Effect of ZrC on the Microstructure and Properties of CrMnFeCoNi High-Entropy Alloy Coatings Prepared by a Plasma Transferred Arc Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
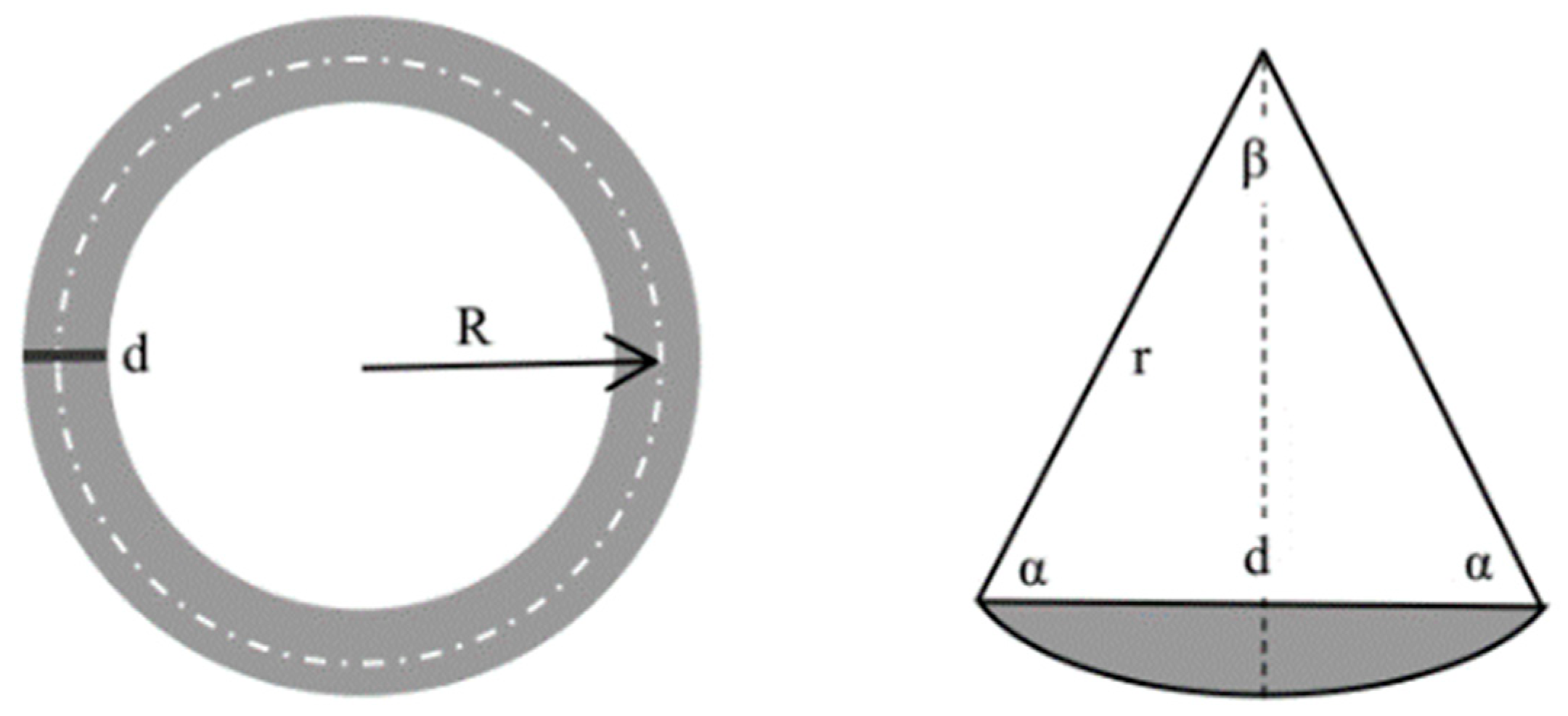
2.2. Experimental Method
3. Results and Discussion
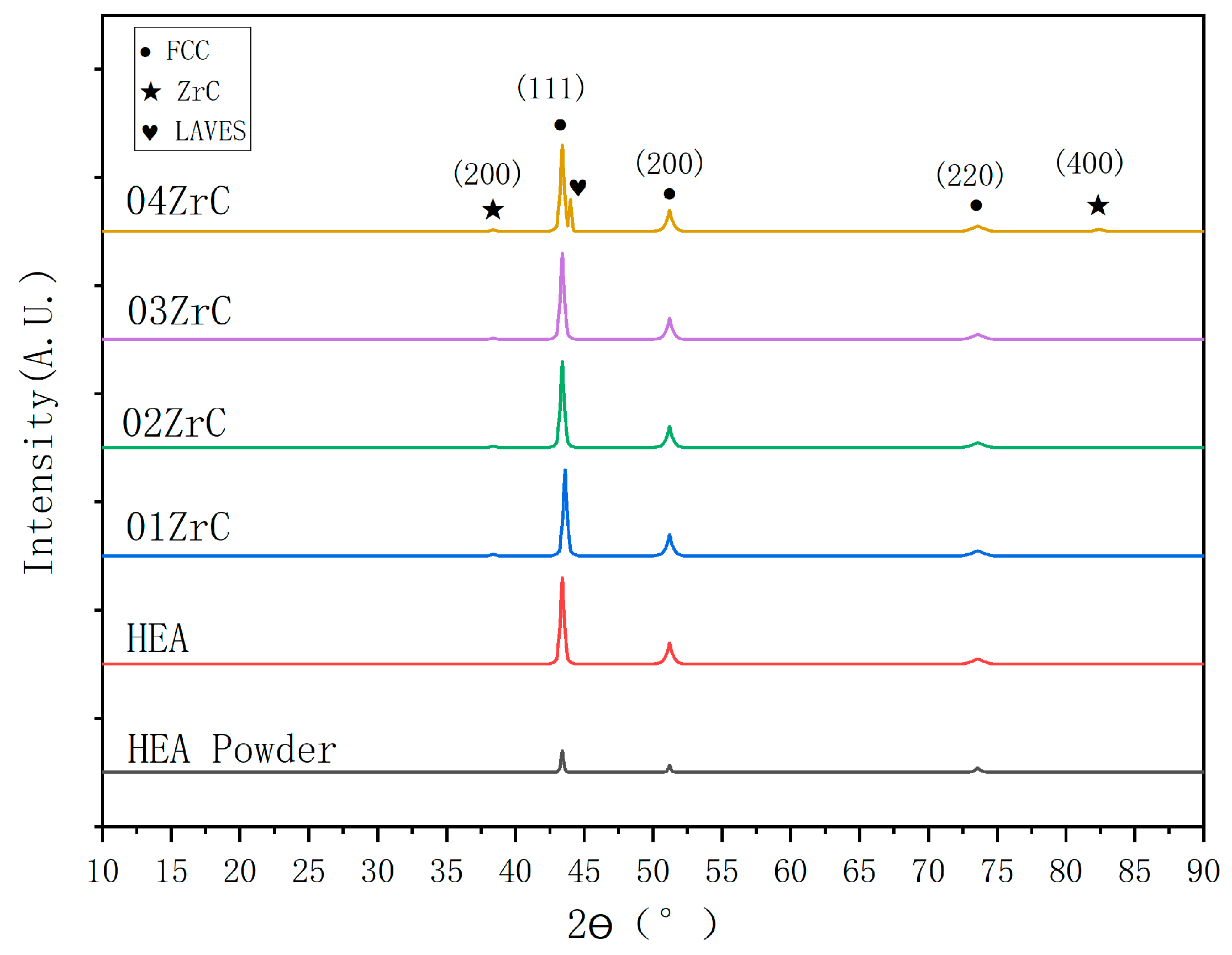
3.1. Phase Composition of the Coating
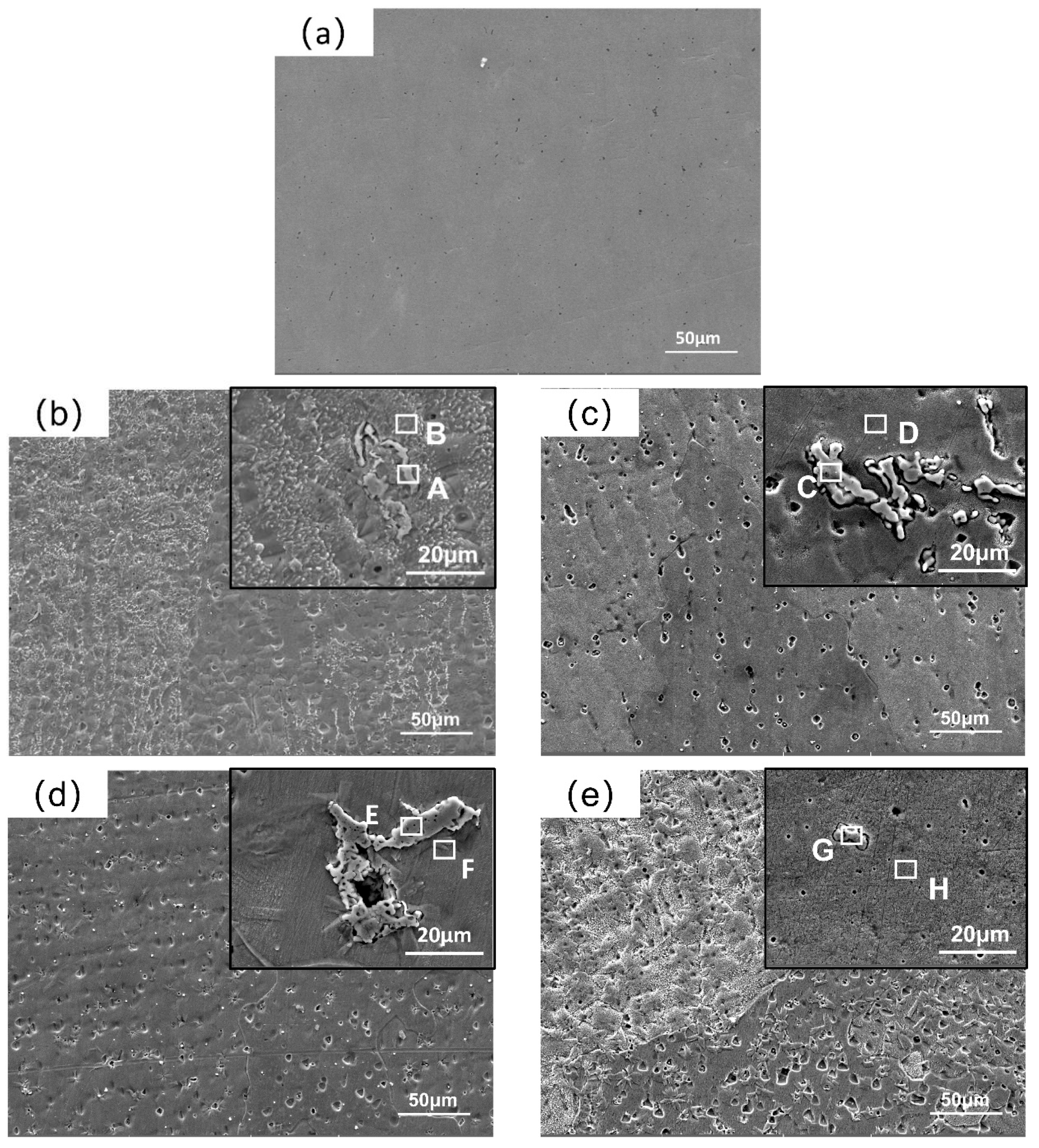
3.2. Morphology and Composition Analysis of the Coatings
3.3. Microhardness
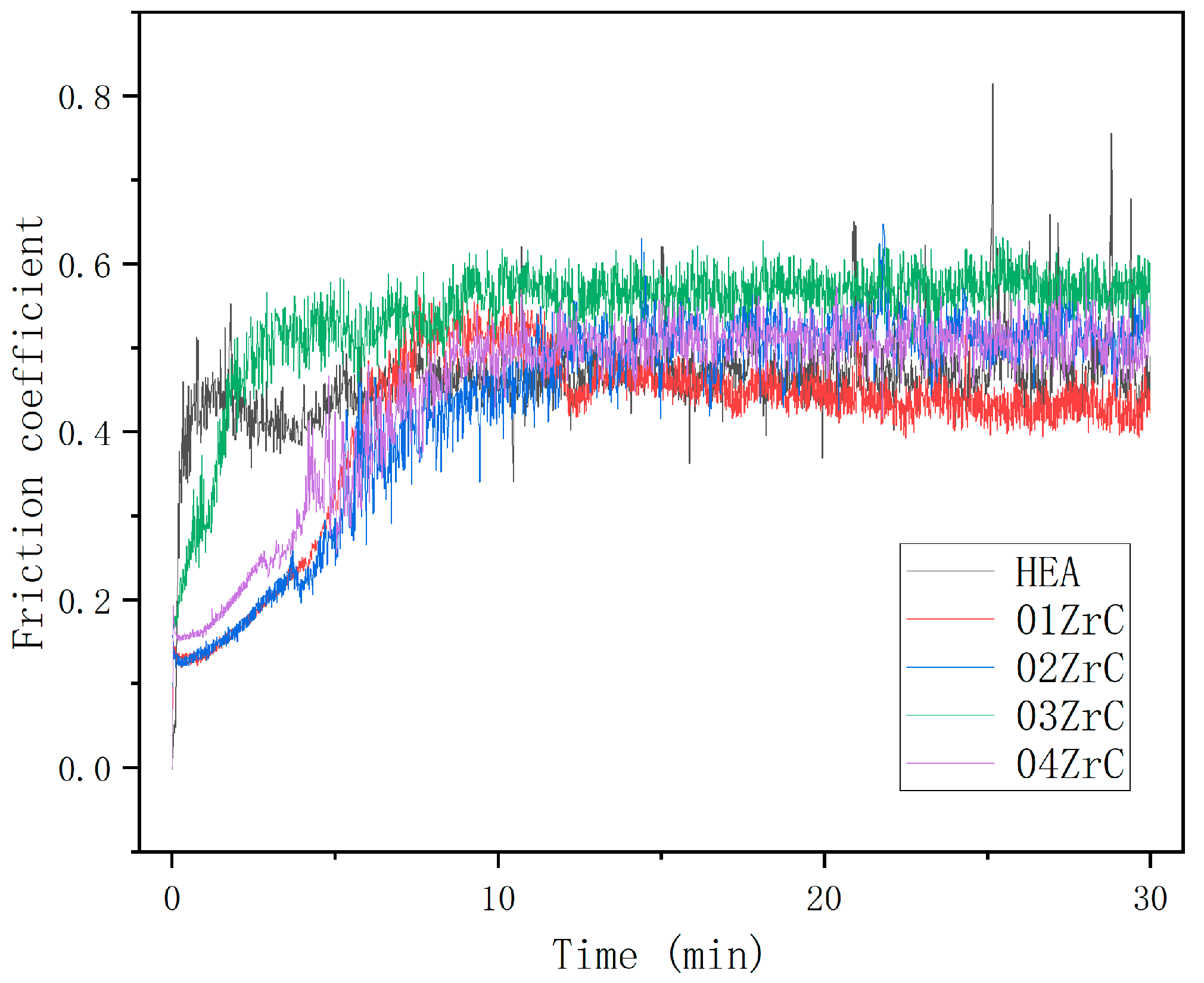
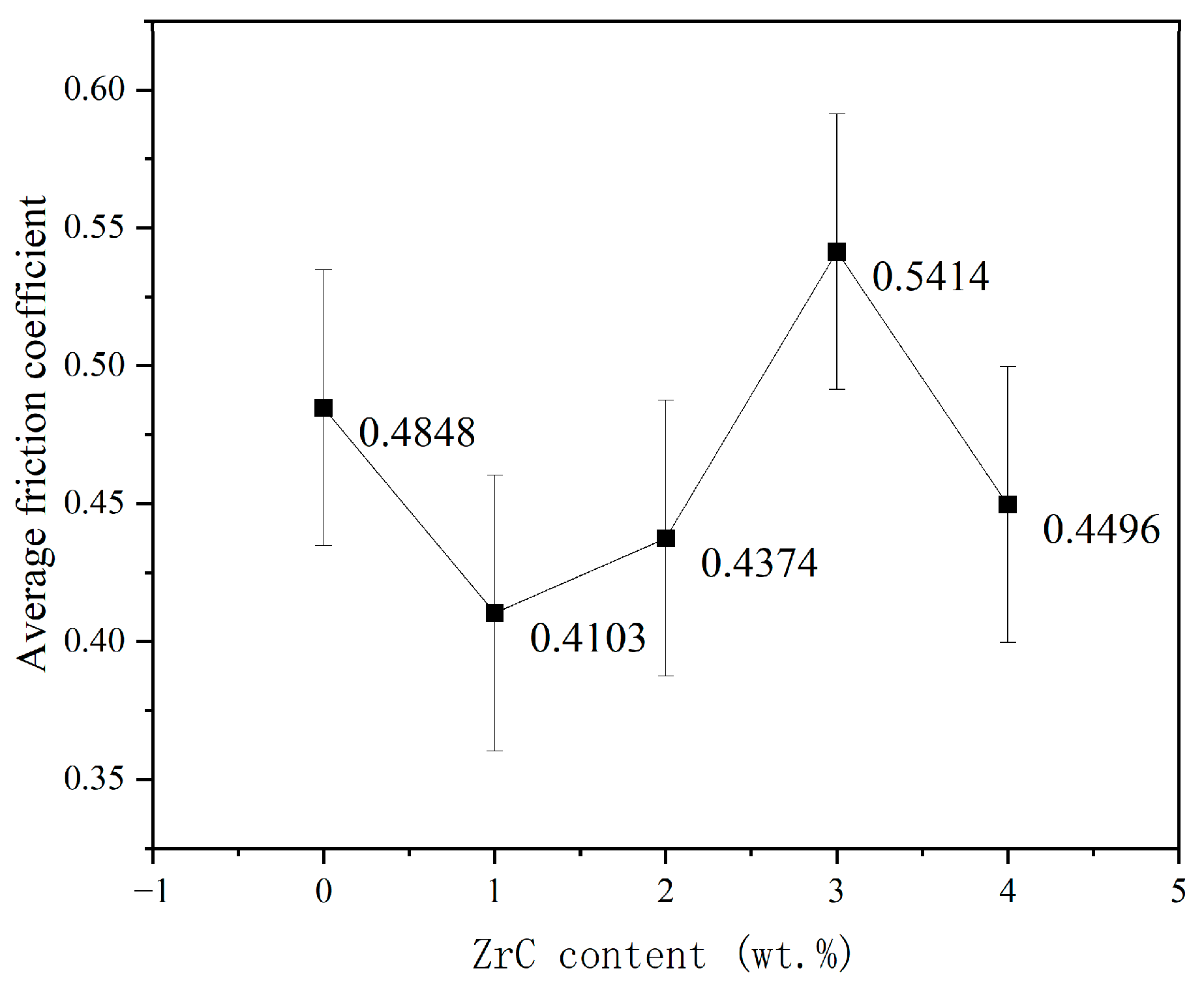
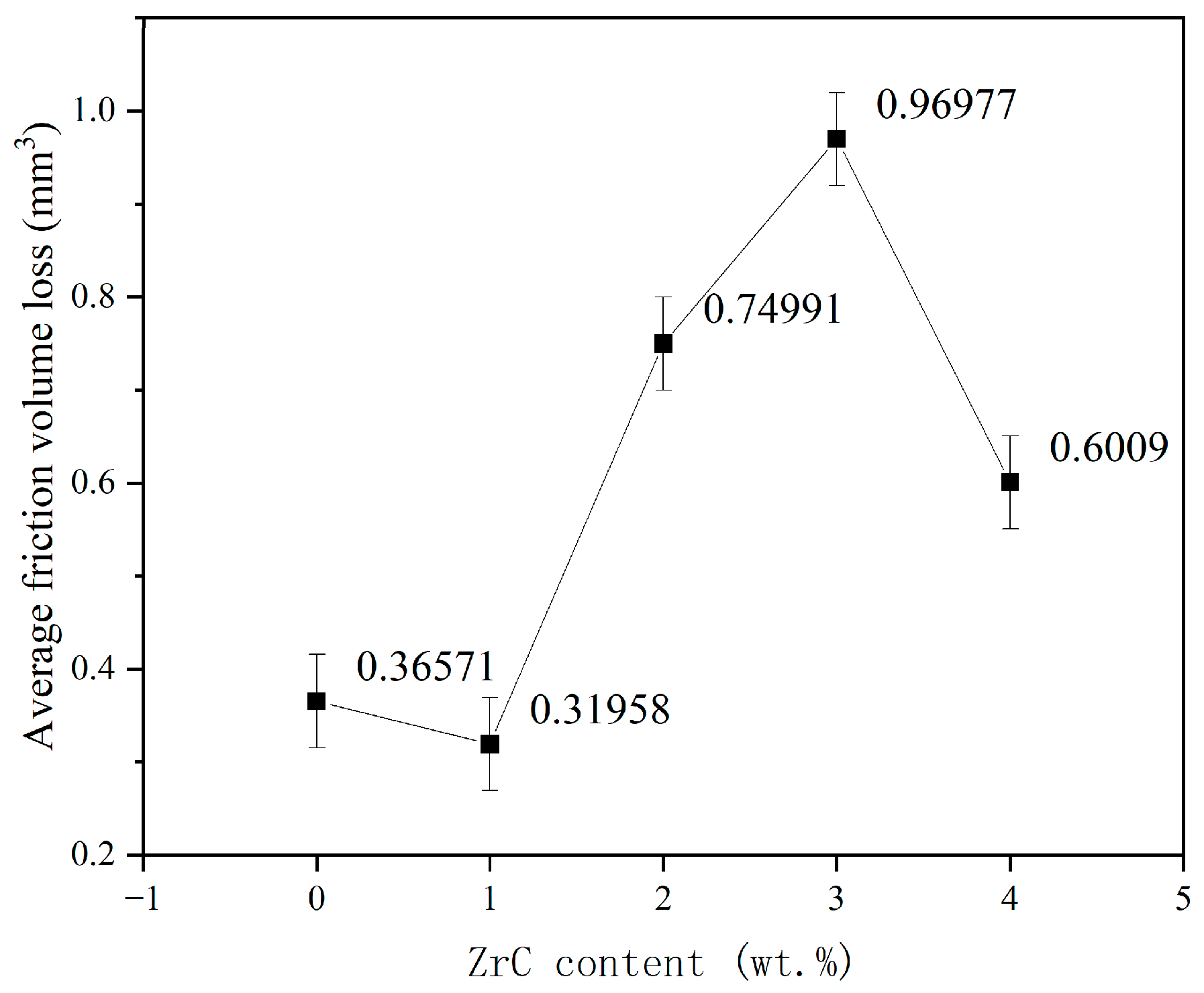
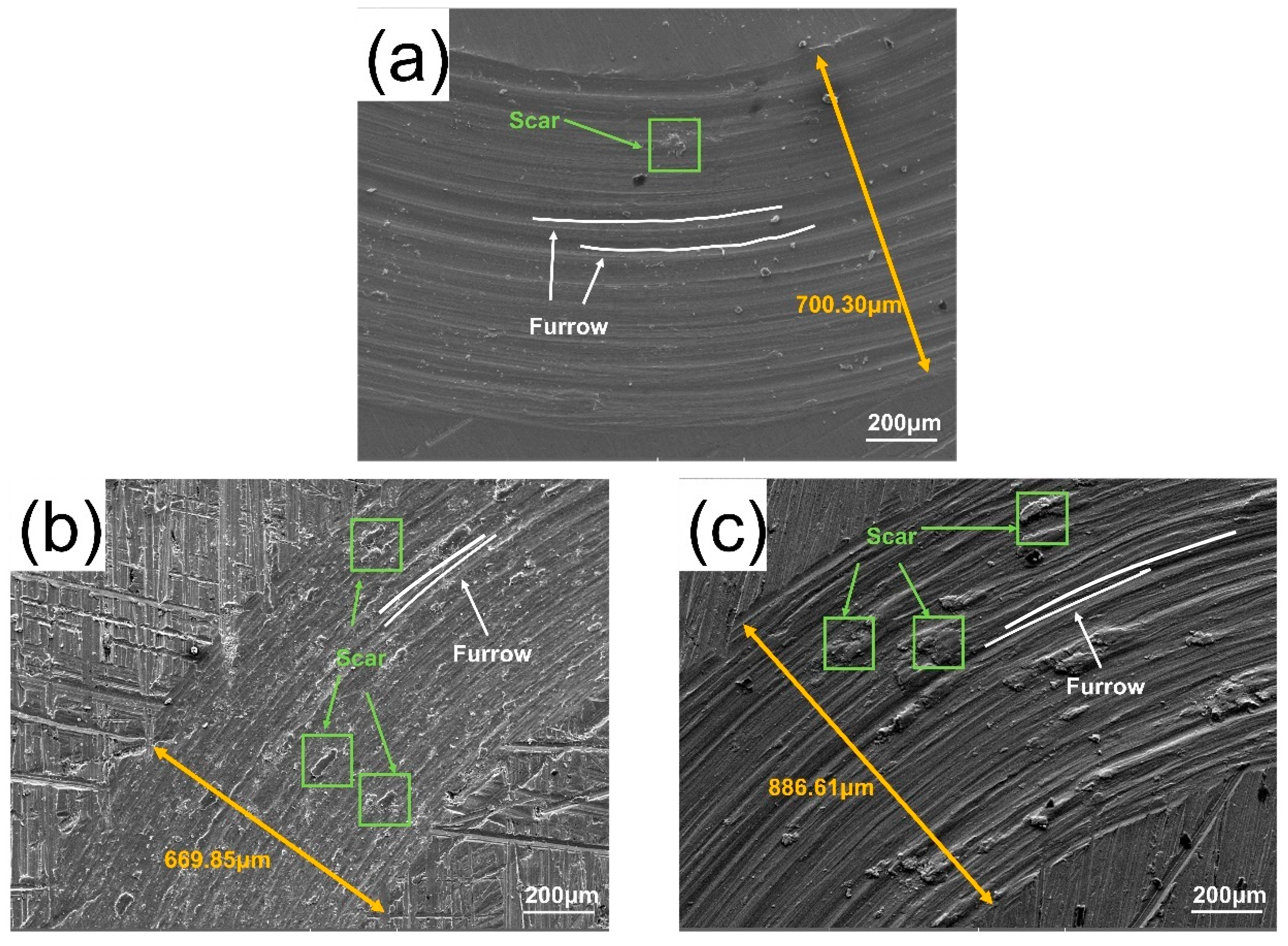
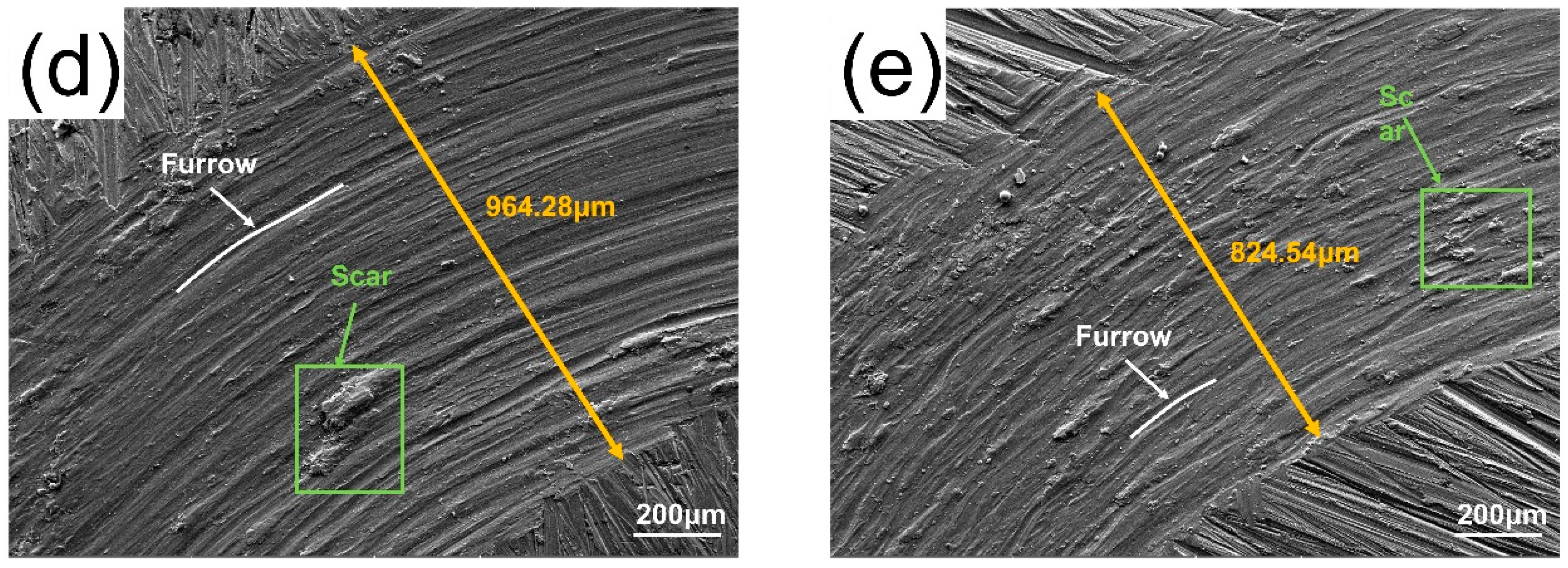
3.4. Friction and Wear Properties
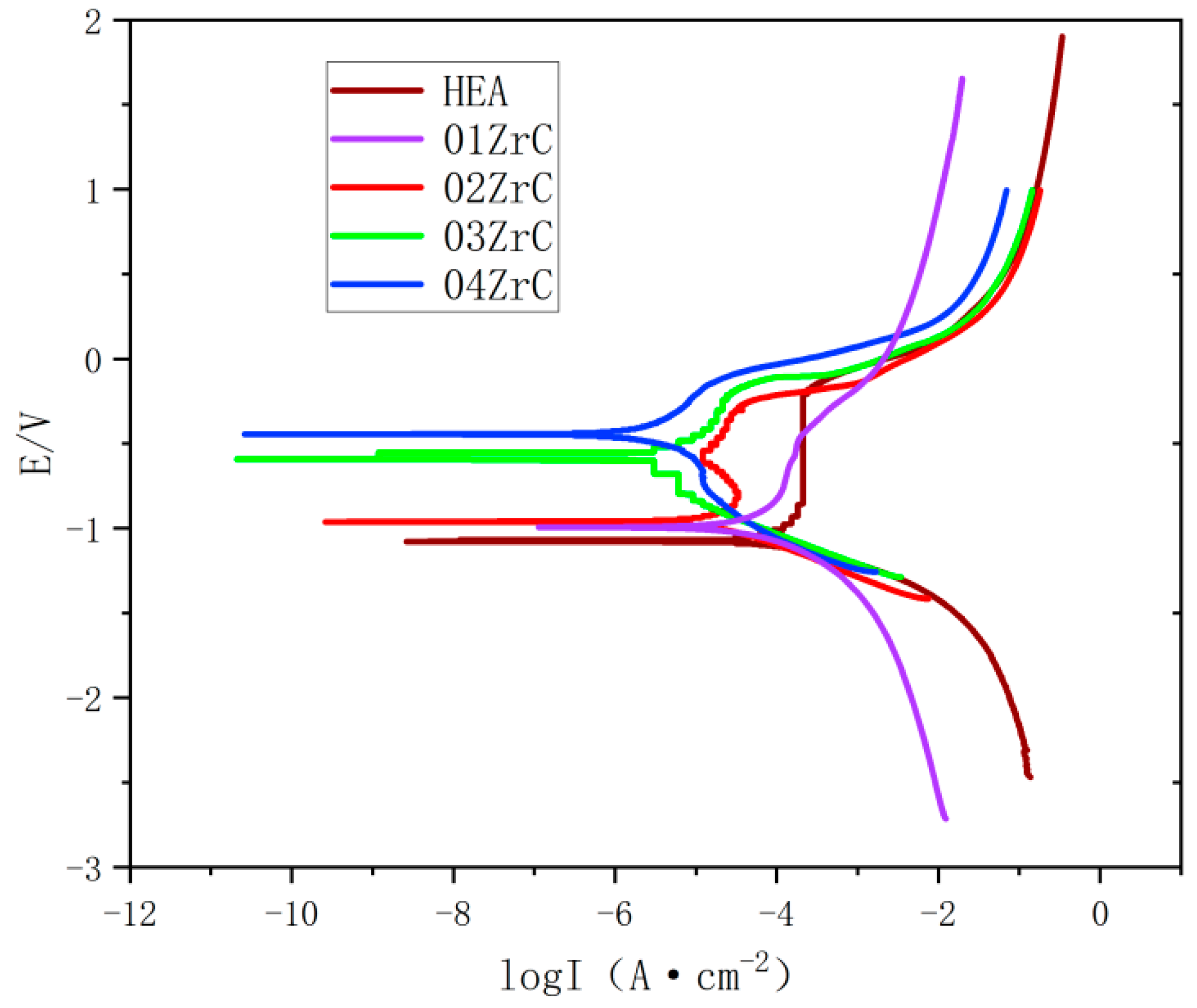
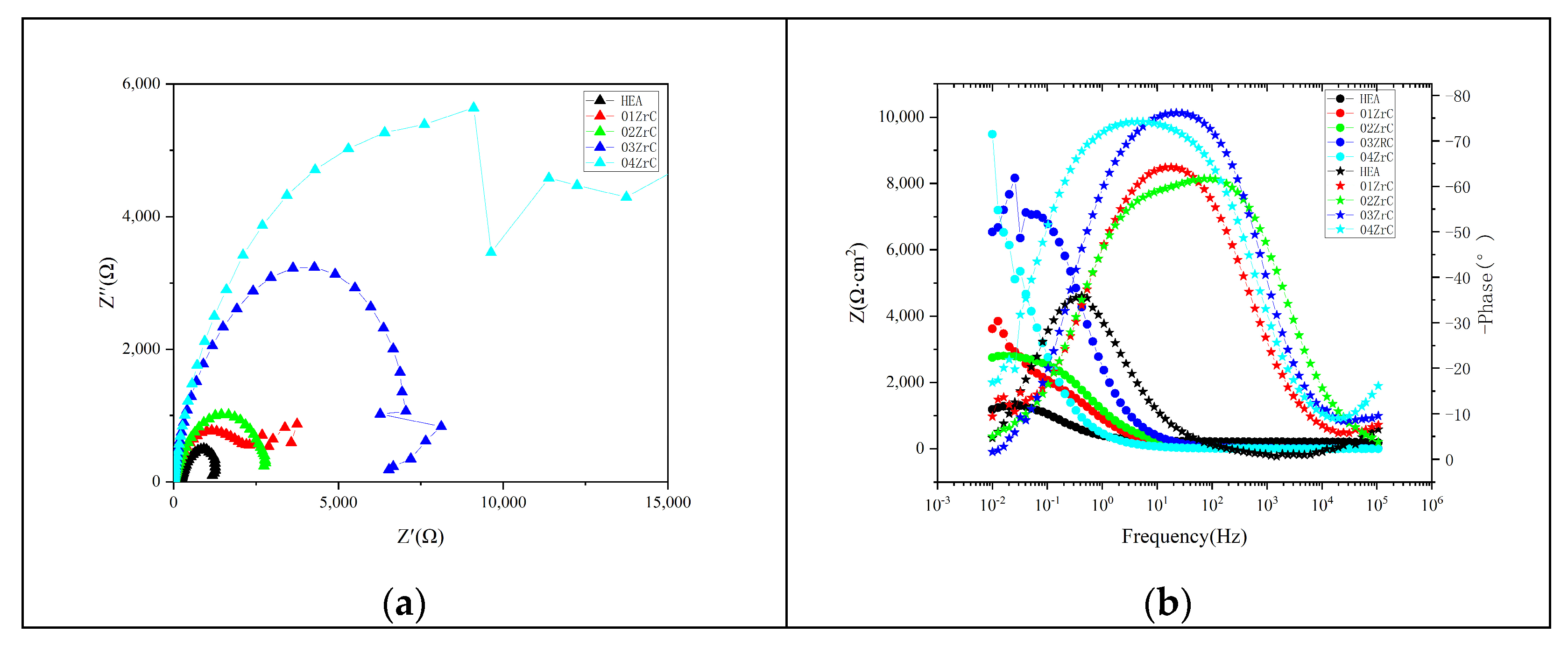
3.5. Corrosion Resistance of the Coating
4. Conclusions
- The microstructure of HEA and coatings with varying ZrC contents consist of dendrites that are perpendicular to the substrate. The crystal structures of the CrMnFeCoNi HEA coatings with different ZrC contents are FCC. The results indicated that the addition of ZrC did not change the microstructure and morphology of the HEA coating. This is due to the fact that ZrC mainly exists in the CrMnFeCoNi HEA coating as a second phase.
- The hardness of the CrMnFeCoNi HEA coating increases gradually with increasing ZrC content, and the hardness of the 01ZrC coating is approximately 20% higher than that of the HEA. However, the increase rate of the hardness decreases when the content of ZrC is more than 1 wt%.
- ZrC had little effect on improving the wear resistance of the CrMnFeCoNi HEA coating. The addition of ZrC did not change the wear mechanism of the CrMnFeCoNi HEA coating. In every case, the wear was a combination of adhesive wear and abrasive wear. The wear resistance of the CrMnFeCoNi HEA coating showed a trend of first increasing, then decreasing, and finally increasing with increasing ZrC content. Although the increase in hardness improved the wear resistance of the 01ZrC coating, the improvement was not remarkable.
- ZrC can effectively improve the corrosion resistance of the CrMnFeCoNi HEA coating, and the corrosion resistance of the CrMnFeCoNi HEA coating increases with increasing ZrC content. The annual corrosion rate of the 04ZrC coating in a 1 mol/L NaCl solution is only 21.5% of that of the HEA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.; Lin, S.; Gan, J.; Chin, T.S.; Shun, T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.; Knight, P.C.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2010, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.; Chang, S.; Hong, Y.; Chen, S.; Lin, S. Anomalous decrease in X-ray diffraction intensities of Cu–Ni–Al–Co–Cr–Fe–Si alloy systems with multi-principal elements. Mater. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Yeh, J. Recent progress in high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Ren, X.; Li, Y.; Qi, Y.; Wang, B. Review on Preparation Technology and Properties of Refractory High Entropy Alloys. Materials 2022, 15, 2931. [Google Scholar] [CrossRef] [PubMed]
- Geambazu, L.E.; Cotruţ, C.M.; Miculescu, F.; Csaki, I. Mechanically Alloyed CoCrFeNiMo0.85 High-Entropy Alloy for Corrosion Resistance Coatings. Materials 2021, 14, 3802. [Google Scholar] [CrossRef] [PubMed]
- Bololoi, A.E.; Geambazu, L.E.; Antoniac, I.V.; Bololoi, R.V.; Manea, C.A.; Cojocaru, V.D.; Pătroi, D. Solid-State Processing of CoCrMoNbTi High-Entropy Alloy for Biomedical Applications. Materials 2023, 16, 6520. [Google Scholar] [CrossRef]
- Tokarewicz, M.; Grądzka-Dahlke, M.; Rećko, K.; Łępicka, M.; Czajkowska, K. Investigation of the Structure and Corrosion Resistance of Novel High-Entropy Alloys for Potential Biomedical Applications. Materials 2022, 15, 3938. [Google Scholar] [CrossRef]
- Bhaskaran Nair, R.; Supekar, R.; Morteza Javid, S.; Wang, W.; Zou, Y.; McDonald, A.G.; Mostaghimi, J.; Stoyanov, P. High-Entropy Alloy Coatings Deposited by Thermal Spraying: A Review of Strengthening Mechanisms, Performance Assessments and Perspectives on Future Applications. Metals 2023, 13, 579. [Google Scholar] [CrossRef]
- Dixit, S.; Rodríguez, S.B.; Jones, M.; Buzby, P.; Dixit, R.; Argibay, N.; DelRio, F.W.; Lim, H.H.; Fleming, D. Refractory High-Entropy Alloy Coatings for High-Temperature Aerospace and Energy Applications. J. Therm. Spray Technol. 2022, 31, 1021–1031. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, C.; Shen, Y.; Feng, X. Microstructure and corrosion property of CrMnFeCoNi high entropy alloy coating on Q235 substrate via mechanical alloying method. Surf. Interfaces 2006, 15, 135–140. [Google Scholar] [CrossRef]
- Chen, C.; Suprianto, S. Microstructure and mechanical properties of AlCuNiFeCr high entropy alloy coatings by mechanical alloying. Surf. Coat. Technol. 2020, 386, 125443. [Google Scholar] [CrossRef]
- Hsu, W.; Yang, Y.; Chen, C.; Yeh, J. Thermal sprayed high-entropy NiCo0.6Fe0.2Cr1.5SiAlTi0.2 coating with improved mechanical properties and oxidation resistance. Intermetallics 2017, 89, 105–110. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Luzin, V.; Schulz, C.; Hall, C.; Murty, B.S.; Kottada, R.S.; Berndt, C.C.; Ang, A.S. Multiscale mechanical performance and corrosion behaviour of plasma sprayed AlCoCrFeNi high-entropy alloy coatings. J. Alloys Compd. 2021, 854, 157140. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, Y.; Yoon, S.; Lee, K. Tuning the Microstructure and Mechanical Properties of Cold Sprayed Equiatomic CoCrFeMnNi High-Entropy Alloy Coating Layer. Metals Mater. Int. 2020, 27, 2406–2415. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, X.; Zhang, L.; Hui, X.; Wu, Y.; Zheng, H.; Ren, Z.; Zhao, Y.; Wang, W.; Zhu, S.; et al. Microstructures, Wear Resistance and Corrosion Resistance of CoCrFeNi High Entropy Alloys Coating on AZ91 Mg Alloy Prepared by Cold Spray. J. Alloys Compd. 2022, 925, 166698. [Google Scholar] [CrossRef]
- Cavaliere, P.D.; Perrone, A.; Silvello, A.; Laska, A.; Blasi, G.D.; Cano, I.G.; Sadeghi, B.; Nagy, S. Cyclic behavior of FeCoCrNiMn high entropy alloy coatings produced through cold spray. J. Alloys Compd. 2023, 931, 167550. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.; Jiao, H. Microstructure and properties of 6FeNiCoSiCrAlTi high-entropy alloy coating prepared by laser cladding. Appl. Surf. Sci. 2011, 257, 2259–2263. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Zeng, M.; Wang, J.; Deng, P.; Wang, Y. In-situ TiC reinforced CoCrCuFeNiSi0.2 high-entropy alloy coatings designed for enhanced wear performance by laser cladding. Mater. Chem. Phys. 2020, 242, 122522. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Fu, H.; Guo, X.; Lin, J. Microstructure and wear resistance of in-situ TiC reinforced AlCoCrFeNi-based coatings by laser cladding. Appl. Surf. Sci. 2022, 585, 152703. [Google Scholar] [CrossRef]
- Yu, K.; Zhao, W.; Li, Z.; Guo, N.; Xiao, G.; Zhang, H. High-temperature oxidation behavior and corrosion resistance of in-situ TiC and Mo reinforced AlCoCrFeNi-based high entropy alloy coatings by laser cladding. Ceram. Int. 2023, 49, 10151–10164. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Juan, Y.F.; Lu, Z.J.; Jia, W. Evolution in microstructure and corrosion behavior of AlCoCrxFeNi high-entropy alloy coatings fabricated by laser cladding. J. Alloys Compd. 2019, 775, 1–14. [Google Scholar] [CrossRef]
- Gu, Z.; Xi, S.; Sun, C. Microstructure and properties of laser cladding and CoCr2.5FeNi2Tix high-entropy alloy composite coatings. J. Alloys Compd. 2020, 819, 152986. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Liu, Q. Microstructure evolution and strengthening mechanism of laser-cladding MoFe CrTiWAlNb refractory high-entropy alloy coatings. J. Alloys Compd. 2020, 834, 155147. [Google Scholar] [CrossRef]
- Sun, S.; Liu, H.; Hao, J.; Yang, H. Microstructural evolution and corrosion behavior of CoCrFeNiAlxMn(1−x) dual-phase high-entropy alloy coatings prepared by laser cladding. J. Alloys Compd. 2021, 886, 161251. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, C. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, H.J.; Mun, S.C.; Jumaev, E.; Hong, S.H.; Song, G.; Kim, J.T.; Park, Y.K.; Kim, K.S.; Jeong, S.; et al. Investigation of structure and mechanical properties of TiZrHfNiCuCo high entropy alloy thin films synthesized by magnetron sputtering. J. Alloys Compd. 2019, 797, 834–841. [Google Scholar] [CrossRef]
- Khan, N.A.; Akhavan, B.; Zhou, C.; Zhou, H.; Chang, L.; Wang, Y.; Liu, Y.; Fu, L.; Bilek, M.M.; Liu, Z. RF magnetron sputtered AlCoCrCu0.5FeNi high entropy alloy (HEA) thin films with tuned microstructure and chemical composition. J. Alloys Compd. 2020, 836, 155348. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Quan, H.; Chen, Y.; Wang, S.; Zhang, S. Effect of Mo addition on structures and properties of FeCoNiCrMn high entropy alloy film by direct current magnetron sputtering. J. Alloys Compd. 2021, 895, 162709. [Google Scholar] [CrossRef]
- Gao, P.H.; Fu, R.; Chen, B.; Zeng, S.; Zhang, B.; Yang, Z.; Guo, Y.; Liang, M.; Li, J.; Lu, Y.; et al. Corrosion Resistance of CoCrFeNiMn High Entropy Alloy Coating Prepared through Plasma Transfer Arc Claddings. Metals 2021, 11, 1876. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, N.; Shi, M.; Xing, B.; Zuo, X.; Yin, S. Microstructure and Tribological Properties of Plasma Cladding FeCoNiCr-x(TiC) Composite Coatings. J. Therm. Spray Technol. 2022, 31, 1649–1661. [Google Scholar] [CrossRef]
- Wang, M.; Lu, Y.; Zhang, G.; Cui, H.; Xu, D.; Wei, N.; Li, T. A novel high-entropy alloy composite coating with core-shell structures prepared by plasma cladding. Vacuum 2021, 184, 109905. [Google Scholar] [CrossRef]
- Xie, Y.; Wen, X.; Huang, B.; Zhuang, J. Microstructure, hardness and corrosion properties of AlCoCrFeNi2.1YHf high-entropy alloy coating prepared by plasma cladding. Mater. Lett. 2023, 330, 133356. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, W.; Li, T.; Zhang, M.; Wang, L.; Hu, S. Microstructures and wear-resistance of WC-reinforced high entropy alloy composite coatings by plasma cladding: Effect of WC morphology. Surf. Eng. 2020, 37, 678–687. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Zhang, B.; Zhang, Z.; Yan, X.D.; Wang, Z. Microstructure and wear behaviour of in-situ TiN-Al2O3 reinforced CoCrFeNiMn high-entropy alloys composite coatings fabricated by plasma cladding. Mater. Lett. 2020, 272, 127870. [Google Scholar] [CrossRef]
- He, Y.; Zhang, S.; He, Y.; Li, H.; Fan, Y.; Zhang, Y.; Xiang, Y.; He, T.; Song, R.; Liu, B.; et al. Strengthening effect of inclusion of ZrC nano-ceramic particles on the corrosion and wear resistance of Ni-P electroless deposits. Thin Solid Films 2022, 756, 139364. [Google Scholar] [CrossRef]
- Kang, X.; Lin, N.; He, Y.; Zhang, M. Influence of ZrC addition on the microstructure, mechanical properties and oxidation resistance of Ti(C,N)-based cermets. Ceram. Int. 2018, 44, 11151–11159. [Google Scholar] [CrossRef]
- Ding, R.; Wang, H.; Jiang, Y.; Liu, R.; Jing, K.; Sun, M.; Zhang, R.; Qiu, S.; Xie, Z.; Deng, H.W.; et al. Effects of ZrC addition on the microstructure and mechanical properties of Fe-Cr-Al alloys fabricated by spark plasma sintering. J. Alloys Compd. 2019, 805, 1025–1033. [Google Scholar] [CrossRef]
- ASTM G99-04; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM international: West Conshohocken, PA, USA, 2017.
- Li, H.; Jiao, L.; Xu, R.; Li, F.; Lu, S.; Qiao, Y.; Li, C.; Zhang, P. Surface Wear Behavior and Friction and Wear Mechanism Studies of A356/3 wt.% Al3Zr Composites. J. Mater. Eng. Perform. 2021, 30, 3892–3902. [Google Scholar] [CrossRef]
- Chen, X.; Qin, G.; Gao, X.; Chen, R.; Song, Q.; Cui, H. Strengthening CoCrFeNi High Entropy Alloy by In-Situ Phases of Laves and ZrC. Met. Mater. Int. 2022, 29, 1390–1398. [Google Scholar] [CrossRef]
- Li, M.; Huang, L.; Zhou, Y.; Zhang, G. Effect of TiC Content on Microstructure and Properties of CrMnFeCoNi High-Entropy Alloy. J. Mater. Eng. Perform. 2023. [Google Scholar] [CrossRef]
- Sun, D.; Cai, Y.; Zhu, L.; Han, J. High-temperature wear behaviour of ZrC/NbC-reinforced CrMnFeCoNi coatings. Surf. Eng. 2022, 38, 778–785. [Google Scholar] [CrossRef]
- Vashishtha, N.; Sapate, S.G.; Gahlot, J.S.; Bagde, P. Effect of Tribo-Oxidation on Friction and Wear Behaviour of HVOF Sprayed WC–10Co–4Cr Coating. Tribol. Lett. 2018, 66, 1–19. [Google Scholar] [CrossRef]
- Liu, P.; Si, W.; Zhang, D.; Dai, S.; Jiang, B.; Shu, D.; Wu, L.; Zhang, C.; Zhang, M. Microstructure and Friction Properties of CoCrFeMnNiTix High-Entropy Alloy Coating by Laser Cladding. Materials 2022, 15, 4669. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, A.H.; Bhlol, M.; Dawood, O.M.; Ghayad, I.M.; Elkady, O.A. Effect of tungsten carbide (WC) on electrochemical corrosion behavior, hardness, and microstructure of CrFeCoNi high entropy alloy. J. Eng. Appl. Sci. 2022, 69, 43. [Google Scholar] [CrossRef]





| C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|
| ≤0.22% | ≤1.4% | ≤0.35% | ≤0.050% | ≤0.045% | Bal. |
| Cr | Mn | Fe | Co | Ni |
|---|---|---|---|---|
| 19.52% | 20.86% | 20.26% | 19.49% | 19.87% |
| Carrier Gas Flow Rate (L/min) | Plasma Gas Flow Rate (L/min) | Powder Feed Rate (g/min) | Electric Current (A) | Deposition Speed (mm/min) | Working Distance to Substrate (mm) |
|---|---|---|---|---|---|
| 5 | 5 | 50 | 180 | 300 | 10 |
| Applied Load (g) | Time (min) | Experimental Temperature (°C) | Rotational Speed (r/min) | The Radius of the Sliding Wear Track (mm) |
|---|---|---|---|---|
| 200 | 30 | 25 | 200 | 3 |
| EIS Test Method | Frequency Range (Hz) | Disturbance Voltage (mV) | Polarization Curve Test Method | Voltage Range (V) | Scanning Speed (mV/s) |
|---|---|---|---|---|---|
| Impedance-frequency scanning | 10−2–10−5 | 10 | Potentiodynamic polarization | −2~2 | 1 |
| FCC | ZrC | LAVES | |
|---|---|---|---|
| HEA powder | 100 | 0 | 0 |
| HEA | 100 | 0 | 0 |
| 01ZrC | 95.3 | 4.7 | 0 |
| 02ZrC | 94.4 | 5.6 | 0 |
| 03ZrC | 93.8 | 6.2 | 0 |
| 04ZrC | 88.8 | 6.3 | 4.9 |
| Point | Cr | Mn | Fe | Co | Ni | Zr | C |
|---|---|---|---|---|---|---|---|
| A | 4.16 | 6.52 | 3.01 | 2.56 | 2.70 | 62.60 | 18.46 |
| B | 16.89 | 22.89 | 18.23 | 17.93 | 21.99 | 0 | 1.89 |
| C | 0.56 | 0.56 | 0.58 | 0.43 | 0.41 | 64.54 | 32.27 |
| D | 17.11 | 15.27 | 22.02 | 18.81 | 17.68 | 3.66 | 5.45 |
| E | 1.63 | 0 | 2.93 | 0 | 0 | 64.40 | 29.38 |
| F | 18.68 | 17.04 | 21.79 | 19.84 | 17.82 | 0.01 | 4.83 |
| G | 4.24 | 5.19 | 8.04 | 3.35 | 3.18 | 51.07 | 22.93 |
| H | 18.22 | 15.64 | 20.48 | 17.54 | 15.68 | 5.48 | 6.98 |
| Specimen | Rp (Ω/cm2) | Ecorr (V) | Icorr (A/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|---|
| HEA | 1076.6 | −0.99367 ± 0.05 | 1.6673 × 10−5 ± 0.3 × 10−5 | 0.1956 ± 0.005 |
| 01ZrC | 1962 | −0.97638 ± 0.05 | 9.3374 × 10−6 ± 0.5 × 10−6 | 0.1095421 ± 0.005 |
| 02ZrC | 2240 | −0.96184 ± 0.05 | 8.0538 × 10−6 ± 0.5 × 10−6 | 0.094269 ± 0.005 |
| 03ZrC | 7488.2 | −0.58537 ± 0.05 | 2.4038 × 10−6 ± 0.5 × 10−6 | 0.024358 ± 0.005 |
| 04ZrC | 18027 | −0.44554 ± 0.05 | 9.9848 × 10−7 ± 0.5 × 10−7 | 0.011731 ± 0.005 |
| Rs (Ω·cm2) | CPE | Y0 (S·Ω−1·cm−2) | Rt (Ω·cm2) | |
|---|---|---|---|---|
| HEA | 5.916 | 0.74374 | 9.4613 × 10−5 | 1286 |
| 01ZrC | 7.597 | 0.75964 | 2.3709 × 10−5 | 3022 |
| 02ZrC | 4.511 | 0.73601 | 1.6096 × 10−5 | 3452 |
| 03ZrC | 6.291 | 0.86851 | 7.1758 × 10−5 | 7524 |
| 04ZrC | 13.66 | 0.84460 | 5.6212 × 10−5 | 13,527 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Li, B.; Xu, B.; Zhou, Y.; Li, M.; Li, C.; Yang, B.; Pan, C.; Zhang, G. Effect of ZrC on the Microstructure and Properties of CrMnFeCoNi High-Entropy Alloy Coatings Prepared by a Plasma Transferred Arc Process. Materials 2023, 16, 7401. https://doi.org/10.3390/ma16237401
Huang L, Li B, Xu B, Zhou Y, Li M, Li C, Yang B, Pan C, Zhang G. Effect of ZrC on the Microstructure and Properties of CrMnFeCoNi High-Entropy Alloy Coatings Prepared by a Plasma Transferred Arc Process. Materials. 2023; 16(23):7401. https://doi.org/10.3390/ma16237401
Chicago/Turabian StyleHuang, Long, Bingyuan Li, Bopin Xu, Yicheng Zhou, Mengzhao Li, Chenglin Li, Bing Yang, Chunxu Pan, and Guodong Zhang. 2023. "Effect of ZrC on the Microstructure and Properties of CrMnFeCoNi High-Entropy Alloy Coatings Prepared by a Plasma Transferred Arc Process" Materials 16, no. 23: 7401. https://doi.org/10.3390/ma16237401
APA StyleHuang, L., Li, B., Xu, B., Zhou, Y., Li, M., Li, C., Yang, B., Pan, C., & Zhang, G. (2023). Effect of ZrC on the Microstructure and Properties of CrMnFeCoNi High-Entropy Alloy Coatings Prepared by a Plasma Transferred Arc Process. Materials, 16(23), 7401. https://doi.org/10.3390/ma16237401








