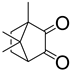
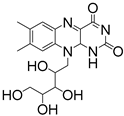
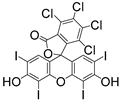
Applications of Light-Based 3D Bioprinting and Photoactive Biomaterials for Tissue Engineering
Abstract
:1. Introduction
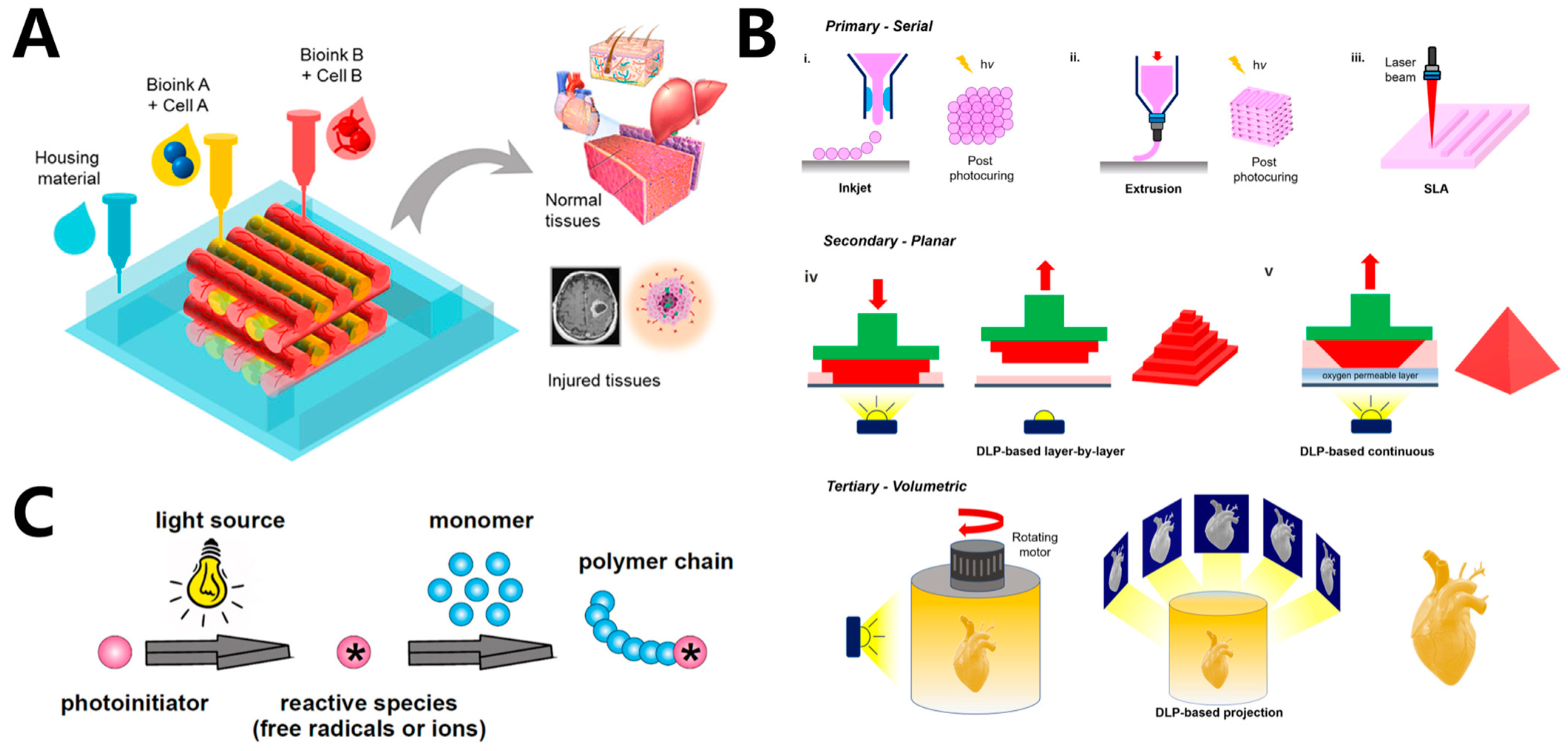
2. Light-Based 3D Bioprinting Methods and Applications
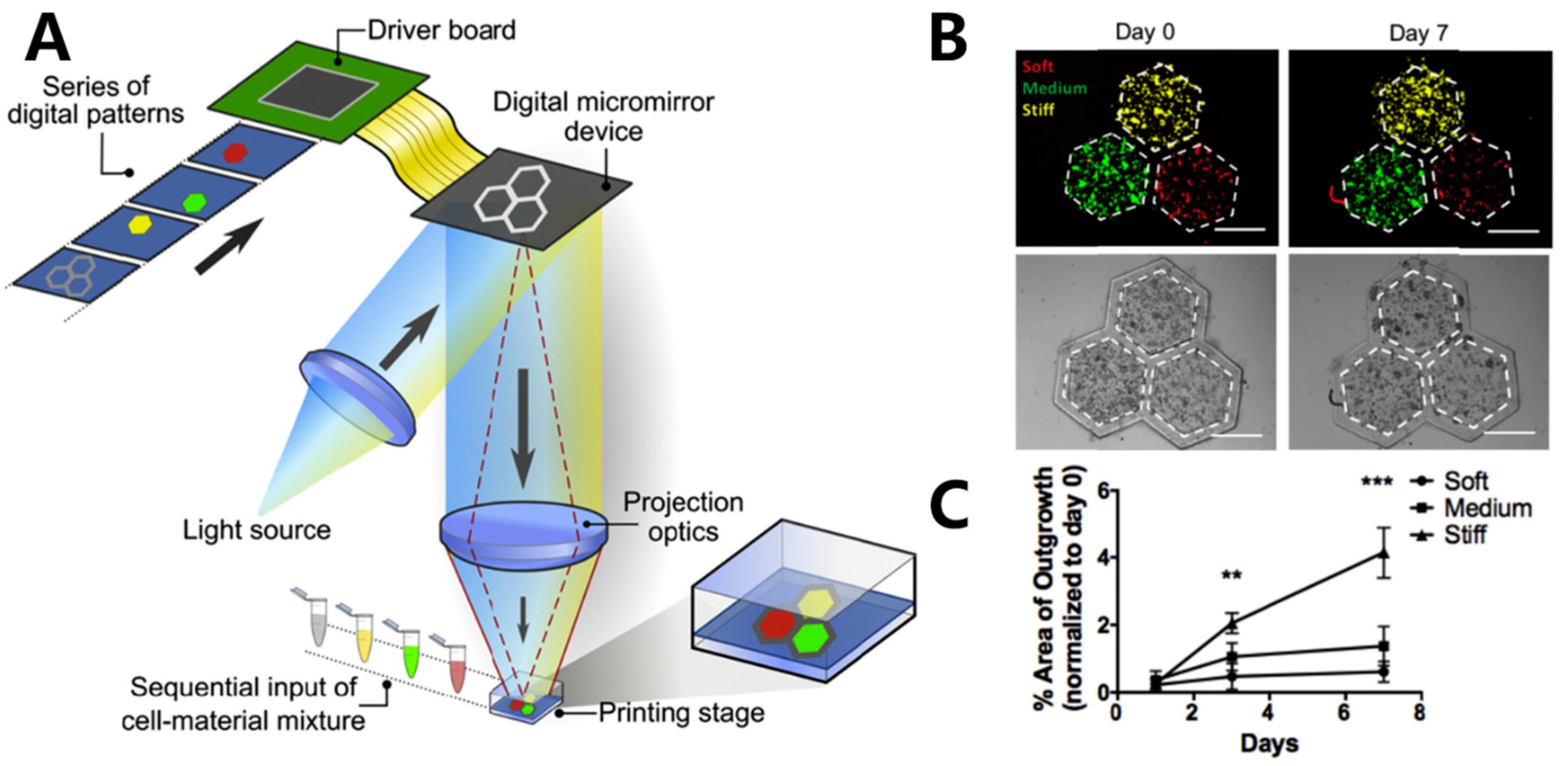
2.1. Light-Based Inkjet 3D Bioprinting
2.2. Light-Based Extrusion 3D Bioprinting
2.3. Suspension Bioprinting
2.4. Stereolithography, SLA
2.5. Digital Light Processing, DLP
2.6. Computed Axial Lithography (CAL)
3. Biological Properties of 3D Bioprinting Hydrogels
3.1. Photo-Initiators (PIs)
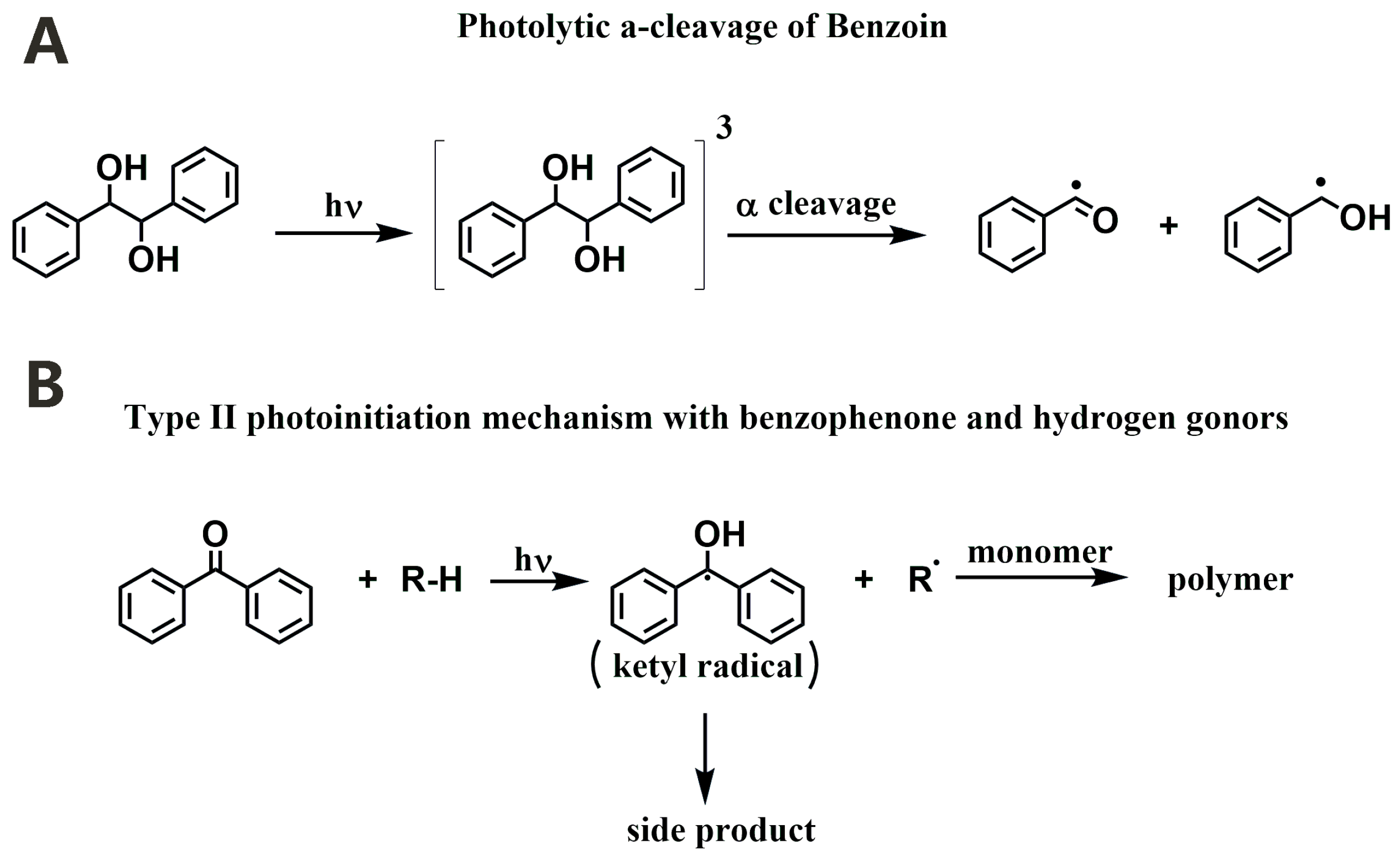
3.1.1. Norrish Type I PIs
| Light-Based Printing Techs | Explanations | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Inkjet-based bioprinting | Ink droplets are propelled out of a microscopic orifice via thermal or piezoelectric actuation and deposited drop by drop on the platform to fabricate a 3D structure. | High printing resolution. Able to print multi-materials. | Difficult to print large-scale structures. Unable to print with bio-inks of high viscosity. Tend to generate satellite droplets during printing. Shear stress that may impact cell viability. | [41,42,43] |
| Extrusion-based bioprinting | Selectively deposit bio-inks layer by layer on the printing platform. | Wide range of bio-ink viscosity. Moderate printing time. Able to print multi-materials. | Shear stress that may impact cell viability. Limited printing resolution. Limited complexity of the printed structures. Limited printing speed. | [32,53,54] |
| Suspension-based bioprinting | Bio-ink is extruded into a gel bath that is immiscible with the printed ink layer by layer, providing adequate support for shaping the bio-ink. After the completion of printing, the gel is washed away. | Provide support for bio-ink with poor mechanical properties. Provide biological environment which supports cell growth. Able to print omnidirectionally. Able to print complex structures with a high aspect ratio. | Limited suspension medium choices. | [48,55,89,90] |
| SLA-based bioprinting | Focused laser is used to selectively solidify the bio-ink layer by layer. | High printing resolution. Able to manufacture complex structures. | Limited in manufacturing scalable products. Unable to print multi-materials. Only suitable for bio-ink with low viscosity. | [58,59] |
| DLP-based bioprinting | A projector based on the digital micromirror device (DMD) or liquid crystal display (LCD) is used to solidify photoactive bio-inks with pre-designed form layer by layer. | High printing resolution. High printing speed. Able to manufacture complex structures. Able to manufacture scalable products. | Limited bio-ink choices. Unable to print multi-materials. Only suitable for bio-ink with low viscosity. | [63,64,66] |
| CAL-based bioprinting | A designed sequence of light patterns is projected onto a rotating printing reservoir containing bio-ink. The bio-ink can be solidified volumetrically. | Able to manufacture complex structures. Rapid printing speed for large constructs. Exceptional fidelity. Smooth surface for the printing structures. Wide range of bio-ink viscosity. | Limited bio-ink choices. Only suitable for transparent bio-inks. Limited printing resolution. | [68,91] |
3.1.2. Norrish Type II PIs
3.2. Biomaterials for Light-Based 3D Bioprinting
3.2.1. Gelatin
3.2.2. Chitosan
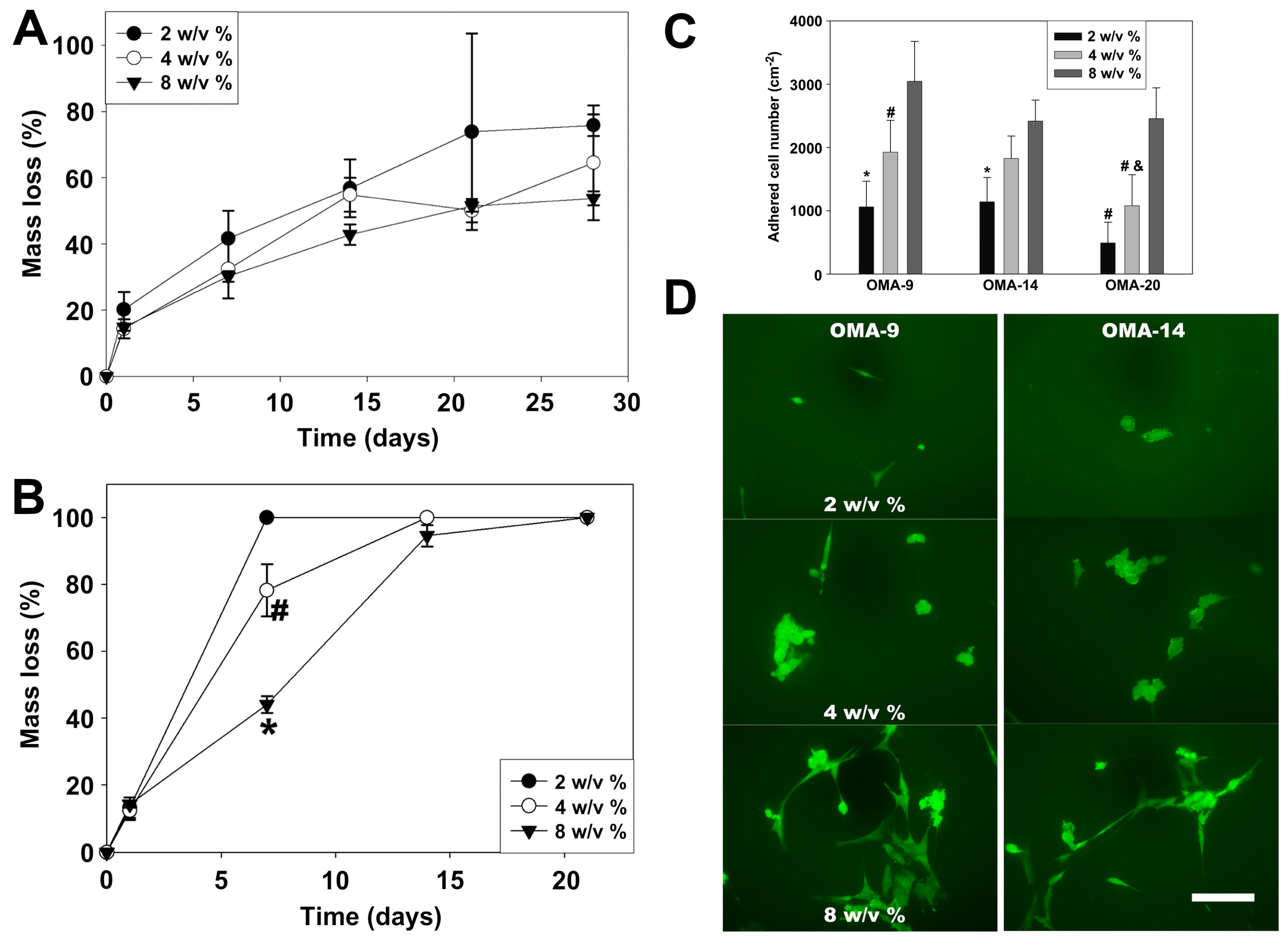
3.2.3. Alginate
3.2.4. Hyaluronic Acid
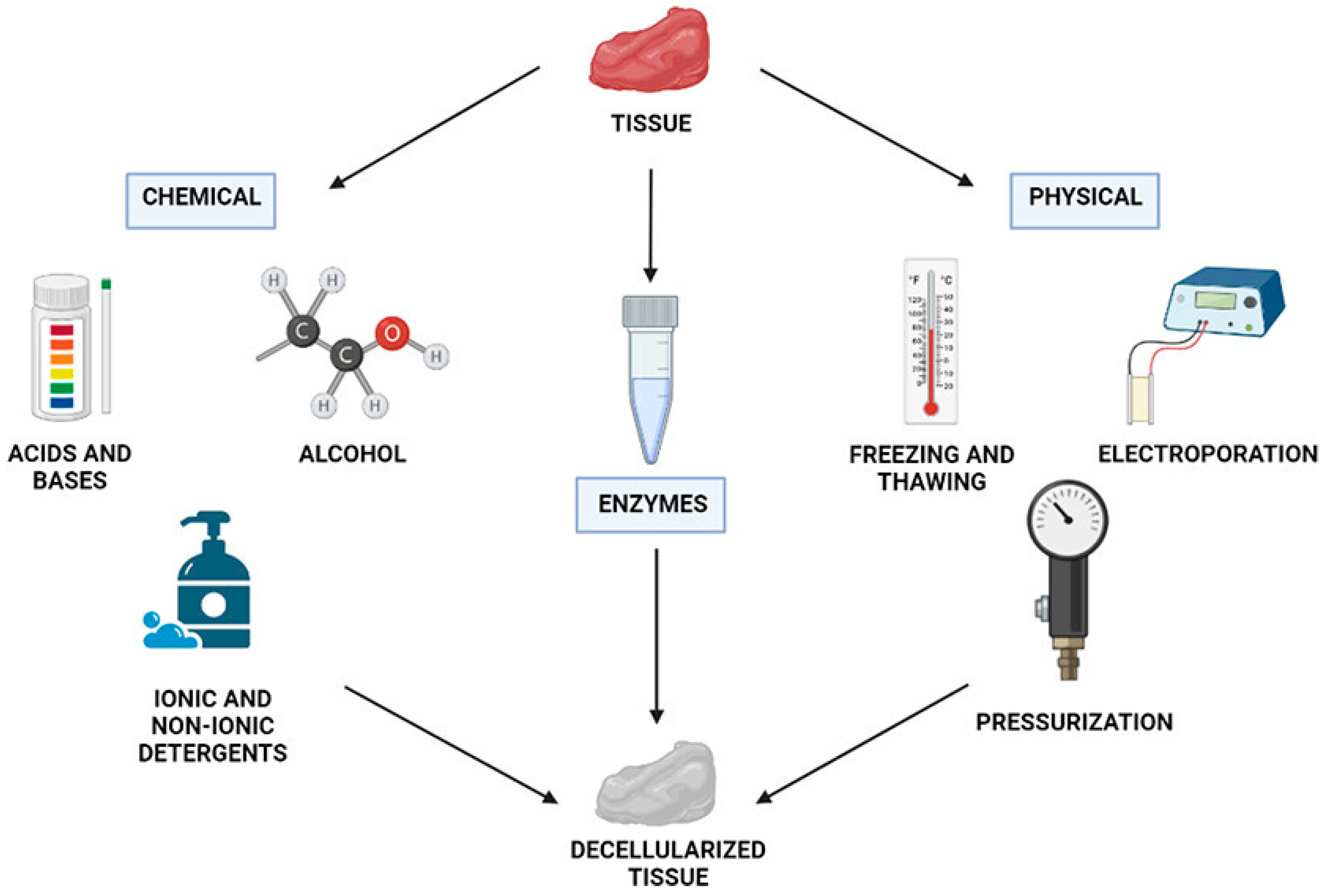
3.2.5. Decellularized Extracellular Matrix (dECM)
3.3. Properties of Bio-Inks
4. Applications of Light-Based Bioprinting
4.1. Liver Tissue Engineering
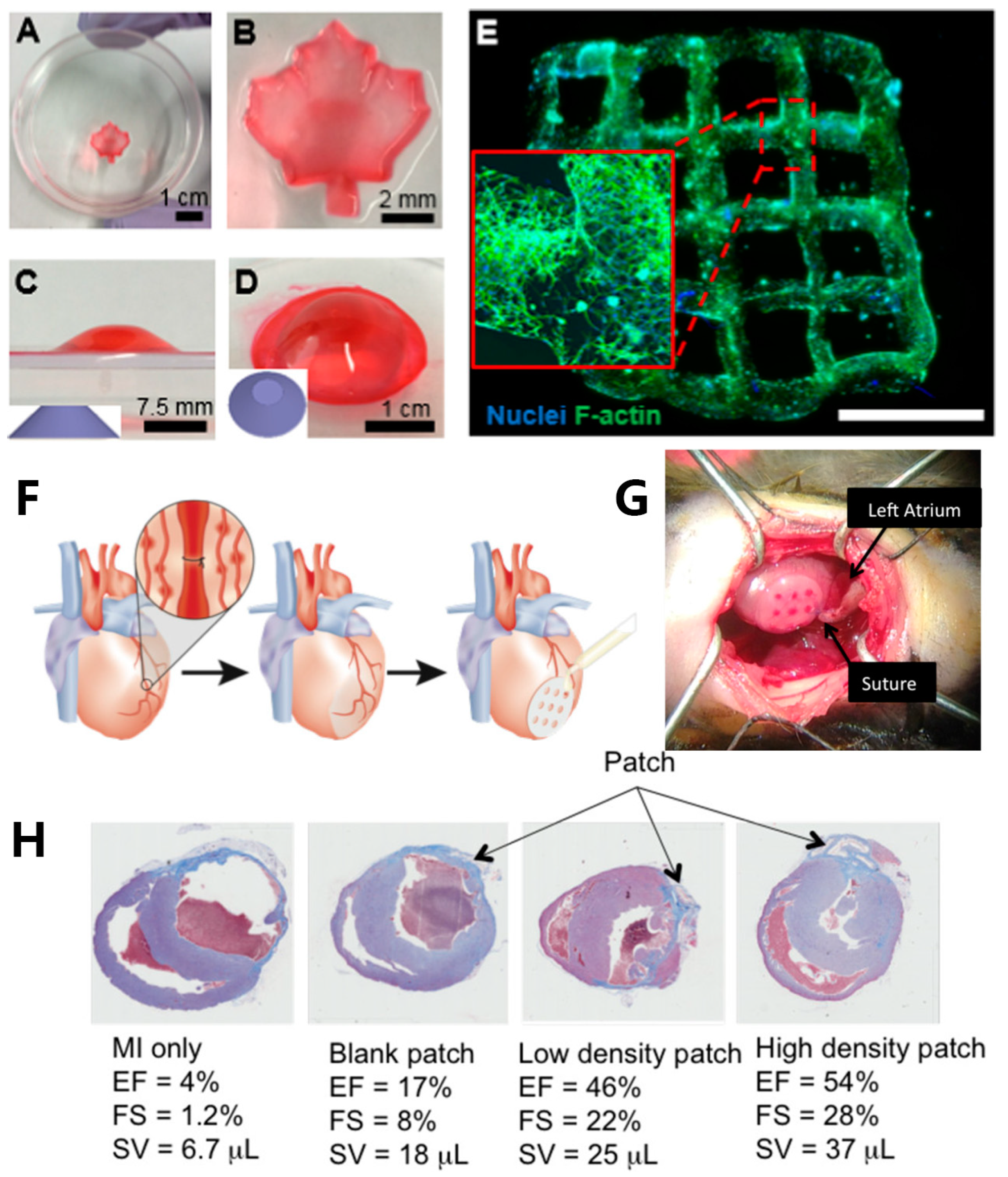
4.2. Cardiovascular Tissue Engineering
4.3. Skin Tissue Regeneration
4.4. Bone Tissue Regeneration
5. Summary and Outlook
- The printing methods have limited printing resolution, up to 20 μm. Thus, the development of novel printers capable of achieving higher printing resolution is quite necessary.
- Although many biomaterials and PIs have been developed recently, the variety of materials for light-based extrusion 3D bioprinting is relatively limited. The selection of bio-inks suitable for SLA and DLP remains constrained. Hence, the development of new bio-inks and a universal bio-ink toolbox for 3D bioprinting is an important direction.
- The production of cell-damaging species, such as initiator fragments, has a negative impact on cell fate and viability. Developing efficient and non-toxic PIs is, therefore, essential. An effective strategy for enhancing the biocompatibility of bio-ink formulations is to develop macromolecular photo-initiators with low mobility.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R. Biomaterials in drug delivery and tissue engineering: One laboratory’s experience. Acc. Chem. Res. 2000, 33, 94–101. [Google Scholar] [CrossRef]
- Sahara, M. Recent Advances in Generation of In Vitro Cardiac Organoids. Int. J. Mol. Sci. 2023, 24, 6244. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Galvez-Monton, C.; Roura, S. Cardiac Tissue Engineering: Lost in Translation or Ready for Translation? J. Am. Coll. Cardiol. 2016, 68, 724–736. [Google Scholar] [CrossRef]
- Acri, T.M.; Shin, K.; Seol, D.; Laird, N.Z.; Song, I.; Geary, S.M.; Chakka, J.L.; Martin, J.A.; Salem, A.K. Tissue Engineering for the Temporomandibular Joint. Adv. Healthc. Mater. 2019, 8, 1801236. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ma, G.; Yang, D.; Nie, J. The effect of the prefrozen process on properties of a chitosan/hydroxyapatite/poly(methyl methacrylate) composite prepared by freeze drying method used for bone tissue engineering. RSC Adv. 2015, 5, 79679–79686. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Onder, O.C.; Batool, S.R.; Nazeer, M.A. Self-assembled silk fibroin hydrogels: From preparation to biomedical applications. Mater. Adv. 2022, 3, 6920–6949. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Jang, J.; Yi, H.G.; Cho, D.W. 3D Printed Tissue Models: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731. [Google Scholar] [CrossRef]
- Ali, N.B.; Khlif, M.; Hammami, D.; Bradai, C. Optimization of structural parameters on hollow spherical cells manufactured by Fused Deposition Modeling (FDM) using Taguchi method. Cell. Polym. 2021, 41, 3–20. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Q.; Wu, H.; Feng, B.; Li, Y.; Bu, L. Synchronized 3D Printing and Corona Charging for One-Step Prototyping of Polarized Polylactic Acid Electrets. Polymers 2023, 15, 2520. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Najihi, I.; Ennawaoui, C.; Hajjaji, A.; Boughaleb, Y. Exploring the piezoelectric porous polymers for energy harvesting: A review. Energy Harvest. Syst. 2023. [Google Scholar] [CrossRef]
- Palaniyappan, S.; Veeman, D.; Sivakumar, N.K.; Natrayan, L. Development and optimization of lattice structure on the walnut shell reinforced PLA composite for the tensile strength and dimensional error properties. Structures 2022, 45, 163–178. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Yoo, J.J.; Atala, A. Solid Organ Bioprinting: Strategies to Achieve Organ Function. Chem. Rev. 2020, 120, 11093–11127. [Google Scholar] [CrossRef]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yuksekkaya, M.; Wan, K.T.; et al. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef]
- Sun, Z. Clinical Applications of Patient-Specific 3D Printed Models in Cardiovascular Disease: Current Status and Future Directions. Biomolecules 2020, 10, 1577. [Google Scholar] [CrossRef]
- Oh, K.C.; Park, J.M.; Shim, J.S.; Kim, J.H.; Kim, J.E.; Kim, J.H. Assessment of metal sleeve-free 3D-printed implant surgical guides. Dent. Mater. 2019, 35, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Najihi, I.; Ennawaoui, C.; Hajjaji, A.; Boughaleb, Y. 3D printed cellular piezoelectric polymers for smart sensors/autonomous energy harvesters. Mater. Today Proc. 2022, 66, 437–440. [Google Scholar] [CrossRef]
- Mueller, E.; Poulin, I.; Bodnaryk, W.J.; Hoare, T. Click Chemistry Hydrogels for Extrusion Bioprinting: Progress, Challenges, and Opportunities. Biomacromolecules 2022, 23, 619–640. [Google Scholar] [CrossRef]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab. Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef] [PubMed]
- Piironen, K.; Haapala, M.; Talman, V.; Jarvinen, P.; Sikanen, T. Cell adhesion and proliferation on common 3D printing materials used in stereolithography of microfluidic devices. Lab. Chip 2020, 20, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Shi, Y.; Zhang, Y.; Zhao, Q.; Wu, J. Reconfigurable Polymer Networks for Digital Light Processing 3D Printing. ACS Appl. Mater. Interfaces 2021, 13, 15584–15590. [Google Scholar] [CrossRef]
- Huang, Z.; Chi-Pong Tsui, G.; Deng, Y.; Tang, C.-Y. Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications. Nanotechnol. Rev. 2020, 9, 1118–1136. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, e2002931. [Google Scholar] [CrossRef]
- Zennifer, A.; Manivannan, S.; Sethuraman, S.; Kumbar, S.G.; Sundaramurthi, D. 3D bioprinting and photocrosslinking: Emerging strategies & future perspectives. Biomater. Adv. 2022, 134, 112576. [Google Scholar]
- Lim, K.S.; Galarraga, J.H.; Cui, X.; Lindberg, G.C.J.; Burdick, J.A.; Woodfield, T.B.F. Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem. Rev. 2020, 120, 10662–10694. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Lim, K.S.; Schon, B.S.; Mekhileri, N.V.; Brown, G.C.J.; Chia, C.M.; Prabakar, S.; Hooper, G.J.; Woodfield, T.B.F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016, 2, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A review on fabricating tissue scaffolds using vat photopolymerization. Acta Biomater. 2018, 74, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, L.; Li, Z.; Wang, S.; Shi, J.; Tang, W.; Li, N.; Yang, J. The recent development of vat photopolymerization: A review. Addit. Manuf. 2021, 48, 102423. [Google Scholar] [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Zheng, F.; Derby, B.; Wong, J. Fabrication of microvascular constructs using high resolution electrohydrodynamic inkjet printing. Biofabrication 2021, 13, 035006. [Google Scholar] [CrossRef]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- Zaupa, A.; Terraza, C.; Abarzua-Illanes, P.N.; Byres, N.; Zavala, G.; Cuenca, J.; Hidalgo, C.; Viafara-Garcia, S.M.; Wolf, B.; Pino-Lagos, K.; et al. A Psychrophilic GelMA: Breaking Technical and Immunological Barriers for Multimaterial High-Resolution 3D Bioprinting. Biomacromolecules 2023, 24, 150–165. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef]
- Mugnaini, G.; Resta, C.; Poggi, G.; Bonini, M. Photopolymerizable pullulan: Synthesis, self-assembly and inkjet printing. J. Colloid. Interface Sci. 2021, 592, 430–439. [Google Scholar] [CrossRef]
- Tigner, T.J.; Rajput, S.; Gaharwar, A.K.; Alge, D.L. Comparison of Photo Cross Linkable Gelatin Derivatives and Initiators for Three-Dimensional Extrusion Bioprinting. Biomacromolecules 2020, 21, 454–463. [Google Scholar] [CrossRef]
- Compaan, A.M.; Song, K.; Huang, Y. Gellan Fluid Gel as a Versatile Support Bath Material for Fluid Extrusion Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jungst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, e1906423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing Based Three-dimensional Printing for Medical Applications. Int. J. Bioprint 2020, 6, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2016, 29, 1604983. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.; Zehnder, S.M.; Rowe, K.G.; Jain, S.; Nixon, R.M.; Sawyer, W.G.; Angelini, T.E. Writing in the granular gel medium. Sci. Adv. 2015, 1, e1500655. [Google Scholar] [CrossRef]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef]
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef]
- Lam, T.; Dehne, T.; Kruger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef]
- Melhem, M.R.; Park, J.; Knapp, L.; Reinkensmeyer, L.; Cvetkovic, C.; Flewellyn, J.; Lee, M.K.; Jensen, T.W.; Bashir, R.; Kong, H.; et al. 3D Printed Stem-Cell-Laden, Microchanneled Hydrogel Patch for the Enhanced Release of Cell-Secreting Factors and Treatment of Myocardial Infarctions. ACS Biomater. Sci. Eng. 2017, 3, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mille, L.S.; Robledo, J.A.; Uribe, T.; Huerta, V.; Zhang, Y.S. Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing. Adv. Healthc. Mater. 2020, 9, e2000156. [Google Scholar] [CrossRef]
- Goodarzi Hosseinabadi, H.; Dogan, E.; Miri, A.K.; Ionov, L. Digital Light Processing Bioprinting Advances for Microtissue Models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience 2023, 26, 106039. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Qian, Y.; Lu, K.; Zhu, Z.; Siow, L.; Zhang, C.; Zhou, S.; Gu, T.; Yin, J.; Yu, M.; et al. Digital light processing (DLP) in tissue engineering: From promise to reality, and perspectives. Biomed. Mater. 2022, 17, 062004. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018, 185, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Hull, S.M.; Lou, J.; Lindsay, C.D.; Navarro, R.S.; Cai, B.; Brunel, L.G.; Westerfield, A.D.; Xia, Y.; Heilshorn, S.C. 3D bioprinting of dynamic hydrogel bioinks enabled by small molecule modulators. Sci. Adv. 2023, 9, eade7880. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jungst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D Printing of Bioinspired Biomaterials for Tissue Regeneration. Adv. Healthc. Mater. 2020, 9, 2000208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Yan, Z.; Du, L.; Tang, W.; Phillips, D.L. Photochemical alpha-Cleavage Reaction of 3′,5′-Dimethoxybenzoin: A Combined Time-Resolved Spectroscopy and Computational Chemistry Study. Molecules 2020, 25, 3548. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Liu, S.; Huang, C.; Nie, J.; He, Y. Significantly improve the photoinitiation ability of hydroxyalkyl-derived polymerizable α-hydroxyalkylacetophenone photoinitiators by blocking hyperconjugation. J. Photochem. Photobiol. A Chem. 2021, 419, 113451. [Google Scholar] [CrossRef]
- Gonsalvi, L.; Peruzzini, M. Novel synthetic pathways for bis(acyl)phosphine oxide photoinitiators. Angew. Chem. Int. Ed. Engl. 2012, 51, 7895–7897. [Google Scholar] [CrossRef] [PubMed]
- Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.A.; Lalevee, J. New Phosphine Oxides as High Performance Near—UV Type I Photoinitiators of Radical Polymerization. Molecules 2020, 25, 1671. [Google Scholar] [CrossRef]
- Nehlig, E.; Schneider, R.; Vidal, L.; Clavier, G.; Balan, L. Silver nanoparticles coated with thioxanthone derivative as hybrid photoinitiating systems for free radical polymerization. Langmuir 2012, 28, 17795–17802. [Google Scholar] [CrossRef]
- Hola, E.; Fiedor, P.; Dzienia, A.; Ortyl, J. Visible-Light Amine Thioxanthone Derivatives as Photoredox Catalysts for Photopolymerization Processes. ACS Appl. Polym. Mater. 2021, 3, 5547–5558. [Google Scholar] [CrossRef]
- Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; González-López, J.A.; Trejo-Carbajal, N.; Herrera-González, A.M. Evaluation of new coinitiators of camphorquinone useful in the radical photopolymerization of dental monomers. J. Photochem. Photobiol. A Chem. 2020, 403, 112844. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Nixon, E.J.; Wang, H.-Y.; Dhawan, U.; Huang, Y.-W.; Huang, S.-H.; Jiang, C.-P.; Kuo, Y.-J.; Chung, R.-J. Photocrosslinked Gelatin Methacryloyl (GelMA)/Hyaluronic Acid Methacryloyl (HAMA) Composite Scaffold Using Anthocyanidin as a Photoinitiator for Bone Tissue Regeneration. ACS Appl. Polym. Mater. 2023, 5, 6012–6021. [Google Scholar] [CrossRef]
- Montazerian, H.; Baidya, A.; Haghniaz, R.; Davoodi, E.; Ahadian, S.; Annabi, N.; Khademhosseini, A.; Weiss, P.S. Stretchable and Bioadhesive Gelatin Methacryloyl-Based Hydrogels Enabled by in Situ Dopamine Polymerization. ACS Appl. Mater. Interfaces 2021, 13, 40290–40301. [Google Scholar] [CrossRef] [PubMed]
- De Moor, L.; Smet, J.; Plovyt, M.; Bekaert, B.; Vercruysse, C.; Asadian, M.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Engineering microvasculature by 3D bioprinting of prevascularized spheroids in photo-crosslinkable gelatin. Biofabrication 2021, 13, 045021. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wu, J.; Reinhart-King, C.A.; Chu, C.C. Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan-polyethylene glycol diacrylate hybrid hydrogels. Acta Biomater. 2010, 6, 3908–3918. [Google Scholar] [CrossRef] [PubMed]
- Amsden, B.G.; Sukarto, A.; Knight, D.K.; Shapka, S.N. Methacrylated glycol chitosan as a photopolymerizable biomaterial. Biomacromolecules 2007, 8, 3758–3766. [Google Scholar] [CrossRef]
- Han, W.T.; Jang, T.; Chen, S.; Chong, L.S.H.; Jung, H.D.; Song, J. Improved cell viability for large-scale biofabrication with photo-crosslinkable hydrogel systems through a dual-photoinitiator approach. Biomater. Sci. 2019, 8, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Benedikt, S.; Wang, J.; Markovic, M.; Moszner, N.; Dietliker, K.; Ovsianikov, A.; Grützmacher, H.; Liska, R. Highly efficient water-soluble visible light photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 473–479. [Google Scholar] [CrossRef]
- Kunwar, P.; Jannini, A.V.S.; Xiong, Z.; Ransbottom, M.J.; Perkins, J.S.; Henderson, J.H.; Hasenwinkel, J.M.; Soman, P. High-Resolution 3D Printing of Stretchable Hydrogel Structures Using Optical Projection Lithography. ACS Appl. Mater. Interfaces 2020, 12, 1640–1649. [Google Scholar] [CrossRef]
- Ghazali, H.S.; Askari, E.; Seyfoori, A.; Naghib, S.M. A high-absorbance water-soluble photoinitiator nanoparticle for hydrogel 3D printing: Synthesis, characterization and in vitro cytotoxicity study. Sci. Rep. 2023, 13, 8577–8589. [Google Scholar] [CrossRef]
- Mirdamadi, E.; Tashman, J.W.; Shiwarski, D.J.; Palchesko, R.N.; Feinberg, A.W. FRESH 3D Bioprinting a Full-Size Model of the Human Heart. ACS Biomater. Sci. Eng. 2020, 6, 6453–6459. [Google Scholar] [CrossRef]
- Mirdamadi, E.; Muselimyan, N.; Koti, P.; Asfour, H.; Sarvazyan, N. Agarose Slurry as a Support Medium for Bioprinting and Culturing Freestanding Cell-Laden Hydrogel Constructs. 3D Print. Addit. Manuf. 2019, 6, 158–164. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, e1904209. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Kirschner, J.; Szillat, F.; Bouzrati-Zerelli, M.; Becht, J.M.; Klee, J.E.; Lalevee, J. Sulfinates and sulfonates as high performance co-initiators in CQ based systems: Towards aromatic amine-free systems for dental restorative materials. Dent. Mater. 2020, 36, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kowsari, K.; Lee, W.; Yoo, S.-S.; Fang, N.X. Scalable visible light 3D printing and bioprinting using an organic light-emitting diode microdisplay. iScience 2021, 24, 103372. [Google Scholar] [CrossRef] [PubMed]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; El-Betany, A.; Menzel, H.; Chen, X. Influence of photoinitiator concentration and irradiation time on the crosslinking performance of visible-light activated pullulan-HEMA hydrogels. Int. J. Biol. Macromol. 2018, 120, 1884–1892. [Google Scholar] [CrossRef]
- Chen, L.; Kenkel, S.M.; Hsieh, P.H.; Gryka, M.C.; Bhargava, R. Freeform Three-Dimensionally Printed Microchannels via Surface-Initiated Photopolymerization Combined with Sacrificial Molding. ACS Appl. Mater. Interfaces 2020, 12, 50105–50112. [Google Scholar] [CrossRef]
- Petta, D.; Grijpma, D.W.; Alini, M.; Eglin, D.; D’Este, M. Three-Dimensional Printing of a Tyramine Hyaluronan Derivative with Double Gelation Mechanism for Independent Tuning of Shear Thinning and Postprinting Curing. ACS Biomater. Sci. Eng. 2018, 4, 3088–3098. [Google Scholar] [CrossRef]
- Piluso, S.; Flores Gomez, D.; Dokter, I.; Moreira Texeira, L.; Li, Y.; Leijten, J.; van Weeren, R.; Vermonden, T.; Karperien, M.; Malda, J. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J. Mater. Chem. B 2020, 8, 9566–9575. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine 2014, 10, 491–501. [Google Scholar] [CrossRef]
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 2462–2471. [Google Scholar] [CrossRef]
- Nur Karakus, F.; Bulgurcuoglu Kuran, S.; Solakoglu, S. Effect of curcumin on sperm parameters after the cryopreservation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 161–166. [Google Scholar] [CrossRef]
- Lin, F.; Fan, W.; Wise, G.E. Eosin Y staining of proteins in polyacrylamide gels. Anal. Biochem. 1991, 196, 279–283. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Chesneau, E. Polymérisation induite sous irradiation laser visible, 4†. Le système éosine/photoamorceur ultra-violet/amine. Die Makromol. Chem. 1991, 192, 245–260. [Google Scholar] [CrossRef]
- Aguirre-Soto, A.; Kim, S.; Kaastrup, K.; Sikes, H.D. On the role of N-vinylpyrrolidone in the aqueous radical-initiated copolymerization with PEGDA mediated by eosin Y in the presence of O2. Polym. Chem. 2019, 10, 926–937. [Google Scholar] [CrossRef]
- Hao, Y.; Shih, H.; Muňoz, Z.; Kemp, A.; Lin, C.-C. Visible light cured thiol-vinyl hydrogels with tunable degradation for 3D cell culture. Acta Biomater. 2014, 10, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, M.H.; Song, J.H.; Kang, C.; Park, W.H. Dual crosslinked alginate hydrogels by riboflavin as photoinitiator. Int. J. Biol. Macromol. 2020, 154, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, S.G.; Previtali, C.M.; Rufs, A.M.; Encinas, M.V. Riboflavin/Triethanolamine as Photoinitiator System of Vinyl Polymerization. A Mechanistic Study by Laser Flash Photolysis. Macromolecules 1999, 32, 2920–2924. [Google Scholar] [CrossRef]
- Grotzinger, C.; Burget, D.; Jacques, P.; Fouassier, J.P. Visible light induced photopolymerization: Speeding up the rate of polymerization by using co-initiators in dye/amine photoinitiating systems. Polymer 2003, 44, 3671–3677. [Google Scholar] [CrossRef]
- Fischer, B.B.; Krieger-Liszkay, A.; Eggen, R.L. Photosensitizers neutral red (type I) and rose bengal (type II) cause light-dependent toxicity in Chlamydomonas reinhardtii and induce the Gpxh gene via increased singlet oxygen formation. Environ. Sci. Technol. 2004, 38, 6307–6313. [Google Scholar] [CrossRef]
- Ahn, D.; Stevens, L.M.; Zhou, K.; Page, Z.A. Rapid High-Resolution Visible Light 3D Printing. ACS Cent. Sci. 2020, 6, 1555–1563. [Google Scholar] [CrossRef]
- Balzani, V.; Ceroni, P.; Credi, A.; Venturi, M. Ruthenium tris(bipyridine) complexes: Interchange between photons and electrons in molecular-scale devices and machines. Coord. Chem. Rev. 2021, 433, 213758. [Google Scholar] [CrossRef]
- Fancy, D.A.; Kodadek, T. Chemistry for the analysis of protein–protein interactions: Rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. USA 1999, 96, 6020–6024. [Google Scholar] [CrossRef]
- Shaukat, U.; Rossegger, E.; Schlogl, S. A Review of Multi-Material 3D Printing of Functional Materials via Vat Photopolymerization. Polymers 2022, 14, 2449. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Leu Alexa, R.; Cucuruz, A.; Ghitulica, C.D.; Voicu, G.; Stamat Balahura, L.R.; Dinescu, S.; Vlasceanu, G.M.; Stavarache, C.; Ianchis, R.; Iovu, H.; et al. 3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 1841. [Google Scholar] [CrossRef]
- Man, K.; Barroso, I.A.; Brunet, M.Y.; Peacock, B.; Federici, A.S.; Hoey, D.A.; Cox, S.C. Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair. Int. J. Mol. Sci. 2022, 23, 832. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R.; et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladaviere, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels incorporating colloids for sustained drug delivery. Carbohydr. Polym. 2022, 275, 118689. [Google Scholar] [CrossRef]
- Deng, L.; Wang, L.; Li, L.; Gong, Z.; Wang, R.; Fei, W.; Zhou, Y.; Wang, F. Bioabsorbable Fibrillar Gauze Dressing Based on N-Carboxyethyl Chitosan Gelling Fibers for Fatal Hemorrhage Control. ACS Appl. Bio Mater. 2023, 6, 899–907. [Google Scholar] [CrossRef]
- Zhang, R.; Chang, S.J.; Jing, Y.; Wang, L.; Chen, C.J.; Liu, J.T. Application of chitosan with different molecular weights in cartilage tissue engineering. Carbohydr. Polym. 2023, 314, 120890. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, J.; Rousseau, D.; Tang, C.-H. Fabrication of chitosan colloidal gels via pH-mediated self-association. Food Hydrocolloids 2023, 135, 108126. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-based inks for 3D printing and bioprinting. Green. Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Hussain, C.M. Current achievements in 3D bioprinting technology of chitosan and its hybrids. New J. Chem. 2021, 45, 10565–10576. [Google Scholar] [CrossRef]
- Yang, D.H.; Seo, D.I.; Lee, D.-W.; Bhang, S.H.; Park, K.; Jang, G.; Kim, C.H.; Chun, H.J. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J. Ind. Eng. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Cao, Y.; Cong, H.; Yu, B.; Shen, Y. A review on the synthesis and development of alginate hydrogels for wound therapy. J. Mater. Chem. B 2023, 11, 2801–2829. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. State-of-the-art of 3D printing technology of alginate-based hydrogels-An emerging technique for industrial applications. Adv. Colloid. Interface Sci. 2021, 293, 102436. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Hasany, M.; Talebian, S.; Sadat, S.; Ranjbar, N.; Mehrali, M.; Wallace, G.G.; Mehrali, M. Synthesis, properties, and biomedical applications of alginate methacrylate (ALMA)-based hydrogels: Current advances and challenges. Appl. Mater. Today 2021, 24, 101150. [Google Scholar] [CrossRef]
- Jeon, O.; Alt, D.S.; Ahmed, S.M.; Alsberg, E. The effect of oxidation on the degradation of photocrosslinkable alginate hydrogels. Biomaterials 2012, 33, 3503–3514. [Google Scholar] [CrossRef]
- Ooi, H.W.; Mota, C.; Ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol-Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Komatani, K.; Taya, M. Glucose-triggered co-enzymatic hydrogelation of aqueous polymer solutions. RSC Adv. 2012, 2, 1502–1507. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Sakai, S.; Taya, M. Characterization of encapsulated cells within hyaluronic acid and alginate microcapsules produced via horseradish peroxidase-catalyzed crosslinking. J. Biomater. Sci. Polym. Ed. 2019, 30, 295–307. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; McDonough, S.L.; Philip, P.A.; Hingorani, S.R.; Lacy, J.; Kortmansky, J.S.; Thumar, J.; Chiorean, E.G.; Shields, A.F.; Behl, D.; et al. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients with Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J. Clin. Oncol. 2019, 37, 1062–1069. [Google Scholar] [CrossRef]
- Kim, H.; Shin, M.; Han, S.; Kwon, W.; Hahn, S.K. Hyaluronic Acid Derivatives for Translational Medicines. Biomacromolecules 2019, 20, 2889–2903. [Google Scholar] [CrossRef]
- Gaffney, J.; Matou-Nasri, S.; Grau-Olivares, M.; Slevin, M. Therapeutic applications of hyaluronan. Mol. Biosyst. 2010, 6, 437–443. [Google Scholar] [CrossRef]
- Chaudhry, G.E.; Akim, A.; Naveed Zafar, M.; Safdar, N.; Sung, Y.Y.; Muhammad, T.S.T. Understanding Hyaluronan Receptor (CD44) Interaction, HA-CD44 Activated Potential Targets in Cancer Therapeutics. Adv. Pharm. Bull. 2021, 11, 426–438. [Google Scholar] [CrossRef]
- Kim, S.J.; Owen, S.C. Hyaluronic acid binding to CD44S is indiscriminate of molecular weight. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183348. [Google Scholar] [CrossRef]
- Hofinger, E.S.; Hoechstetter, J.; Oettl, M.; Bernhardt, G.; Buschauer, A. Isoenzyme-specific differences in the degradation of hyaluronic acid by mammalian-type hyaluronidases. Glycoconj. J. 2008, 25, 101–109. [Google Scholar] [CrossRef]
- Shigefuji, M.; Tokudome, Y. Nanoparticulation of hyaluronic acid: A new skin penetration enhancing polyion complex formulation: Mechanism and future potential. Materialia 2020, 14, 100879. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Wu, L.; Qu, H.; Song, D.; Huang, H.; Wu, C.; Xu, M. Rational design of porous starch/hyaluronic acid composites for hemostasis. Int. J. Biol. Macromol. 2020, 158, 1319–1329. [Google Scholar] [CrossRef]
- Smeds, K.A.; Grinstaff, M.W. Photocrosslinkable polysaccharides forin situ hydrogel formation. J. Biomed. Mater. Res. 2001, 54, 115–121. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef]
- Fan, P.; Dong, Q.; Yang, J.; Chen, Y.; Yang, H.; Gu, S.; Xu, W.; Zhou, Y. Flexible dual-functionalized hyaluronic acid hydrogel adhesives formed in situ for rapid hemostasis. Carbohydr. Polym. 2023, 313, 120854. [Google Scholar] [CrossRef]
- Loebel, C.; Broguiere, N.; Alini, M.; Zenobi-Wong, M.; Eglin, D. Microfabrication of Photo-Cross-Linked Hyaluronan Hydrogels by Single- and Two-Photon Tyramine Oxidation. Biomacromolecules 2015, 16, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Nakamoto, T.; Kawazoe, N.; Chen, G. Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells. Biomaterials 2015, 52, 199–207. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Zhou, B.; Duan, X.; Weng, W.; Cheng, K.; Wang, H.; Lin, J. Cell-Sheet-Derived ECM Coatings and Their Effects on BMSCs Responses. ACS Appl. Mater. Interfaces 2018, 10, 11508–11518. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.A., Jr.; Hoehn, J.G. Use of commercial porcine skin for wound dressings. Plast. Reconstr. Surg. 1973, 52, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Gilbert, T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Spang, M.T.; Christman, K.L. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater. 2018, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Dziki, J.; Sobieski, E.; Smoulder, A.; Castleton, A.; Turner, N.; White, L.J.; Badylak, S.F. Restoring Mucosal Barrier Function and Modifying Macrophage Phenotype with an Extracellular Matrix Hydrogel: Potential Therapy for Ulcerative Colitis. J. Crohns Colitis 2017, 11, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96. [Google Scholar] [CrossRef]
- De Paula, A.G.P.; de Lima, J.D.; Bastos, T.S.B.; Czaikovski, A.P.; Dos Santos Luz, R.B.; Yuasa, B.S.; Smanioto, C.C.S.; Robert, A.W.; Braga, T.T. Decellularized Extracellular Matrix: The Role of This Complex Biomaterial in Regeneration. ACS Omega 2023, 8, 22256–22267. [Google Scholar] [CrossRef]
- Dabaghi, M.; Saraei, N.; Carpio, M.B.; Nanduri, V.; Ungureanu, J.; Babi, M.; Chandiramohan, A.; Noble, A.; Revill, S.D.; Zhang, B.; et al. A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture. Cells 2021, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T. Decellularized Extracellular Matrix for Cell Biology. Curr. Protoc. 2021, 1, e318. [Google Scholar] [CrossRef]
- Biehl, A.; Gracioso Martins, A.M.; Davis, Z.G.; Sze, D.; Collins, L.; Mora-Navarro, C.; Fisher, M.B.; Freytes, D.O. Towards a standardized multi-tissue decellularization protocol for the derivation of extracellular matrix materials. Biomater. Sci. 2023, 11, 641–654. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, N.; Zamanian, A. Decellularized extracellular matrix bioinks and their application in skin tissue engineering. Bioprinting 2020, 20, e00095. [Google Scholar] [CrossRef]
- Bejleri, D.; Davis, M.E. Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, e1801217. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Li, T.; Javed, R.; Ao, Q. Xenogeneic Decellularized Extracellular Matrix-based Biomaterials for Peripheral Nerve Repair and Regeneration. Curr. Neuropharmacol. 2021, 19, 2152–2163. [Google Scholar] [CrossRef]
- Lewis, P.L.; Su, J.; Yan, M.; Meng, F.; Glaser, S.S.; Alpini, G.D.; Green, R.M.; Sosa-Pineda, B.; Shah, R.N. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci. Rep. 2018, 8, 12220. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Nam, S.A.; Yi, J.; Kim, J.Y.; Lee, J.Y.; Park, S.Y.; Sen, T.; Choi, Y.M.; Lee, J.Y.; Kim, H.L.; et al. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv. Sci. 2022, 9, e2103526. [Google Scholar] [CrossRef]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and dECM/PCL resins for 3D printing of acellular in vitro tissue scaffolds by stereolithography. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110958. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, K.; Nie, J.; Sun, M.; Fu, J.; Wang, H.; He, Y. Modeling the printability of photocuring and strength adjustable hydrogel bioink during projection-based 3D bioprinting. Biofabrication 2021, 13, 035032. [Google Scholar] [CrossRef]
- Vurat, M.T.; Ergun, C.; Elçin, A.E.; Elçin, Y.M. 3D Bioprinting of Tissue Models with Customized Bioinks. Adv. Exp. Med. Biol. 2020, 1249, 67–84. [Google Scholar]
- Kannurpatti, A.R.; Anseth, J.W.; Bowman, C.N. A study of the evolution of mechanical properties and structural heterogeneity of polymer networks formed by photopolymerizations of multifunctional (meth)acrylates. Polymer 1998, 39, 2507–2513. [Google Scholar] [CrossRef]
- Xin, S.; Chimene, D.; Garza, J.E.; Gaharwar, A.K.; Alge, D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019, 7, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lum, N.; Seow, L.; Lim, P.; Tan, L. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef]
- Rodríguez-Rivero, C.; Hilliou, L.; Martín del Valle, E.M.; Galán, M.A. Rheological characterization of commercial highly viscous alginate solutions in shear and extensional flows. Rheol. Acta 2014, 53, 559–570. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Liska, R.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. Cross-Linkable Gelatins with Superior Mechanical Properties Through Carboxylic Acid Modification: Increasing the Two-Photon Polymerization Potential. Biomacromolecules 2017, 18, 3260–3272. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Janarthanan, G.; Noh, I. Nano-biomaterials for designing functional bioinks towards complex tissue and organ regeneration in 3D bioprinting. Addit. Manuf. 2021, 37, 101639. [Google Scholar] [CrossRef]
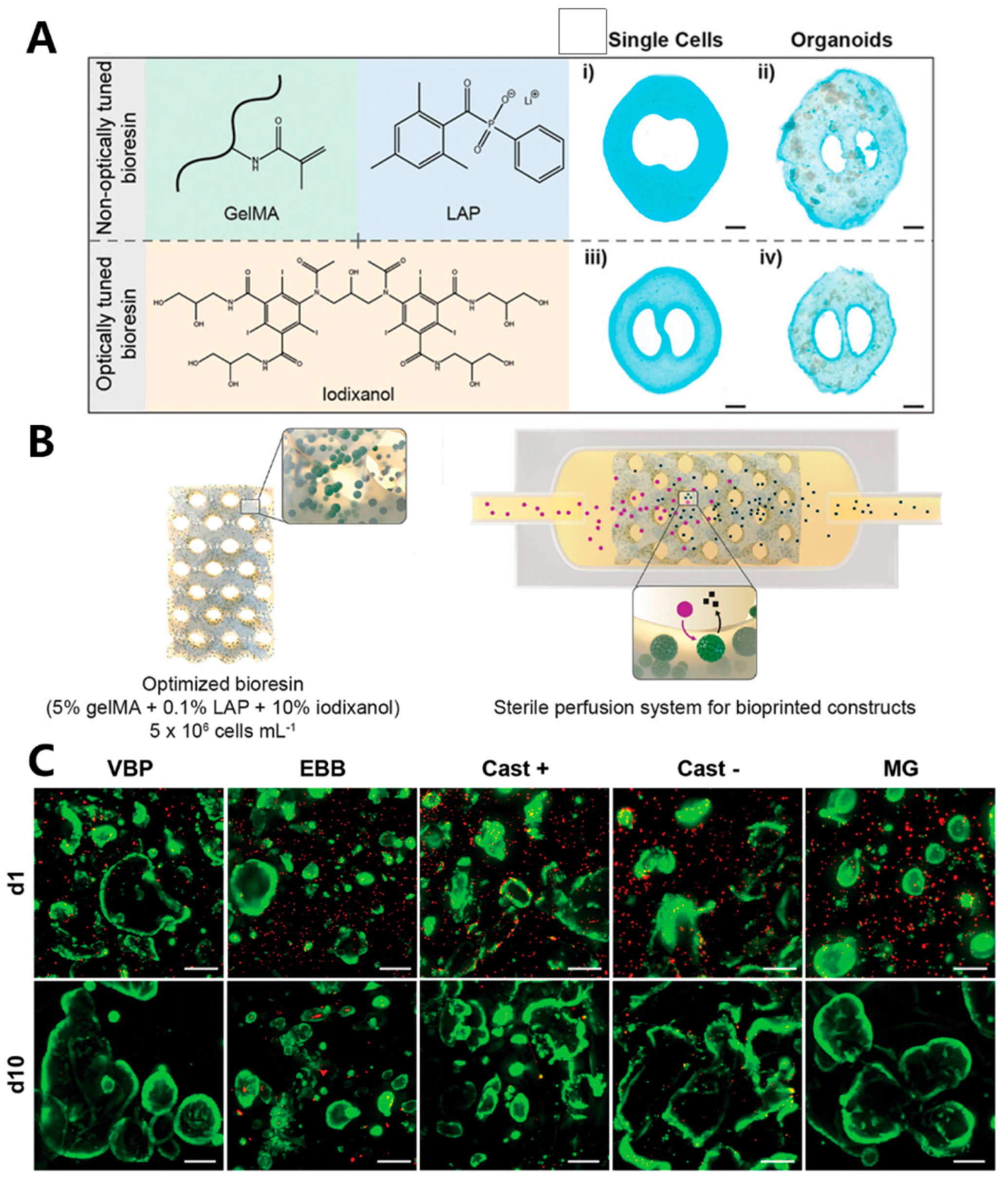
- Bernal, P.N.; Bouwmeester, M.; Madrid-Wolff, J.; Falandt, M.; Florczak, S.; Rodriguez, N.G.; Li, Y.; Grossbacher, G.; Samsom, R.A.; van Wolferen, M.; et al. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv. Mater. 2022, 34, e2110054. [Google Scholar] [CrossRef]
- Han, X.; Courseaus, J.; Khamassi, J.; Nottrodt, N.; Engelhardt, S.; Jacobsen, F.; Bierwisch, C.; Meyer, W.; Walter, T.; Weisser, J.; et al. Optimized vascular network by stereolithography for tissue engineered skin. Int. J. Bioprint 2018, 4, 134. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Designing functional hyaluronic acid-based hydrogels for cartilage tissue engineering. Mater. Today Bio 2022, 17, 100495. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Sphabmixay, P.; Raredon, M.S.B.; Wang, A.J.; Lee, H.; Hammond, P.T.; Fang, N.X.; Griffith, L.G. High resolution stereolithography fabrication of perfusable scaffolds to enable long-term meso-scale hepatic culture for disease modeling. Biofabrication 2021, 13, 045024. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.L.; Shah, R.N. 3D Printing for Liver Tissue Engineering: Current Approaches and Future Challenges. Curr. Transplant. Rep. 2016, 3, 100–108. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Tang, F.; Jiang, H.; Zhou, Z.; Hao, X.; Zhang, J.M. Application of 3D Bioprinting in Liver Diseases. Micromachines 2023, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, J.; Kurzatkowska, M.; Sobczak, M.; Piotrowska, U. Utilization of 3D bioprinting technology in creating human tissue and organoid models for preclinical drug research—State-of-the-art. Int. J. Pharm. 2023, 644, 123313. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Zhang, S.; Yang, H.; Mao, Y. 3D bioprinted liver tissue and disease models: Current advances and future perspectives. Biomater. Adv. 2023, 152, 213499. [Google Scholar] [CrossRef]
- Kufelt, O.; El-Tamer, A.; Sehring, C.; Schlie-Wolter, S.; Chichkov, B.N. Hyaluronic acid based materials for scaffolding via two-photon polymerization. Biomacromolecules 2014, 15, 650–659. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2021, 31, 2006967. [Google Scholar] [CrossRef]
- Rajabi, M.; Cabral, J.D.; Saunderson, S.; Ali, M.A. 3D printing of chitooligosaccharide-polyethylene glycol diacrylate hydrogel inks for bone tissue regeneration. J. Biomed. Mater. Res. A 2023, 111, 1468–1481. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in Vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Luo, B.; Wang, H.; Bian, Y.; Li, X.; Li, S.; Wang, H. Precise and Arbitrary Deposition of Biomolecules onto Biomimetic Fibrous Matrices for Spatially Controlled Cell Distribution and Functions. Adv. Mater. 2017, 29, 1701154. [Google Scholar] [CrossRef] [PubMed]
- Cidonio, G.; Alcala-Orozco, C.R.; Lim, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.H.; Dawson, J.I.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks. Biofabrication 2019, 11, 035027. [Google Scholar] [CrossRef]
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P.W. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Chapman, D.; Babyn, P.; Chen, X.; Kelly, M.E. UV-Assisted 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering. Tissue Eng. Part. C Methods 2018, 24, 74–88. [Google Scholar] [CrossRef]
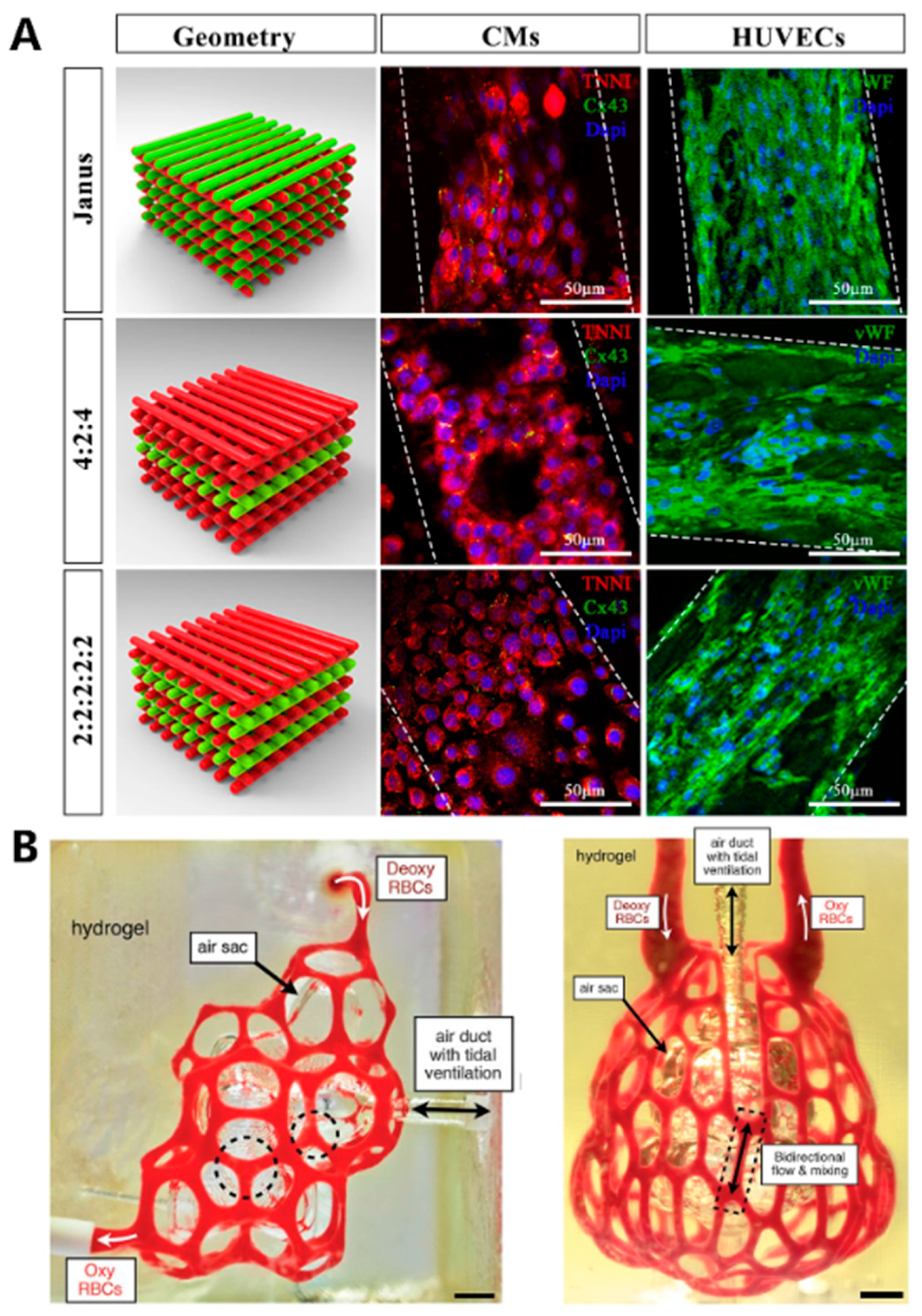
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivi, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Swieszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef]
- Barros, N.R.; Kim, H.J.; Gouidie, M.J.; Lee, K.; Bandaru, P.; Banton, E.A.; Sarikhani, E.; Sun, W.; Zhang, S.; Cho, H.J.; et al. Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication 2021, 13, 035030. [Google Scholar] [CrossRef]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part. A 2012, 18, 1304–1312. [Google Scholar] [CrossRef]
- Phull, A.R.; Eo, S.H.; Abbas, Q.; Ahmed, M.; Kim, S.J. Applications of Chondrocyte-Based Cartilage Engineering: An Overview. Biomed. Res. Int. 2016, 2016, 1879837. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Pothiawala, A.; Lee, J.Y.; Matthias, N.; Umeda, K.; Ang, B.K.; Huard, J.; Huang, Y.; Sun, D. Human pluripotent stem cell-derived chondroprogenitors for cartilage tissue engineering. Cell Mol. Life Sci. 2020, 77, 2543–2563. [Google Scholar] [CrossRef]
- Sun, A.X.; Lin, H.; Beck, A.M.; Kilroy, E.J.; Tuan, R.S. Projection Stereolithographic Fabrication of Human Adipose Stem Cell-Incorporated Biodegradable Scaffolds for Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 115–119. [Google Scholar] [CrossRef]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110625. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.W.; Kwon, S.M.; Cho, D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Li, J.; Dong, X.H.; Cui, Y.T.; Cui, Y.F.; Ban, T.; Huo, R. The role of BRG1 in epigenetic regulation of cardiovascular diseases. Eur. J. Pharmacol. 2023, 957, 176039. [Google Scholar] [CrossRef]
- Ullah, M.; Bibi, A.; Wahab, A.; Hamayun, S.; Rehman, M.U.; Khan, S.U.; Awan, U.A.; Riaz, N.U.; Naeem, M.; Saeed, S.; et al. Shaping the Future of Cardiovascular Disease by 3D Printing Applications in Stent Technology and its Clinical Outcomes. Curr. Probl. Cardiol. 2023, 49 Pt A, 102039. [Google Scholar] [CrossRef]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.; Mondal, P.; Yadav, P.; Chatterjee, K. Light-based 3D bioprinting of bone tissue scaffolds with tunable mechanical properties and architecture from photocurable silk fibroin. Int. J. Biol. Macromol. 2022, 202, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Yang, D.H.; Ha, M.Y.; Kim, H.J.; Kim, C.H.; Kim, S.H.; Choi, J.W.; Chun, H.J. 3D printing of cell-laden visible light curable glycol chitosan bioink for bone tissue engineering. Carbohydr. Polym. 2022, 287, 119328. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Fan, P.; Zhang, M.; Shi, K.; Chen, X.; Yang, H.; Liu, X.; Xu, W.; Zhou, Y. Multiple Crosslinking Hyaluronic Acid Hydrogels with Improved Strength and 3D Printability. ACS Appl. Bio Mater. 2022, 5, 334–343. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Pahoff, S.; Bock, N.; Hutmacher, D.W. Gelatin Methacryloyl Hydrogels Control the Localized Delivery of Albumin-Bound Paclitaxel. Polymers 2020, 12, 501. [Google Scholar] [CrossRef]
- Zanon, M.; Chiappone, A.; Garino, N.; Canta, M.; Frascella, F.; Hakkarainen, M.; Pirri, C.F.; Sangermano, M. Microwave-assisted methacrylation of chitosan for 3D printable hydrogels in tissue engineering. Mater. Adv. 2022, 3, 514–525. [Google Scholar] [CrossRef]
- Picard, J.; Giraudier, S.; Larreta-Garde, V. Controlled remodeling of a protein-polysaccharide mixed gel: Examples of gelatin-hyaluronic acid mixtures. Soft Matter. 2009, 5, 4198–4205. [Google Scholar] [CrossRef]
- Salisu, A.; Sanagi, M.M.; Abu Naim, A.; Wan Ibrahim, W.A.; Abd Karim, K.J. Removal of lead ions from aqueous solutions using sodium alginate-graft-poly(methyl methacrylate) beads. Desalination Water Treat. 2015, 57, 15353–15361. [Google Scholar] [CrossRef]
- Loebel, C.; D’Este, M.; Alini, M.; Zenobi-Wong, M.; Eglin, D. Precise tailoring of tyramine-based hyaluronan hydrogel properties using DMTMM conjugation. Carbohydr. Polym. 2015, 115, 325–333. [Google Scholar] [CrossRef] [PubMed]





| PI | Structure | λmax/nm | Solubility g/L | Toxicity LC50 [mmol/dm3] | Ref. |
|---|---|---|---|---|---|
| Irgacure 2959 | 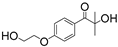 | 296 | 5 | 9.0 | [86] |
| APi-180 | 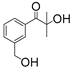 | 329 | 74 | 8.7 | [86] |
| LAP | 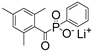 | 375 | 47 | 3.1 | [86] |
| Na-TPO | 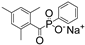 | 380 | 29 | ˂0.56 | [86] |
| BAPO-OLi |  | 383 | 54 | 2.6 | [86] |
| BAPO-ONa |  | 383 | 60 | 2.8 | [86] |
| Tissue or Organ Type | Light-Based Bioprinting Technology | Applications | Development Directions | Ref. |
|---|---|---|---|---|
| Liver | Extrusion | Fabrication of biological livers and in vitro liver models for purposes such as liver regeneration, drug screening, metabolism research, and the study of hepatotoxicity | The construction of a perfusable high-density biomimetic vascular network in the printed liver tissue. | [66,179,183,184,185,186,187] |
| SLA | ||||
| DLP | ||||
| CAL | ||||
| Bone | Inkjet | Fabrication of a framework to improve cell adhesion, proliferation, and differentiation, then to further integrate with the native tissue. | Developing bio-inks with improved mechanical properties for seamless integration with native bone tissue. | [188,189,190,191,192,193,194] |
| Extrusion | ||||
| SLA | ||||
| DLP | ||||
| CAL | ||||
| Cardiac tissue | Extrusion | Fabrication of cardiac tissue capable of the regeneration of different structures in a human heart. Building in vitro cardiac models for studying cardiovascular diseases | To develop materials with good flexibility and elasticity suitable for the encapsulation of related cells. | [39,40,61,195,196,197,198] |
| Suspension | ||||
| SLA | ||||
| DLP | ||||
| Skin | Inkjet | Printing skin replicas for the repair of skin damage. Fabricating skin models to study the pathophysiology of skin diseases. | Developing skin-specific biomimetic bio-inks and the regeneration of skin appendages. Developing multilayered complex skin models. | [180,199,200,201] |
| Extrusion | ||||
| SLA | ||||
| DLP | ||||
| Cartilage | Inkjet | Printing cartilage implants for the regeneration of damaged cartilage. Building models for drug testing | Fabrication cartilage structures exhibit mechanical compatibility with the damaged sites. Constructing integrated bone-cartilage tissues and grafts | [60,181,202,203,204,205,206] |
| Extrusion | ||||
| SLA | ||||
| DLP | ||||
| CAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, X.; Li, Y.; Zhang, Y. Applications of Light-Based 3D Bioprinting and Photoactive Biomaterials for Tissue Engineering. Materials 2023, 16, 7461. https://doi.org/10.3390/ma16237461
Zhang X, Zhang X, Li Y, Zhang Y. Applications of Light-Based 3D Bioprinting and Photoactive Biomaterials for Tissue Engineering. Materials. 2023; 16(23):7461. https://doi.org/10.3390/ma16237461
Chicago/Turabian StyleZhang, Xueqin, Xin Zhang, Ying Li, and Yuxuan Zhang. 2023. "Applications of Light-Based 3D Bioprinting and Photoactive Biomaterials for Tissue Engineering" Materials 16, no. 23: 7461. https://doi.org/10.3390/ma16237461
APA StyleZhang, X., Zhang, X., Li, Y., & Zhang, Y. (2023). Applications of Light-Based 3D Bioprinting and Photoactive Biomaterials for Tissue Engineering. Materials, 16(23), 7461. https://doi.org/10.3390/ma16237461