Effect of Different Cellulose Fillers on the Properties of Xanthan-Based Composites for Soil Conditioning Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (S1) A top soil layer sampled from a garden in the Department of Industrial Engineering of the University of Trento (46.06° N, 11.15° E, altitude 398 asl) and characterized by Fondazione Edmund Mach (San Michele all’Adige, Trento, Italy). The main properties of this soil are listed in Table 2.
- (S2) A top soil layer sampled in Alpine forests from the Ljubelj area (Slovenia, E(D96/TM) 443431, N (D96/TM) 144159, altitude 1100 asl) [69]. This is fine-grained soil and can be classified according to USCS (ASTM D2487-17 [70]) as organic silt of high plasticity (OH). The average value of natural gravimetric water content, determined according to ISO 17892-1 [71], is 42.9%. The main properties of this soil are listed in Table 2.
| Type of Filler | Basic Raw Material | Cellulose Content (%) | Oxide Ash (850 °C, 4 h) (%) | Average Fiber Length (µm) | Aspect Ratio |
|---|---|---|---|---|---|
| Arbocel R | Pure cellulose | >99 | 0.5 | 200–300 | 9.9 |
| Arbocel FT 400 | Technical cellulose | 75 | 2 | 1200 | 34.4 |
| Arbocel ZZC 500 | Technical raw cellulose | 80 | 15 | 400 | 8.8 |
| Cellugrün | Technical raw cellulose | 80 | 15 | 1400 | 31.1 |
| Arbocel ZZ 8-2 CA1 | Technical raw cellulose | 50 | 50 | 1000 | 22.2 |
| Arbocel Adsorb 2 | Cellulose dextrose derivate | - | - | 30–250 (particles) | - * |
| STEICO flex 036 (milled) | Wood fibers | - | - | 9000–30,000 | 25 |
| Determination | Soil (S1) | Soil (S2) |
|---|---|---|
| Sand (2.0–0.05 mm) | 412 g/kg | 202 g/kg |
| Silt (0.05–0.002 mm) | 458 g/kg | 493 g/kg |
| Clay (<0.002 mm) | 130 g/kg | 305 g/kg |
| pH (in water ratio 1:2.5) | 8.1 | 7.5 |
| Total limestone | 349 g/kg CaCO3 | 17 g/kg CaCO3 |
| Active limestone | 15 g/kg CaCO3 | - |
| Organic substance | 33 g/kg | 82 g/kg |
| Assimilable phosphorus | 27 mg/kg P2O5 | <60 mg/kg P2O5 |
| Potassium | 166 mg/kg K2O | <100 mg/kg K2O |
| Magnesium | 317 mg/kg MgO | - |
2.2. Soil Conditioners Preparation
2.3. Experimental Techniques
2.3.1. Rheological Properties
2.3.2. Fourier-Transformed Infrared Spectroscopy (FT-IR)
2.3.3. Light Microscopy
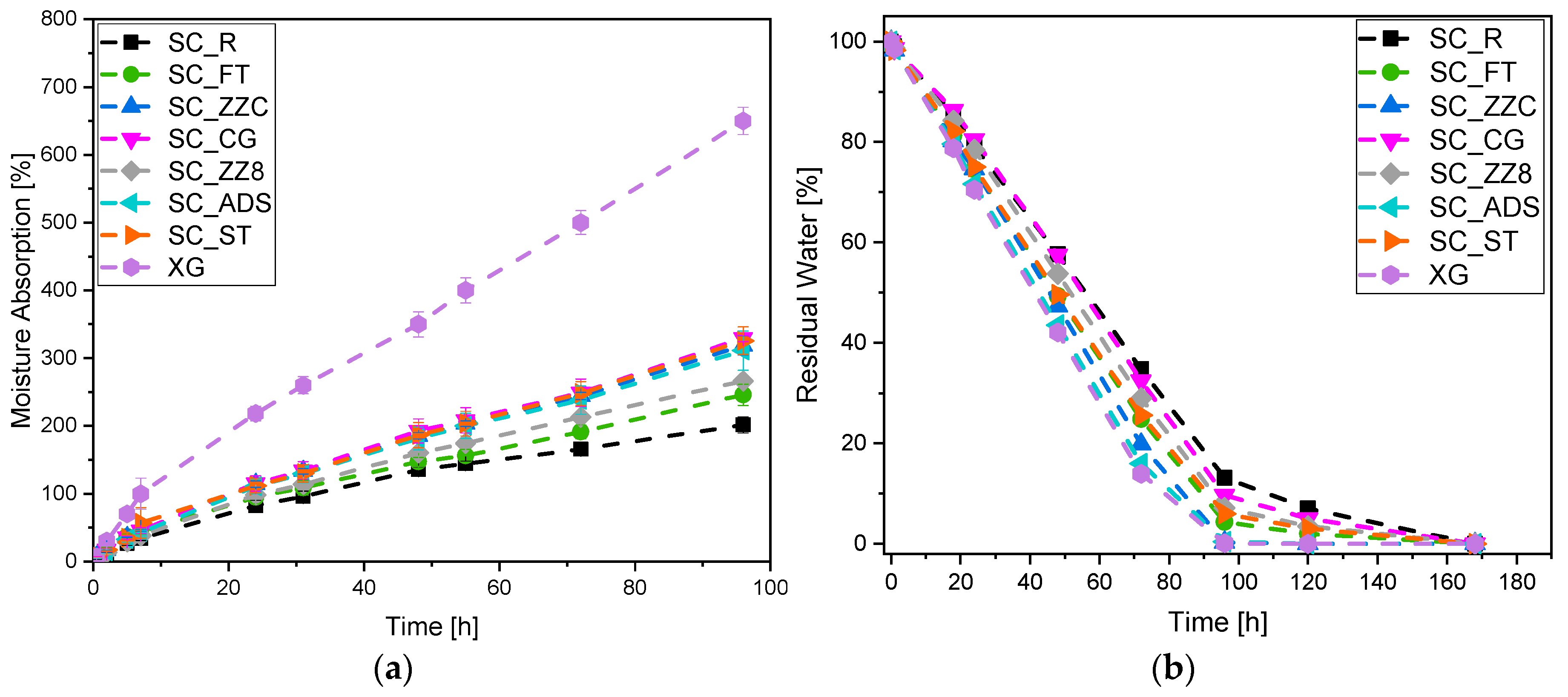
2.3.4. Moisture Absorption and Water Retention Capability
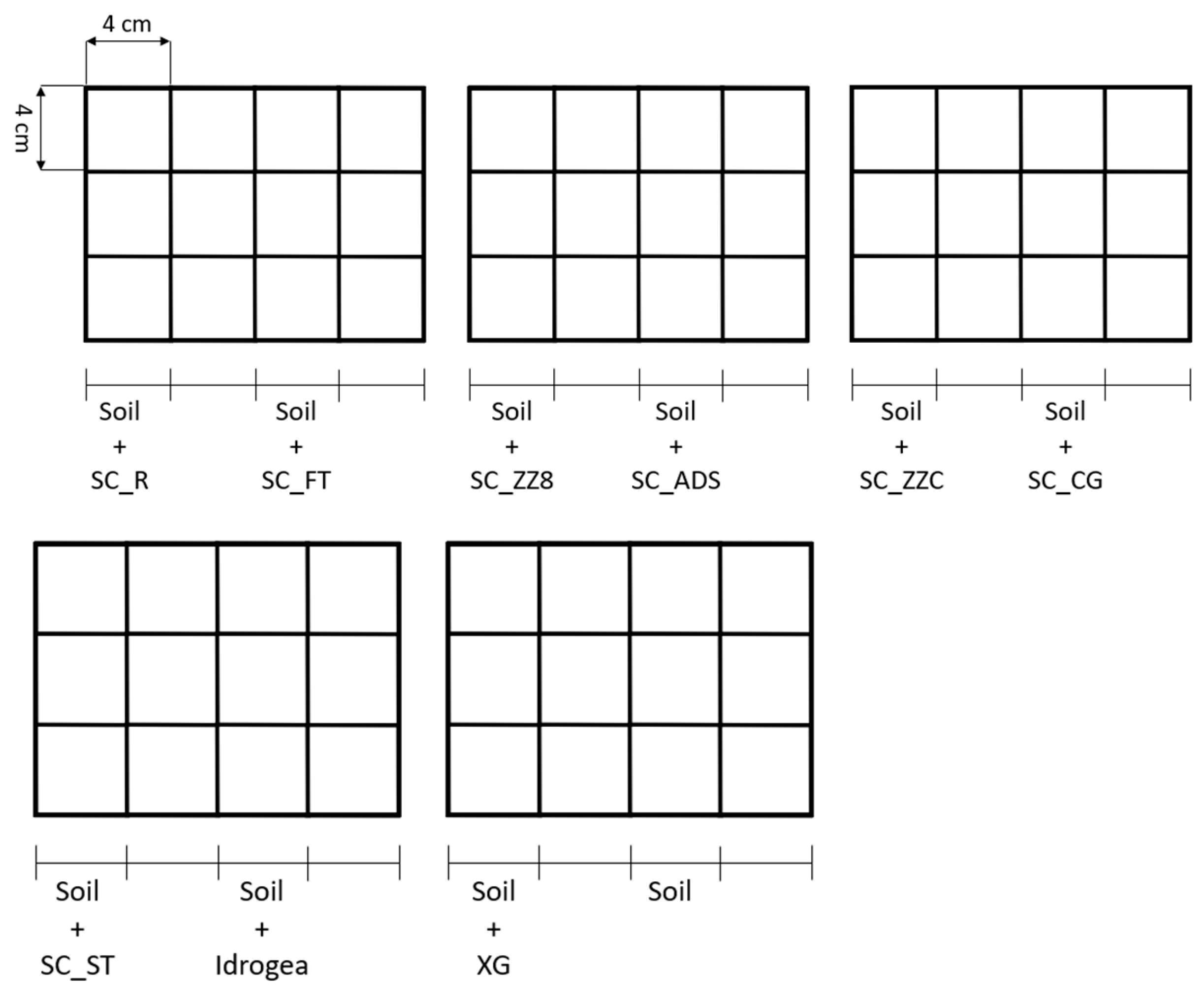
2.3.5. Application on Soil
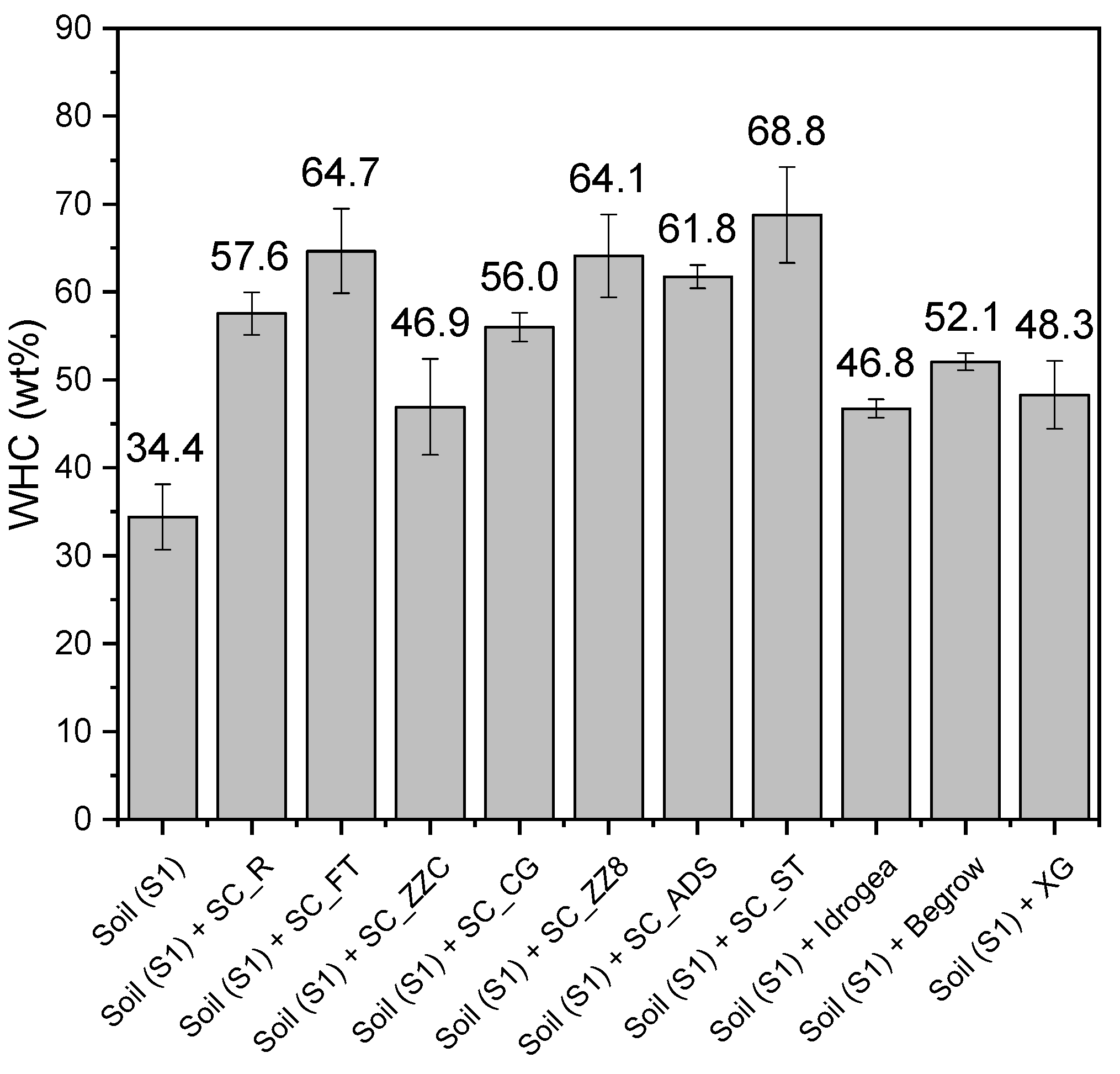
Evaluation of the Water Holding Capacity (WHC) of Soil S1
Evaluation of the Water Retention Capacity of the Soil S1
Evaluation of the Water Absorption (wA) of Soil S2
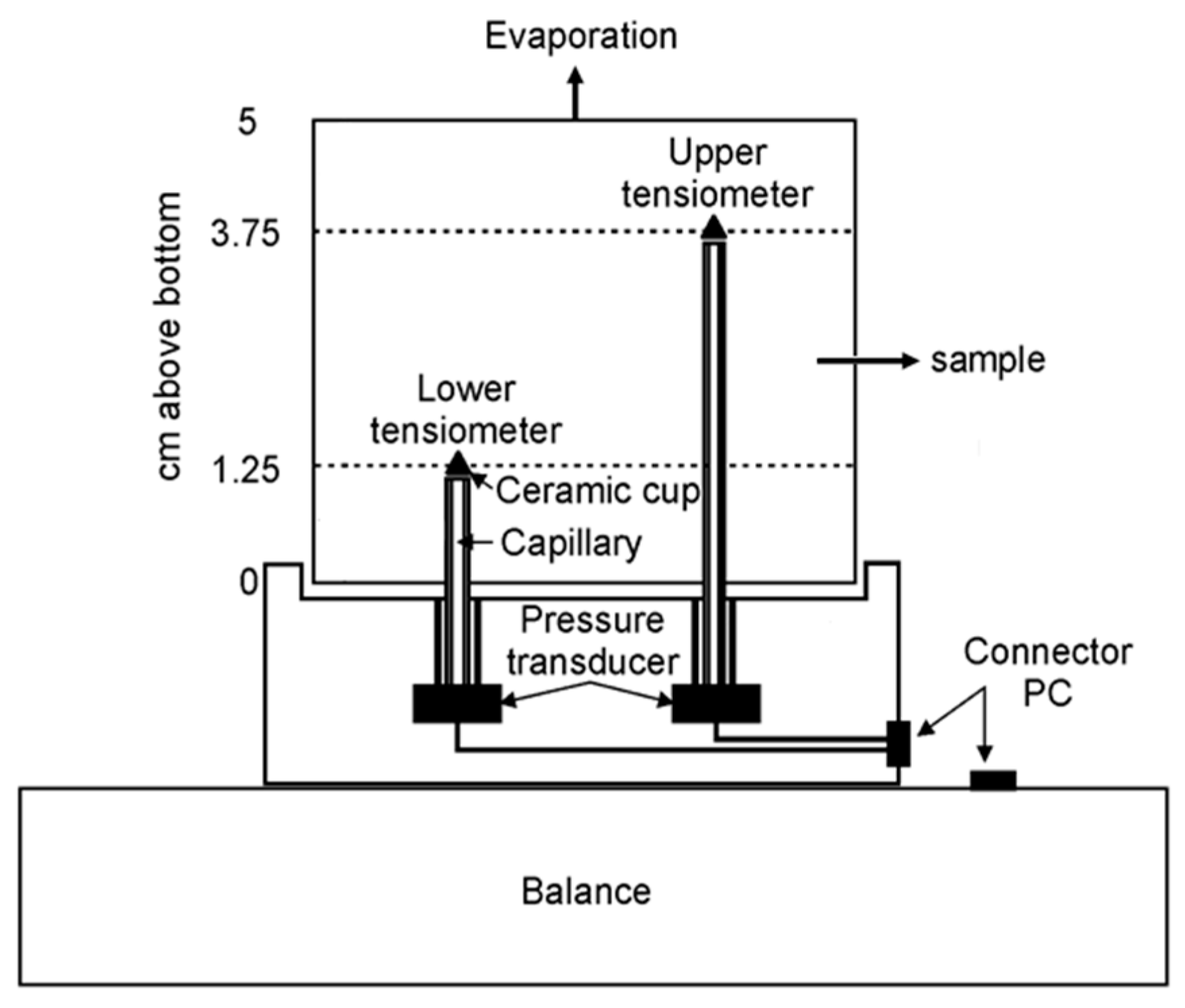
Determination of the Soil Water Retention Curve (SWRC) of Soil S2
Case Study Application
Evaluation of the grass germination in soil S1
3. Results and Discussion
3.1. Rheological Properties
3.2. FT-IR Spectroscopy
3.3. Light Microscopy
3.4. Moisture Absorption and Water Retention Capability
3.5. Application on Soil
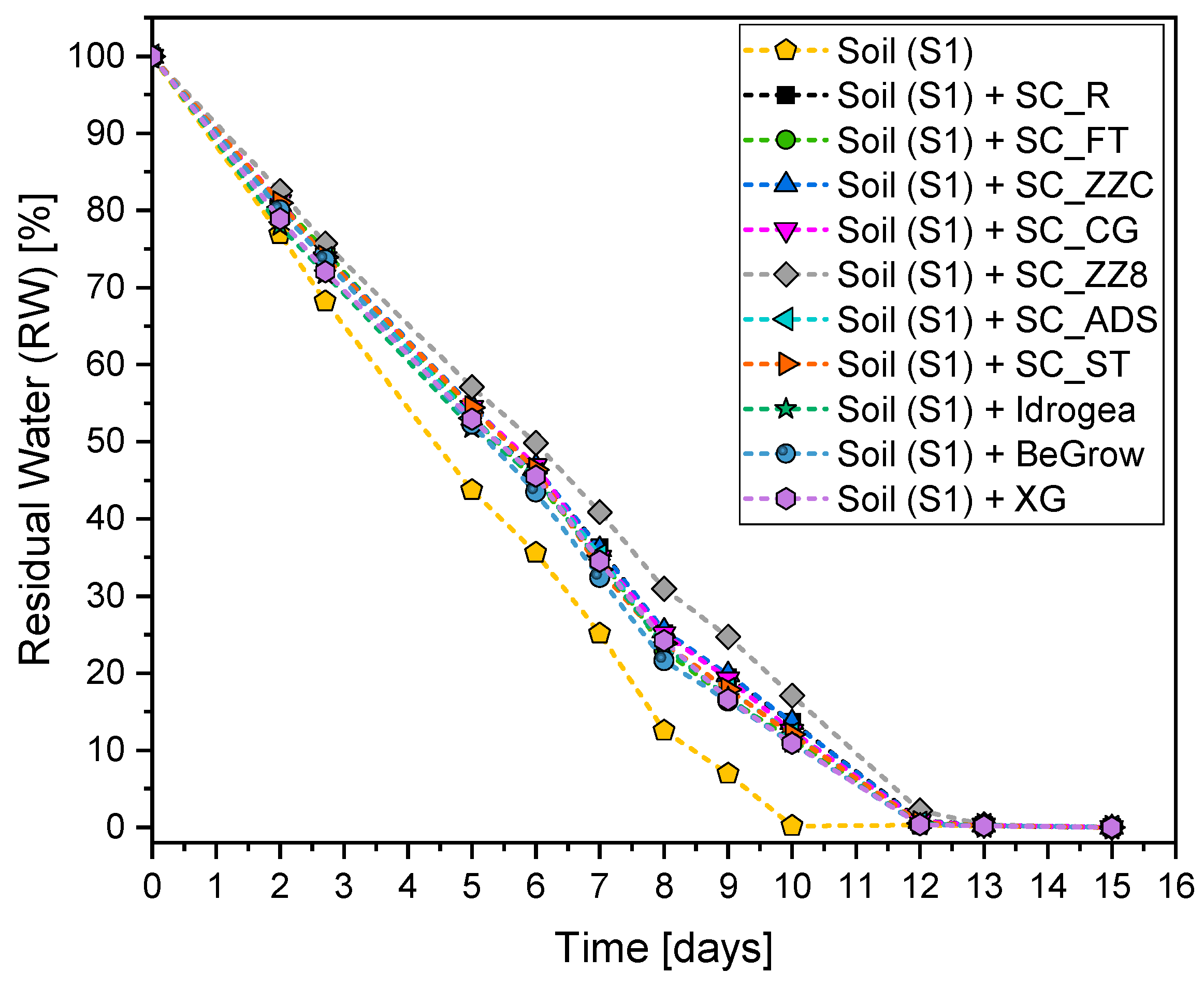
3.5.1. Evaluation of the Water Holding Capacity (WHC) of the Soil S1
3.5.2. Evaluation of the Water Retention Capacity of the Soil S1
3.5.3. Evaluation of the Water Absorption (wA) of Soil S2
3.5.4. Determination of the Soil Water Retention Curve (SWRC) of Soil S2
3.5.5. Case Study Application
Evaluation of the Grass Germination in Soil S1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrarin, C.; Lionello, P.; Orlic, M.; Raicich, F.; Salvadori, G. Venice as a paradigm of coastal flooding under multiple compound drivers. Sci. Rep. 2022, 12, 5754. [Google Scholar] [CrossRef] [PubMed]
- Coppi, A.; Lazzaro, L.; Selvi, F. Plant mortality on ultramafic soils after an extreme heat and drought event in the Mediterranean area. Plant. Soil. 2021, 471, 123–139. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018. [Google Scholar]
- Berardi, D.; Picarelli, A.; Tettamanzi, M. Siccità e Alluvioni, Così Diverse ma Anche Così Simili; Laboratorio Ref Ricerche: Milan, Italy, 2023; pp. 1–28. [Google Scholar]
- Prigent, O.; Wynn Owen, P.; Homrich Hickmann, M.; Bryan, K.; Caruda Ruiz, A.; Huth, J.; Bortnowschi, R.; Friel, C.; Gilson, V.; Roberts, G. Combating desertification in the EU: A growing threat in need of more action. Eur. Court. Audit. 2018, 33, 65. [Google Scholar]
- Neave, M.; Rayburg, S. A field investigation into the effects of progressive rainfall-induced soil seal and crust development on runoff and erosion rates: The impact of surface cover. Geomorphology 2007, 87, 378–390. [Google Scholar] [CrossRef]
- European Environment Agency. What Is Soil Sealing and Why Is It Important to Monitor It? Available online: https://www.eea.europa.eu/help/faq/what-is-soil-sealing-and (accessed on 20 July 2023).
- Mirzabaev, A.; Wu, J.; Evans, J.; García-Oliva, F.; Hussein, I.A.G.; Iqbal, M.H.; Kimutai, J.; Knowles, T.; Meza, F.; Nedjraoui, D.; et al. Desertification. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019; Volume 3, pp. 249–343. [Google Scholar]
- Zhang, Z.; Huisingh, D. Combating desertification in China: Monitoring, control, management and revegetation. J. Clean. Prod. 2018, 182, 765–775. [Google Scholar] [CrossRef]
- Huebner, L.; Al-Quraishi, A.M.F.; Branch, O.; Gaznayee, H.A.A. Sahel Afforestation and Simulated Risks of Heatwaves and Flooding Versus Ecological Revegetation That Combines Planting and Succession. J. Geosci. Environ. Prot. 2022, 10, 94–108. [Google Scholar] [CrossRef]
- Gillner, S.; Vogt, J.; Tharang, A.; Dettmann, S.; Roloff, A. Role of street trees in mitigating effects of heat and drought at highly sealed urban sites. Landsc. Urban. Plann. 2015, 143, 33–42. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Rodríguez Pleguezuelo, C.R. Soil-erosion and runoff prevention by plant covers. A review. Agron. Sustain. Dev. 2008, 28, 65–86. [Google Scholar] [CrossRef]
- Li, D.; Bou-Zeid, E.; Oppenheimer, M. The effectiveness of cool and green roofs as urban heat island mitigation strategies. Environ. Res. Lett. 2014, 9, 55002. [Google Scholar] [CrossRef]
- Fini, A.; Frangi, P.; Mori, J.; Donzelli, D.; Ferrini, F. Nature based solutions to mitigate soil sealing in urban areas: Results from a 4-year study comparing permeable, porous, and impermeable pavements. Environ. Res. 2017, 156, 443–454. [Google Scholar] [CrossRef]
- Kowsar, S. Desertification control through floodwater harvesting: The current state of know-how. In Proceedings of the Future of Drylands: International Scientific Conference on Desertification and Drylands Research, Tunis, Tunisia, 19–21 June 2006; pp. 229–241. [Google Scholar]
- Interreg Central Europe. City Water Circles: Transnational Online Handbook on Circular Urban Water Management and Use. Available online: https://programme2014-20.interreg-central.eu/Content.Node/CWC-Transnational-on-line-handbook-with-pilot-project-s-less.pdf (accessed on 18 July 2023).
- Dennis, E.S.; Dolferus, R.; Ellis, M.; Rahman, M.; Wu, Y.; Hoeren, F.U.; Grover, A.; Ismond, K.P.; Good, A.G.; Peacock, W.J. Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 2000, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef]
- Preece, N.D.; van Oosterzee, P.; Lawes, M.J. Reforestation success can be enhanced by improving tree planting methods. J. Environ. Manag. 2023, 336, 117645. [Google Scholar] [CrossRef] [PubMed]
- Coello, J.; Piqué, M. Soil Conditioners and Groundcovers for Sustainable and Cost-Efficient Tree Planting in Europe and the Mediterranean-Technical Guide; Centre Tecnològic Forestal de Catalunya: Solsona, Spain, 2016; p. 60. [Google Scholar]
- Coello, J.; Ameztegui, A.; Rovira, P.; Fuentes, C.; Piqué, M. Innovative soil conditioners and mulches for forest restoration in semiarid conditions in northeast Spain. Ecol. Eng. 2018, 118, 52–65. [Google Scholar] [CrossRef]
- Celik, I.; Ortas, I.; Kilic, S. Effects of compost, mycorrhiza, manure and fertilizer on some physical properties of a Chromoxerert soil. Soil. Tillage Res. 2004, 78, 59–67. [Google Scholar] [CrossRef]
- Srivastava, V.; de Araujo, A.S.F.; Vaish, B.; Bartelt-Hunt, S.; Singh, P.; Singh, R.P. Biological response of using municipal solid waste compost in agriculture as fertilizer supplement. Rev. Environ. Sci. Biotechnol. 2016, 15, 677–696. [Google Scholar] [CrossRef]
- Schroder, J. Revisiting the agronomic benefits of manure: A correct assessment and exploitation of its fertilizer value spares the environment. Bioresour. Technol. 2005, 96, 253–261. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef]
- Sojka, R.; Bjorneberg, D.; Entry, J.; Lentz, R.; Orts, W. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Ritonga, H.; Nurfadillah, A.; Rembon, F.S.; Ramadhan, L.; Nurdin, M. Preparation of Chitosan-EDTA hydrogel as soil conditioner for soybean plant (Glycine max). Groundw. Sustain. Dev. 2019, 9, 100277. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Water sorption kinetics of superabsorbent hydrogels of saponified cassava starch-graft-poly(acrylamide). Starke 2012, 64, 803–812. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hosseinzadeh, H. Synthesis and superswelling behavior of carboxymethylcellulose–poly(sodium acrylate-co-acrylamide) hydrogel. J. Appl. Polym. Sci. 2008, 108, 1142–1151. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Samadi, M.; Ghasemzadeh, H. Fast-swelling Superabsorbent Hydrogels from Poly(2-hydroxy ethyl acrylate-co-sodium acrylate) Grafted on Starch. Starke 2008, 60, 79–86. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Fajardo, A.R.; Muniz, E.C.; Tambourgi, E.B. Superabsorbent hydrogel based on modified polysaccharide for removal of Pb2+ and Cu2+ from water with excellent performance. J. Appl. Polym. Sci. 2007, 105, 2903–2909. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Zhang, L.; Zhan, H. Superabsorbent nanocomposite hydrogels made of carboxylated cellulose nanofibrils and CMC-g-p(AA-co-AM). Carbohydr. Polym. 2013, 97, 429–435. [Google Scholar] [CrossRef]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Theodoropoulos, A.G.; Drossopoulos, J.B. Designing synthetic polymers as soil conditioners. Commun. Soil. Sci. Plant. Anal. 2008, 26, 1455–1480. [Google Scholar] [CrossRef]
- Orts, W.J.; Sojka, R.E.; Glenn, G.M. Biopolymer additives to reduce erosion-induced soil losses during irrigation. Ind. Crops Prod. 2000, 11, 19–29. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Shin, H.-D.; Cho, G.-C. Soil treatment using microbial biopolymers for anti-desertification purposes. Geoderma 2015, 253–254, 39–47. [Google Scholar] [CrossRef]
- Dearfield, K.L.; Abernathy, C.O.; Ottley, M.S.; Brantner, J.H.; Hayes, P.F. Acrylamide: Its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutat. Res.-Rev. Genet. Toxicol. 1988, 195, 45–77. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.H.; Breiting, V.B.; Aasted, A.; Jørgensen, A.; Kebuladze, I. Long-Term Effects of Polyacrylamide Hydrogel on Human Breast Tissue. Plast. Reconstr. Surg. 2003, 111, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Zou, H.-C.; Lü, Z.-R.; Zou, F.; Park, Y.-D.; Yan, Y.-B.; Yao, S.-J. Effects of Acrylamide on the Activity and Structure of Human Brain Creatine Kinase. Int. J. Mol. Sci. 2009, 10, 4210–4222. [Google Scholar] [CrossRef]
- Cabalar, A.F.; Awraheem, M.H.; Khalaf, M.M. Geotechnical Properties of a Low-Plasticity Clay with Biopolymer. J. Mater. Civ. Eng. 2018, 30, 04018170. [Google Scholar] [CrossRef]
- Lentz, R.D. Polyacrylamide and biopolymer effects on flocculation, aggregate stability, and water seepage in a silt loam. Geoderma 2015, 241–242, 289–294. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.-M.; Im, J.; Cho, G.-C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuang, J.; Wang, Y.; Jia, Y.; Niu, P.; Jia, K. Improvement of loess characteristics using sodium alginate. Bull. Eng. Geol. Environ. 2019, 79, 1879–1891. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Langford, S.J.; Ahmad, M.B.; Lim, Y.Y.; Hashim, K. Eco-friendly smart hydrogels for soil conditioning and sustain release fertilizer. Int. J. Environ. Sci. Technol. 2017, 15, 2059–2074. [Google Scholar] [CrossRef]
- Heise, K.; Kirsten, M.; Schneider, Y.; Jaros, D.; Keller, H.; Rohm, H.; Kalbitz, K.; Fischer, S. From Agricultural Byproducts to Value-Added Materials: Wheat Straw-Based Hydrogels as Soil Conditioners? ACS Sustain. Chem. Eng. 2019, 7, 8604–8612. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Chung, M.-K.; Cho, G.-C. Bovine casein as a new soil strengthening binder from diary wastes. Constr. Build. Mater. 2018, 160, 1–9. [Google Scholar] [CrossRef]
- Dorigato, A.; Negri, M.; Pegoretti, A. Ultrathin wood laminae-polyvinyl alcohol biodegradable composites. Polym. Compos. 2018, 39, 1116–1124. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Dussin, A.; Xanthopoulou, E.; Bikiaris, D.N.; Botta, L.; Fiore, V.; Pegoretti, A. Compatibilization of Polylactide/Poly(ethylene 2,5-furanoate) (PLA/PEF) Blends for Sustainable and Bioderived Packaging. Molecules 2022, 27, 6371. [Google Scholar] [CrossRef] [PubMed]
- Fambri, L.; Dorigato, A.; Pegoretti, A. Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide). Appl. Sci. 2020, 10, 6731. [Google Scholar] [CrossRef]
- Dorigato, A.; Negri, M.; Pegoretti, A. Ultrathin Wood Laminae–Thermoplastic Starch Biodegradable Composites. J. Renew. Mater. 2018, 6, 493–503. [Google Scholar] [CrossRef]
- Valentini, F.; Dorigato, A.; Rigotti, D.; Pegoretti, A. Polyhydroxyalkanoates/Fibrillated Nanocellulose Composites for Additive Manufacturing. J. Polym. Environ. 2019, 27, 1333–1341. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.u.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2021, 9, 104702. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.-C. Effects of Xanthan gum biopolymer on soil strengthening. Constr. Build. Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Vydehi, K.V. State-of-the-art review on efficacy of xanthan gum and guar gum inclusion on the engineering behavior of soils. Innov. Infrastruct. Solut. 2021, 6, 108. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; Gonzalez Lopez, O. Water-soluble polymers in agriculture: Xanthan gum as eco-friendly alternative to synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Gils, P.S.; Ray, D.; Sahoo, P.K. Characteristics of xanthan gum-based biodegradable superporous hydrogel. Int. J. Biol. Macromol. 2009, 45, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Katzen, F.; Pühler, A.; Ielpi, L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Garcıa-Ochoa, F.; Santos, V.; Casas, J.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Moorthy, N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Pan, S.; Ragauskas, A.J. Preparation of superabsorbent cellulosic hydrogels. Carbohydr. Polym. 2012, 87, 1410–1418. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X.L. Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos. Sci. Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Sreekala, M.S.; Goda, K.; Devi, P.V. Sorption characteristics of water, oil and diesel in cellulose nanofiber reinforced corn starch resin/ramie fabric composites. Compos. Interfaces 2008, 15, 281–299. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; Halal, S.; Rosa, G.S.D.; Dias, A.R.G.; Zavareze, E.D.R. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules 2023, 28, 1952. [Google Scholar] [CrossRef] [PubMed]
- ONEforest Project—H2020. Available online: https://www.oneforest.eu/ (accessed on 19 February 2023).
- ASTM D2487-17; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 17892-1:2014; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 1: Determination of Water Content. European Commission for Standardization: Bruxelles, Belgium, 2014.
- Rettenmaier & Söhne Gmbh. Available online: https://www.jrs.de/de/ (accessed on 15 January 2023).
- STEICO. Available online: https://www.steico.com/it/ (accessed on 15 January 2023).
- Macosko, C.W. Rheology Principles; VCH Publishers Inc.: New York, NY, USA, 1994; pp. 1–174. [Google Scholar]
- Yu, J.; Shi, J.G.; Dang, P.F.; Mamedov, A.I.; Shainberg, I.; Levy, G.J. Soil and Polymer Properties Affecting Water Retention by Superabsorbent Polymers under Drying Conditions. Soil. Sci. Soc. Am. J. 2012, 76, 1758–1767. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009, 155, 892–898. [Google Scholar] [CrossRef]
- DIN 18132:2012-04; Soil, Testing Procedures and Testing Equipment—Determination of Water Absorption. Deutsches Institut für Normung: Berlin, Germany, 2012.
- Maček, M.; Smolar, J.; Petkovšek, A. Extension of measurement range of dew-point potentiometer and evaporation method. In Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering, Paris, France, 2–6 September 2013; pp. 1137–1142. [Google Scholar]
- ASTM D6836-16; Standard Test Methods for Determination of the Soil Water Characteristic Curve for Desorption Using Hanging Column, Pressure Extractor, Chilled Mirror Hygrometer, or Centrifuge. ASTM International: West Conshohocken, PA, USA, 2016.
- Schindler, U.; Durner, W.; von Unold, G.; Mueller, L.; Wieland, R. The evaporation method: Extending the measurement range of soil hydraulic properties using the air-entry pressure of the ceramic cup. J. Plant Nutr. Soil Sci. 2010, 173, 563–572. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Tarres, Q.; Mutje, P.; Balea, A.; Campano, C.; Sanchez-Salvador, J.L.; Negro, C.; Delgado-Aguilar, M. Correlation between rheological measurements and morphological features of lignocellulosic micro/nanofibers from different softwood sources. Int. J. Biol. Macromol. 2021, 187, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Rahbar, N.; Calvert, P.D. Strong fiber-reinforced hydrogel. Acta Biomater. 2013, 9, 5313–5318. [Google Scholar] [CrossRef]
- Barrientos-Sanhueza, C.; Mondaca, P.; Tamayo, M.; Álvaro, J.E.; Díaz-Barrera, A.; Cuneo, I.F. Enhancing the mechanical and hydraulic properties of coarse quartz sand using a water-soluble hydrogel based on bacterial alginate for novel application in agricultural contexts. Soil. Sci. Soc. Am. J. 2021, 85, 1880–1893. [Google Scholar] [CrossRef]
- Shortt, R.; Verhallen, A.; Fisher, P. Monitoring Soil Moisture to Improve Irrigation Decisions; Ministry of Agriculture, Food and Rural Affairs: Ontario, ON, Canada, 2011; pp. 1–12. [Google Scholar]
- Chang, I.; Im, J.; Cho, G.-C. Introduction of Microbial Biopolymers in Soil Treatment for Future Environmentally-Friendly and Sustainable Geotechnical Engineering. Sustainability 2016, 8, 251–274. [Google Scholar] [CrossRef]






| Sample | Type of Filler |
|---|---|
| SC_R | Arbocel R |
| SC_FT | Arbocel FT 400 |
| SC_ZZC | Arbocel ZZC 500 |
| SC_CG | Cellugrün |
| SC_ZZ8 | Arbocel ZZ 8-2 CA 1 |
| SC_ADS | Arbocel Adsorb 2 |
| SC_ST | STEICO flex 036 |
| XG | - |
| Sample | Yield Stress [Pa] |
|---|---|
| SC_R | 38.5 ± 1.6 |
| SC_FT | 56.9 ± 2.3 |
| SC_ZZC | 51.3 ± 2.5 |
| SC_CG | 44.2 ± 2.7 |
| SC_ZZ8 | 37.7 ± 1.2 |
| SC_ADS | 35.5 ± 3.1 |
| SC_ST | 53.8 ± 1.8 |
| XG | 42.6 ± 2.2 |
| Specimen | Dosage of SC | wA 24h (%) | wA max (%) * |
|---|---|---|---|
| Soil (S2) (untreated) | 87–89 | 87–89 | |
| Soil (S2) + SC_R | Low | 91–99 | Not measured |
| Soil (S2) + SC_R | High | 154–162 | 166–176 |
| Soil (S2) + SC_CG | High | 140–150 | 174–201 |
| Soil (S2) + SC_ZZC | High | 131–139 | 140–175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorze, A.; Valentini, F.; Smolar, J.; Logar, J.; Pegoretti, A.; Dorigato, A. Effect of Different Cellulose Fillers on the Properties of Xanthan-Based Composites for Soil Conditioning Applications. Materials 2023, 16, 7285. https://doi.org/10.3390/ma16237285
Sorze A, Valentini F, Smolar J, Logar J, Pegoretti A, Dorigato A. Effect of Different Cellulose Fillers on the Properties of Xanthan-Based Composites for Soil Conditioning Applications. Materials. 2023; 16(23):7285. https://doi.org/10.3390/ma16237285
Chicago/Turabian StyleSorze, Alessandro, Francesco Valentini, Jasna Smolar, Janko Logar, Alessandro Pegoretti, and Andrea Dorigato. 2023. "Effect of Different Cellulose Fillers on the Properties of Xanthan-Based Composites for Soil Conditioning Applications" Materials 16, no. 23: 7285. https://doi.org/10.3390/ma16237285
APA StyleSorze, A., Valentini, F., Smolar, J., Logar, J., Pegoretti, A., & Dorigato, A. (2023). Effect of Different Cellulose Fillers on the Properties of Xanthan-Based Composites for Soil Conditioning Applications. Materials, 16(23), 7285. https://doi.org/10.3390/ma16237285











