Evaluation of Cytotoxicity, Cell Attachment, and Elemental Characterization of Three Calcium Silicate-Based Sealers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Elemental Composition Analysis
2.3. SEM/EDX Characterization
2.4. FTIR Characterization
2.5. Cytotoxicity
2.6. Cell Attachment
2.7. Statistical Analysis
3. Results
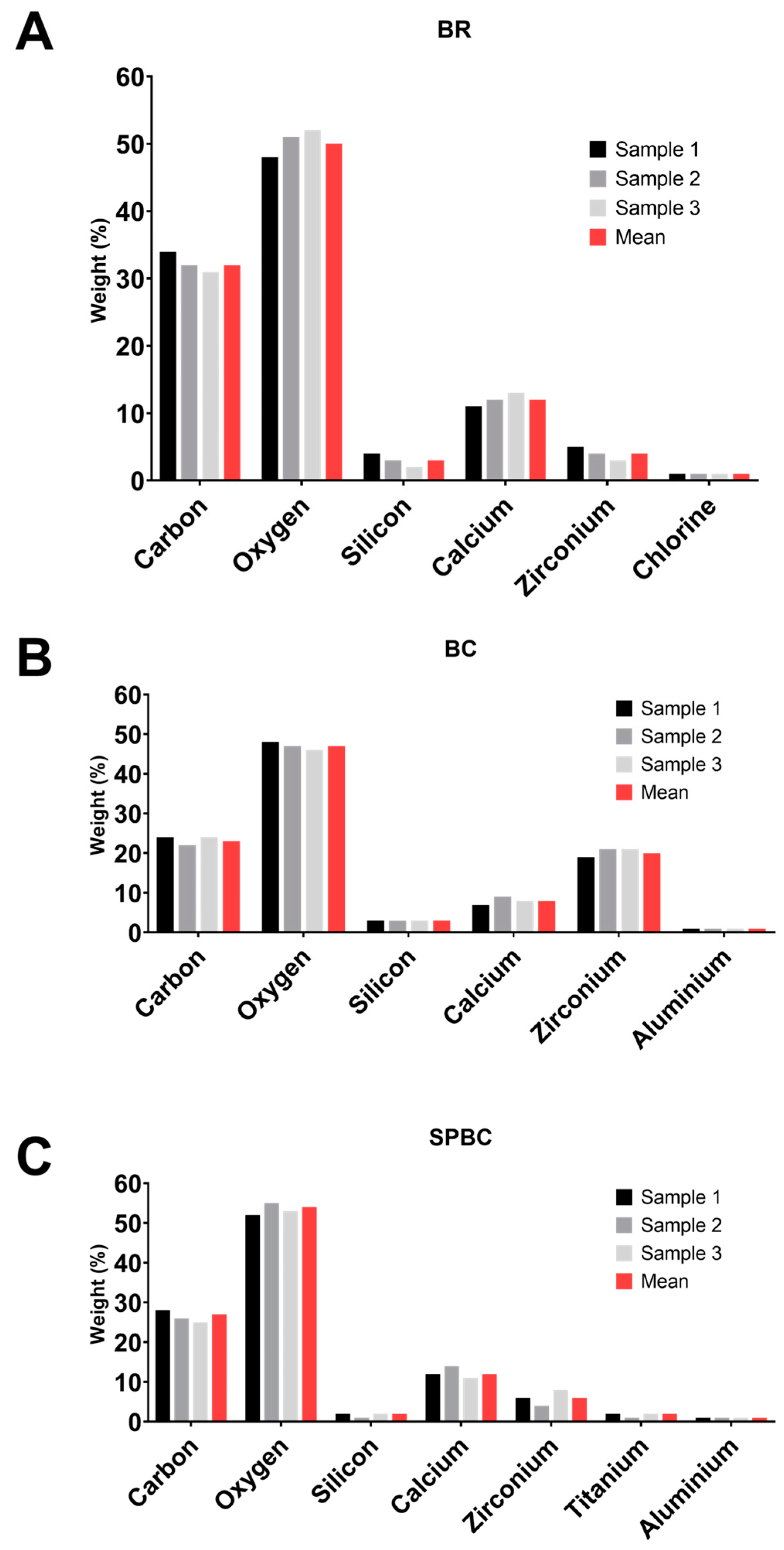
3.1. XRF Analysis
3.2. SEM-EDX Analysis
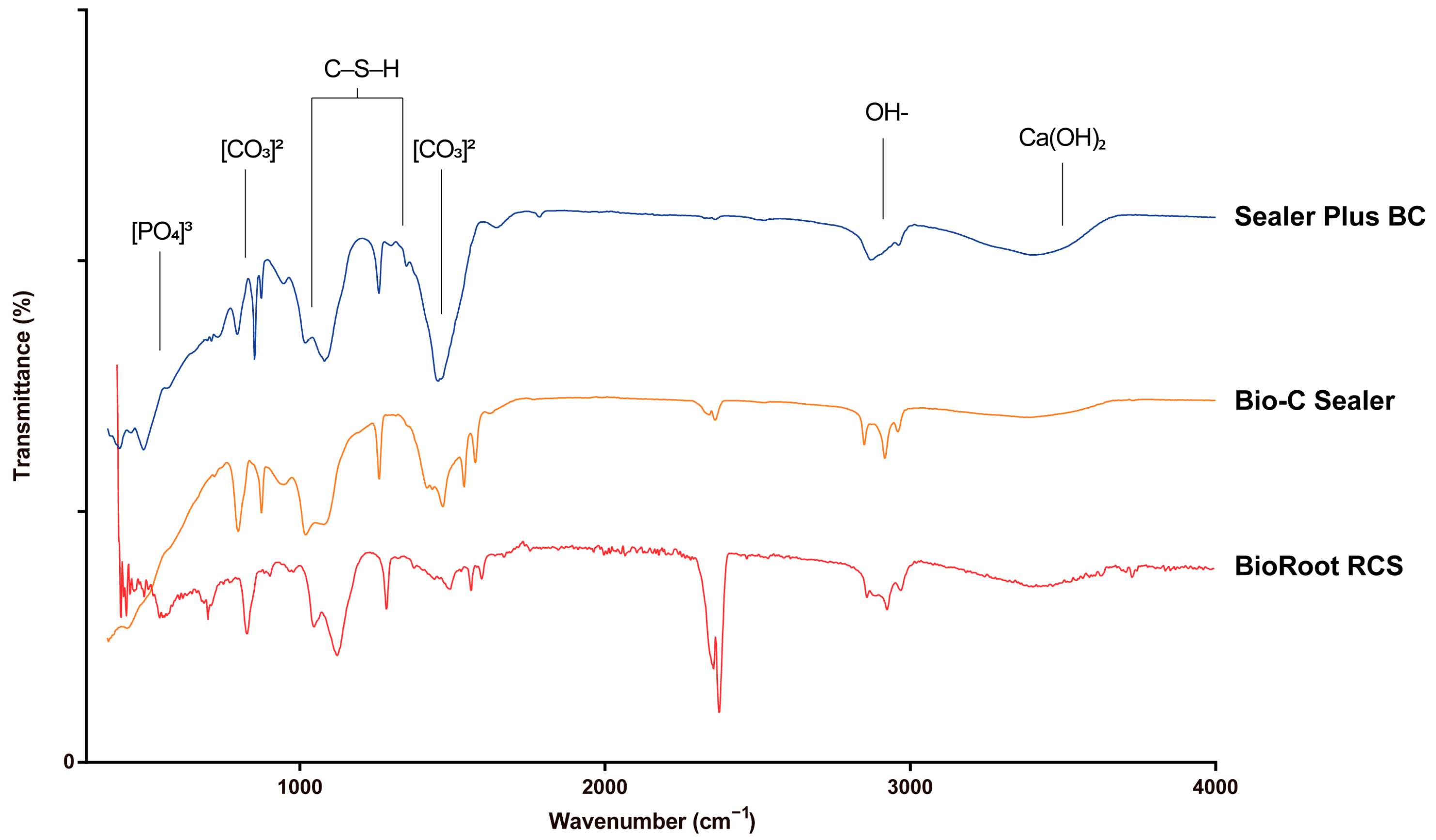
3.3. FTIR Analysis
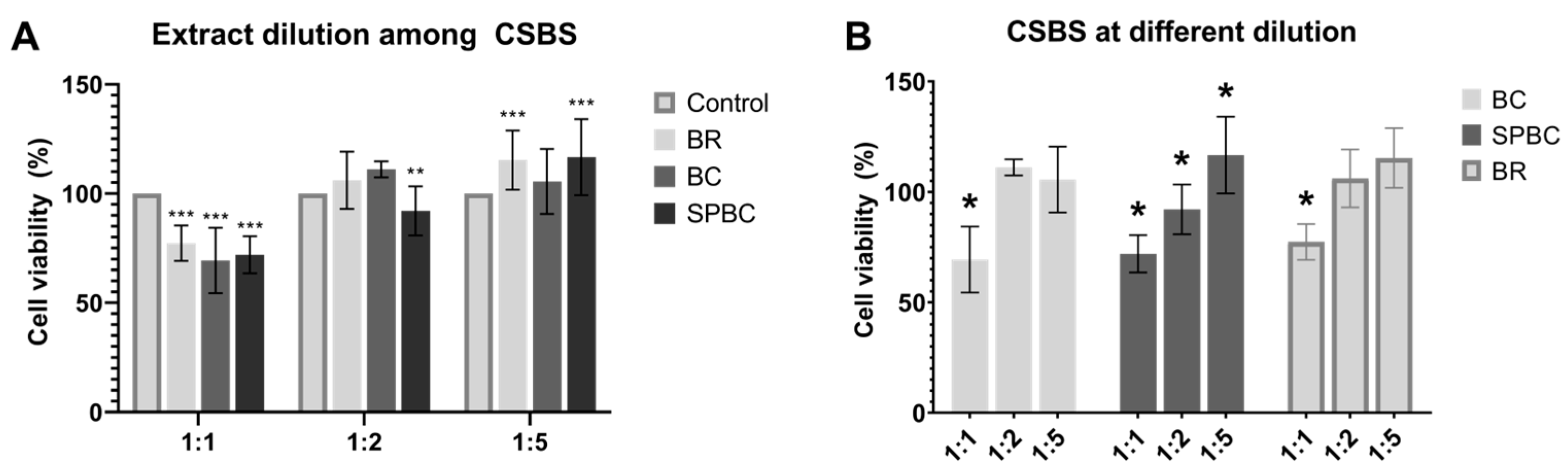
3.4. Cell Morphology, Adhesion and Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camilleri, J.; Atmeh, A.; Li, X.; Meschi, N. Present status and future directions: Hydraulic materials for endodontic use. Int. Endod. J. 2022, 55 (Suppl. S3), 710–777. [Google Scholar] [CrossRef] [PubMed]
- Janini, A.C.P.; Pelepenko, L.E.; Gomes, B.; Marciano, M.A. Physico-chemical properties of calcium silicate-based sealers in powder/liquid and ready-to-use forms. Braz. Dent. J. 2022, 33, 18–25. [Google Scholar] [CrossRef]
- Sanz, J.L.; Guerrero-Girones, J.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Melo, M. Biological interactions between calcium silicate-based endodontic biomaterials and periodontal ligament stem cells: A systematic review of in vitro studies. Int. Endod. J. 2021, 54, 2025–2043. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Coelho, C.M.; Sequeira, D.B.; Marques, J.A.; Pereira, J.F.; Sousa, V.; Palma, P.J.; Santos, A.C. Subcutaneous Implantation Assessment of New Calcium-Silicate Based Sealer for Warm Obturation. Biomedicines 2021, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Orangi, J.; Asatourian, A.; Gutmann, J.L.; Garcia-Godoy, F.; Lotfi, M.; Sheibani, N. Calcium silicate-based cements and functional impacts of various constituents. Dent. Mater. J. 2017, 36, 8–18. [Google Scholar] [CrossRef]
- Santos, G.; Carvalho, C.N.; Tavares, R.R.J.; Silva, P.G.B.; Candeiro, G.T.M.; Maia Filho, E.M. Tissue repair capacity of bioceramic endodontic sealers in rat subcutaneous tissue. Braz. Dent. J. 2023, 34, 25–32. [Google Scholar] [CrossRef]
- Song, M.; Park, M.G.; Kwak, S.W.; Kim, R.H.; Ha, J.H.; Kim, H.C. Pilot evaluation of sealer-based root canal obturation using epoxy-resin-based and calcium-silicate-based sealers: A randomized clinical trial. Materials 2022, 15, 5146. [Google Scholar] [CrossRef]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Silva, E.C.A.; Tanomaru-Filho, M.; da Silva, G.F.; Delfino, M.M.; Cerri, P.S.; Guerreiro-Tanomaru, J.M. Biocompatibility and bioactive potential of new calcium silicate-based endodontic sealers: Bio-C Sealer and Sealer Plus BC. J. Endod. 2020, 46, 1470–1477. [Google Scholar] [CrossRef]
- Souza, G.L.; Rosatto, C.M.P.; Silva, M.J.B.; Silva, M.V.; Rocha Rodrigues, D.B.; Moura, C.C.G. Evaluation of apoptosis/necrosis and cytokine release provoked by three root canal sealers in human polymorphonuclears and monocytes. Int. Endod. J. 2019, 52, 629–638. [Google Scholar] [CrossRef]
- Lopez-Garcia, S.; Pecci-Lloret, M.R.; Guerrero-Girones, J.; Pecci-Lloret, M.P.; Lozano, A.; Llena, C.; Rodriguez-Lozano, F.J.; Forner, L. Comparative cytocompatibility and mineralization potential of Bio-C Sealer and TotalFill BC Sealer. Materials 2019, 12, 3087. [Google Scholar] [CrossRef]
- Collado-Gonzalez, M.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Lozano, A.; Forner, L.; Llena, C.; Rodriguez-Lozano, F.J. Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int. Endod. J. 2017, 50, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Tomson, P.L.; Grover, L.M.; Lumley, P.J.; Sloan, A.J.; Smith, A.J.; Cooper, P.R. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J. Dent. 2007, 35, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Tomson, P.L.; Lumley, P.J.; Smith, A.J.; Cooper, P.R. Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int. Endod. J. 2017, 50, 281–292. [Google Scholar] [CrossRef]
- Martorano, A.S.; Messias, N.S.; Bighetti-Trevisan, R.L.; de Oliveira, P.T.; de Castro Raucci, L.M.S.; Raucci Neto, W. In vitro inflammatory modulation of bioceramic endodontic sealer in macrophages stimulated by bacterial lipopolysaccharide. Int. Endod. J. 2023, 56, 213–226. [Google Scholar] [CrossRef]
- Long, J.; Kreft, J.U.; Camilleri, J. Antimicrobial and ultrastructural properties of root canal filling materials exposed to bacterial challenge. J. Dent. 2020, 93, 103283. [Google Scholar] [CrossRef] [PubMed]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 2017, 50 (Suppl. S2), e120–e136. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.F.E.; Zordan-Bronzel, C.L.; Guerreiro-Tanomaru, J.M.; Chavez-Andrade, G.M.; Pinto, J.C.; Tanomaru-Filho, M. Effect of immersion in distilled water or phosphate-buffered saline on the solubility, volumetric change and presence of voids within new calcium silicate-based root canal sealers. Int. Endod. J. 2020, 53, 385–391. [Google Scholar] [CrossRef]
- Zordan-Bronzel, C.L.; Esteves Torres, F.F.; Tanomaru-Filho, M.; Chavez-Andrade, G.M.; Bosso-Martelo, R.; Guerreiro-Tanomaru, J.M. Evaluation of physicochemical properties of a new calcium silicate-based sealer, Bio-C Sealer. J. Endod. 2019, 45, 1248–1252. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity, 3rd ed. ISO: Geneva, Switzerland, 2009; pp. 1–34.
- Zhang, J.; Zhu, L.; Yan, P.; Peng, B. Effect of Bioaggregate on receptor activator of nuclear factor-kappa B ligand-induced osteoclastogenesis from murine macrophage cell line in vitro. J. Endod. 2015, 41, 1265–1271. [Google Scholar] [CrossRef]
- de Souza, G.L.; Moura, C.C.G.; Silva, A.C.A.; Marinho, J.Z.; Silva, T.R.; Dantas, N.O.; Bonvicini, J.F.S.; Turrioni, A.P. Effects of zinc oxide and calcium-doped zinc oxide nanocrystals on cytotoxicity and reactive oxygen species production in different cell culture models. Restor. Dent. Endod. 2020, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Haapasalo, M.; Mobuchon, C.; Li, X.; Ma, J.; Shen, Y. Cytotoxicity and the effect of temperature on physical properties and chemical composition of a new calcium silicate-based root canal sealer. J. Endod. 2020, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Okamura, T.; Chen, L.; Tsumano, N.; Ikeda, C.; Komasa, S.; Tominaga, K.; Hashimoto, Y. Biocompatibility of a high-plasticity, calcium silicate-based, ready-to-use material. Materials 2020, 13, 4770. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Gong, H.; Jiang, X.; Wang, H.; Li, K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010, 203, 315–321. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ciapetti, G.; Prati, C. Development of the foremost light-curable calcium-silicate MTA cement as root-end in oral surgery. Chemical-physical properties, bioactivity and biological behavior. Dent. Mater. 2011, 27, e134–e157. [Google Scholar] [CrossRef]
- Taddei, P.; Modena, E.; Tinti, A.; Siboni, F.; Prati, C.; Gandolfi, M. Vibrational investigation on the in vitro bioactivity of commercial and experimental calcium-silicate cements for root-end endodontic therapy. In Book of Abstract EUCMOS 2010; University of Florence: Florence, Italy, 2011. [Google Scholar]
- Camilleri, J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent. Mater. 2011, 27, 836–844. [Google Scholar] [CrossRef]
- Kim, J.R.; Nosrat, A.; Fouad, A.F. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J. Dent. 2015, 43, 241–247. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Ibing, M.; Burklein, S.; Weber, I.; Reitze, M.P.; Schafer, E. Physico-chemical investigation of endodontic sealers exposed to simulated intracanal heat application: Hydraulic calcium silicate-based sealers. Materials 2021, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Jafari, S. Composition and physicochemical properties of calcium silicate based sealers: A review article. J. Clin. Exp. Dent. 2017, 9, e1249–e1255. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Arntz, Y.; Eid, A.; Zghal, J.; Sauro, S.; Haikel, Y.; Mancino, D. Physicochemical and antibacterial properties of novel, premixed calcium silicate-based sealer compared to powder-liquid bioceramic sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef]
- ISO 6876; Root Canal Sealing Materials. British Standards Institution: London, UK, 2012.
- Silva, E.J.N.L.; Cardoso, M.L.; Rodrigues, J.P.; De-Deus, G.; Fidalgo, T. Solubility of bioceramic- and epoxy resin-based root canal sealers: A systematic review and meta-analysis. Aust. Endod. J. 2021, 47, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Staining potential of Neo MTA Plus, MTA Plus, and Biodentine used for pulpotomy procedures. J. Endod. 2015, 41, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.K.; Schembri Wismayer, P.; Camilleri, J. Influence of environment on testing of hydraulic sealers. Sci. Rep. 2017, 7, 17927. [Google Scholar] [CrossRef]
- Braga, J.M.; Oliveira, R.R.; Martins, R.C.; Ribeiro Sobrinho, A.P. The effects of a mineral trioxide aggregate-based sealer on the production of reactive oxygen species, nitrogen species and cytokines by two macrophage subtypes. Int. Endod. J. 2014, 47, 909–919. [Google Scholar] [CrossRef]
- Benetti, F.; de Azevedo Queiroz, I.O.; Oliveira, P.H.C.; Conti, L.C.; Azuma, M.M.; Oliveira, S.H.P.; Cintra, L.T.A. Cytotoxicity and biocompatibility of a new bioceramic endodontic sealer containing calcium hydroxide. Braz. Oral. Res. 2019, 33, e042. [Google Scholar] [CrossRef]
- Kuramoto, M.; Kawashima, N.; Tazawa, K.; Nara, K.; Fujii, M.; Noda, S.; Hashimoto, K.; Nozaki, K.; Okiji, T. Mineral trioxide aggregate suppresses pro-inflammatory cytokine expression via the calcineurin/nuclear factor of activated T cells/early growth response 2 pathway in lipopolysaccharide-stimulated macrophages. Int. Endod. J. 2020, 53, 1653–1665. [Google Scholar] [CrossRef]
- Wang, L.; Jin, H.; Ye, D.; Wang, J.; Ao, X.; Dong, M.; Niu, W. Enterococcus faecalis lipoteichoic acid-induced NLRP3 inflammasome via the activation of the nuclear factor kappa B pathway. J. Endod. 2016, 42, 1093–1100. [Google Scholar] [CrossRef]


| Groups | Materials | Manufacturer | Composition | Proportion |
|---|---|---|---|---|
| BR | BioRoot RCS | Septodont, Saint-Maur-des-Fossés, France | Powder: tricalcium silicate, zirconium oxide and povidone Liquid: aqueous solution of calcium chloride and polycarboxylate | 1 spoon of powder + 6 drops of liquid |
| BC | Bio-C Sealer | Angelus, Londrina, PR, Brazil | Tricalcium silicate, dicalcium silicate, tricalcium aluminate, calcium oxide, zirconium oxide, silicon oxide, polyethylene glycol, iron oxide | Ready to use |
| SPBC | Sealer Plus BC | MK Life, Porto Alegre, RS, Brazil | Calcium disilicate, calcium trisilicate, zirconium oxide, calcium hydroxide, propylene glycol | Ready to use |
| CSBS | ||||
|---|---|---|---|---|
| Elements Identified | BR | BC | SPBC | p |
| Calcium (Ca) | 3200 | 200 | 800 | 0.0201 |
| Silicon (Si) | 19 | 20 | 12 | 0.5302 |
| Zirconium (Zr) | NI | 4 | NI | 0.4219 |
| Wavenumber (cm−1) | BioRoot RCS | Bio-C Sealer | Sealer Plus BC Sealer | Reference |
|---|---|---|---|---|
| 878 | [CO3]2− | [CO3]2 | [CO3]2 | [23,24,28] |
| ~1400 | [CO3]2 | [CO3]2 | [CO3]2 | [23,24,28] |
| ~900–1200 | C–S–H | C–S–H | C–S–H | [23,24] |
| 1350 | C–S–H | C–S–H | C–S–H | [23,24] |
| ~3640 | Ca(OH)2 | Ca(OH)2 | * Ca(OH)2 | [25,26,27] |
| 560 | [PO4]3− | [PO4]3− | [PO4]3− | [23] |
| 600 | [PO4]3− | [PO4]3− | [PO4]3− | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, A.d.P.; de Rosatto, C.M.P.; Ferraz, D.C.; de Souza, G.L.; Moura, C.C.G. Evaluation of Cytotoxicity, Cell Attachment, and Elemental Characterization of Three Calcium Silicate-Based Sealers. Materials 2023, 16, 6705. https://doi.org/10.3390/ma16206705
Melo AdP, de Rosatto CMP, Ferraz DC, de Souza GL, Moura CCG. Evaluation of Cytotoxicity, Cell Attachment, and Elemental Characterization of Three Calcium Silicate-Based Sealers. Materials. 2023; 16(20):6705. https://doi.org/10.3390/ma16206705
Chicago/Turabian StyleMelo, Anahi de Paula, Camila Maria Peres de Rosatto, Danilo Cassiano Ferraz, Gabriela Leite de Souza, and Camilla Christian Gomes Moura. 2023. "Evaluation of Cytotoxicity, Cell Attachment, and Elemental Characterization of Three Calcium Silicate-Based Sealers" Materials 16, no. 20: 6705. https://doi.org/10.3390/ma16206705
APA StyleMelo, A. d. P., de Rosatto, C. M. P., Ferraz, D. C., de Souza, G. L., & Moura, C. C. G. (2023). Evaluation of Cytotoxicity, Cell Attachment, and Elemental Characterization of Three Calcium Silicate-Based Sealers. Materials, 16(20), 6705. https://doi.org/10.3390/ma16206705







