Influence of Molecular Weight on the Enzymatic Degradation of PLA Isomer Blends by a Langmuir System
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PLA
2.3. Measurements
2.4. Enzymatic Degradation
2.5. Stereocomplex Effect
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Reeve, M.S.; McCarthy, S.P.; Downey, M.J.; Gross, R.A. Polylactide stereochemistry: Effect on enzymic degradability. Macromolecules 1994, 27, 825–831. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Monteiro, R.C.P.; do Sul, J.A.I.; Costa, M.F. Plastic pollution in islands of the Atlantic Ocean. Environ. Pollut. 2018, 238, 103–110. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Do Sul, J.A.I.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Ha, C.-S.; Gardella, J.A. Surface chemistry of biodegradable polymers for drug delivery systems. Chem. Rev. 2005, 105, 4205–4232. [Google Scholar] [CrossRef]
- Li, S.; McCarthy, S. Influence of crystallinity and stereochemistry on the enzymatic degradation of poly(lactide)s. Macromolecules 1999, 32, 4454–4456. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanoclay and crystallinity effects on the hydrolytic degradation of polylactides. Polym. Degrad. Stab. 2008, 93, 1450–1459. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Chen, W.; Qi, C.; Li, Y.; Tao, H. The degradation investigation of biodegradable PLA/PBAT blend: Thermal stability, mechanical properties and PALS analysis. Radiat. Phys. Chem. 2021, 180, 109239. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and biological application of polylactic acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, M.; Biela, T. Micro-and nanostructures of polylactide stereocomplexes and their biomedical applications. Polym. Int. 2015, 64, 1667–1675. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Im, D.; Gavande, V.; Park, E.; Kim, D.; Lee, W.-K. Synthesis and selective enzymatic degradation of polylactide/poly (butylene succinate) mixtures. Mol. Cryst. Liq. Cryst. 2022, 758, 64–70. [Google Scholar] [CrossRef]
- Kwon, Y.; Gavande, V.; Kim, S.; Im, D.; Lee, W.-K. Renewable triblock copolymers of polylactide and polyether polyol: Degradation behaviors of their monolayers and films. Mol. Cryst. Liq. Cryst. 2023, 1–9. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Gavande, V.; Kim, G.; Kim, B.; Jin, Y.; Jang, S.-H.; Chun, J.H.; Lee, W.-K. Effect of molecular weight on stereocomplexation of enantiomeric polylactide mixtures and their degradation behaviors by Langmuir technique. Mol. Cryst. Liq. Cryst. 2022, 742, 133–138. [Google Scholar] [CrossRef]
- Park, H.-S.; Hong, C.-K. Relationship between the Stereocomplex Crystallization Behavior and Mechanical Properties of PLLA/PDLA Blends. Polymers 2021, 13, 1851. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, R.J.; Melveger, A.J.; Dolegiewitz, L.J. Morphological and structural changes in a copolymer of glycolide and lactide occurring as a result of hydrolysis. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 57–66. [Google Scholar] [CrossRef]
- Ariga, K. Chemistry of Materials Nanoarchitectonics for Two-Dimensional Films: Langmuir–Blodgett, Layer-by-Layer Assembly, and Newcomers. Chem. Mater. 2023. [Google Scholar] [CrossRef]
- Oliveira, O.N., Jr.; Caseli, L.; Ariga, K. The past and the future of Langmuir and Langmuir–Blodgett films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef]
- Im, D.; Gavande, V.; Lee, H.; Iwata, T.; Lee, W.-K. Compatibility and hydrolytic behaviors of polylactide isomer/poly (butylene succinate) mixtures by the Langmuir technique. Polym. Degrad. Stab. 2021, 186, 109517. [Google Scholar] [CrossRef]
- Paul, G.P.; Virivinti, N. An outlook on recent progress in poly (lactic acid): Polymerization, modeling, and optimization. Iran. Polym. J. 2022, 31, 59–81. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly (lactic Acid): A versatile biobased polymer for the future with multifunctional properties—From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Pan, P.; Han, L.; Bao, J.; Xie, Q.; Shan, G.; Bao, Y. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly (l-lactic acid)/poly (d-lactic acid) racemic blends: Molecular weight effects. J. Phys. Chem. B 2015, 119, 6462–6470. [Google Scholar] [CrossRef]
- Shao, J.; Sun, J.; Bian, X.; Cui, Y.; Li, G.; Chen, X. Investigation of poly (lactide) stereocomplexes: 3-armed poly (l-lactide) blended with linear and 3-armed enantiomers. J. Phys. Chem. B 2012, 116, 9983–9991. [Google Scholar] [CrossRef]
- Bao, R.-Y.; Yang, W.; Wei, X.-F.; Xie, B.-H.; Yang, M.-B. Enhanced formation of stereocomplex crystallites of high molecular weight poly (l-lactide)/poly (d-lactide) blends from melt by using poly (ethylene glycol). ACS Sustain. Chem. Eng. 2014, 2, 2301–2309. [Google Scholar] [CrossRef]
- Li, Y.; Xin, S.; Bian, Y.; Dong, Q.; Han, C.; Xu, K.; Dong, L. Stereocomplex crystallite network in poly (d,l-lactide): Formation, structure and the effect on shape memory behaviors and enzymatic hydrolysis of poly (d,l-lactide). RSC Adv. 2015, 5, 24352–24362. [Google Scholar] [CrossRef]
- Tsuji, H.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. 4. Differential scanning calorimetric studies on precipitates from mixed solutions of poly (d-lactic acid) and poly (l-lactic acid). Macromolecules 1991, 24, 5657–5662. [Google Scholar] [CrossRef]
- Shao, J.; Xiang, S.; Bian, X.; Sun, J.; Li, G.; Chen, X. Remarkable melting behavior of PLA stereocomplex in linear PLLA/PDLA blends. Ind. Eng. Chem. Res. 2015, 54, 2246–2253. [Google Scholar] [CrossRef]
- Lee, W.-K.; Iwata, T.; Gardella, J.A. Hydrolytic behavior of enantiomeric poly (lactide) mixed monolayer films at the air/water interface: Stereocomplexation effects. Langmuir 2005, 21, 11180–11184. [Google Scholar] [CrossRef]
- Schneider, M.; Fritzsche, N.; Puciul-Malinowska, A.; Baliś, A.; Mostafa, A.; Bald, I.; Zapotoczny, S.; Taubert, A. Surface etching of 3D printed poly (lactic acid) with NaOH: A systematic approach. Polymers 2020, 12, 1711. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Kawai, F.; Nakadai, K.; Nishioka, E.; Nakajima, H.; Ohara, H.; Masaki, K.; Iefuji, H. Different enantioselectivity of two types of poly (lactic acid) depolymerases toward poly (l-lactic acid) and poly (D-lactic acid). Polym. Degrad. Stab. 2011, 96, 1342–1348. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Regnell Andersson, S.; Hakkarainen, M.; Inkinen, S.; Södergård, A.; Albertsson, A.-C. Customizing the hydrolytic degradation rate of stereocomplex PLA through different PDLA architectures. Biomacromolecules 2012, 13, 1212–1222. [Google Scholar] [CrossRef]
- Tsuji, H.; Horii, F.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. 2. Stereocomplex formation in concentrated solutions. Macromolecules 1991, 24, 2719–2724. [Google Scholar] [CrossRef]
- Tsuji, H.; Hyon, S.H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. 3. Calorimetric studies on blend films cast from dilute solution. Macromolecules 1991, 24, 5651–5656. [Google Scholar] [CrossRef]

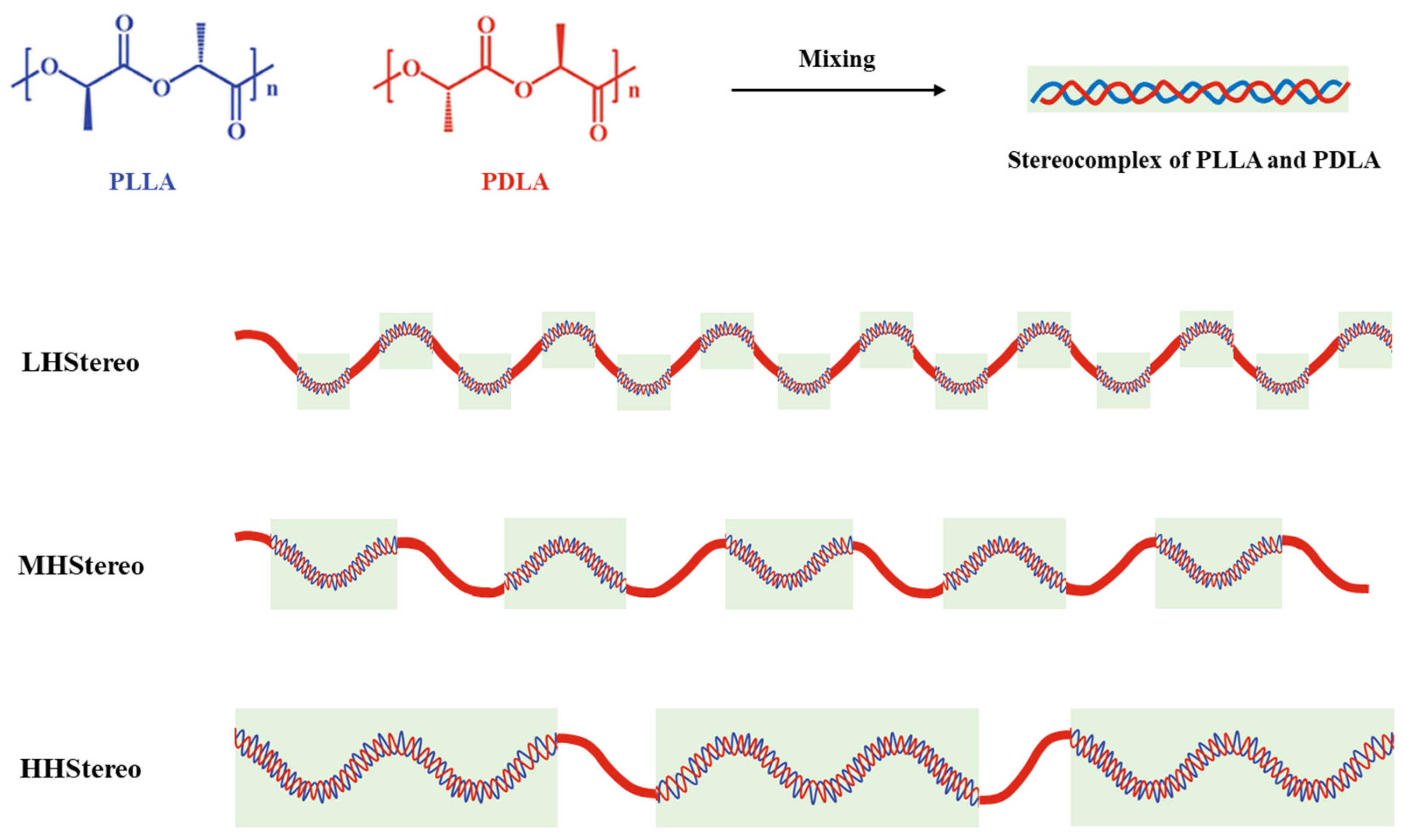
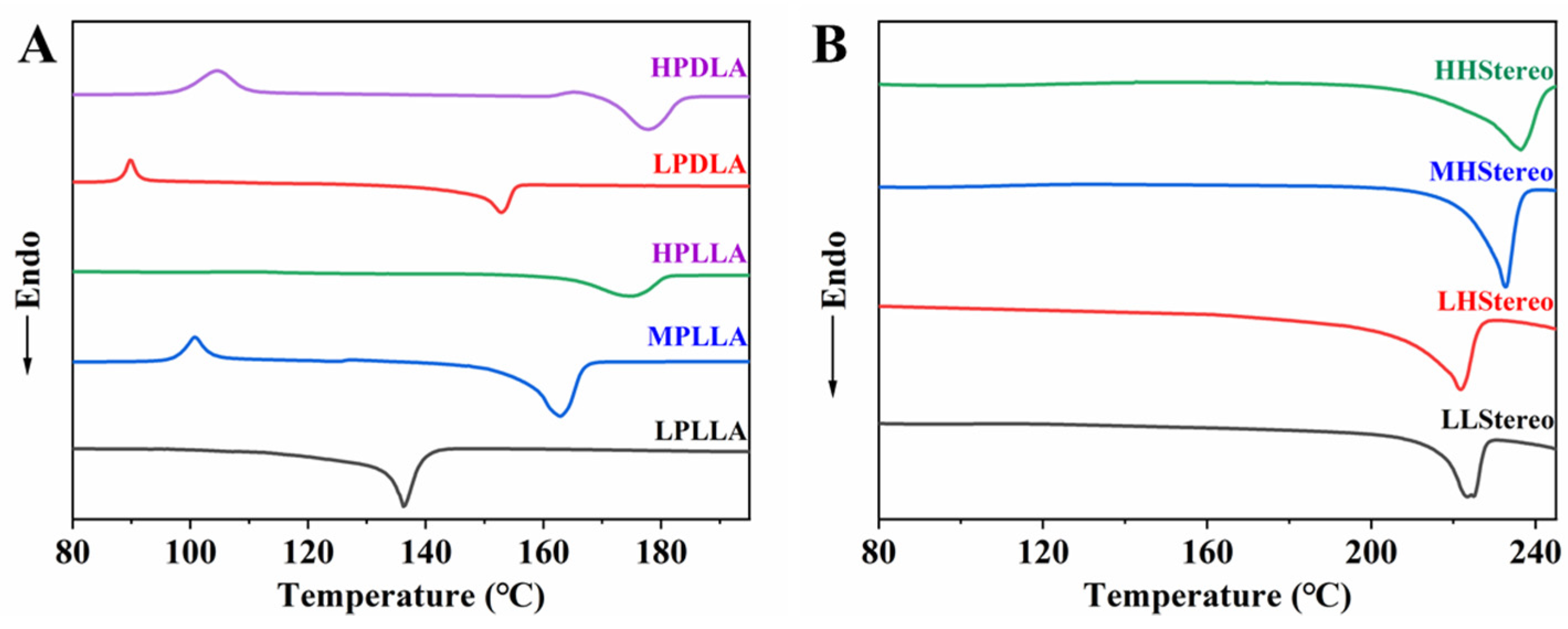
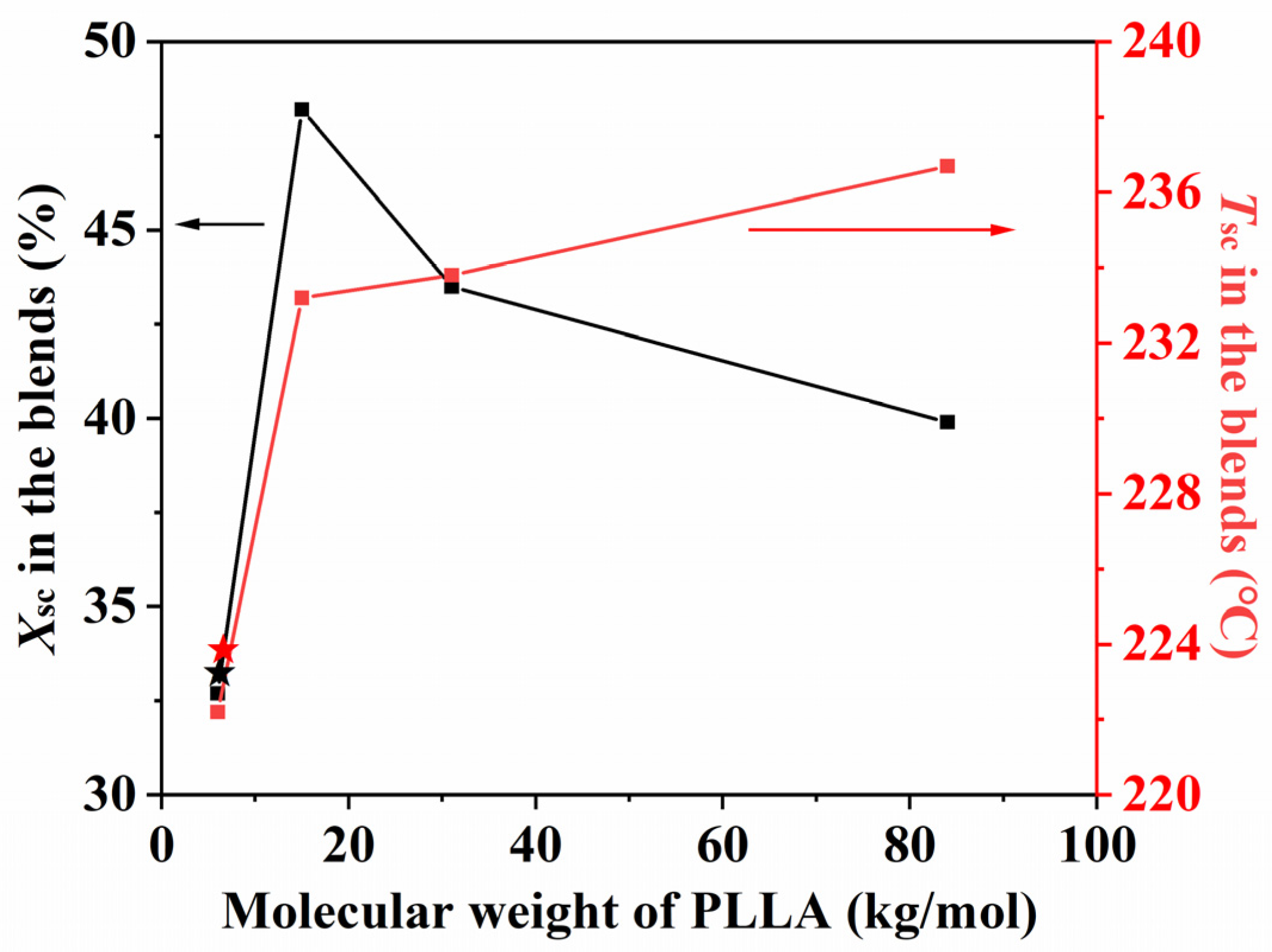
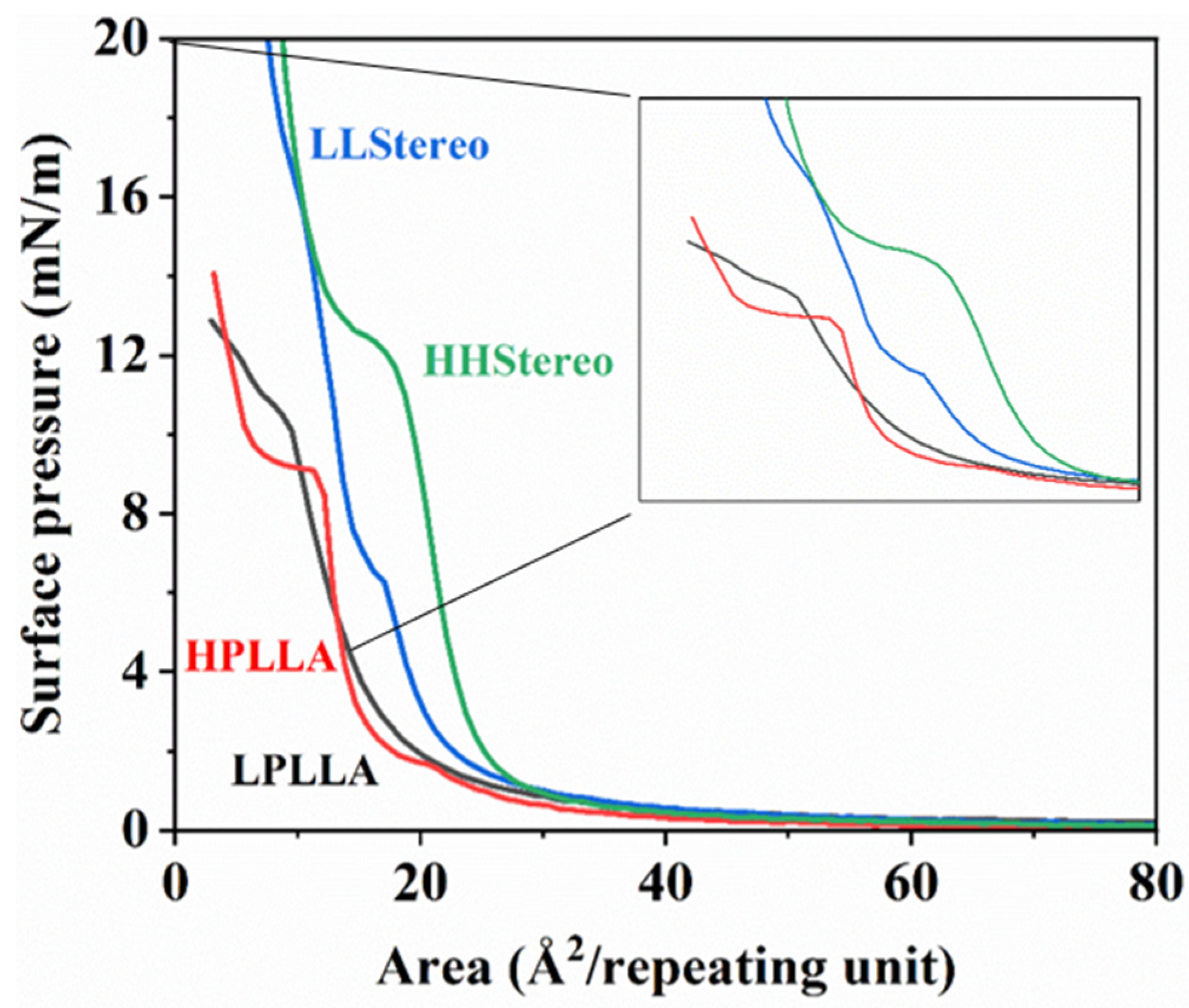
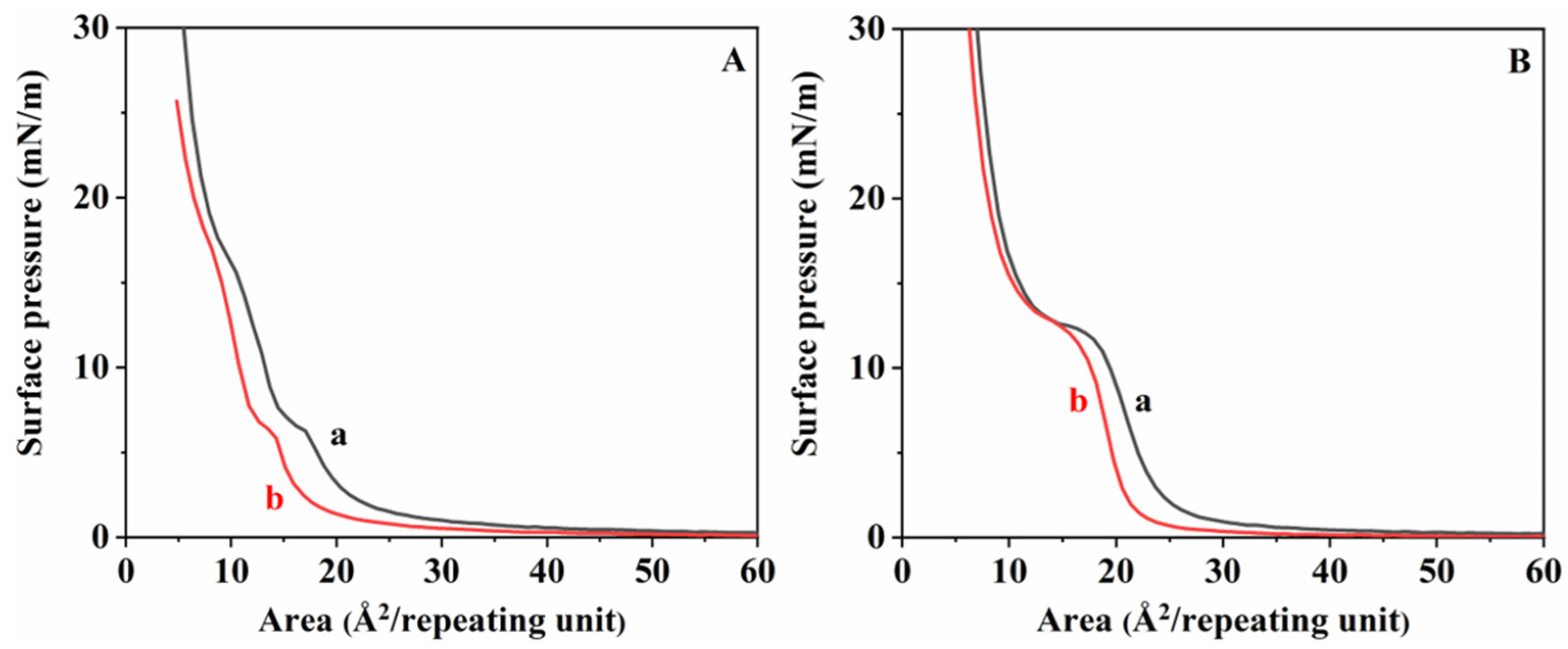
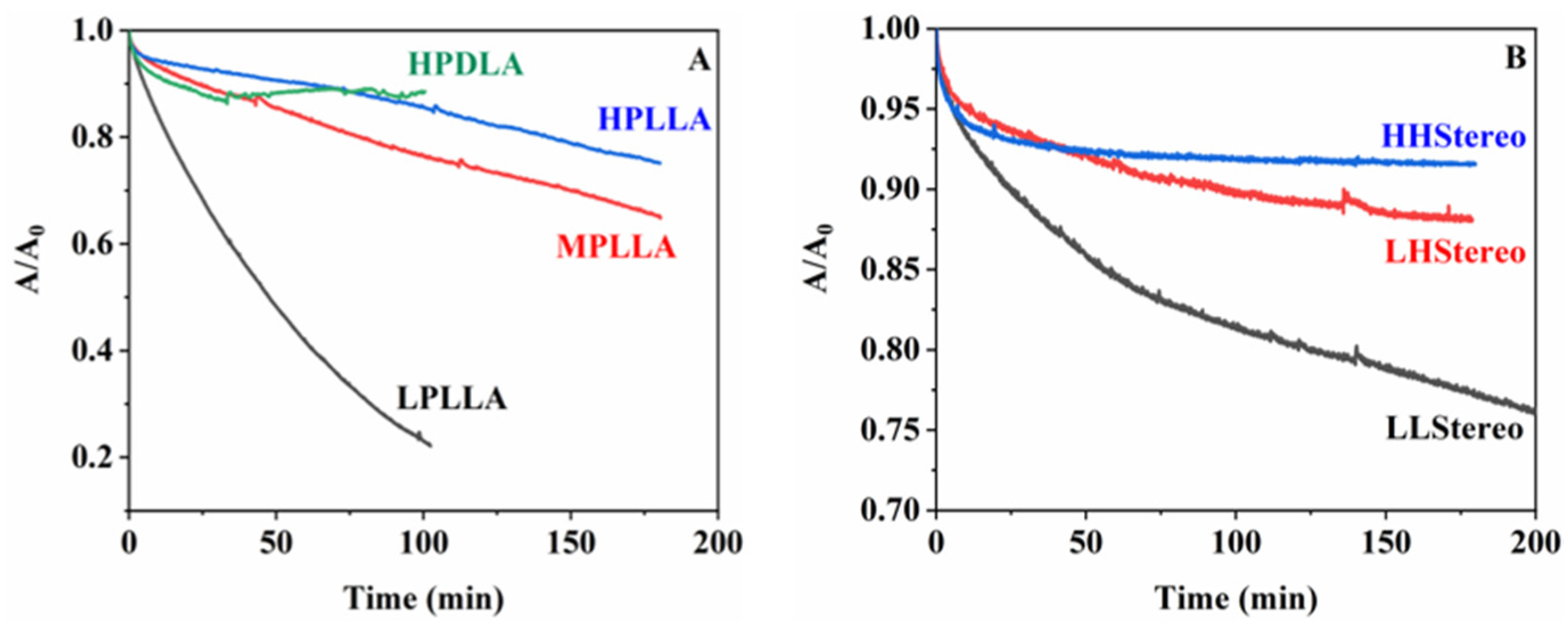

| Catalyst Amount (wt%) | Reaction Time (h) | Temperature (°C) | Mn | Mw | PDI | Tg (°C) | Tm (°C) | |
|---|---|---|---|---|---|---|---|---|
| PLLA5 (LPLLA) | 0.2 | 24 | 100 | 5.5 k | 6.9 k | 1.26 | 27.6 | 136.0 |
| PLLA62 | 0.7 | 24 | 100 | 62 k | 96 k | 1.54 | ||
| PLLA84 (HPLLA) | 1 | 24 | 100 | 84 k | 140 k | 1.66 | 61.6 | 175.2 |
| PLLA83 | 3 | 24 | 100 | 83 k | 142 k | 1.7 | ||
| - | 5 | 3 | 70 | - | - | - | ||
| PLLA15 (MPLLA) | 5 | 12 | 100 | 15 k | 19 k | 1.28 | 53.4 | 163.3 |
| PLLA28 | 5 | 24 | 100 | 28 k | 46 k | 1.63 | ||
| PDLA9 (LPDLA) | 0.5 | 24 | 100 | 9 k | - | - | 50.12 | 153.2 |
| PDLA90 (HPDLA) | 1 | 24 | 100 | 90 k | 150 k | 1.65 | 62.9 | 178.3 |
| PDLA66 | 3 | 24 | 100 | 66 k | 107 k | 1.64 | ||
| PDLA74 | 5 | 24 | 100 | 74 k | 126 k | 1.7 | ||
| PDLA37 | 7 | 24 | 100 | 37 k | 52 k | 1.41 |
| Sample Code | Tm (°C) | Sources |
|---|---|---|
| LLStereo | 223.8 | LL-6K/LD-9K (50/50 by wt%) |
| LHStereo | 222.2 | LL-6K/HD-90K (50/50 by wt%) |
| MHStereo | 233.2 | ML-15K/HD-90K (50/50 by wt%) |
| HHStereo | 236.7 | HL-84K/HD-90K (50/50 by wt%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, D.; Gavande, V.; Lee, H.Y.; Lee, W.-K. Influence of Molecular Weight on the Enzymatic Degradation of PLA Isomer Blends by a Langmuir System. Materials 2023, 16, 5087. https://doi.org/10.3390/ma16145087
Im D, Gavande V, Lee HY, Lee W-K. Influence of Molecular Weight on the Enzymatic Degradation of PLA Isomer Blends by a Langmuir System. Materials. 2023; 16(14):5087. https://doi.org/10.3390/ma16145087
Chicago/Turabian StyleIm, Donghyeok, Vishal Gavande, Hak Yong Lee, and Won-Ki Lee. 2023. "Influence of Molecular Weight on the Enzymatic Degradation of PLA Isomer Blends by a Langmuir System" Materials 16, no. 14: 5087. https://doi.org/10.3390/ma16145087
APA StyleIm, D., Gavande, V., Lee, H. Y., & Lee, W.-K. (2023). Influence of Molecular Weight on the Enzymatic Degradation of PLA Isomer Blends by a Langmuir System. Materials, 16(14), 5087. https://doi.org/10.3390/ma16145087









