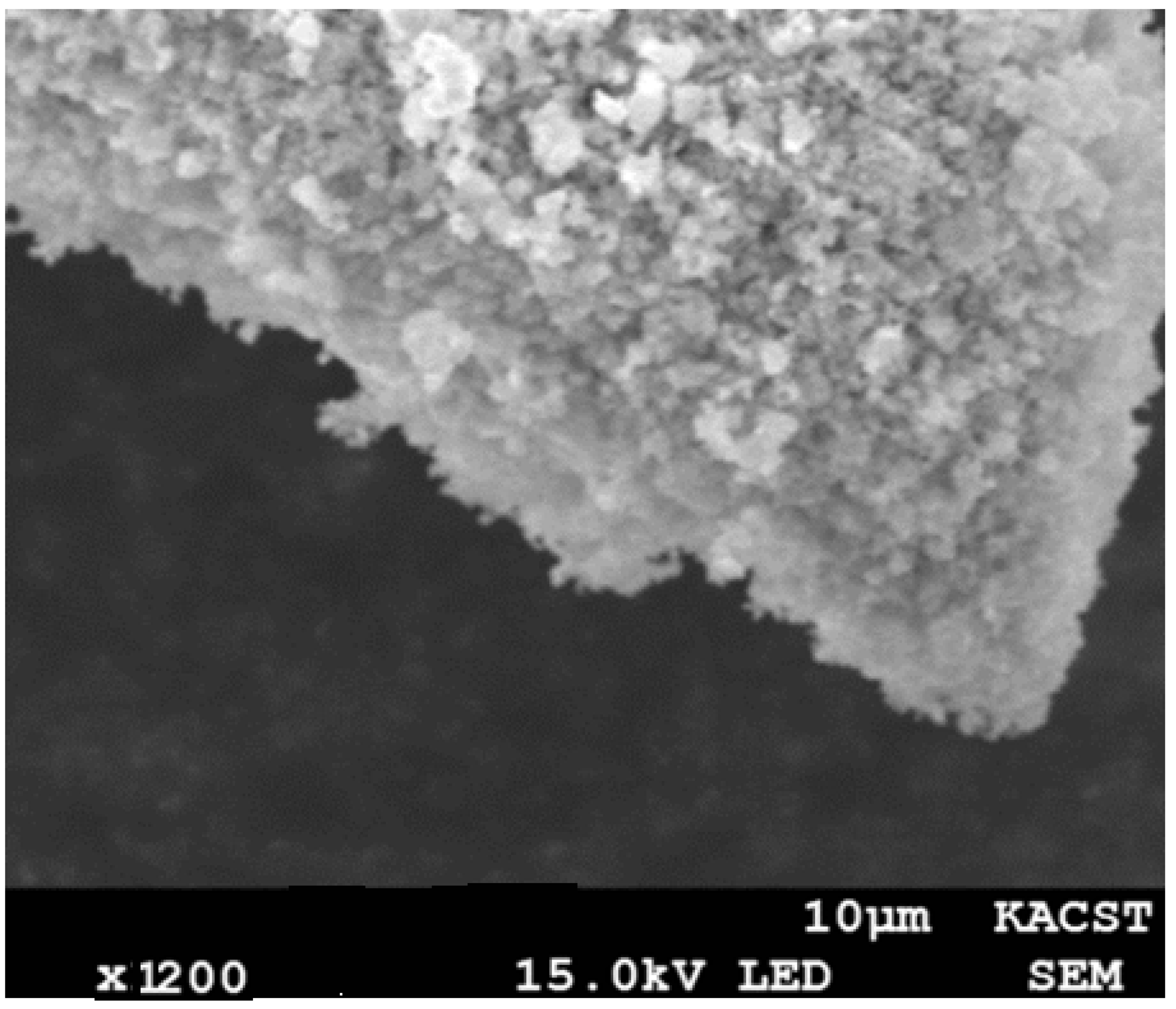
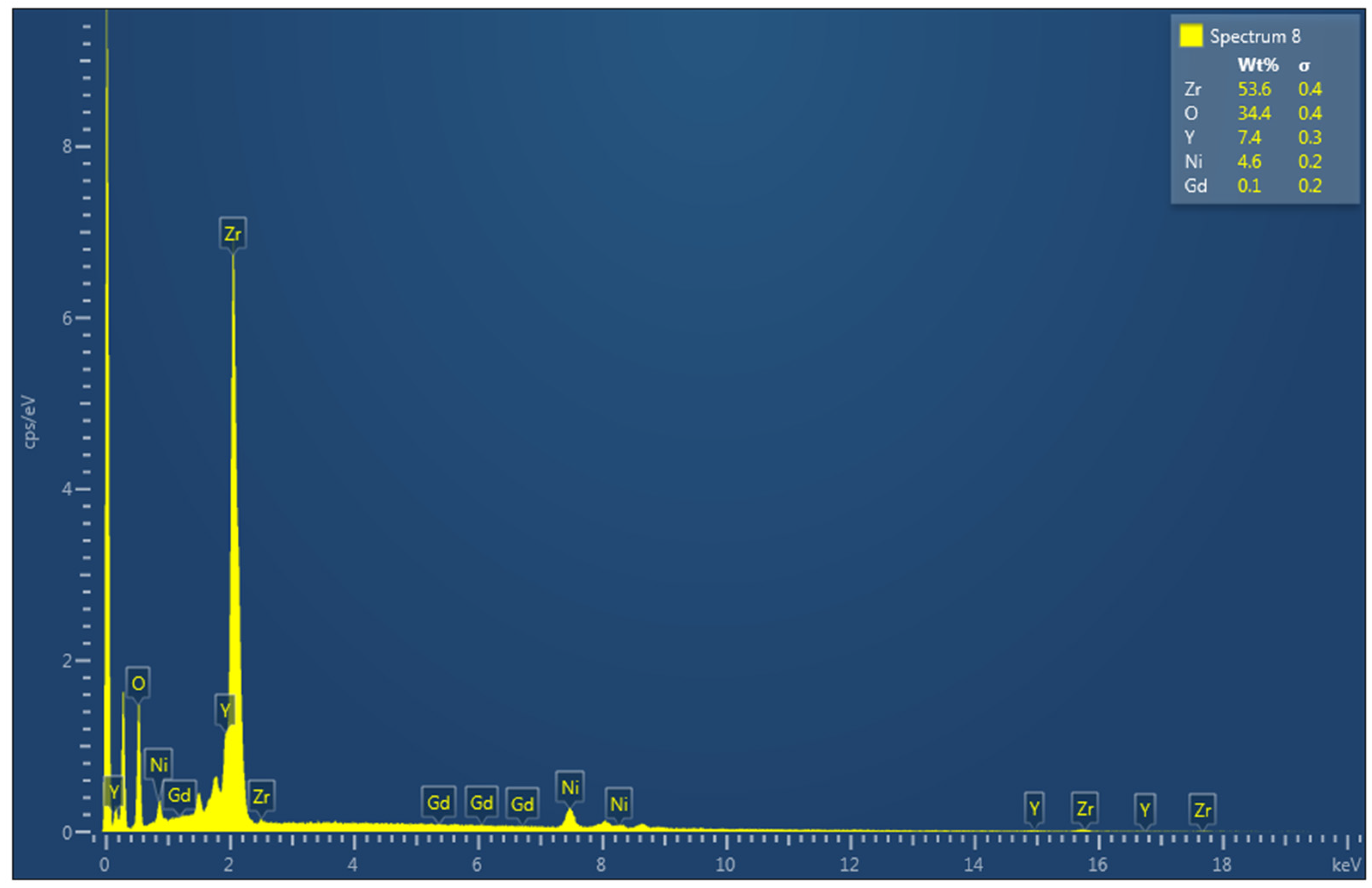
3.7. Scanning Electron Microscope (SEM)
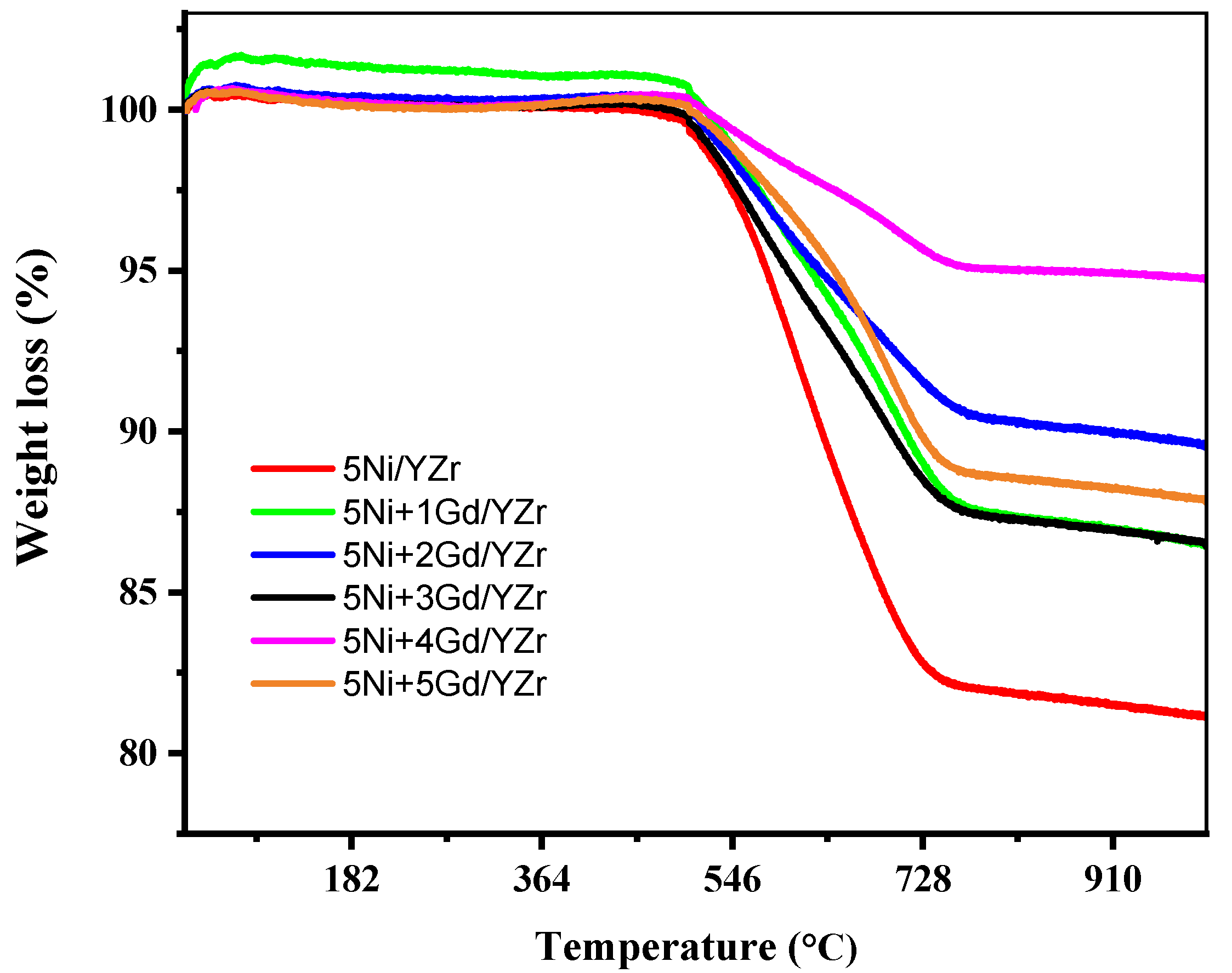
3.8. Thermogravimetric Analysis (TGA) of the Spent Catalyst
3.9. Plausible Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, R.; Costa, D.; Junior, R.; Santos, M.; Rodella, C.; Fréty, R.; Beretta, A.; Brandão, S. Dry Reforming of Methane over NiLa-Based Catalysts: Influence of Synthesis Method and Ba Addition on Catalytic Properties and Stability. Catalysts 2019, 9, 313–323. [Google Scholar] [CrossRef]
- Steinhauer, B.; Kasireddy, M.R.; Radnik, J.; Martin, A. Development of Ni-Pd Bimetallic Catalysts for the Utilization of Carbon Dioxide and Methane by Dry Reforming. Appl. Catal. A Gen. 2009, 366, 333–341. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.P.; Gallachóir, B.P.Ó. The Role of Hydrogen in Low Carbon Energy Futures—A Review of Existing Perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- Burgt, M.J.V.D.; Van Leeuwen, C.J.; Del’Amico, J.J.; Sie, S.T. The Shell Middle Distillate Synthesis Process. Stud. Surf. Sci. Catal. 1988, 36, 473–482. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Hu, Y.H. Catalysts for CO2 reforming of CH4: A Review. J. Mater. Chem. A 2021, 9, 12495–12520. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Chatterjee, S.; Huang, K.-W. The Insignificant Role of Dry Reforming of Methane in CO2 Emission Relief. ACS Energy Lett. 2020, 5, 2881–2885. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Hydrogen Production from Carbon Dioxide Reforming of Methane over Ni–Co/MgO–ZrO2 Catalyst: Process Optimization. Int. J. Hydrogen Energy 2011, 36, 4875–4886. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Qureshi, M.N.; Liu, S.Y.; Wang, R. Accelerating Photocatalytic Hydrogen Production and Pollutant Degradation by Functionalizing G-C3N4 With SnO2. Front. Chem. 2020, 7, 941. [Google Scholar] [CrossRef]
- Hayat, A.; Taha, T.A.M.; Alenad, A.M.; Yingjin, L.; Mane, S.K.B.; Hayat, A.; Khan, M.; Rehman, A.U.; Khan, W.U.; Shaishta, N. Organic Conjugation of Polymeric Carbon Nitride for Improved Photocatalytic CO2 Conversion and H2 Fixation. Energy Technol. 2021, 9, 91. [Google Scholar] [CrossRef]
- Lanre, M.S.; Abasaeed, A.E.; Fakeeha, A.H.; Ibrahim, A.A.; Al-awadi, A.S.; Bin Jumah, A.; Al-mubaddel, F.S.; Al-fatesh, A.S. Lanthanum–Cerium-Modified Nickel Catalysts for Dry Reforming of Methane. Catalysts 2022, 12, 715–724. [Google Scholar] [CrossRef]
- Lanre, M.S.; Abasaeed, A.E.; Fakeeha, A.H.; Ibrahim, A.A.; Alquraini, A.A.; Alreshaidan, S.B.; Al-fatesh, A.S. Substituting Yttrium with Zirconia in Dry Reforming of Methane. Materials 2022, 15, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.H. Progress in Petrochemical Science A Mini-Review on CO2 Reforming of Methane. Prog. Petrochem. Sci. 2018, 2, 161–165. [Google Scholar] [CrossRef]
- Lanre, M.S.; Al-Fatesh, A.S.; Fakeeha, A.H.; Kasim, S.O.; Ibrahim, A.A.; Al-Awadi, A.S.; Al-Zahrani, A.A.; Abasaeed, A.E. Catalytic Performance of Lanthanum Promoted Ni/ZrO2 for Carbon Dioxide Reforming of Methane. Processes 2020, 8, 1502–1513. [Google Scholar] [CrossRef]
- de Araújo Moreira, T.G.; de Carvalho Filho, J.F.S.; Carvalho, Y.; de Almeida, J.M.A.R.; Nothaft Romano, P.; Falabella Sousa-Aguiar, E. Highly Stable Low Noble Metal Content Rhodium-Based Catalyst for the Dry Reforming of Methane. Fuel 2021, 287, 119536–1195349. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Calvino, J.J.; Rodríguez-Izquierdo, J.M.; Blanco, G.; Arias, D.C.; Pérez-Omil, J.A.; Hernández-Garrido, J.C.; González-Leal, J.M.; Cauqui, M.A.; Yeste, M.P. Highly Stable Ceria-Zirconia-Yttria Supported Ni Catalysts for Syngas Production by CO2 Reforming of Methane. Appl. Surf. Sci. 2017, 426, 864–873. [Google Scholar] [CrossRef]
- Chen, H.W.; Wang, C.Y.; Yu, C.H.; Tseng, L.T.; Liao, P.H. Carbon Dioxide Reforming of Methane Reaction Catalyzed by Stable Nickel Copper Catalysts. In Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2004; Volume 97, pp. 173–180. [Google Scholar]
- Jeong, H.; Kim, K.I.; Kim, D.; Song, I.K. Effect of Promoters in the Methane Reforming with Carbon Dioxide to Synthesis Gas over Ni/HY Catalysts. J. Mol. Catal. A Chem. 2006, 246, 43–48. [Google Scholar] [CrossRef]
- Daza, C.E.; Gallego, J.; Mondragón, F.; Moreno, S.; Molina, R. High Stability of Ce-Promoted Ni/Mg–Al Catalysts Derived from Hydrotalcites in Dry Reforming of Methane. Fuel 2010, 89, 592–603. [Google Scholar] [CrossRef]
- Stagg-Williams, S.M.; Noronha, F.B.; Fendley, G.; Resasco, D.E. CO2 Reforming of CH4 over Pt/ZrO2 Catalysts Promoted with La and Ce Oxides. J. Catal. 2000, 194, 240–249. [Google Scholar] [CrossRef]
- Faroldi, B.; Múnera, J.; Falivene, J.M.; Ramos, I.R.; García, Á.G.; Fernández, L.T.; Carrazán, S.G.; Cornaglia, L. Well-Dispersed Rh Nanoparticles with High Activity for the Dry Reforming of Methane. Int. J. Hydrogen Energy 2017, 42, 16127–16138. [Google Scholar] [CrossRef]
- Damyanova, S.; Shtereva, I.; Pawelec, B.; Mihaylov, L.; Fierro, J.L.G. Characterization of None and Yttrium-Modified Ni-Based Catalysts for Dry Reforming of Methane. Appl. Catal. B Environ. 2020, 278, 119335. [Google Scholar] [CrossRef]
- Orak, C.; Yüksel, A. Box–Behnken Design for Hydrogen Evolution from Sugar Industry Wastewater Using Solar-Driven Hybrid Catalysts. ACS Omega 2022, 7, 42489–42498. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, X.; Gao, G.; Wang, J.; Han, C.; Liang, X.; Li, C.; Li, Y.; Zhang, W.; Chen, X. Metal (Fe, Co, Ce or La) Doped Nickel Catalyst Supported on ZrO2 Modified Mesoporous Clays for CO and CO2 Methanation. Fuel 2016, 183, 335–344. [Google Scholar] [CrossRef]
- Mustu, H.; Yasyerli, S.; Yasyerli, N.; Dogu, G.; Dogu, T.; Djinović, P.; Pintar, A. Effect of Synthesis Route of Mesoporous Zirconia Based Ni Catalysts on Coke Minimization in Conversion of Biogas to Synthesis Gas. Int. J. Hydrog. Energy 2015, 40, 3217–3228. [Google Scholar] [CrossRef]
- Reddy, G.K.; Loridant, S.; Takahashi, A.; Delichère, P.; Reddy, B.M. Reforming of Methane with Carbon Dioxide over Pt/ZrO2/SiO2 Catalysts—Effect of Zirconia to Silica Ratio. Appl. Catal. A Gen. 2010, 389, 92–100. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Zhang, J.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Methane Decomposition and Carbon Deposition over Ni/ZrO2 Catalysts: Comparison of Amorphous, Tetragonal, and Monoclinic Zirconia Phase. Int. J. Hydrog. Energy 2019, 44, 17887–17899. [Google Scholar] [CrossRef]
- Santamaria, L.; Arregi, A.; Alvarez, J.; Artetxe, M.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Performance of a Ni/ZrO2 Catalyst in the Steam Reforming of the Volatiles Derived from Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2018, 136, 222–231. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S. Promotional Effect of Gd over Ni/Y2O3 Catalyst Used in Dry Reforming of CH4 for H2 Production. Int. J. Hydrogen Energy 2017, 42, 18805–18816. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Khatri, J.; Kumar, R.; Kumar Srivastava, V.; Osman, A.I.; AlGarni, T.S.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H.; Rooney, D.W. Role of Ca, Cr, Ga and Gd Promotor over Lanthana-Zirconia–Supported Ni Catalyst towards H2-Rich Syngas Production through Dry Reforming of Methane. Energy Sci. Eng. 2022, 10, 866–880. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Hanan atia; Ibrahim, A.A.; Fakeeha, A.H.; Singh, S.K.; Labhsetwar, N.K.; Shaikh, H.; Qasim, S.O. CO2 Reforming of CH4: Effect of Gd as Promoter for Ni Supported over MCM-41 as Catalyst. Renew. Energy 2019, 140, 658–667. [Google Scholar] [CrossRef]
- García, V.; Fernández, J.J.; Ruíz, W.; Mondragón, F.; Moreno, A. Effect of MgO Addition on the Basicity of Ni/ZrO2 and on Its Catalytic Activity in Carbon Dioxide Reforming of Methane. Catal. Commun. 2009, 11, 240–246. [Google Scholar] [CrossRef]
- Naeem, M.A.; Al-Fatesh, A.S.; Abasaeed, A.E.; Fakeeha, A.H. Activities of Ni-Based Nano Catalysts for CO2-CH4 Reforming Prepared by Polyol Process. Fuel Process. Technol. 2014, 122, 141–152. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Ni Loadings on the Activity and Coke Formation of MgO-Modified Ni/Al2O3 Nanocatalyst in Dry Reforming of Methane. J. Energy Chem. 2014, 23, 633–638. [Google Scholar] [CrossRef]
- Lindo, M.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Ethanol Steam Reforming on Ni/Al-SBA-15 Catalysts: Effect of the Aluminium Content. Int. J. Hydrogen Energy 2010, 35, 5895–5901. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Nada, A.A.; Bekheet, M.F.; Roualdes, S.; Riedel, W.; Iatsunskyi, I.; Coy, E.; Gurlo, A.; Bechelany, M. Coaxial Nanofibers of Nickel/Gadolinium Oxide/Nickel Oxide as Highly Effective Electrocatalysts for Hydrogen Evolution Reaction. J. Colloid Interface Sci. 2021, 587, 457–466. [Google Scholar] [CrossRef]
- Au, C.T.; Chen, K.D.; Ng, C.F. The Modification of Gd2O3 with BaO for the Oxidative Coupling of Methane Reactions. Appl. Catal. A Gen. 1998, 170, 81–92. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Z.; Gao, J.; Zheng, X. Syngas Production via Combined Oxy-CO2 Reforming of Methane over Gd2O3-Modified Ni/SiO2 Catalysts in a Fluidized-Bed Reactor. Fuel 2008, 87, 1348–1354. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Bagabas, A.A.; Lanre, M.S.; Osman, A.I.; Kasim, S.O.; Ibrahim, A.A.; Arasheed, R.; Alkhalifa, A.; Elnour, A.Y.; Abasaeed, A.E.; et al. Catalytic Performance of Metal Oxides Promoted Nickel Catalysts Supported on Mesoporous γ-Alumina in Dry Reforming of Methane. Processes 2020, 8, 522–531. [Google Scholar] [CrossRef]
- Guo, J.; Gao, J.; Chen, B.; Hou, Z.; Fei, J.; Lou, H.; Zheng, X. Catalytic Conversion of CH4 and CO2 to Synthesis Gas on Ni/SiO2 Catalysts Containing Gd2O3 Promoter. Int. J. Hydrogen Energy 2009, 34, 8905–8911. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Ibrahim, A.A.; Osman, A.I.; Albaqi, F.; Arasheed, R.; Francesco, F.; Serena, T.; Anojaid, K.; Lanre, M.S.; Abasaeed, A.E.; et al. Optimizing Barium Oxide Promoter for Nickel Catalyst Supported on Yttria–Stabilized Zirconia in Dry Reforming of Methane. SSRN Electron. J. 2022, 1–29. [Google Scholar] [CrossRef]
- Arevalo, R.L.; Aspera, S.M.; Escaño, M.C.S.; Nakanishi, H.; Kasai, H. Tuning Methane Decomposition on Stepped Ni Surface: The Role of Subsurface Atoms in Catalyst Design. Sci. Rep. 2017, 7, 13963. [Google Scholar] [CrossRef]
- Chen, Q.; Lua, A.C. Kinetic Reaction and Deactivation Studies on Thermocatalytic Decomposition of Methane by Electroless Nickel Plating Catalyst. Chem. Eng. J. 2020, 389, 124366–124375. [Google Scholar] [CrossRef]
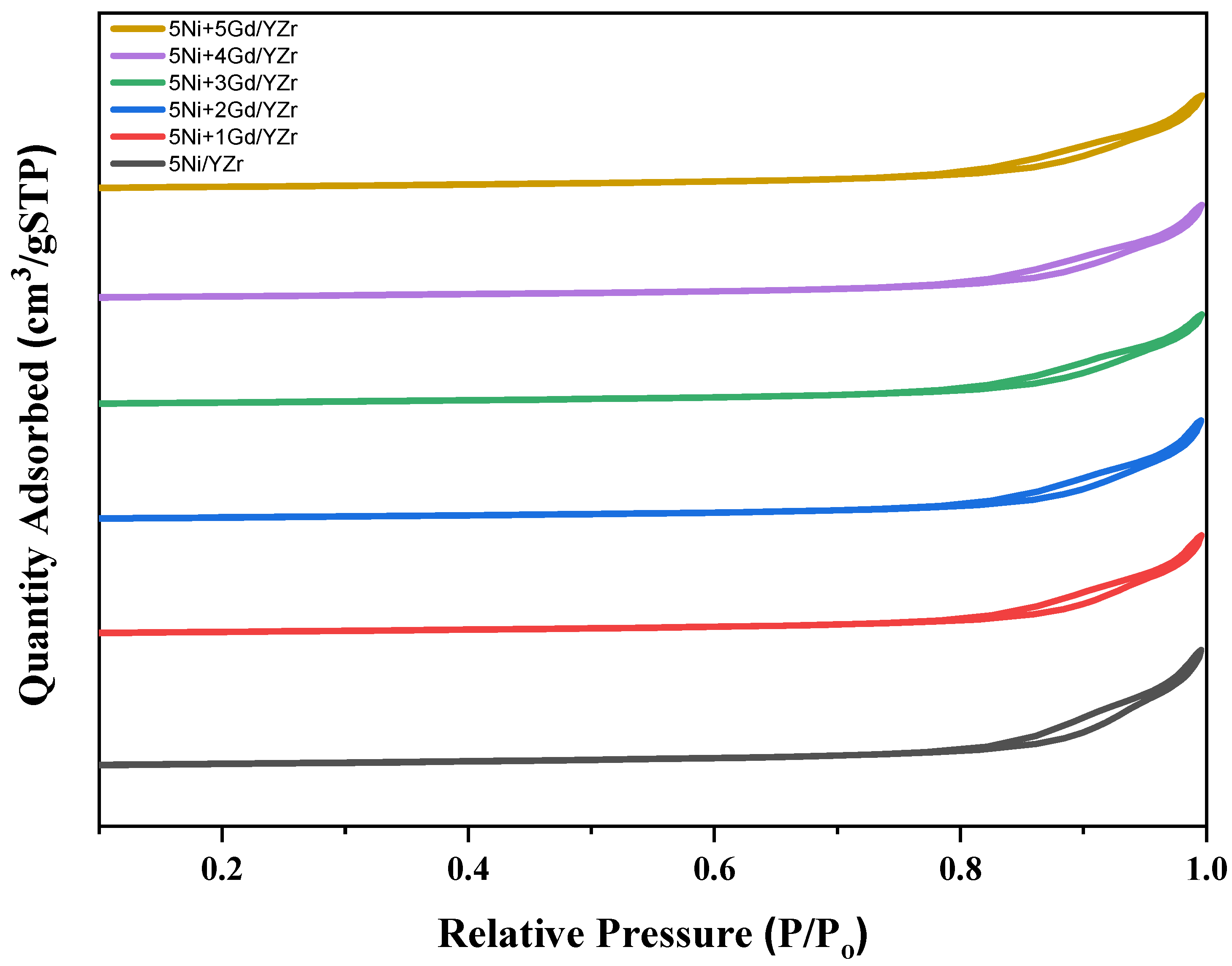
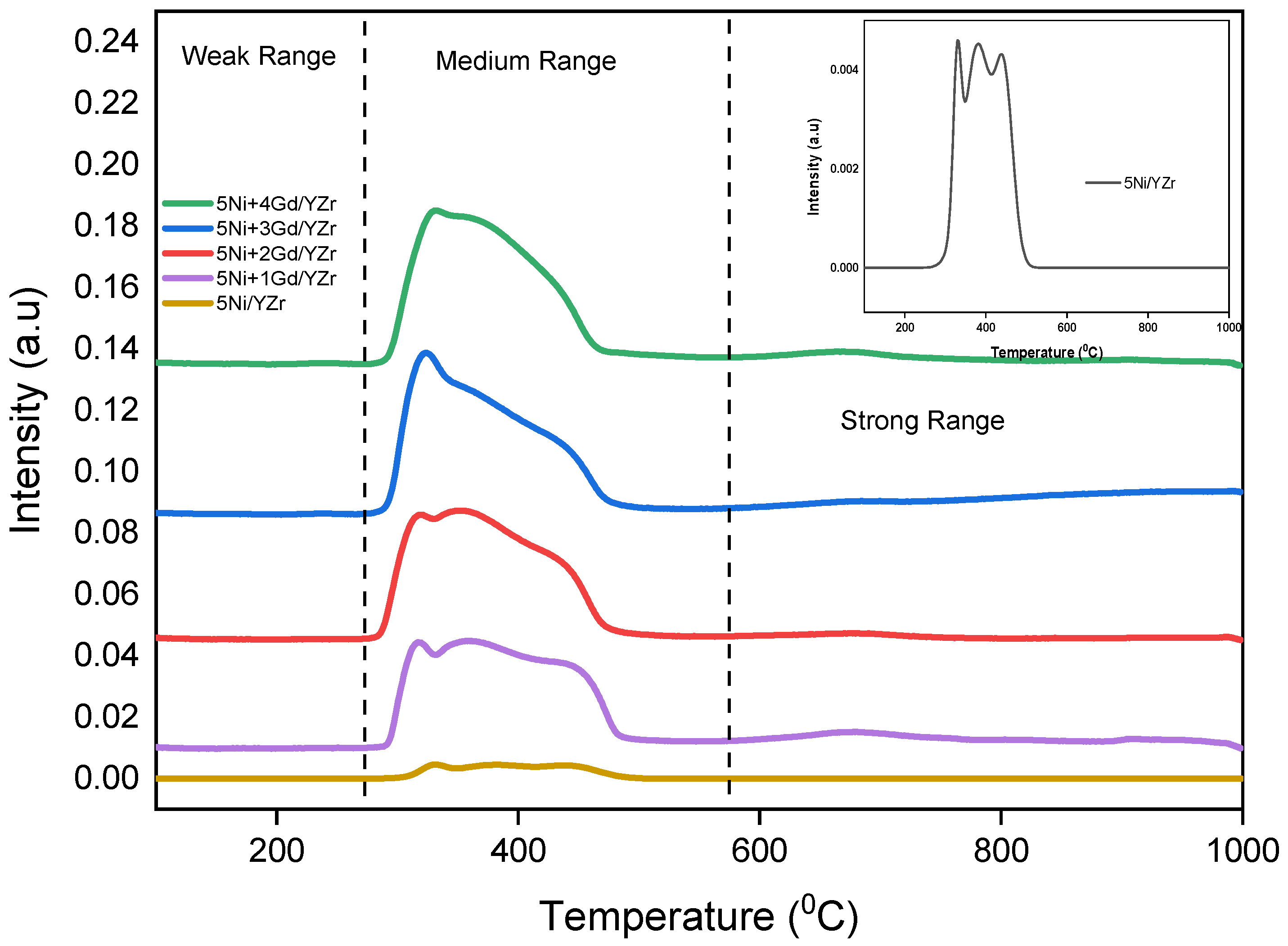
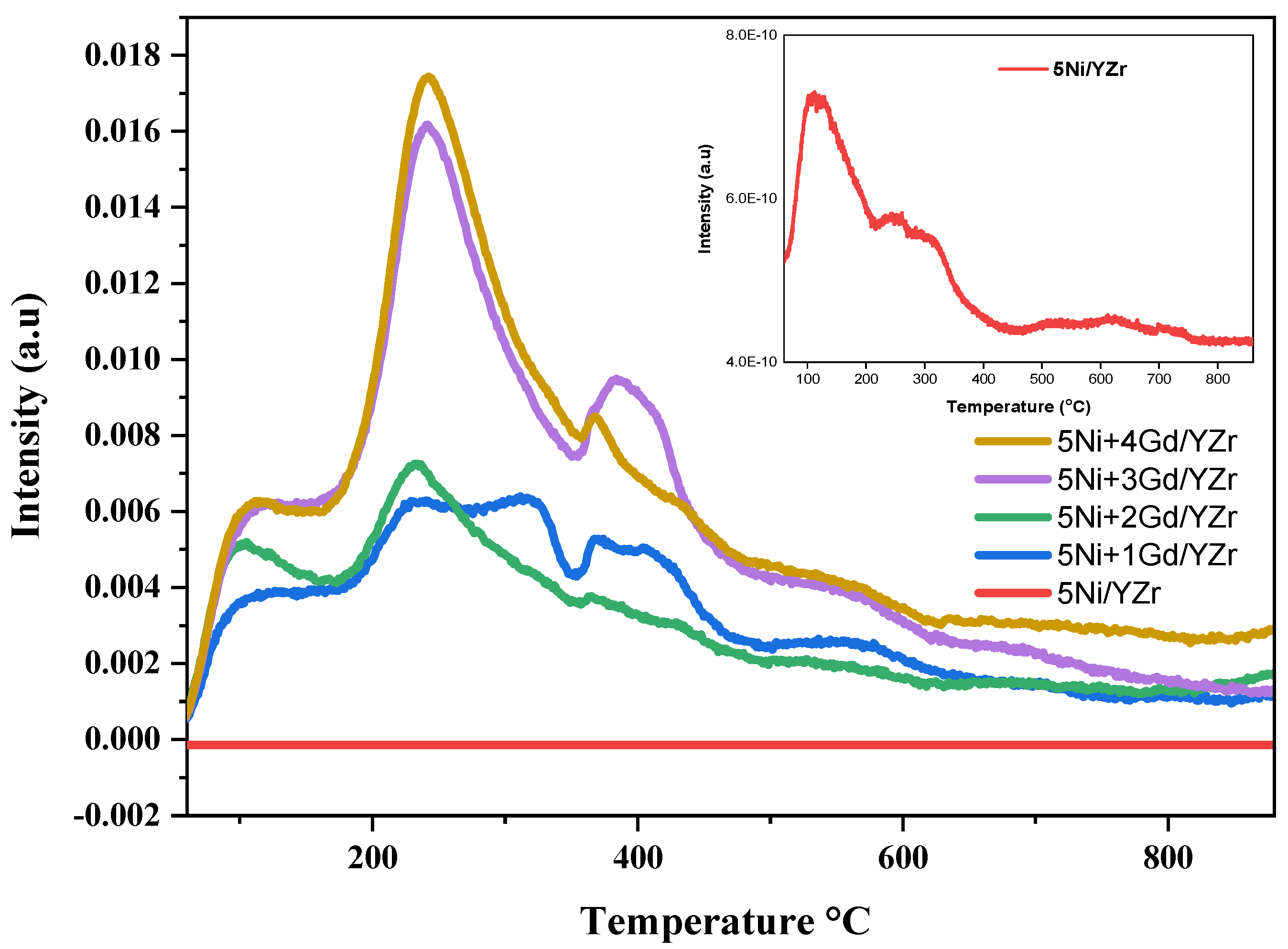
| Catalyst | SBET, m2/g | Pv, cm3/g | Pd, nm |
|---|---|---|---|
| 5Ni/YZr | 31 | 0.19 | 24.78 |
| 5Ni+1Gd/YZr | 27 | 0.16 | 23.49 |
| 5Ni+2Gd/YZr | 26 | 0.16 | 24.05 |
| 5Ni+3Gd/YZr | 27 | 0.14 | 21.36 |
| 5Ni+4Gd/YZr | 26 | 0.15 | 22.28 |
| 5Ni+5Gd/YZr | 27 | 0.15 | 21.36 |
| Catalyst | Gd2O3 (wt.%) | 2θ (°) | d-Spacing for (111), Å | 2θ (°) | d-Spacing for (220), Å |
|---|---|---|---|---|---|
| 5Ni/YZr | 0.0 | 30.04 | 2.9727 | 50.15 | 1.8176 |
| 5Ni+1Gd/YZr | 1.0 | 30.04 | 2.9721 | 50.16 | 1.8172 |
| 5Ni+2Gd/YZr | 2.0 | 30.05 | 2.9717 | 50.17 | 1.8169 |
| 5Ni+3Gd/YZr | 3.0 | 30.07 | 2.9699 | 50.18 | 1.8166 |
| 5Ni+4Gd/YZr | 4.0 | 30.11 | 2.9652 | 50.19 | 1.8162 |
| 5Ni+5Gd/YZr | 5.0 | 30.19 | 2.9614 | 50.24 | 1.8145 |
| Catalyst | Gd2O3 (wt.%) | 2θ (°) | d-Spacing for (111), Å | 2θ (°) | d-Spacing for (220), Å |
|---|---|---|---|---|---|
| 5Ni/YZr | 0.0 | 30.00 | 2.9762 | 50.02 | 1.8220 |
| 5Ni+1Gd/YZr | 1.0 | 30.15 | 2.9617 | 50.24 | 1.8145 |
| 5Ni+2Gd/YZr | 2.0 | 30.32 | 2.9455 | 50.49 | 1.8061 |
| 5Ni+3Gd/YZr | 3.0 | 30.09 | 2.9675 | 50.14 | 1.8179 |
| 5Ni+4Gd/YZr | 4.0 | 30.66 | 2.9136 | 51.00 | 1.7893 |
| 5Ni+5Gd/YZr | 5.0 | 30.27 | 2.9503 | 50.40 | 1.8092 |
| Cat | Wt.% | GHSV, L/(h·g) | Rx. Temp., °C | Conversion, % | Mole Ratio | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| NiO | Gd2O3 | CH4 | CO2 | CH4/CO2 | H2/CO | ||||
| 5Ni+1Gd/Al | 5 | 1 | 29.9 | 700 | 83 | 89 | 1:1 | 1 | [38] |
| Gd0.45Ni/SiO2 | 6.36 | 0.52 | 9.0 | 750 | 86.9 | 75.1 | 1:0.4 | 1.42 | [37] |
| 3Gd+10Ni/Y2O3 | 12.72 | 3 | 8 | 700 | 84 | 82 | 1:1 | - | [28] |
| 0.1Gd5NiMCM41 | 6.36 | 0.12 | 39 | 800 | 87 | 91 | 1:1 | 0.9 | [30] |
| NiGd0.45/SiO2 | 6.36 | 0.52 | 9.0 | 700 | 67.3 | 72.4 | 1:1 | - | [39] |
| 5Ni+4Gd/YZr | 5 | 4 | 42 | 800 | 80 | 86 | 1:1 | 0.9 | This work |
| Catalyst | d-Spacing Calculated from HRTEM, nm | d-Spacing in Bulk YZr, nm | Miller Indices (hkl) Assignment |
|---|---|---|---|
| Fresh | 0.248 | 0.248 | 411 |
| Spent | 0.258 | 0.263 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alreshaidan, S.B.; Al-Fatesh, A.; Lanre, M.S.; Alanazi, Y.M.; Ibrahim, A.A.; Fakeeha, A.H.; Albaqi, F.; Anojaidi, K.; Bagabas, A. Effect of Adding Gadolinium Oxide Promoter on Nickel Catalyst over Yttrium-Zirconium Oxide Support for Dry Reforming of Methane. Materials 2023, 16, 1158. https://doi.org/10.3390/ma16031158
Alreshaidan SB, Al-Fatesh A, Lanre MS, Alanazi YM, Ibrahim AA, Fakeeha AH, Albaqi F, Anojaidi K, Bagabas A. Effect of Adding Gadolinium Oxide Promoter on Nickel Catalyst over Yttrium-Zirconium Oxide Support for Dry Reforming of Methane. Materials. 2023; 16(3):1158. https://doi.org/10.3390/ma16031158
Chicago/Turabian StyleAlreshaidan, Salwa B., Ahmed Al-Fatesh, Mahmud S. Lanre, Yousef M. Alanazi, Ahmed A. Ibrahim, Anis H. Fakeeha, Fahad Albaqi, Khalid Anojaidi, and Abdulaziz Bagabas. 2023. "Effect of Adding Gadolinium Oxide Promoter on Nickel Catalyst over Yttrium-Zirconium Oxide Support for Dry Reforming of Methane" Materials 16, no. 3: 1158. https://doi.org/10.3390/ma16031158
APA StyleAlreshaidan, S. B., Al-Fatesh, A., Lanre, M. S., Alanazi, Y. M., Ibrahim, A. A., Fakeeha, A. H., Albaqi, F., Anojaidi, K., & Bagabas, A. (2023). Effect of Adding Gadolinium Oxide Promoter on Nickel Catalyst over Yttrium-Zirconium Oxide Support for Dry Reforming of Methane. Materials, 16(3), 1158. https://doi.org/10.3390/ma16031158









