Mechanical Performance, Microstructure, and Porosity Evolution of Fly Ash Geopolymer after Ten Years of Curing Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Mixing of the Geopolymer Aggregate
2.2. Characterization Techniques
2.2.1. Compressive Measurement and Microstructural Characterization
2.2.2. X-ray Diffraction (XRD) Analysis
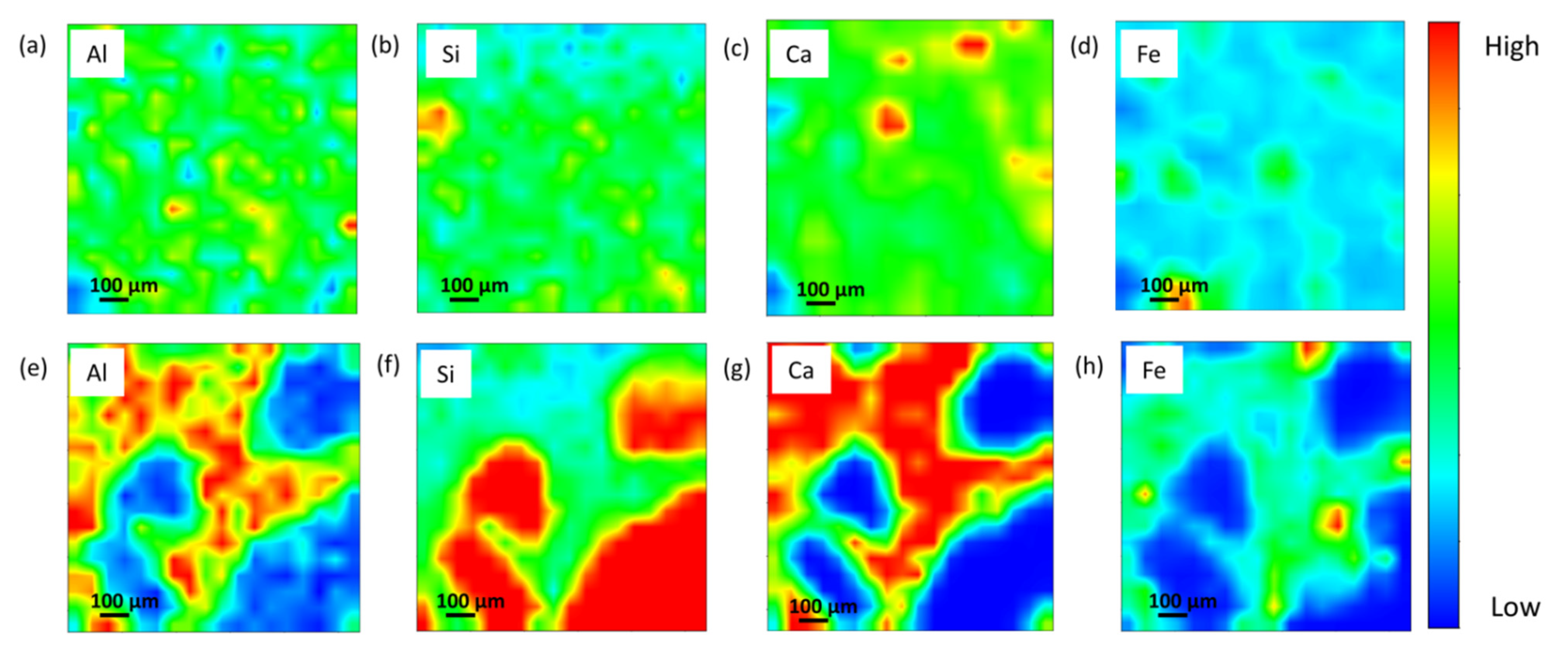
2.2.3. Synchrotron Micro X-ray Fluorescence (µ-XRF) and X-ray Tomographic Microscopy (SXTM)
3. Results and Discussion
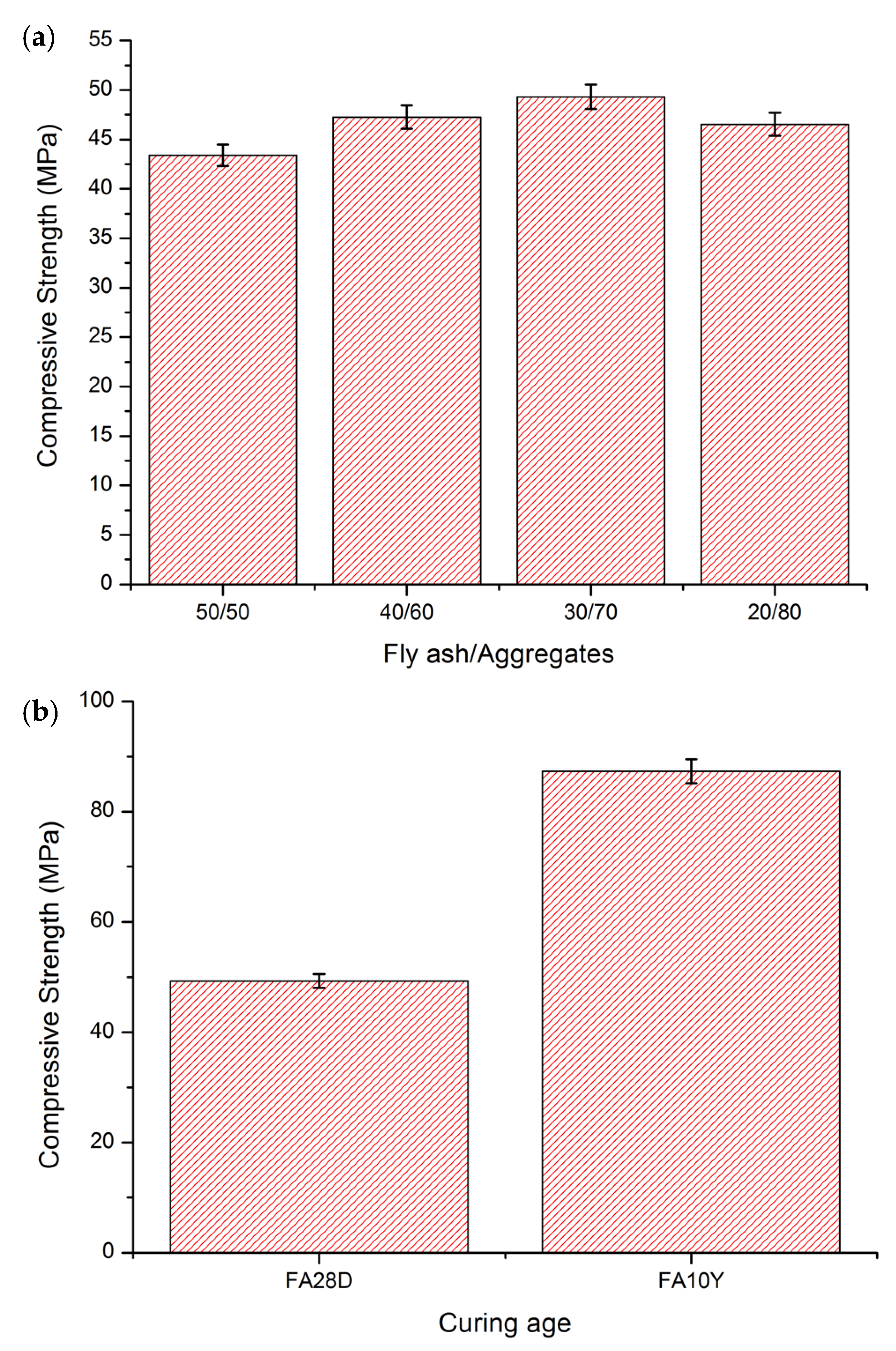
3.1. Compressive Strength Measurement
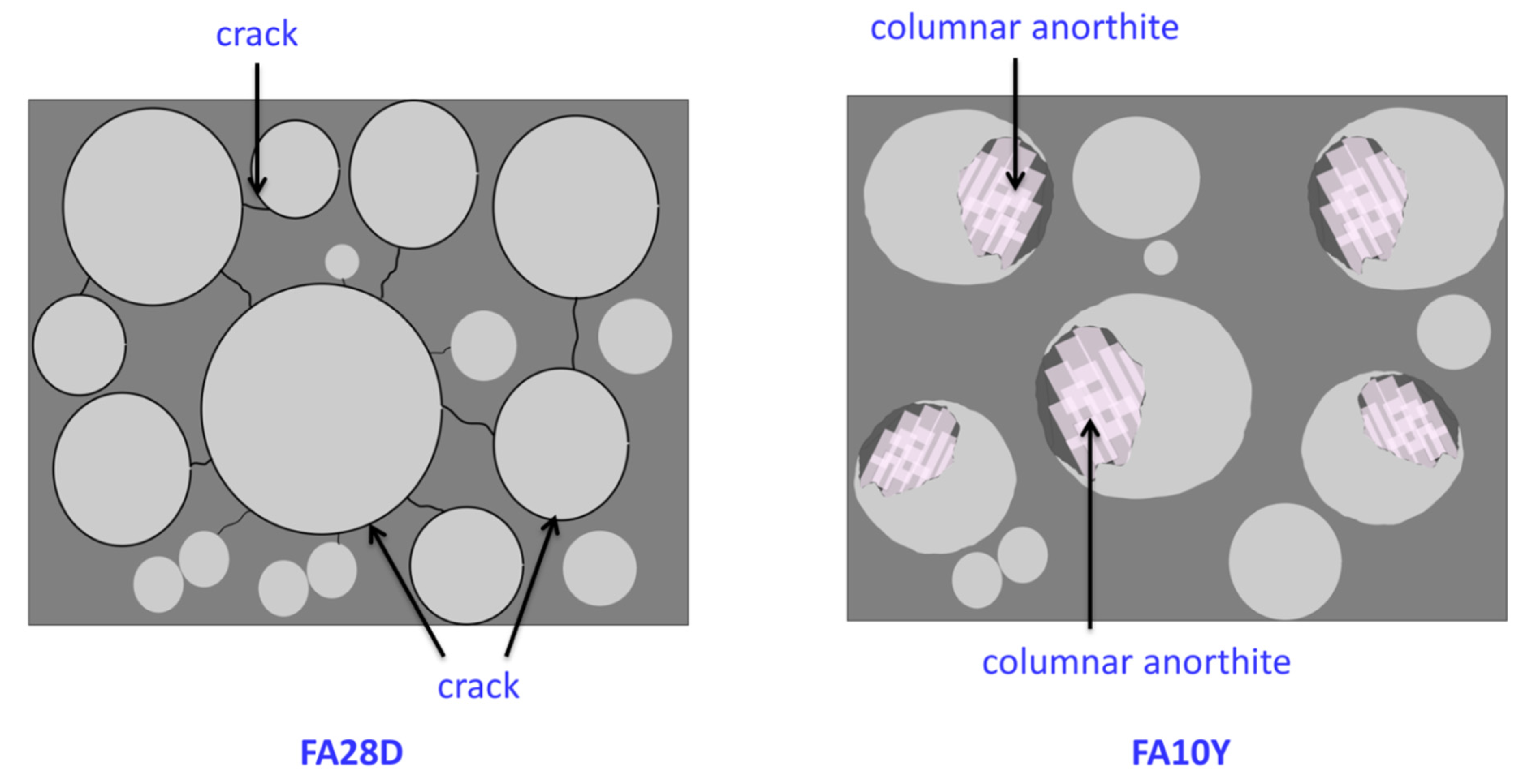
3.2. SEM
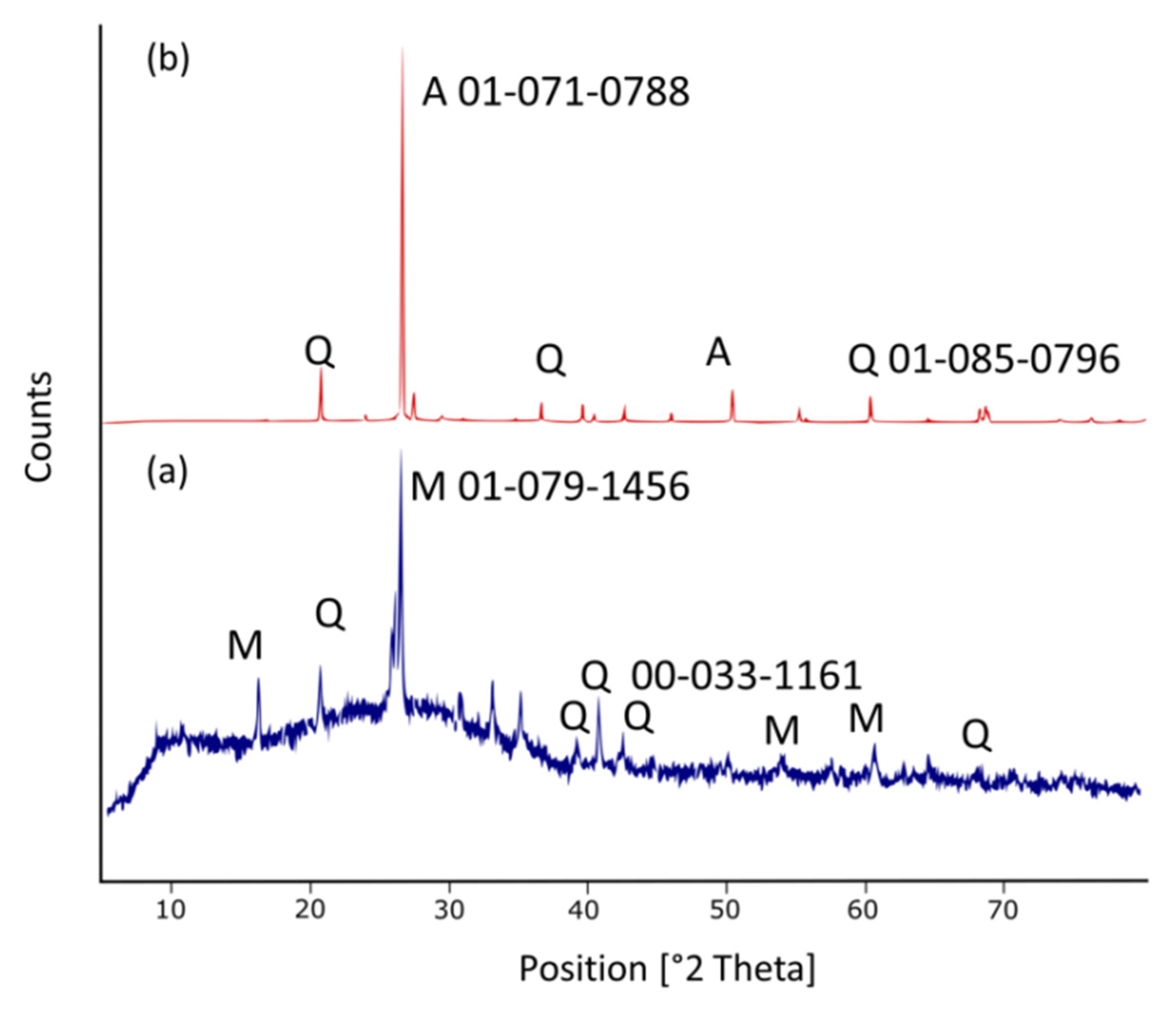
3.3. XRD and u-XRF
3Ca2Al2SiO7 (gehlenite) + Al6Si2O13 + 7SiO2 (mullite) → 6CaAl2Si2O8 (anorthite)
3.4. XTM
4. Conclusions
- The compressive strength for FA10Y was high compared to FA28D. Compared to FA28D, the compressive strength of FA10Y was approximately ~74% higher than the compressive strength of FA28D.
- There are increasing densification surfaces and less total porosity within FA10Y compared to FA28D. The columnar anorthite crystal was obtained inside the fly ash spherical particles which contributed to the strength development.
- The synchrotron micro X-ray tomography revealed a lower range (5–20 µm) of pore distribution and higher localized Al, Si, and Ca elements after 10 years of curing age.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahedan, N.F.; Abdullah, M.M.A.B.; Mahmed, N.; Kusbiantoro, A.; Tammas-Williams, S.; Li, L.-Y.; Aziz, I.H.; Vizureanu, P.; Wysłocki, J.J.; Błoch, K. Properties of a New Insulation Material Glass Bubble in Geopolymer Concrete. Materials 2021, 14, 809. [Google Scholar] [CrossRef] [PubMed]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Nabiałek, M.; Sandu, A.V.; Szmidla, J.; Jurczyńska, A.; Razak, R.A.; Aziz, I.H.A.; Jamil, N.H. Assessment of the suitability of ceramic waste in geopolymer composites: An appraisal. Materials 2021, 14, 3279. [Google Scholar] [CrossRef] [PubMed]
- Kheimi, M.; Aziz, I.H.; Abdullah, M.M.A.B.; Almadani, M.; Razak, R.A. Waste Material via Geopolymerization for Heavy-Duty Application: A Review. Materials 2022, 15, 3205. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, A.S.; Molin, D.C.D.; Vilela, A.C.; da Silva, F.J.; Pavao, B.; Veit, H. The effects of Na2O/SiO2 molar ratio, curing temperature and age on compressive strength, morphology and microstructure of alkali-activated fly ash-based geopolymers. Cement Concr. Compos. 2011, 33, 653–660. [Google Scholar] [CrossRef]
- Santa, R.A.A.B.; Padoin, N.; Soares, C.; Riella, H.G. Microstructural characteristics of geopolymer materials with twenty eight days of curing and after eight years stored at room temperature. J. Clean. Prod. 2021, 278, 123437. [Google Scholar] [CrossRef]
- Sekou, T.; Sine, D.; Lanciné, T.D.; Bakaridjan, C. Synthesis and characterization of a red mud and rice husk based geopolymer for engineering applications. In Macromolecular Symposia; Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wong, V.; Jervis, W.; Fishburn, B.; Numata, T.; Joe, W.; Rawal, A.; Sorrell, C.C.; Koshy, P. Long-Term Strength Evolution in Ambient-Cured Solid-Activator Geopolymer Compositions. Minerals 2021, 11, 143. [Google Scholar] [CrossRef]
- Alhazmi, H.; Shah, S.A.R.; Anwar, M.K.; Raza, A.; Ullah, M.K.; Iqbal, F. Utilization of Polymer Concrete Composites for a Circular Economy: A Comparative Review for Assessment of Recycling and Waste Utilization. Polymers 2021, 13, 2135. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.M.; Yoriya, S.; Chaiprapa, J.; Rojviriya, C.; Li, L.Y. Microstructure and porosity evolution of alkali activated slag at various heating temperatures. J. Mater. Res. Technol. 2020, 9, 15894–15907. [Google Scholar] [CrossRef]
- Ramli, M.I.I.; Salleh, M.A.A.M.; Abdullah, M.M.A.B.; Aziz, I.H.; Ying, T.C.; Shahedan, N.F.; Kockelmann, W.; Fedrigo, A.; Sandu, A.V.; Vizureanu, P. The Influence of Sintering Temperature on the Pore Structure of an Alkali-Activated Kaolin-Based Geopolymer Ceramic. Materials 2022, 15, 2667. [Google Scholar] [CrossRef]
- Yong-Sing, N.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Pakawanit, P.; Vizureanu, P.; Khalid, M.S.; Hui-Teng, N.; Yong-Jie, H.; Nabiałek, M. Improvements of Flexural Properties and Thermal Performance in Thin Geopolymer Based on Fly Ash and Ladle Furnace Slag Using Borax Decahydrates. Materials 2022, 15, 4178. [Google Scholar] [CrossRef]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2015, 48, 721–731. [Google Scholar] [CrossRef]
- Lee, W.-H.; Lin, K.-L.; Chang, T.-H.; Ding, Y.-C.; Cheng, T.-W. Sustainable development and performance evaluation of marble-waste-based geopolymer concrete. Polymers 2020, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.Y.; Javed, S.; Baig, R.U. Optimization of Alkaline Activator on the Strength Properties of Geopolymer Concrete. Polymers 2022, 14, 2434. [Google Scholar] [CrossRef] [PubMed]
- Kankia, M.U.; Baloo, L.; Danlami, N.; Mohammed, B.S.; Haruna, S.; Abubakar, M.; Jagaba, A.H.; Sayed, K.; Abdulkadir, I.; Salihi, I.U. Performance of Fly Ash-Based Inorganic Polymer Mortar with Petroleum Sludge Ash. Polymers 2021, 13, 4143. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. Aggregates. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: Warrendale, PA, USA, 2013. [Google Scholar]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B Atom. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Tiyasatkulkovit, W.; Promruk, W.; Rojviriya, C.; Pakawanit, P.; Chaimongkolnukul, K.; Kengkoom, K.; Teerapornpuntakit, J.; Panupinthu, N.; Charoenphandhu, N. Impairment of bone microstructure and upregulation of osteoclastogenic markers in spontaneously hypertensive rats. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kyrieleis, A.; Ibison, M.; Titarenko, V.; Withers, P.J. Image stitching strategies for tomographic imaging of large objects at high resolution at synchrotron sources. Nucl. Instrum. Methods Phys. Res. Sect. A Accelerat. Spectrometr. Detect. Assoc. Equip. 2009, 607, 677–684. [Google Scholar] [CrossRef]
- Desrues, J.; Viggiani, G.; Besuelle, P. Advances in X-ray Tomography for Geomaterials; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 118. [Google Scholar]
- Lee, S.; Jou, H.-T.; van Riessen, A.; Rickard, W.D.; Chon, C.-M.; Kang, N.-H. Three-dimensional quantification of pore structure in coal ash-based geopolymer using conventional electron tomography. Construct. Build. Mater. 2014, 52, 221–226. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C.; Yang, S. Recycling of lime mud and fly ash for fabrication of anorthite ceramic at low sintering temperature. Ceram. Int. 2015, 41, 5648–5655. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.M.; Azimi, E.A.; Chaiprapa, J.; Sandu, A.V. Strength development of solely ground granulated blast furnace slag geopolymers. Construct. Build. Mater. 2020, 250, 118720. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, S.; Sun, H.; Zhang, W.; Wang, B. Mineral transition of high-temperature sintering confirmed in CaAl2O4-Ca2SiO4 non-equilibrium binary system. Construct. Build. Mater. 2020, 234, 117402. [Google Scholar] [CrossRef]


| Elemental Oxide (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | TiO2 | MgO | K2O | Na2O | P2O5 | LOI |
| 26.4 | 9.25 | 21.6 | 30.13 | 3.07 | 0.78 | 2.58 | 0.42 | 1.31 | 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, I.H.A.; Abdullah, M.M.A.B.; Razak, R.A.; Yahya, Z.; Salleh, M.A.A.M.; Chaiprapa, J.; Rojviriya, C.; Vizureanu, P.; Sandu, A.V.; Tahir, M.F.; et al. Mechanical Performance, Microstructure, and Porosity Evolution of Fly Ash Geopolymer after Ten Years of Curing Age. Materials 2023, 16, 1096. https://doi.org/10.3390/ma16031096
Aziz IHA, Abdullah MMAB, Razak RA, Yahya Z, Salleh MAAM, Chaiprapa J, Rojviriya C, Vizureanu P, Sandu AV, Tahir MF, et al. Mechanical Performance, Microstructure, and Porosity Evolution of Fly Ash Geopolymer after Ten Years of Curing Age. Materials. 2023; 16(3):1096. https://doi.org/10.3390/ma16031096
Chicago/Turabian StyleAziz, Ikmal Hakem A., Mohd Mustafa Al Bakri Abdullah, Rafiza Abd Razak, Zarina Yahya, Mohd Arif Anuar Mohd Salleh, Jitrin Chaiprapa, Catleya Rojviriya, Petrica Vizureanu, Andrei Victor Sandu, Muhammad FaheemMohd Tahir, and et al. 2023. "Mechanical Performance, Microstructure, and Porosity Evolution of Fly Ash Geopolymer after Ten Years of Curing Age" Materials 16, no. 3: 1096. https://doi.org/10.3390/ma16031096
APA StyleAziz, I. H. A., Abdullah, M. M. A. B., Razak, R. A., Yahya, Z., Salleh, M. A. A. M., Chaiprapa, J., Rojviriya, C., Vizureanu, P., Sandu, A. V., Tahir, M. F., Abdullah, A., & Jamaludin, L. (2023). Mechanical Performance, Microstructure, and Porosity Evolution of Fly Ash Geopolymer after Ten Years of Curing Age. Materials, 16(3), 1096. https://doi.org/10.3390/ma16031096









