Processing and Properties of ZrB2-Copper Matrix Composites Produced by Ball Milling and Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Raw Materials
2.2. Fabrication of the Cu–ZrB2 Composites
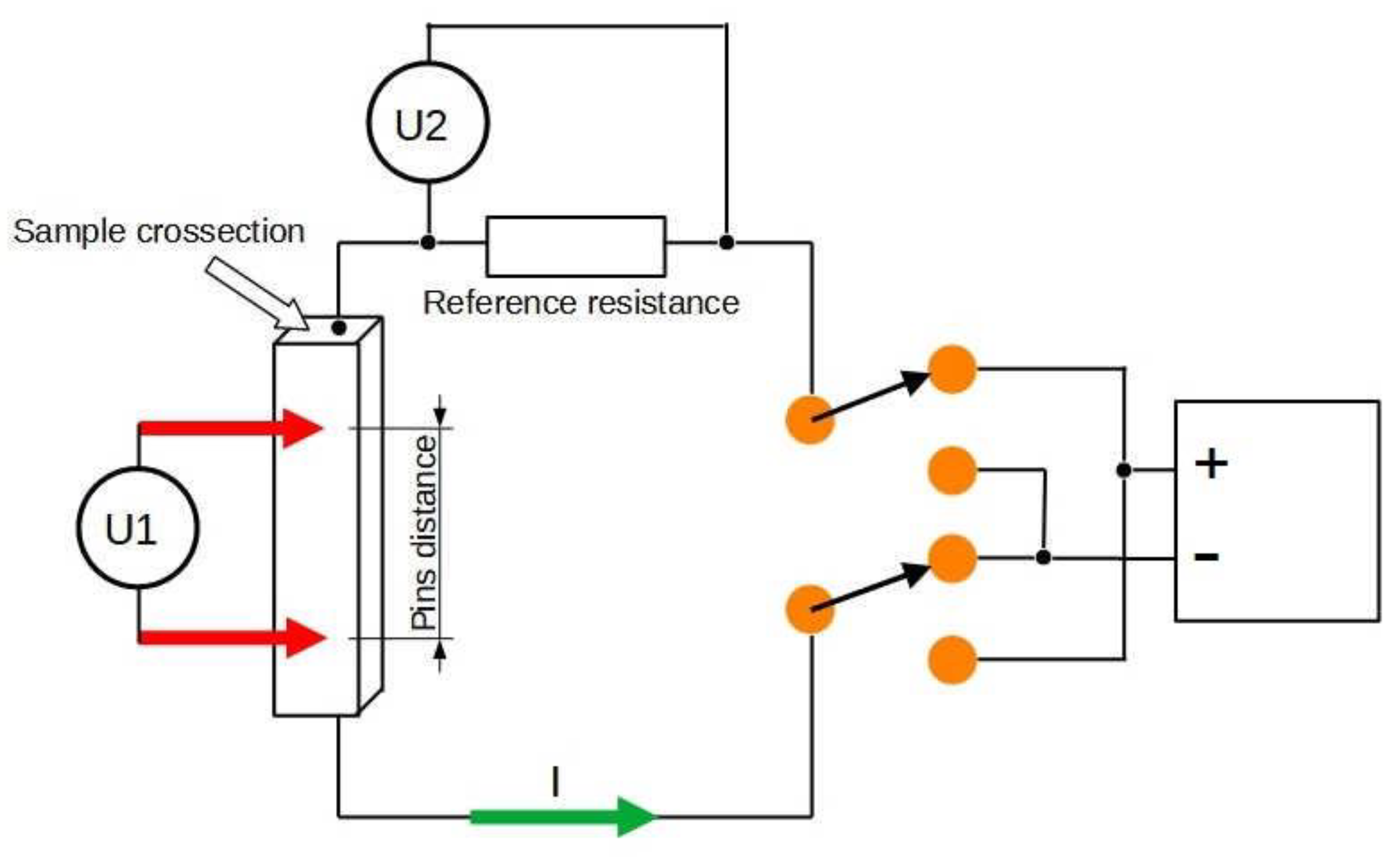
2.3. Material Characterisation
3. Research Results
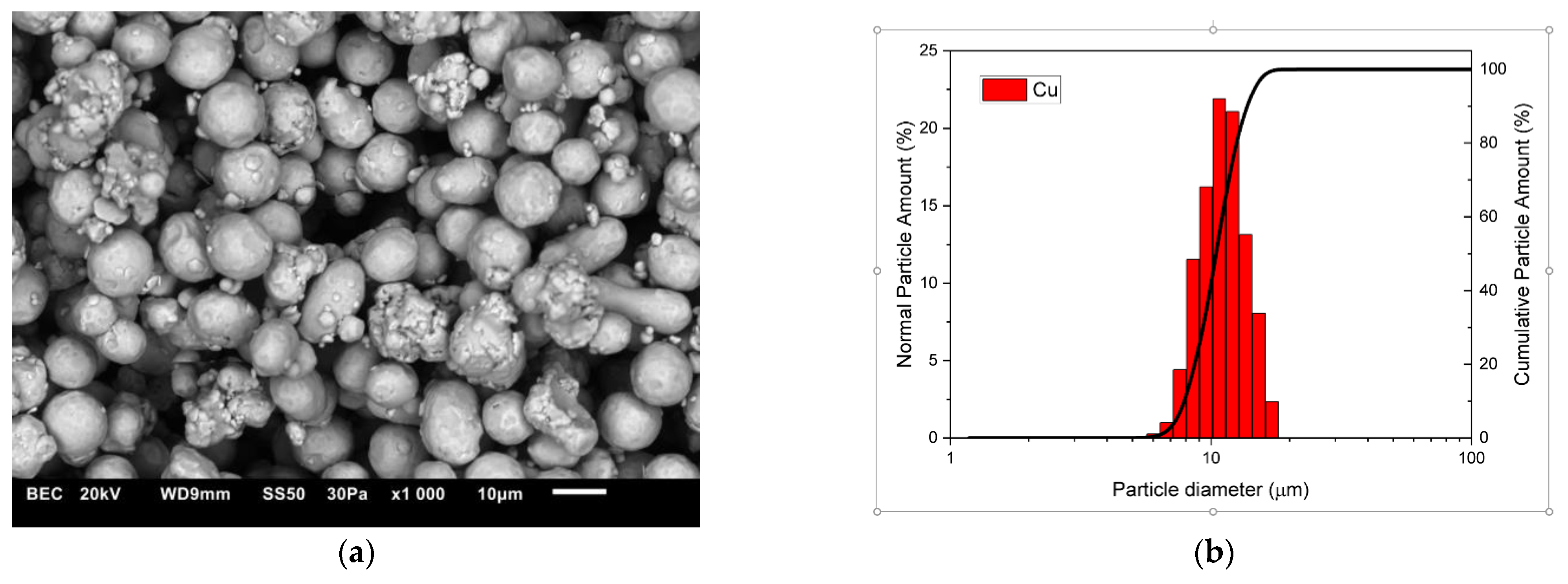
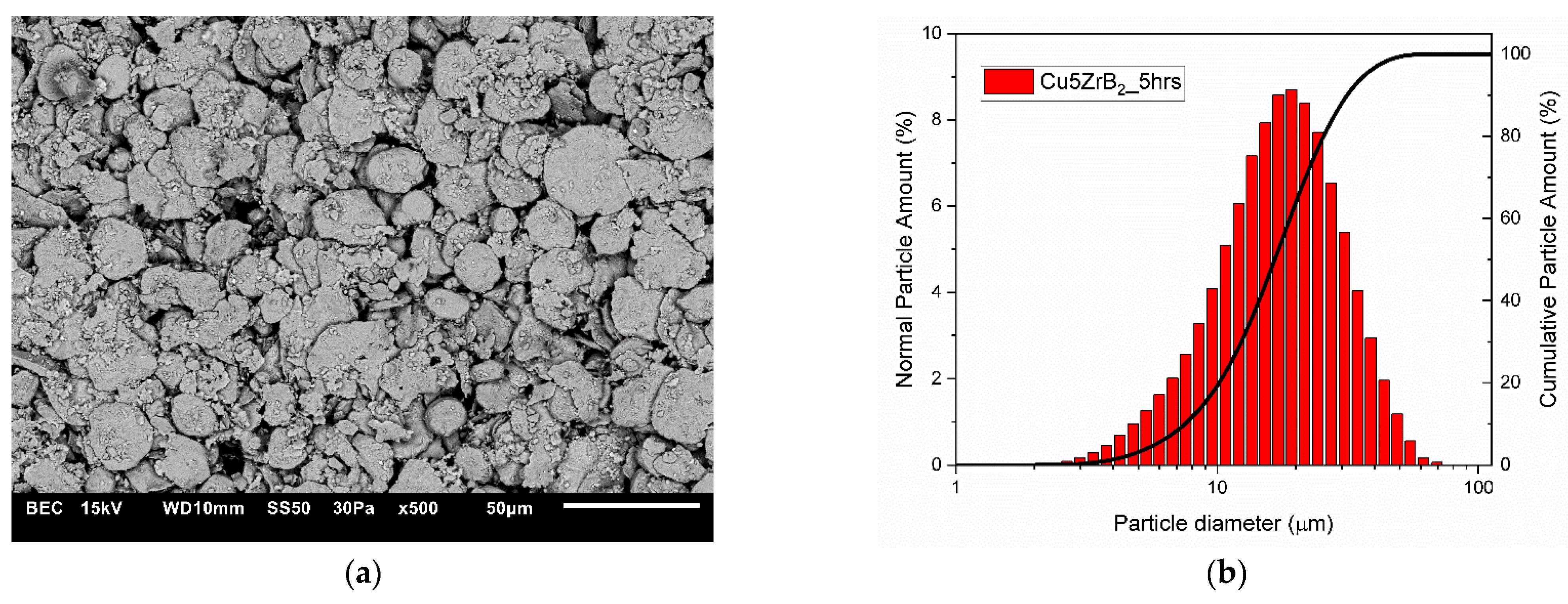
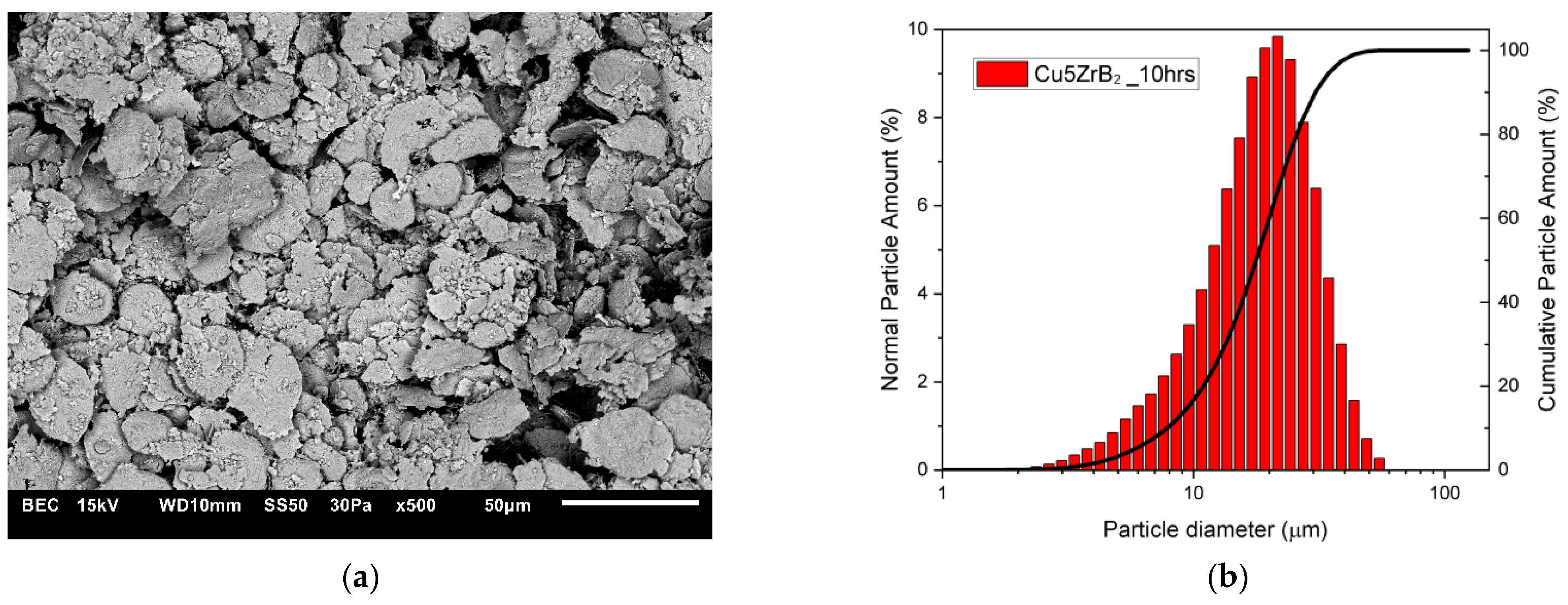
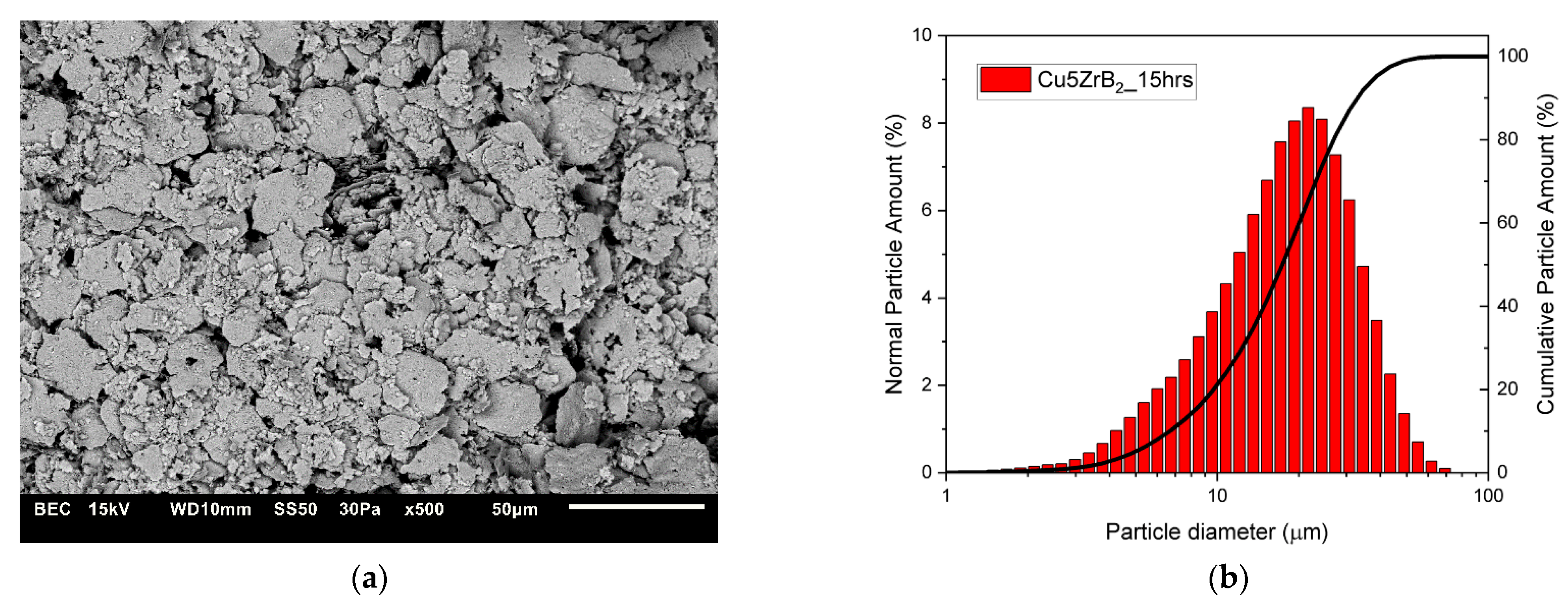
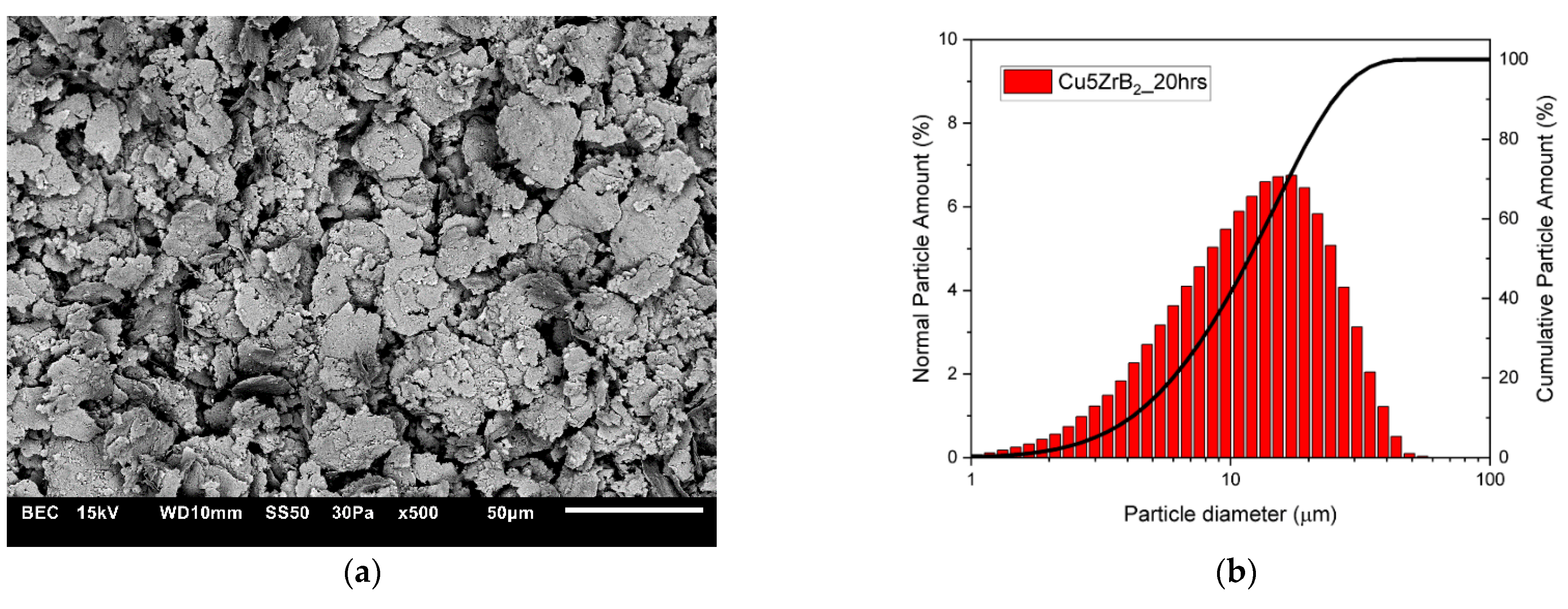
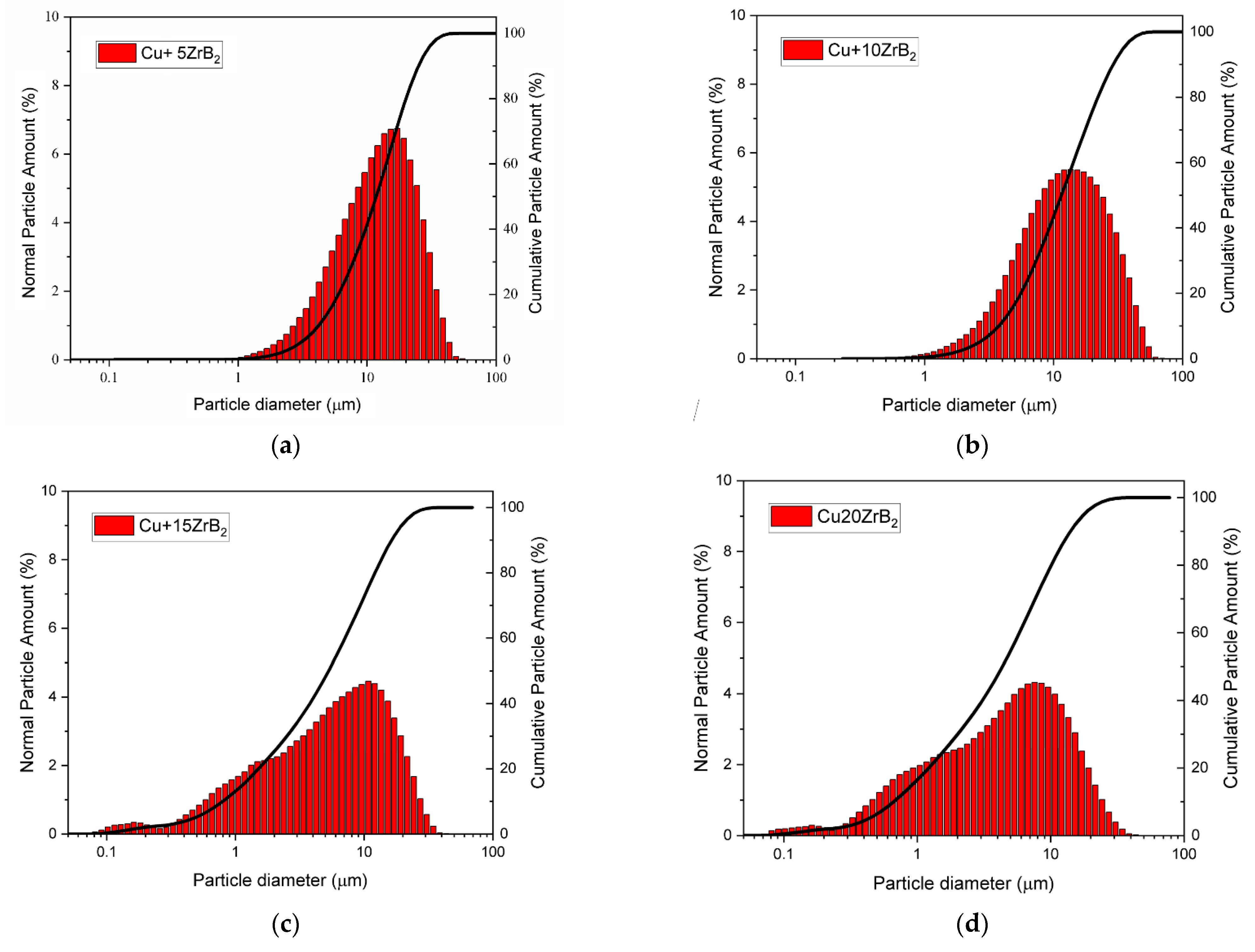
3.1. Characterisation of Composite Powder Mixtures
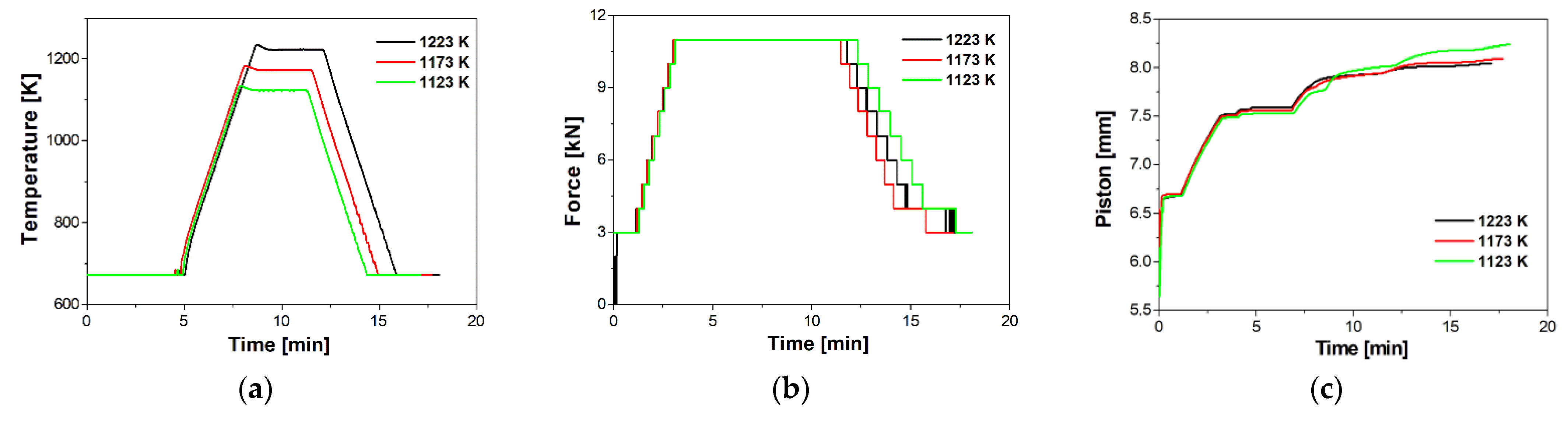
3.2. Characterisation of Sintered Cu–ZrB2 Composites
4. Discussion
- σ—electrical conductivity tensor,
- E—vector of electric field intensity,
- and
- κx—thermal conductivity coefficient along the x axis,
- dT/dx—temperature gradient.
- j—flux density vector of the appropriate amount (size) of internal energy for thermal conduction or charge for electrical conduction,
- β—coefficient of proportionality of thermal or electrical conductivity,
- A—scalar quantity whose gradient causes a given phenomenon (temperature, electric potential) [64].
5. Conclusions
- A change in SPS temperature from 1123–1223 K led to an increase in the density, Young’s modulus, and hardness. The best physical and mechanical properties were obtained for the composites that were sintered at 1223 K–35 MPa.
- Furthermore, the influence of the ZrB2 content on the microstructure, physical, mechanical, and electrical properties of sintered composites was studied. The results showed that the increase in the ZrB2 content in the copper matrix improves Young’s modulus and hardness while reducing the density and electrical conductivity. The addition of the reinforcing phase in an amount greater than 10 wt% negatively affects the properties of the copper matrix composites.
- The addition of 20 wt% ZrB2 causes the beginning of the formation of particle conglomerates and, consequently, disturbs their homogeneous distribution in the copper matrix.
- A strong decrease in the electrical conductivity of composites containing 5 and 10 wt% ZrB2, as well as stabilization of the conductivity in larger amounts, indicates the partial diffusion of boron into the copper matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renwick, S. Copper Alloys: Processes, Applications and Developments. In Copper and Copper Base Alloys: The Physical and Mechanical Properties of Copper and Its Commercial Alloys in Wrought Form; Forgotten Books; New York Research Press: New York, NY, USA, 2018. [Google Scholar]
- Wang, Q.; Chen, M.; Shan, Z.; Sui, C.; Zhang, L.; Zhu, S.; Wang, F. Comparative study of mechanical and wear behavior of Cu/WS2 composites fabricated by spark plasma sintering and hot pressing. J. Mater. Sci. Technol. 2017, 33, 1416–1423. [Google Scholar] [CrossRef]
- Zhou, D.S.; Zhang, D.L.; Kong, C.; Munroe, P. Factors controlling the tensile properties of ultrafine structured Cu–5vol%Al2O3 nanocomposite prepared by high energy mechanical milling and powder compact extrusion. Mater. Sci. Eng. A 2013, 584, 67–72. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, J.H.; Park, Y.H.; Cho, K.M.; Choi, I.D.; Park, I.M. Manufacturing of Cu-TiB2 composites by turbulent in situ mixing process. Mater. Sci. Eng. A 2007, 449–451, 1018–1021. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, W.; Dong, L.; Li, H.; Fu, Y. Enhancing copper infiltration into alumina using spark plasma sintering to achieve high performance Al2O3/Cu composites. Ceram. Int. 2018, 44, 57–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, Z.; Jia, C.; Liu, G.; Wan, F.; Zhan, Q. Influence of lanthanum on enhancement of mechanical and electrical properties of Cu-Al2O3 composites. J. Rare Earths 2019, 37, 534–540. [Google Scholar] [CrossRef]
- Efe, G.C.; Altinsoy, I.; Yener, T.; Ipek, M.; Zeytin, S.; Bindal, C. Characterization of cemented Cu matrix composites reinforced with SiC. Vacuum 2010, 85, 643–647. [Google Scholar] [CrossRef]
- Aal, M.I.A.E. Effect of high-pressure torsion processing on the microstructure evolution and mechanical properties of consolidated micro size Cu and Cu-SiC powders. Adv. Powder Technol. 2017, 28, 2135–2150. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, H.; Huang, J.; Li, J.; Xie, Z. Processing and characterization of in-situ ultrafine TiB2-Cu composites from Ti-B-Cu system. Powder Technol. 2017, 320, 66–72. [Google Scholar] [CrossRef]
- Ruzic, J.; Stasic, J.; Rajkovic, V.; Bozic, D. Synthesis, microstructure and mechanical properties of ZrB2 nano and microparticle reinforced copper matrix composite by in situ processing’s. Mater. Des. 2014, 62, 409–415. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, A.; Sengupta, P.R.; Dutta, P.K.; Mathur, R.B. Improving the mechanical and thermal properties of semi-coke based carbon/copper composites reinforced using carbon nanotubes. Adv. Mater. Lett. 2014, 5, 265–271. [Google Scholar] [CrossRef]
- Yang, L.; Sun, L.; Bai, W.; Li, L. Thermal conductivity of Cu-Ti/diamond composites via spark plasma sintering. Diam. Relat. Mater. 2019, 94, 37–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Xu, Y.; Cao, F.; Liang, S. Fabrication and properties of in situ heterogeneous Cu/TiB2 composites with a harmonic structure. Mater. Lett. 2020, 263, 127–132. [Google Scholar] [CrossRef]
- Xie, J.; Zeng, W.; Zhou, D.; Zhang, D. Microstructure and properties of a nanocrystalline Cu-Al-NbC composite with high strength and good conductivity. Mater. Lett. 2018, 214, 174–177. [Google Scholar] [CrossRef]
- Gao, X.; Yue, H.; Guo, E.; Zhang, H.; Lin, X.; Yao, L.; Wang, B. Mechanical properties and thermal conductivity of graphene reinforced copper matrix composites. Powder Technol. 2016, 301, 601–607. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, Y.J.; Erb, U. Thermal conductivity of copper-diamond composite materials produced by electrodeposition and the effect of TiC coatings on diamond particles. Compos. Part B 2018, 155, 197–203. [Google Scholar] [CrossRef]
- Safari, F.; Khosroshahi, R.A.; Zolriasatein, A. Wear behavior of copper matrix composites reinforced by γ-Cu5Zn8 nanoparticles. Powder Technol. 2017, 318, 549–557. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Chaira, D. Fabrication and characterization of Cu–B4C metal matrix composite by powder metallurgy: Effect of B4C on microstructure, mechanical properties and electrical conductivity. Transit. Indian Inst. Met. 2018, 72, 673–684. [Google Scholar] [CrossRef]
- Monteverde, F.; Bellosi, A.; Guicciardi, S. Processing and properties of zirconium diboride-based composites, The Journal of the European. Ceram. Soc. 2002, 22, 279–288. [Google Scholar] [CrossRef]
- Wilkins, J.M.L. Boron and Refractory Borides; Matkovich, V.I., Ed.; Springer: New York, NY, USA, 1977; p. 633. [Google Scholar]
- Mroz, C. Zirconium diboride. J. Am. Ceram. Soc. Bull. 1994, 73, 141–142. [Google Scholar]
- Fan, X.; Liu, Q.; Ding, H.; Wang, H.; Hao, C. The microstructures and properties of in-situ ZrB2 reinforced Cu matrix composites. Results Phys. 2019, 14, 102494. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Zhang, Z.; Li, W. Fabrication, Interfacial characteristics and strengthening mechanisms of ZrB2 microparticles reinforced Cu composites prepared by hot-pressed sintering. J. Alloys Compd. 2018, 748, 546–552. [Google Scholar] [CrossRef]
- Guan, C.; Chen, G.; Kai, X.; Gao, X.; Huang, L.; Cao, R.; Qian, W.; Zhao, Y. Transformation of dislocation strengthening mechanisms induced by graphene nanoplates and ZrB2 nanoparticle in nanolaminated Al matrix composites. Powder Technol. 2021, 377, 723–732. [Google Scholar] [CrossRef]
- Rino, J.J.; Prabu, S.B.; Paskaramoorthy, R. Comparison of thermal and mechanical properties of Al-5wt.%TiB2 and Al-5wt.%ZrB2 composites processed through salt-melt reaction route. Mater. Today Proc. 2017, 8, 8739–8750. [Google Scholar] [CrossRef]
- Çamurlu, H.E.; Maglia, F. Self-propagating high-temperature synthesis of ZrB2 or TiB2 reinforced Ni-Al composite powder. J. Alloys Compd. 2009, 478, 721–725. [Google Scholar] [CrossRef]
- Jiang, S.; An, Q.; Cui, X.; Xiang, X.; Huang, L.; Zhang, R.; Sun, Y.; Geng, L. Fabrication and mechanical properties of the titanium matrix composites based on Ti6Al4V-ZrB2-(Si) system. Mater. Sci. Eng. A 2021, 819, 141488. [Google Scholar] [CrossRef]
- Davis, J.R. Copper and Copper Alloy; ASM International: Materials Park, OH, USA, 2001. [Google Scholar]
- Ding, H.; Miao, W.; Huang, X.; Liu, Q.; Fan, X.; Wang, H.; Chu, K.; Li, C. Influence of Al addition on microstructures of Cu-B alloys and Cu-ZrB2 composites. Trans. Nonferrous Met. Soc. China 2020, 30, 1335–1346. [Google Scholar] [CrossRef]
- Shaik, M.A.; Golla, B.R. Two body abrasion wear behaviour of Cu–ZrB2 composites against SiC emery paper. Wear 2020, 450–451, 203260. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Zhou, S.; Guo, B.; Zhang, Z.; Yu, Z.; Li, W. Effect of sintering temperature of the microstructure and properties of high-strength and highly conductive 5 wt.% ZrB2/Cu composite. Powder Metall. Met. Ceram. 2023, 61, 9–10. [Google Scholar]
- Zhang, X.; Qian, L.; Bin, L.; Xi, C.; You, L.; Zhen, H.; Huan, L. Enhanced softening resistance and mechanical properties of Mo2C particle-reinforced Cu-matrix composites. Compos. Struct. 2023, 305, 116503. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wang, X.; Koizumi, Y.; Kenta, Y.; Chiba, A. In-situ fabrication and characterization of ultrafine structured Cu-TiC composites with high strength and high conductivity by mechanical milling. J. Alloys Compd. 2016, 657, 122–132. [Google Scholar] [CrossRef]
- Dash, K.; Ray, B.C.; Chaira, D. Synthesis and characterization of copper–alumina metal matrix composite by conventional and spark plasma sintering. J. Alloys Compd. 2012, 516, 78–84. [Google Scholar] [CrossRef]
- Hyjek, P.; Stępień, M.; Kowalik, R.; Sulima, I. Corrosion Resistance of Nickel-Aluminum Sinters Produced by High-Pressure HPHT/SPS Method. Materials 2023, 16, 1907. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qin, J.; Wang, J.; Jin, P.; Li, P. Significant strain hardening ability of AZ91 magnesium alloy fabricated by spark plasma sintering. Mater. Today Commun. 2023, 35, 105670. [Google Scholar] [CrossRef]
- Venkateswaran, T.; Basu, B.; Raju, G.B.; Kim, D.Y. Densification and properties of transition metal borides-based cermets via spark plasma sintering. J. Eur. Ceram. Soc. 2006, 26, 2431–2440. [Google Scholar] [CrossRef]
- Shi, X.; Shao, G.; Duan, X.; Yuan, R. Study on the diamond/ultrafine WC-Co cermets interface formed in a SPS consolidated composite. Rare Met. 2006, 25, 150–155. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mallick, A.R.; Debnath, D.; Das, P.K. Densification, mechanical and tribological properties of ZrB2 by SPS, Effect of pulsed current. Int. J. Refract. Met. Hard Mater. 2015, 4, 150–156. [Google Scholar] [CrossRef]
- Hocquet, S.; Dupont, V.; Cambier, F.; Ludewig, F.; Vandewalle, N. Densification of complex shape ceramics parts by SPS. J. Eur. Ceram. Soc. 2020, 40, 2586–2596. [Google Scholar] [CrossRef]
- Sulima, I.; Putyra, P.; Hyjek, P.; Tokarski, T. Effect of SPS parameters on densification and properties of steel matrix composites. Adv. Powder Technol. 2015, 26, 1152–1161. [Google Scholar] [CrossRef]
- Fujii, T.; Tohgo, K.; Iwao, M.; Shimamura, Y. Fabrication of alumina-titanium composites by spark plasma sintering and their mechanical properties. J. Alloys Compd. 2018, 744, 759–768. [Google Scholar] [CrossRef]
- Sun, H.Y.; Cheng, L.; Lu, Y.L.; Ma, M.; Liu, W.C. A technique for improving deformability of the 6061Al-TiCp composites by spark plasma sintering and hot rolling. Mater. Lett. 2024, 355, 135290. [Google Scholar] [CrossRef]
- Olumor, I.D.; Wiśniewska, M.; Torresani, E.; Olevsky, E.A. Additive manufacturing and spark plasma sintering as effective routes for manufacturing of AISI 316L austenitic stainless steel—WC composites. J. Mater. Res. Technol. 2023, 26, 3234–3244. [Google Scholar] [CrossRef]
- Tokita, M. Trends in Advanced SPS Spark Plasma Sintering Systems and Technology. J. Soc. Powder Technol. Jpn. 1993, 30, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Omori, M. Sintering, consolidation, reaction and crystal growth by the spark plasma system (SPS). Mater. Sci. Eng. A 2000, 287, 183–188. [Google Scholar] [CrossRef]
- Mamedov, V. Spark plasma sintering as advanced PM sintering method. Powder Metall. 2002, 45, 322–328. [Google Scholar] [CrossRef]
- Guillon, O.; Dargatz, B.; Kessel, T.; Schierning, G.; Rathel, J.; Herrmann, M. Field-Assisted Sintering Technology/Spark Plasma Sintering: Mechanism. Mater. Technol. Dev. 2014, 16, 830–849. [Google Scholar]
- Smits, F.M. Measurement of Sheet Resisitivities with the Four-Point Probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Boczkal, G.; Perek-Nowak, M. Reconfiguration of point defects in FCC and HCP metals at initial stage of recovery process. Arch. Metall. Mater. 2014, 59, 1733–3490. [Google Scholar] [CrossRef]
- Yu, P.F.; Zhang, L.J.; Cheng, H.; Zhang, H.; Ma, M.Z.; Li, Y.C.; Li, G.; Liaw, P.K.; Liu, R.P. The high-entropy alloys with high hardness and soft magnetic property prepared by mechanical alloying and high-pressure sintering. Intermetallics 2016, 70, 82–87. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Ružić, J.; Stašić, J.; Marković, S.; Raić, K.; Božić, D. Synthesis and Characterization of Cu-ZrB2 Alloy Produced by PM Techniques. Sci. Sinter. 2014, 46, 217–224. [Google Scholar] [CrossRef]
- Fan, Z.; Tsakiropoulos, P.; Miodownik, A.P. Prediction of Young’s modulus of particulate two phase composites. Mater. Sci. Technol. 1992, 8, 922–929. [Google Scholar] [CrossRef]
- Prusa, V.; Trnka, L. Mechanical response of elastic materials with density dependent Young modulus. Appl. Eng. Sci. 2023, 14, 100126. [Google Scholar]
- Swikker, K.R.J.; Kanagasabapathy, H.; Manickam, I.N.; Ponraj Nadar, N.V.; Alwin, S. Effect of sintering temperature on the microstructure and properties of graphite/copper matrix composite. Nonferrous Met. Sci. Eng. 2020, 11, 51–59. [Google Scholar]
- Su, Y.; Jiang, F.; Long, M.; Wu, F.; Xiao, Z.; Wu, M. Microstructure and frictional properties of copper-tin composites containing graphite and MoS2 by rapid hot-press sintering. Tribol. Int. 2023, 183, 108392. [Google Scholar] [CrossRef]
- Soloviova, T.O.; Solodkyi, I.V.; Loboda, P.I. Spark Plasma Sintering of Cu-(LaB6-TiB2) Metal-Ceramic Composite and Its Physical-Mechanical Properties. J. Superhard Mater. 2019, 41, 213–220. [Google Scholar] [CrossRef]
- Wei, H.; Zou, J.; Li, X.; Zhan, W.; Li, F. Friction and wear behavior of copper/graphite/Ti2SnC composites fabricated by spark plasma sintering (SPS). Wear 2023, 512–513, 204530. [Google Scholar] [CrossRef]
- Morz, C. Annual mineral review. Zirconium diboride. Am. Ceram. Soc. Bull. 1995, 74, 165–166. [Google Scholar]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Li, M.; Zinkle, S.J. Physical and mechanical properties of copper and copper alloys. Compr. Nucl. Mater. 2012, 4, 667–690. [Google Scholar]
- Azimi, M.; Akbari, G.H. Development of nano-structure Cu–Zr alloys by the mechanical alloying process. J. Alloys Compd. 2011, 509, 27–32. [Google Scholar] [CrossRef]
- Robert, A. (Ed.) Encyclopaedia of Physical Science and Technology, Reference Work, 3rd ed.; Meyers, Ramtech, Inc.: Tarzana, CA, USA, 2001; ISBN 978-0-12-227410-7. [Google Scholar]
- Chakrabarti, D.J.; Laughlin, D.E. The B-Cu (Boron-Copper) system. Bull. Alloy Phase Diagr. 1982, 3, 564–570. [Google Scholar] [CrossRef]

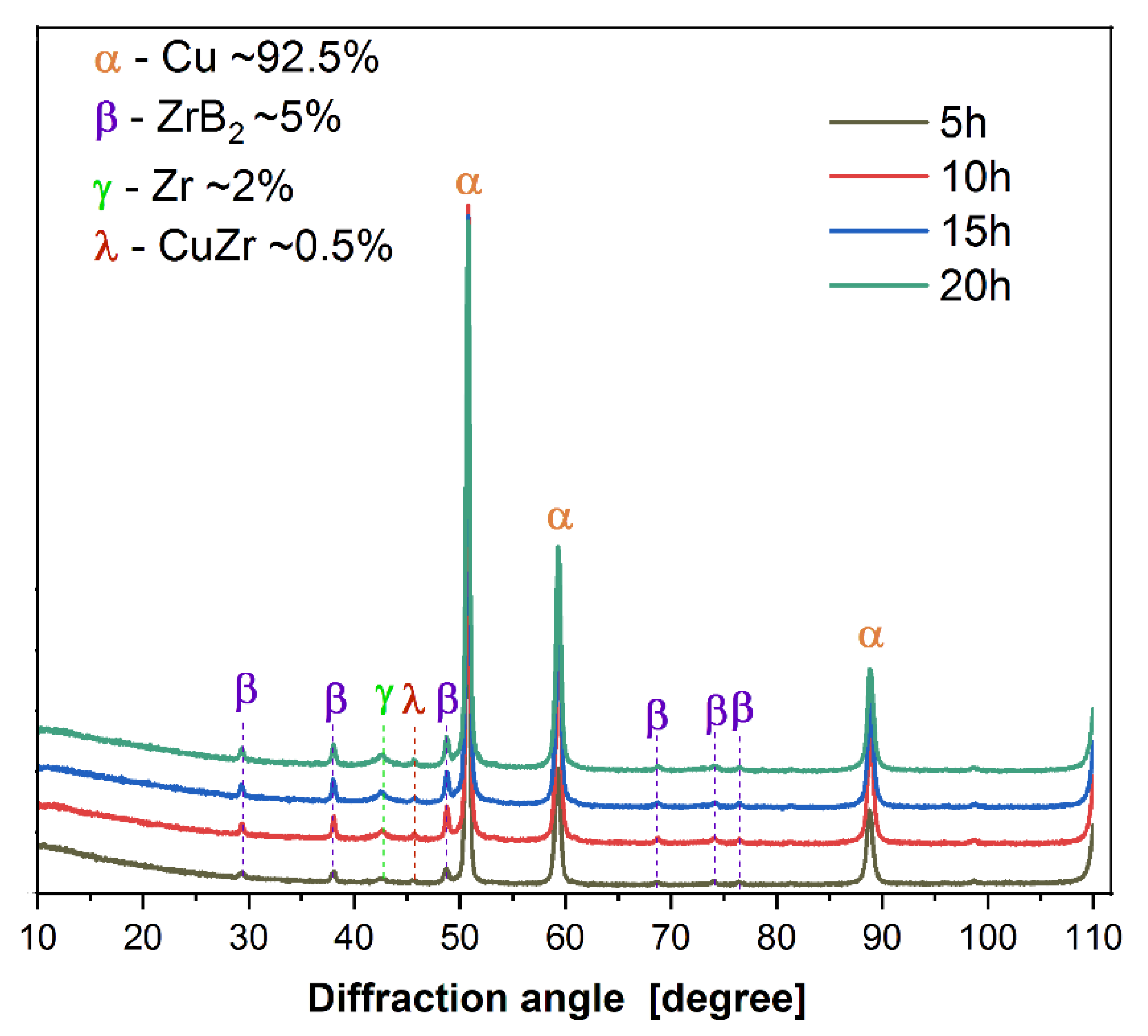
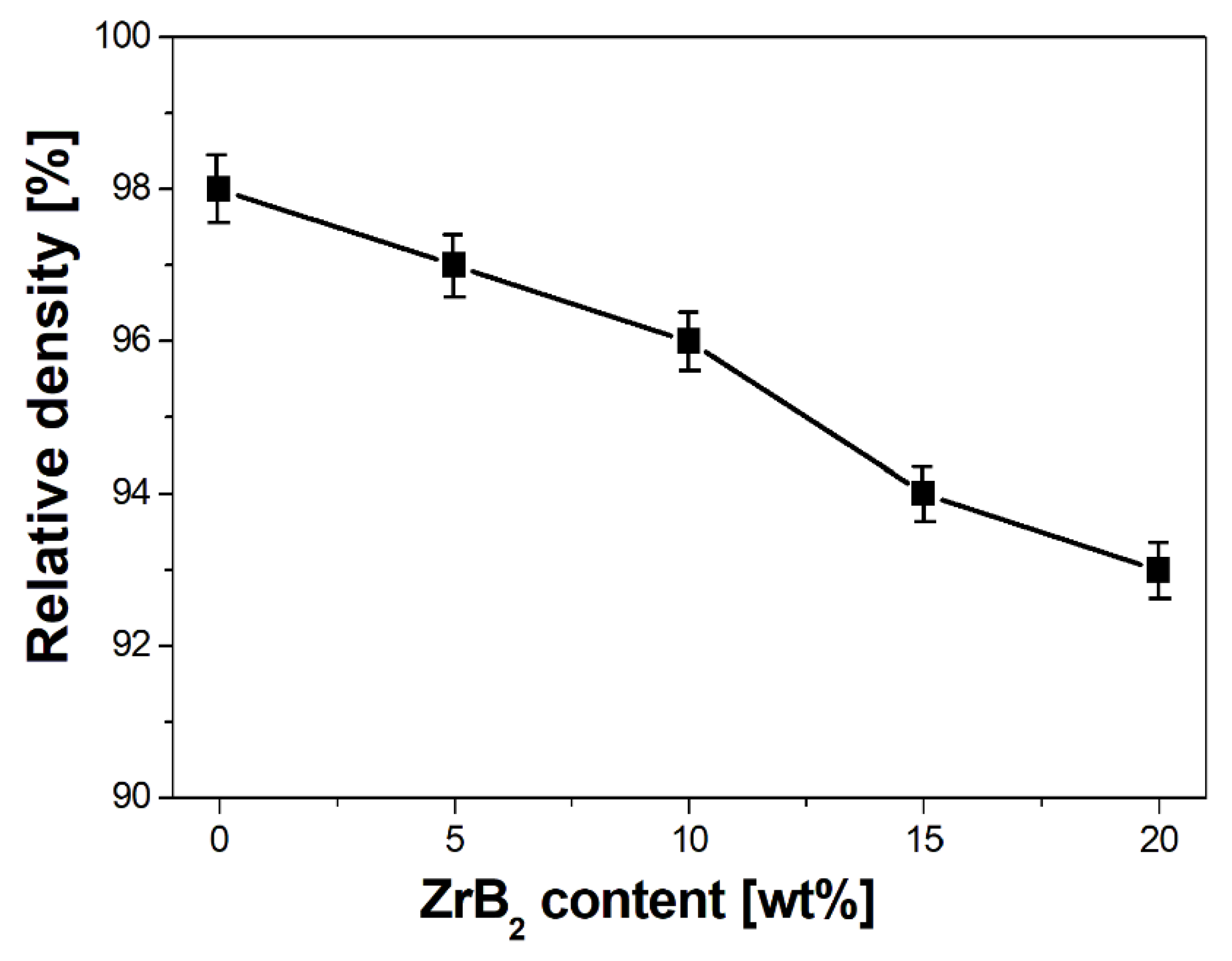
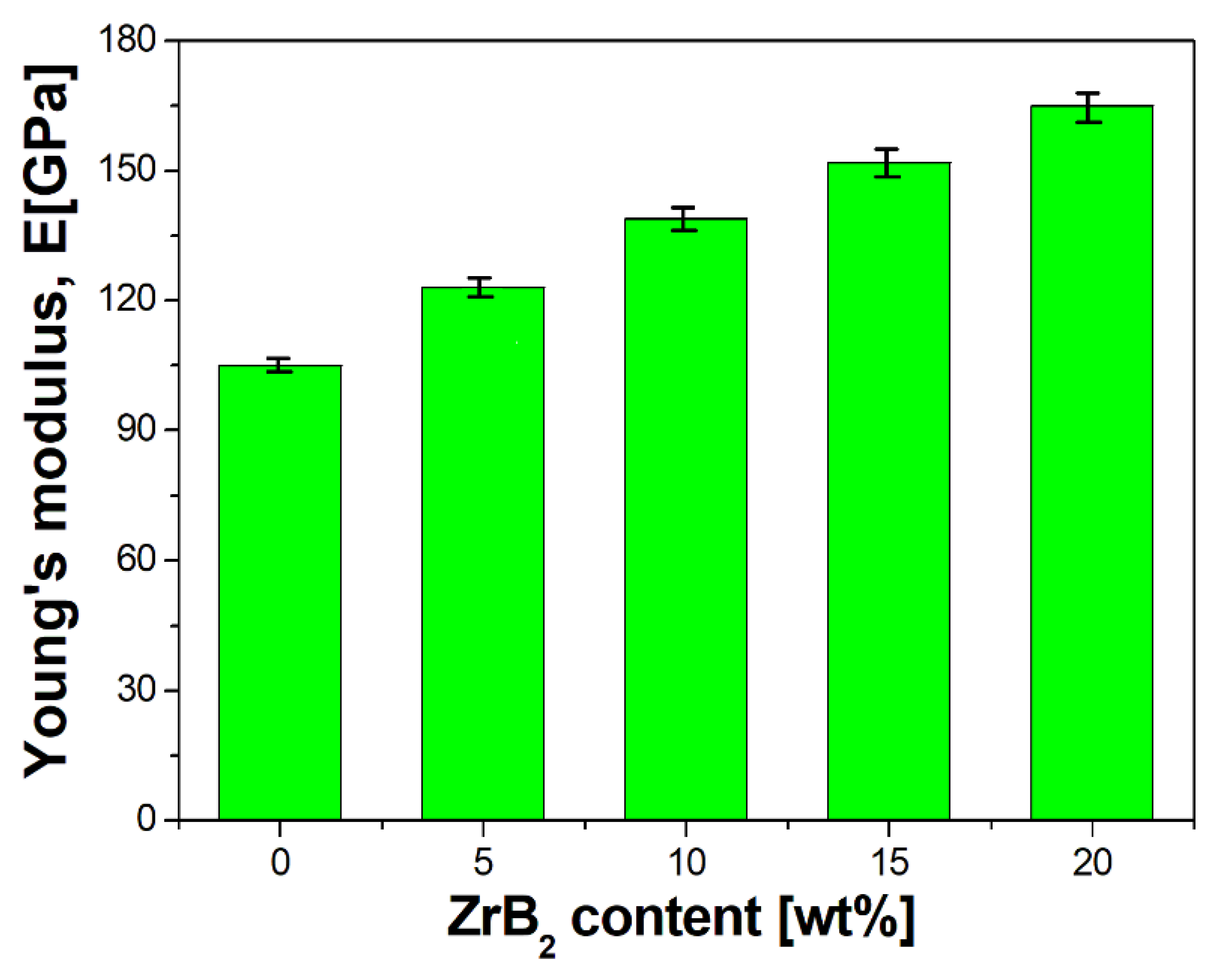
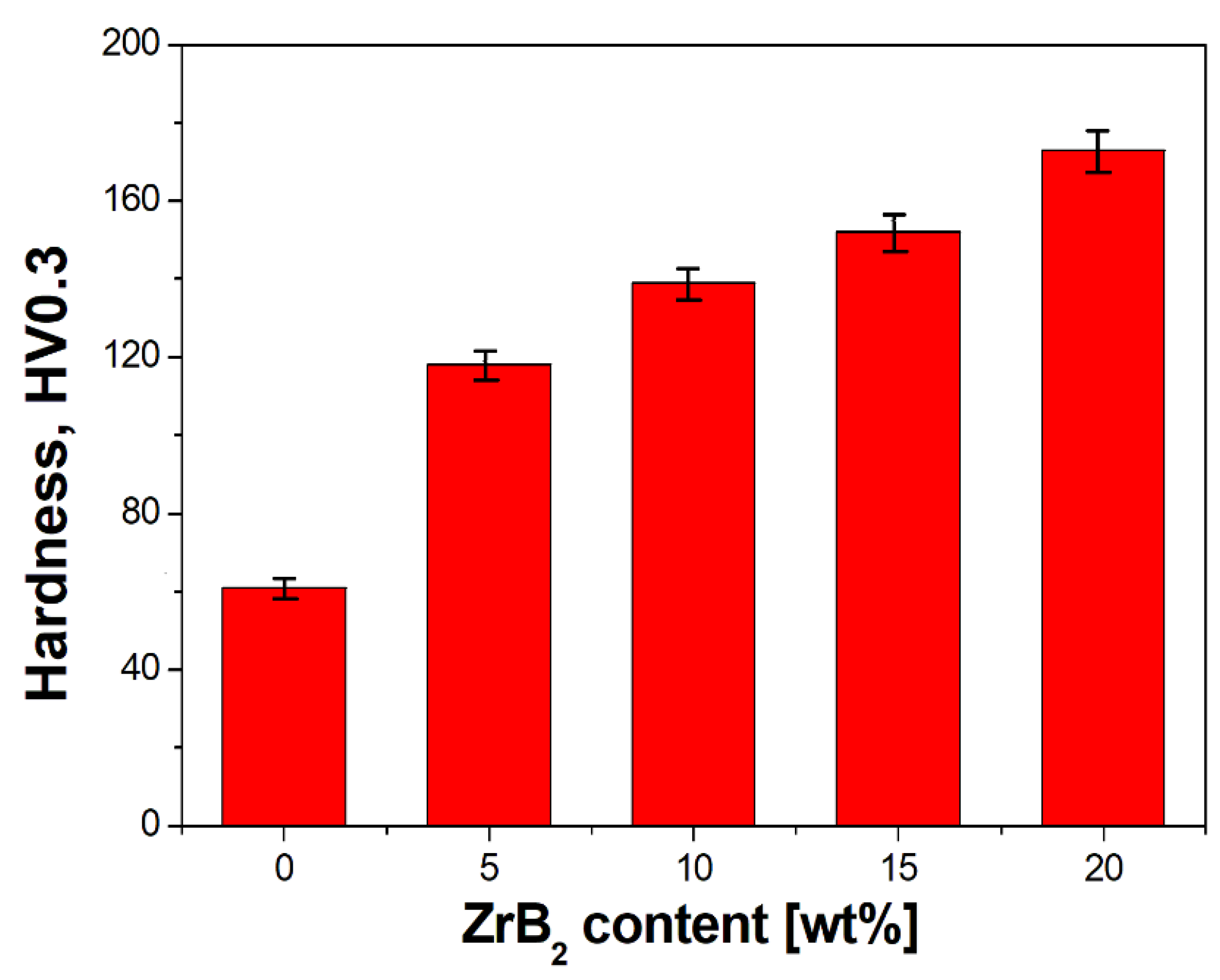
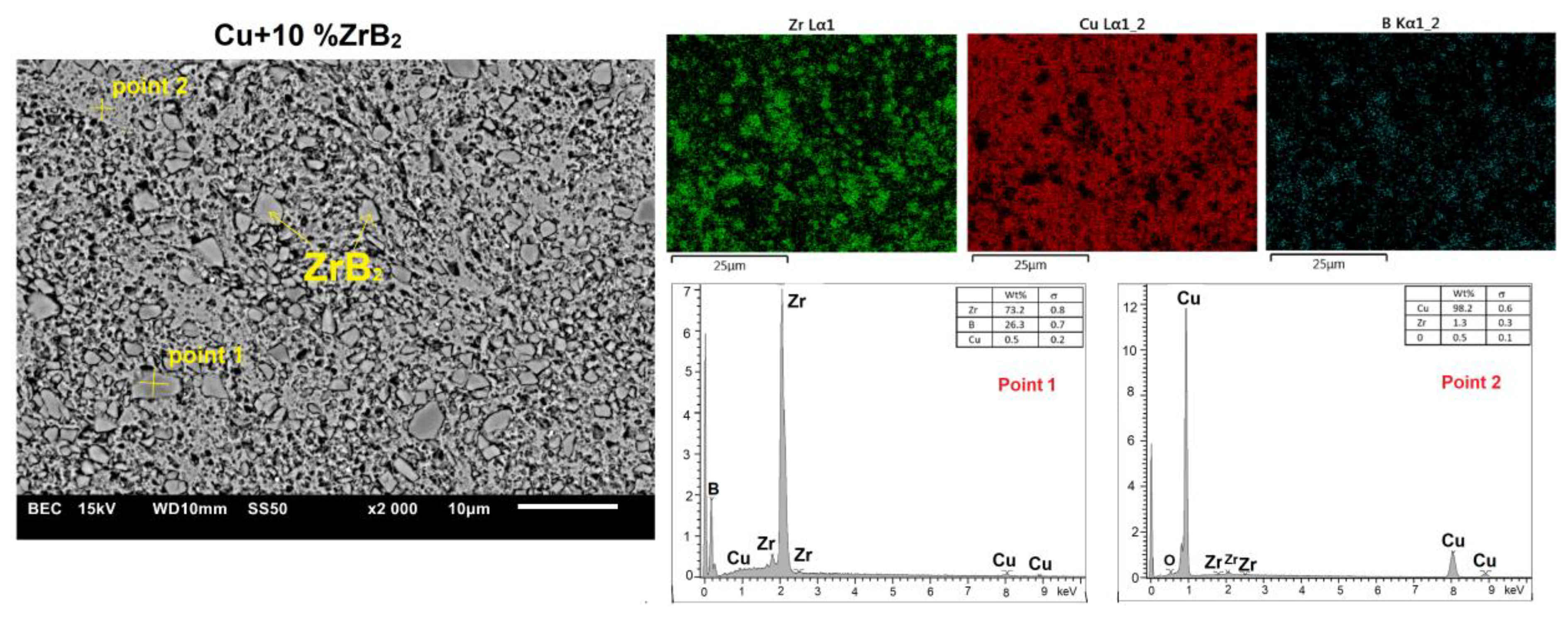
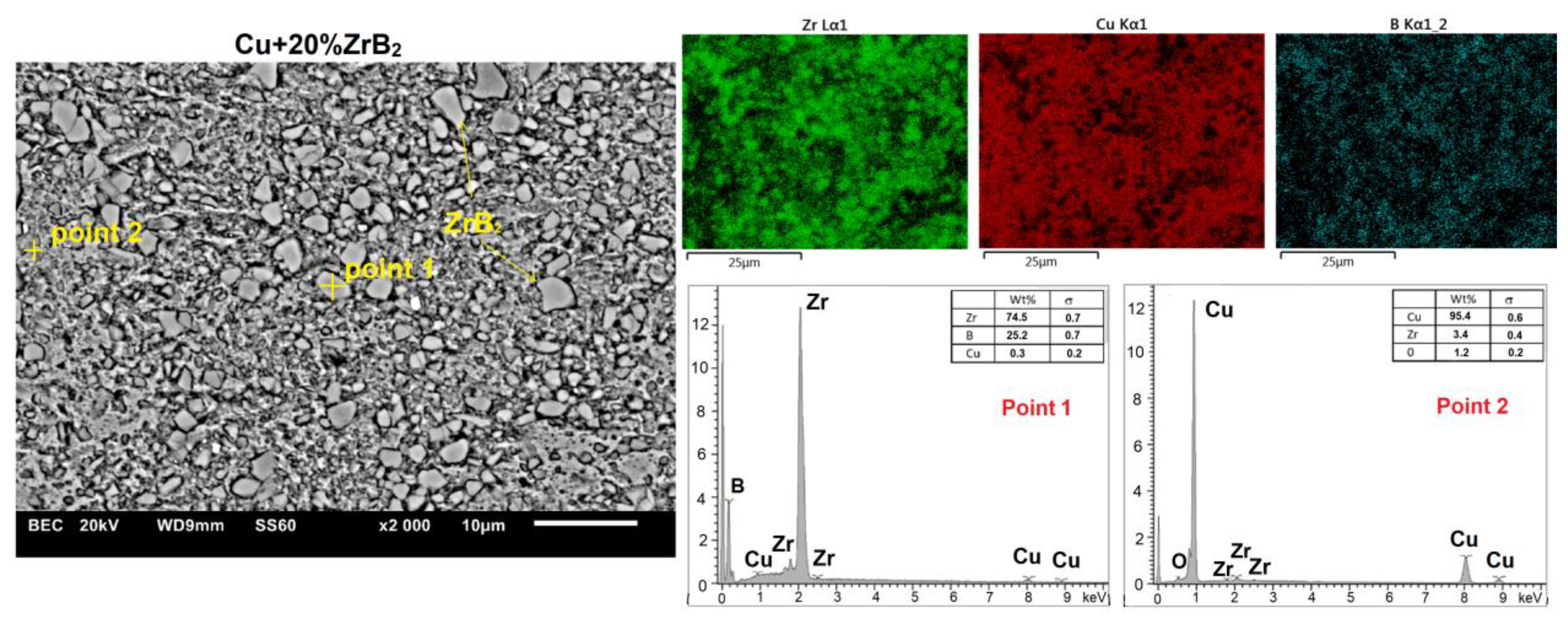

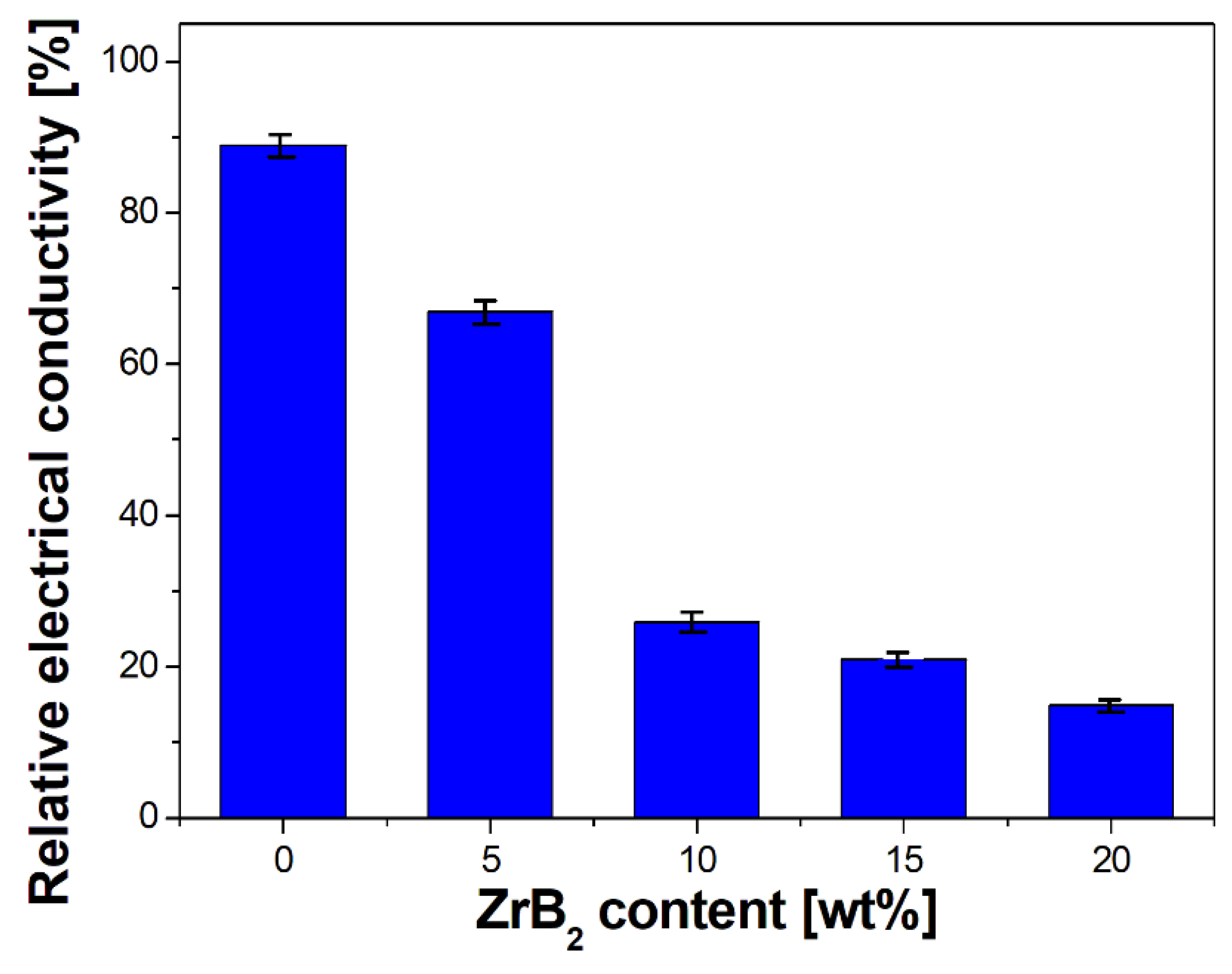
| Cu-Based Composites | Sintering Condition | Density | Hardness | Electrical Conductivity | Ref. |
|---|---|---|---|---|---|
| Cu–1 wt% ZrB2 | Hot-pressed sintering; 840 °C; 25 MPa; 2 h; 10 °C/min | 96% | 69 HV0.2 | 96% IACS | [23] |
| Cu–3 wt% ZrB2 | 95% | 84 HV0.2 | 93% IACS | ||
| Cu–5 wt% ZrB2 | 92.5% | 93 HV0.2 | 88% IACS | ||
| Cu–7 wt% ZrB2 | 91.8% | 100.8 HV0.2 | 83% IACS | ||
| Cu–9 wt% ZrB2 | 91.3% | 82 HV0.2 | 58% IACS | ||
| Cu–5 wt% ZrB2 | Hot-pressed sintering; 760, 800, 840, 880, 920 °C; 25 MPa; 2 h; 10 °C/min | 83–94% | 80–92 HV0.2 | 85–93% IACS | [31] |
| Cu–2 wt% Mo2C | Hot-pressed sintering; 880 °C; 20 MPa; 10 min | 91.5% | 58.9 HV | 83.5% IACS | [32] |
| Cu–5 wt% Mo2C | 91.2% | 65.8 HV | 77.1% IACS | ||
| Cu–7 wt% Mo2C | 91.3% | 69.6 HV | 74.7% IACS | ||
| Cu–5%vol TiC | SPS; 800 °C; 10–80 MPa; 5 min | 7.0–8.6 g/cm3 | 125-268 HV1 | 30-53% ISCS | [33] |
| Cu–1%vol Al2O3 | SPS; 700 °C; 10–50 MPa; 5 min; 80 °C/min | 93.2% | 77 HV0.3 | --- | [34] |
| Cu–5%vol Al2O3 | 92.8% | 125 HV0.3 | --- | ||
| Cu–7%vol Al2O3 | 86.1% | 75 HV0.3 | --- |
| Mass ratio of ball mass/powder mass | 5:1 |
| Material of milling balls | WC (tungsten carbide) |
| Diameter of milling balls | 10 mm |
| Total milling time | 20 h |
| Milling time/cooling time in one cycle | 20 min/10 min |
| Rotational speeds | 200 rpm |
| Powders | Median D (μm) | Modal D (μm) |
|---|---|---|
| Cu + 5ZrB2 (5 h) | 16.587 | 17.138 |
| Cu + 5ZrB2 (10 h) | 17.677 | 21.633 |
| Cu + 5ZrB2 (15 h) | 17.304 | 20.952 |
| Cu5 + ZrB2 (20 h) | 11.752 | 13.277 |
| Powders | Median D (μm) | Modal D (μm) |
|---|---|---|
| Cu + 5ZrB2 | 11.752 | 13.277 |
| Cu + 10ZrB2 | 10.308 | 11.556 |
| Cu + 15ZrB2 | 4.454 | 6.750 |
| Cu5 + 20ZrB2 | 3.402 | 5.348 |
| Sintered Materials | Temperature [K] | Pressure [MPa] | Apparent Density [g/cm3] | Relative Density [%] | Open Porosity [%] | Young’s Modulus [GPa] | Relative Young’s Modulus [%] |
|---|---|---|---|---|---|---|---|
| Cu + 5%ZrB2 | 1123 | 20 | 7.45 | 84 | 11.34 | 88 | 68 |
| 1173 | 7.68 | 87 | 9.89 | 92 | 72 | ||
| 1223 | 7.89 | 89 | 6.95 | 106 | 82 | ||
| 1123 | 35 | 8.35 | 95 | 2.54 | 113 | 88 | |
| 1173 | 8.46 | 96 | 1.78 | 117 | 91 | ||
| 1223 | 8.52 | 97 | 1.12 | 123 | 97 |
| Sintered Materials | Temperature [K] | Apparent Density [g/cm3] | Relative Density [%] | Open Prosity [%] | Young’s Modulus [GPa] | Relative Young’s Modulus [%] |
|---|---|---|---|---|---|---|
| Cu | 1123 | 8.75 | 98 | 0.12 | 107 | 97 |
| 1173 | 8.74 | 98 | 0.13 | 107 | 97 | |
| 1223 | 8.76 | 98 | 0.12 | 105 | 98 | |
| Cu + 5%ZrB2 | 1123 | 8.35 | 95 | 2.54 | 113 | 88 |
| 1173 | 8.46 | 96 | 1.78 | 117 | 91 | |
| 1223 | 8.52 | 97 | 1.12 | 123 | 97 | |
| Cu + 10%ZrB2 | 1123 | 7.62 | 88 | 9.78 | 112 | 76 |
| 1173 | 7.94 | 92 | 3.18 | 126 | 85 | |
| 1223 | 8.18 | 94 | 1.89 | 139 | 96 | |
| Cu + 15%ZrB2 | 1123 | 7.41 | 87 | 10.67 | 119 | 71 |
| 1173 | 7.53 | 88 | 8.71 | 132 | 79 | |
| 1223 | 7.86 | 92 | 3.21 | 154 | 94 | |
| Cu + 20%ZrB2 | 1123 | 7.22 | 86 | 10.54 | 129 | 72 |
| 1173 | 7.31 | 87 | 9.73 | 143 | 80 | |
| 1223 | 7.57 | 90 | 3.83 | 165 | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulima, I.; Boczkal, G. Processing and Properties of ZrB2-Copper Matrix Composites Produced by Ball Milling and Spark Plasma Sintering. Materials 2023, 16, 7455. https://doi.org/10.3390/ma16237455
Sulima I, Boczkal G. Processing and Properties of ZrB2-Copper Matrix Composites Produced by Ball Milling and Spark Plasma Sintering. Materials. 2023; 16(23):7455. https://doi.org/10.3390/ma16237455
Chicago/Turabian StyleSulima, Iwona, and Grzegorz Boczkal. 2023. "Processing and Properties of ZrB2-Copper Matrix Composites Produced by Ball Milling and Spark Plasma Sintering" Materials 16, no. 23: 7455. https://doi.org/10.3390/ma16237455
APA StyleSulima, I., & Boczkal, G. (2023). Processing and Properties of ZrB2-Copper Matrix Composites Produced by Ball Milling and Spark Plasma Sintering. Materials, 16(23), 7455. https://doi.org/10.3390/ma16237455






