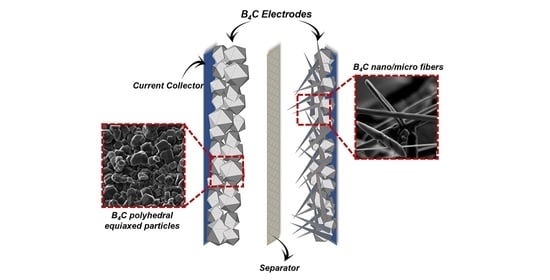
Boron Carbide as an Electrode Material: Tailoring Particle Morphology to Control Capacitive Behaviour
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Sol–Gel Synthesis of Boron Carbide
3.2. Electrochemical Performance of Boron Carbide Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Liang, J.; Li, X.; Yue, L.; Liu, Q.; Lu, S.; Asiri, A.M.; Hu, J.; Luo, Y.; Sun, X. Recent advances in perovskite oxides as electrode materials for supercapacitors. Chem. Commun. 2021, 57, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and opportunities for supercapacitors. APL Mater. 2019, 7, 100901. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, M.A.K.; Humayun, M.; Usman, M.; Shah, S.S.; Bibi, S.; Hasnain, B.S.U.; Ahmad, S.M.; Khan, A.; Shah, N.; et al. A Review of Supercapacitors: Materials Design, Modification, and Applications. Energies 2021, 14, 7779. [Google Scholar] [CrossRef]
- Verma, S.; Arya, S.; Gupta, V.; Mahajan, S.; Furukawa, H.; Khosla, A. Performance analysis, challenges and future perspectives of nickel based nanostructured electrodes for electrochemical supercapacitors. J. Mater. Res. Technol. 2021, 11, 564–599. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Shi, X.; Zhu, Y.; Wu, Z.-S. Recent status and future perspectives of ultracompact and customizable micro-supercapacitors. Nano Res. Energy 2022, 1, e9120018. [Google Scholar] [CrossRef]
- Yue, L.; Wu, D.; Wu, Z.; Zhao, W.; Wang, D.; Zhong, B.; Liu, Q.; Liu, Y.; Gao, S.; Asiri, A.M.; et al. A MnS/FeS 2 heterostructure with a high degree of lattice matching anchored into carbon skeleton for ultra-stable sodium-ion storage. J. Mater. Chem. A 2021, 9, 24024–24035. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhang, X.-Q.; Zhou, M.-Y.; Huang, J.-Q. Mechanism, quantitative characterization, and inhibition of corrosion in lithium batteries. Nano Res. Energy 2022, 1–18. [Google Scholar] [CrossRef]
- Wang, G.; Chen, T.; Gómez-García, C.J.; Zhang, F.; Zhang, M.; Ma, H.; Pang, H.; Wang, X.; Tan, L. A High-Capacity Negative Electrode for Asymmetric Supercapacitors Based on a PMo12 Coordination Polymer with Novel Water-Assisted Proton Channels. Small 2020, 16, e2001626. [Google Scholar] [CrossRef]
- Zdolšek, N.; Perović, I.; Brković, S.; Tasić, G.; Milović, M.; Vujković, M. Deep Eutectic Solvent for Facile Synthesis of Mn3O4@N-Doped Carbon for Aqueous Multivalent-Based Supercapacitors: New Concept for Increasing Capacitance and Operating Voltage. Materials 2022, 15, 8540. [Google Scholar] [CrossRef]
- Attia, S.Y.; Mohamed, S.G.; Barakat, Y.F.; Hassan, H.H.; Zoubi, W.A. Supercapacitor electrode materials: Addressing challenges in mechanism and charge storage. Rev. Inorg. Chem. 2021, 42, 53–88. [Google Scholar] [CrossRef]
- Gogotsi, Y. What nano can do for energy storage. ACS Nano 2014, 8, 5369–5371. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y.; Dunn, B. Materials science. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.K.; Shahi, S.; Gnawali, C.L.; Adhikari, M.P.; Rajbhandari, R.; Pokharel, B.P.; Ma, R.; Shrestha, R.G.; Ariga, K. Phyllanthus emblica Seed-Derived Hierarchically Porous Carbon Materials for High-Performance Supercapacitor Applications. Materials 2022, 15, 8335. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Xu, L. Core-Shell Carbon Nanofibers@Ni(OH)2/NiO Composites for High-Performance Asymmetric Supercapacitors. Materials 2022, 15, 8377. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Wu, Y.; Nguyen, T.T.; Wu, J.; Guo, M.; Du, C. Inspired by Skeletal Muscles: Study of the Physical and Electrochemical Properties of Derived Lignocellulose-Based Carbon Fibers. Materials 2022, 15, 8068. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Rychagov, A.Y.; Sosenkin, V.E.; Baskakov, S.A.; Kabachkov, E.N.; Shulga, Y.M. Supercapacitor Properties of rGO-TiO2 Nanocomposite in Two-component Acidic Electrolyte. Materials 2022, 15, 7856. [Google Scholar] [CrossRef]
- Ullah, K.; Kim, I.-J.; Yang, S.-H.; Oh, W.-C. Preparation of highly expanded graphene with large surface area and its additional conductive effect for EDLC performance. J. Mater. Sci. Mater. Electron. 2015, 26, 6945–6953. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not to Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Yue, L.; Ma, C.; Yan, S.; Wu, Z.; Zhao, W.; Liu, Q.; Luo, Y.; Zhong, B.; Zhang, F.; Liu, Y.; et al. Improving the intrinsic electronic conductivity of NiMoO4 anodes by phosphorous doping for high lithium storage. Nano Res. 2022, 15, 186–194. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, X.; Yue, L.; Zhang, L.; Luo, Y.; Ren, Y.; Zhao, X.-E.; Li, N.; Tang, B.; Liu, Q.; et al. A gradient hexagonal-prism Fe 3 Se 4 @SiO 2 @C configuration as a highly reversible sodium conversion anode. J. Mater. Chem. A 2022, 10, 4087–4099. [Google Scholar] [CrossRef]
- Shen, Q.; Gómez-García, C.J.; Sun, W.; Lai, X.; Pang, H.; Ma, H. Improving the photocatalytic H 2 evolution activity of Keggin polyoxometalates anchoring copper-azole complexes. Green Chem. 2021, 23, 3104–3114. [Google Scholar] [CrossRef]
- Tian, Y.; Chang, Z.-H.; Wang, X.-L.; Lin, H.-Y.; Zhang, Y.-C.; Liu, Q.Q.; Chen, Y.-Z. Pseudocapacitance improvement of polymolybdates-based metal–organic complexes via modification with hydrogen molybdenum bronze by electrochemical treatment. Chem. Eng. J. 2022, 428, 132380. [Google Scholar] [CrossRef]
- Alper, J.P.; Kim, M.S.; Vincent, M.; Hsia, B.; Radmilovic, V.; Carraro, C.; Maboudian, R. Silicon carbide nanowires as highly robust electrodes for micro-supercapacitors. J. Power Sources 2013, 230, 298–302. [Google Scholar] [CrossRef]
- Lee, C.-P.; Murti, B.-T.; Yang, P.-K.; Rossi, F.; Carraro, C.; Maboudian, R. Cobalt Oxide-Decorated Silicon Carbide Nano-Tree Array Electrode for Micro-Supercapacitor Application. Materials 2021, 14, 4514. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Blackford, M.G. Electrochemical characteristics of B4C or BN added MnO2 cathode material for alkaline batteries. Mater. Chem. Phys. 2010, 123, 700–705. [Google Scholar] [CrossRef]
- Yang, T.; Liu, H.-J.; Bai, F.; Wang, E.-H.; Chen, J.-H.; Chou, K.-C.; Hou, X.-M. Supercapacitor electrode based on few-layer h-BNNSs/rGO composite for wide-temperature-range operation with robust stable cycling performance. Int. J. Miner. Metall. Mater. 2020, 27, 220–231. [Google Scholar] [CrossRef]
- Maity, C.K.; Santra, D.K.; Verma, K.; Sahoo, S.; Cotts, S.; Akinwande, D.; Berry, V.; Chandra Nayak, G. Induced conducting energy-levels in a boron nitride nano-framework for asymmetric supercapacitors in high charge-mobility ionic electrolytes. Compos. Part B Eng. 2021, 212, 108728. [Google Scholar] [CrossRef]
- Song, S.; Yu, L.; Ruan, Y.; Sun, J.; Chen, B.; Xu, W.; Zhang, J.-G. Highly efficient Ru/B4C multifunctional oxygen electrode for rechargeable Li O2 batteries. J. Power Sources 2019, 413, 11–19. [Google Scholar] [CrossRef]
- Song, S.; Xu, W.; Zheng, J.; Luo, L.; Engelhard, M.H.; Bowden, M.E.; Liu, B.; Wang, C.-M.; Zhang, J.-G. Complete Decomposition of Li2CO3 in Li-O2 Batteries Using Ir/B4C as Noncarbon-Based Oxygen Electrode. Nano Lett. 2017, 17, 1417–1424. [Google Scholar] [CrossRef]
- Song, N.; Gao, Z.; Zhang, Y.; Li, X. B4C nanoskeleton enabled, flexible lithium-sulfur batteries. Nano Energy 2019, 58, 30–39. [Google Scholar] [CrossRef]
- Jiang, H.R.; Shyy, W.; Wu, M.C.; Wei, L.; Zhao, T.S. Highly active, bi-functional and metal-free B 4 C-nanoparticle-modified graphite felt electrodes for vanadium redox flow batteries. J. Power Sources 2017, 365, 34–42. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, X.; Ma, M.; Mu, C.; Li, P.; Li, L.; Li, M.; Nie, A.; Xiang, J.; Zhao, Z.; et al. Application of hard ceramic materials B4C in energy storage: Design B4C@C core-shell nanoparticles as electrodes for flexible all-solid-state micro-supercapacitors with ultrahigh cyclability. Nano Energy 2020, 75, 104947. [Google Scholar] [CrossRef]
- Balcı, Ö.; Buldu, M.; Ammar, A.U.; Kiraz, K.; Somer, M.; Erdem, E. Defect-induced B4C electrodes for high energy density supercapacitor devices. Sci. Rep. 2021, 11, 11627. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron Carbide: Structure, Properties, and Stability under Stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Kim, T.-S.; Yeo, J.-H.; Nam, K.-B.; Kim, M.J.; Yoo, J.-B. Boron carbide coating to improve the chemical stability of nm-thick graphite films. Thin Solid Film. 2020, 704, 138002. [Google Scholar] [CrossRef]
- Avcıoğlu, S.; Buldu, M.; Kaya, F.; Üstündağ, C.B.; Kam, E.; Menceloğlu, Y.Z.; Kaptan, H.Y.; Kaya, C. Processing and properties of boron carbide (B4C) reinforced LDPE composites for radiation shielding. Ceram. Int. 2020, 46, 343–352. [Google Scholar] [CrossRef]
- Avcioglu, S.; Kaya, F.; Kaya, C. Effect of elemental nano boron on the transformation and morphology of boron carbide (B4C) powders synthesized from polymeric precursors. Ceram. Int. 2020, 46, 17938–17950. [Google Scholar] [CrossRef]
- Avcıoğlu, S.; Kaya, F.; Kaya, C. Morphological evolution of boron carbide particles: Sol-gel synthesis of nano/micro B4C fibers. Ceram. Int. 2021, 47, 26651–26667. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Matsuura, H.; Sashiwa, H.; Saimoto, H. Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. Int. J. Biol. Macromol. 1996, 18, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Siqueira, R.L.; Yoshida, I.V.P.; Pardini, L.C.; Schiavon, M.A. Poly(borosiloxanes) as precursors for carbon fiber ceramic matrix composites. Mat. Res. 2007, 10, 147–151. [Google Scholar] [CrossRef]
- Chen, X.W.; Dong, S.M.; Kan, Y.M.; Zhou, H.J.; Hu, J.B.; Ding, Y.S. Effect of glycerine addition on the synthesis of boron carbide from condensed boric acid–polyvinyl alcohol precursor. RSC Adv. 2016, 6, 9338–9343. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Stalla, D.; Tekeei, A.; Suppes, G.; Jalisatgi, S.; Lee, M.; Hawthorne, F.; Robertson, J.D.; Firlej, L.; et al. Infrared study of boron–carbon chemical bonds in boron-doped activated carbon. Carbon 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, J.; Cui, L.; Song, S.; Zhang, S.; Yu, L.; Zhang, P. Tribological characteristic and mechanism analysis of borate ester as a lubricant additive in different base oils. RSC Adv. 2017, 7, 7944–7953. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Wang, S.; Xing, P.; Kong, J.; Yang, G. A low cost, low energy, environmentally friendly process for producing high-purity boron carbide. Ceram. Int. 2019, 45, 3101–3110. [Google Scholar] [CrossRef]
- Kocakuşak, S.; Akçay, K.; Ayok, T.; Koöroǧlu, H.J.; Koral, M.; Savaşçi, Ö.T.; Tolun, R. Production of anhydrous, crystalline boron oxide in fluidized bed reactor. Chem. Eng. Process. Process Intensif. 1996, 35, 311–317. [Google Scholar] [CrossRef]
- Smith, R.A. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2000. [Google Scholar]
- Avcıoğlu, S.; Buldu, M.; Kaya, F.; Kaya, C. Synthesis of nuclear-grade nano-sized boron carbide powders and its application in LDPE matrix composites for neutron shielding. In Composite Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 543–579. [Google Scholar]
- Tekoğlu, E.; İmer, C.; Ağaoğulları, D.; Lütfi Öveçoğlu, M. Synthesis of LaB6–Al2O3 nanocomposite powders via ball milling-assisted annealing. J. Mater. Sci. 2018, 53, 13538–13549. [Google Scholar] [CrossRef]
- Avcioglu, S.; Kaya, F.; Kaya, C. Non-catalytic synthesis of boron carbide (B4C) nano structures with various morphologies by sol-gel process. Mater. Lett. 2019, 249, 201–205. [Google Scholar] [CrossRef]
- Najib, S.; Bakan, F.; Abdullayeva, N.; Bahariqushchi, R.; Kasap, S.; Franzò, G.; Sankir, M.; Demirci Sankir, N.; Mirabella, S.; Erdem, E. Tailoring morphology to control defect structures in ZnO electrodes for high-performance supercapacitor devices. Nanoscale 2020, 12, 16162–16172. [Google Scholar] [CrossRef] [PubMed]
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. High-performance supercapacitors based on the carbon nanotubes, graphene and graphite nanoparticles electrodes. Heliyon 2018, 4, e00862. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, M.; Pour, G.B. Design and Fabrication of Ultracapacitor based on Paper Substrate and BaTiO3/PEDOT: PSS Separator Film. Recent Pat. Nanotechnol. 2018, 12, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.N.; Anitha, V.C.; Joo, S.W. Improved electrochemical properties of morphology-controlled titania/titanate nanostructures prepared by in-situ hydrothermal surface modification of self-source Ti substrate for high-performance supercapacitors. Sci. Rep. 2017, 7, 13227. [Google Scholar] [CrossRef] [PubMed]
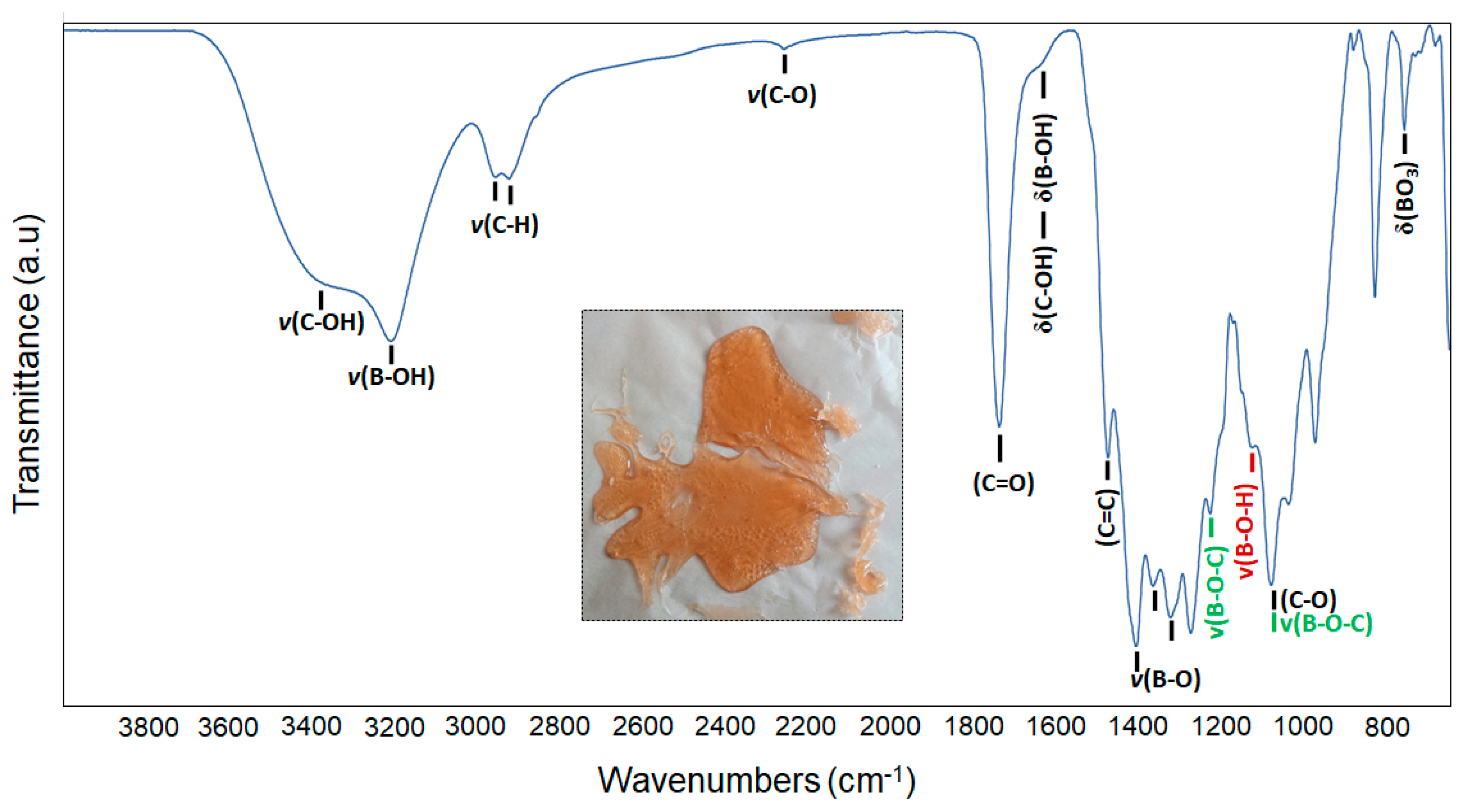
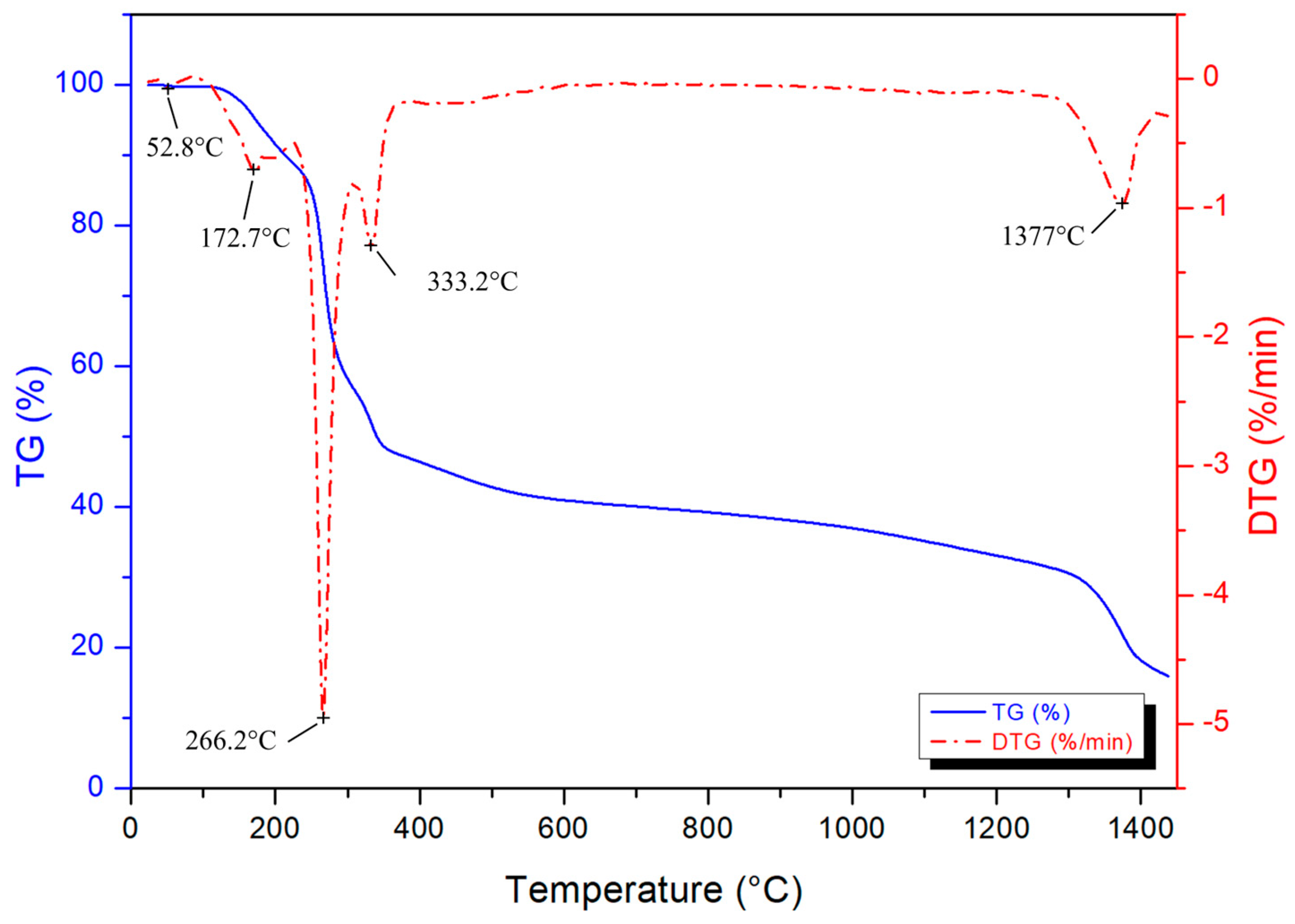

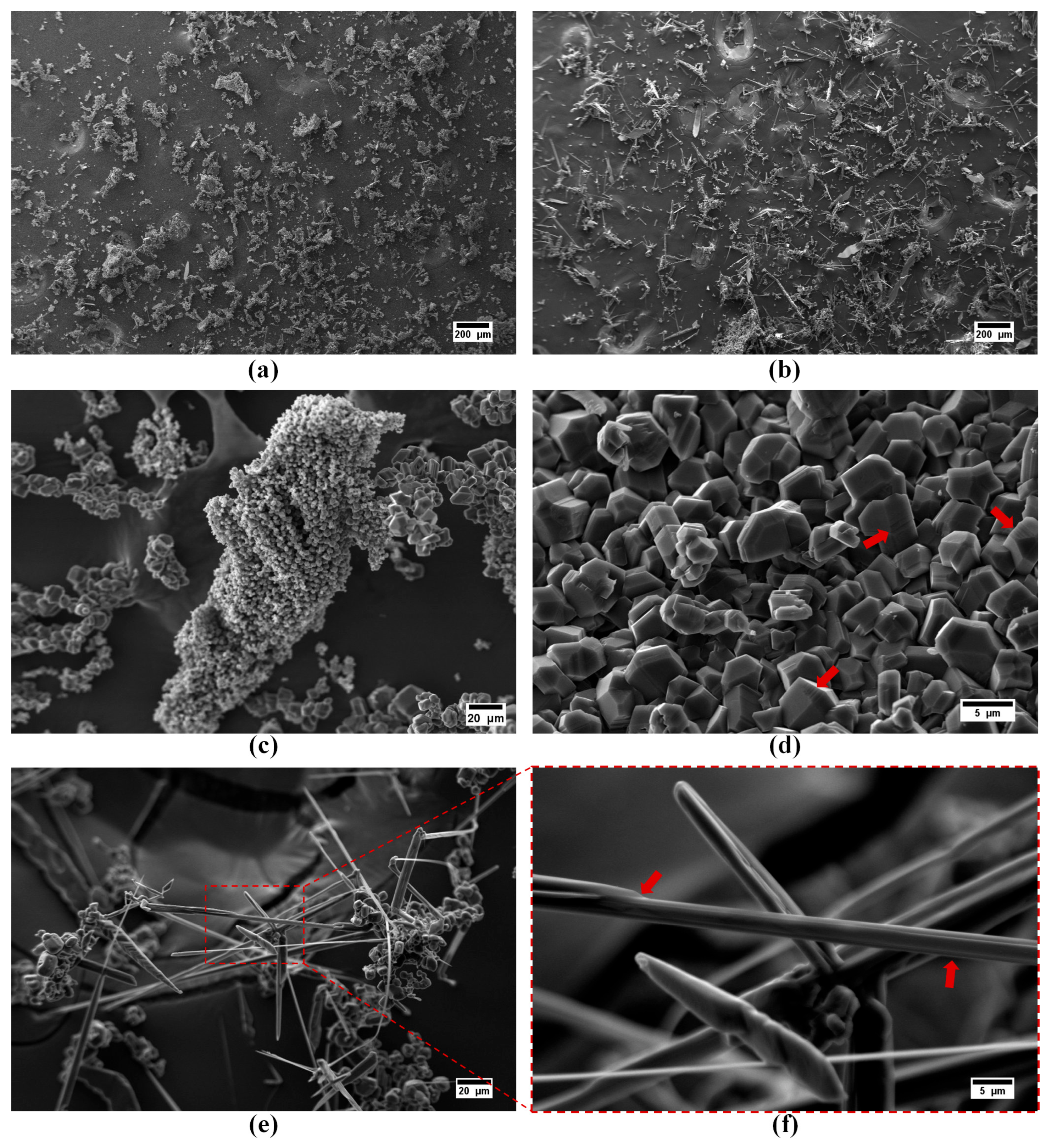
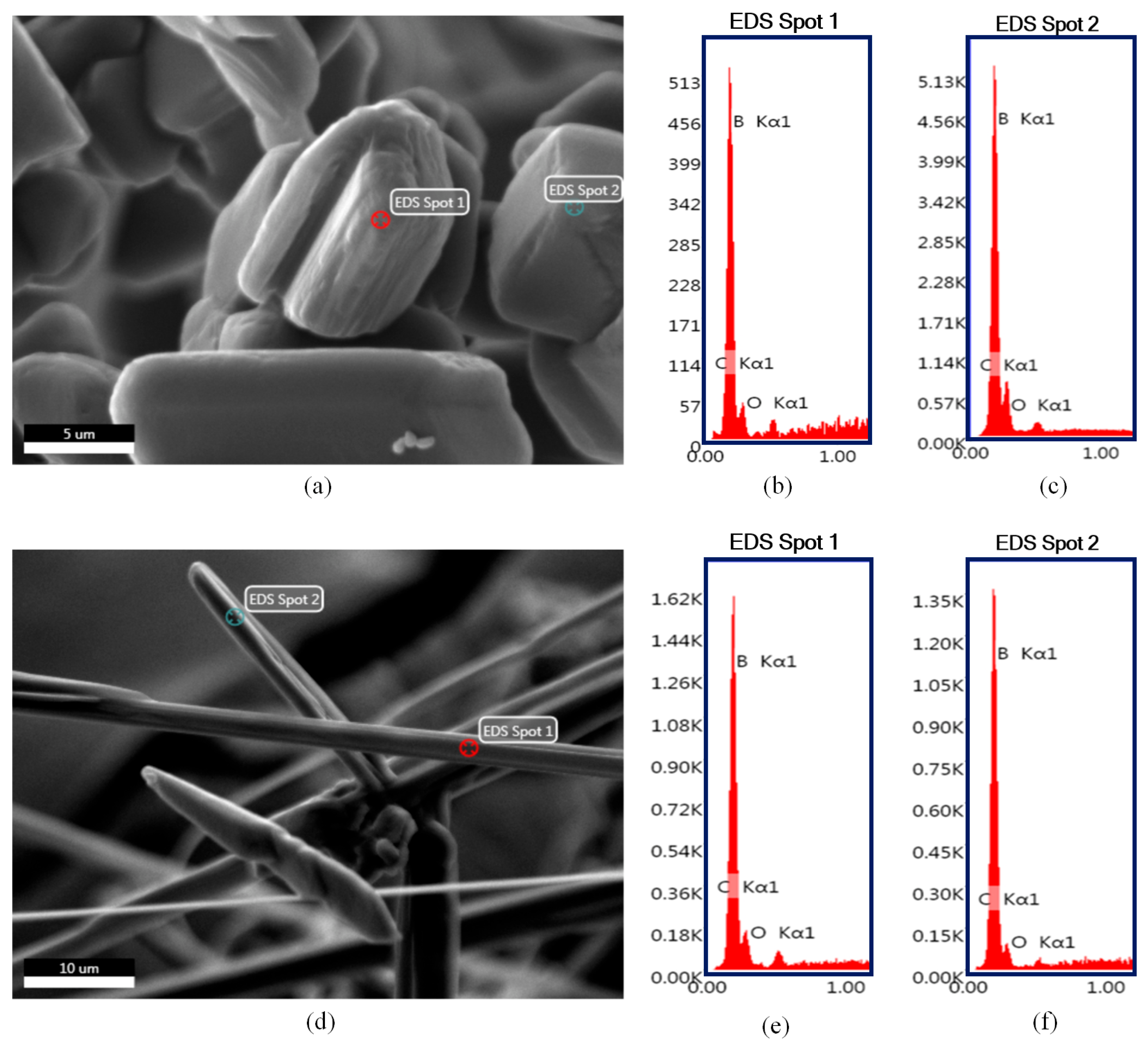
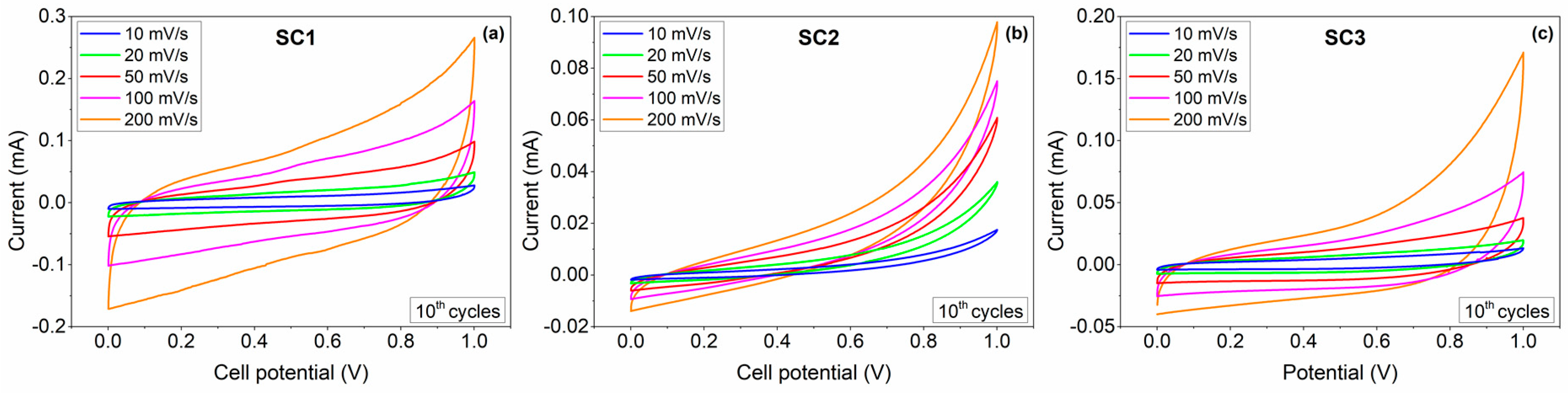
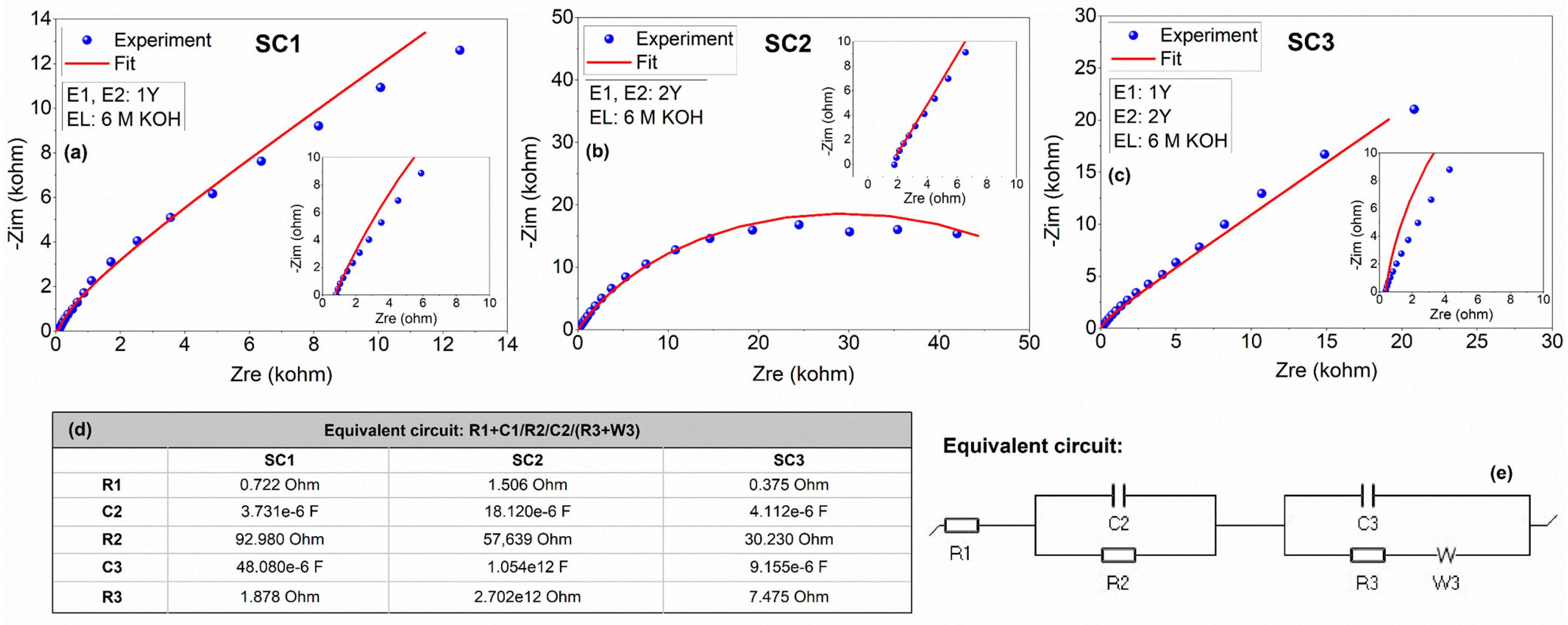
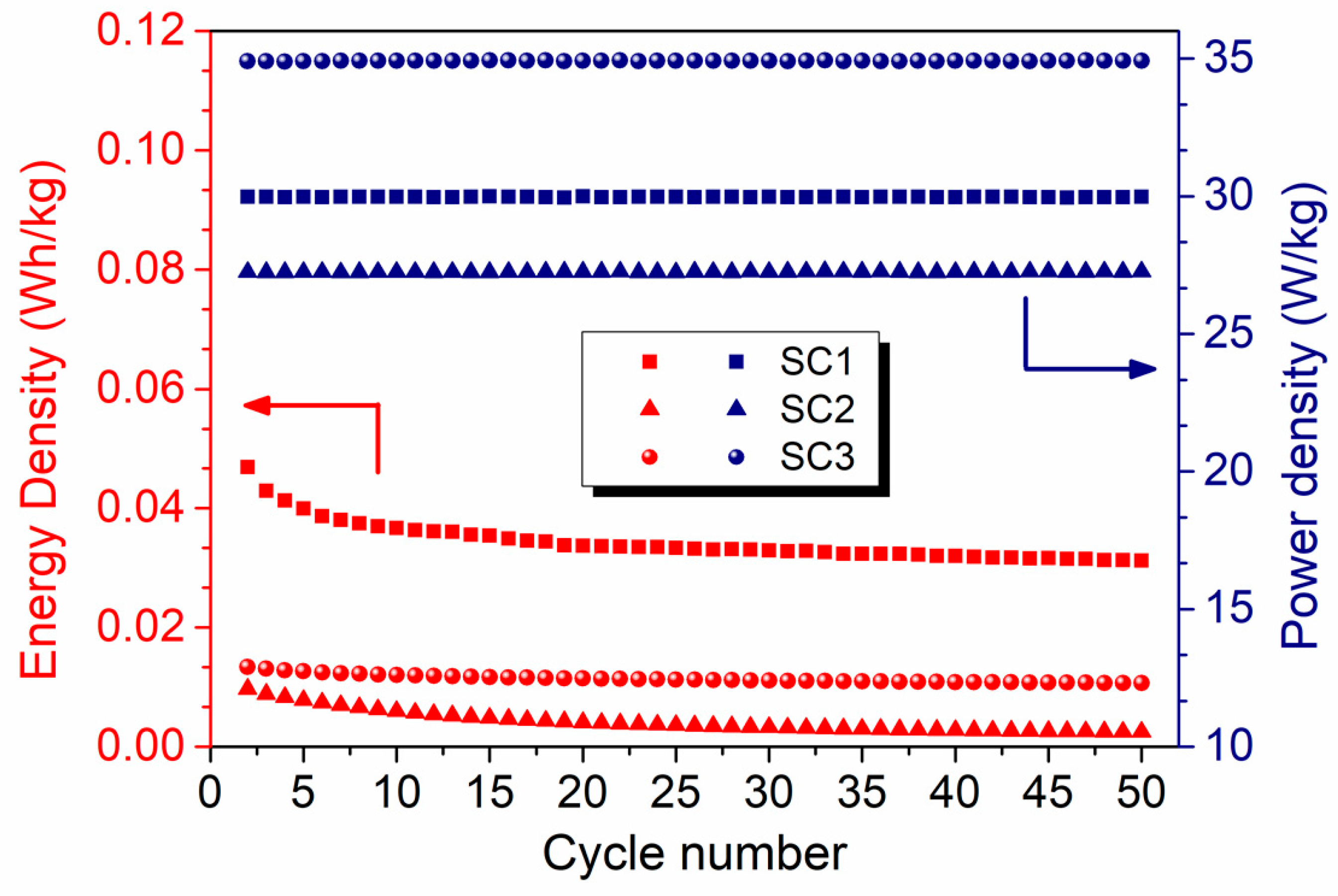
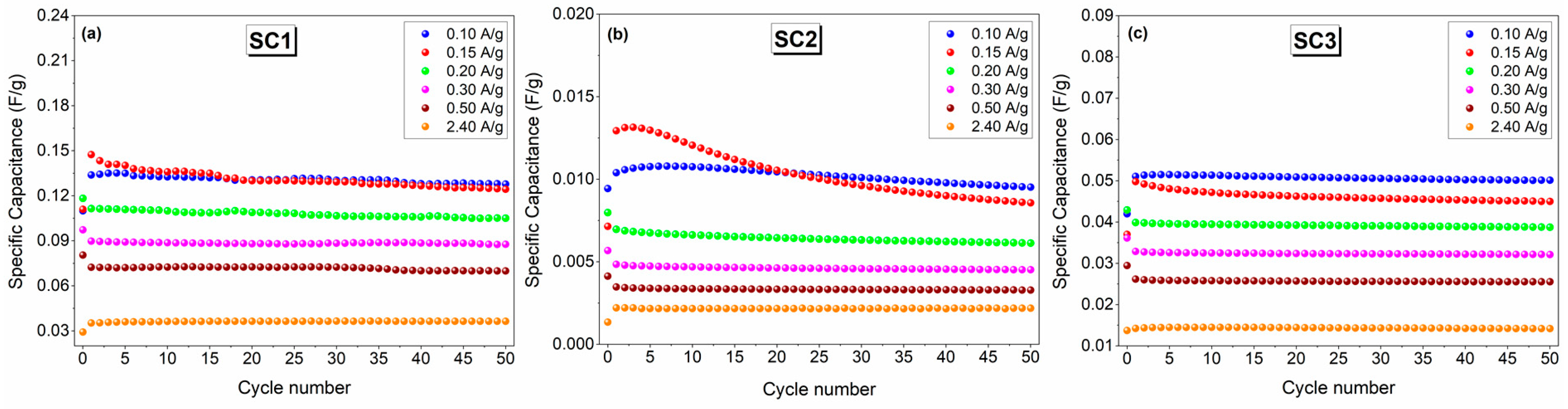
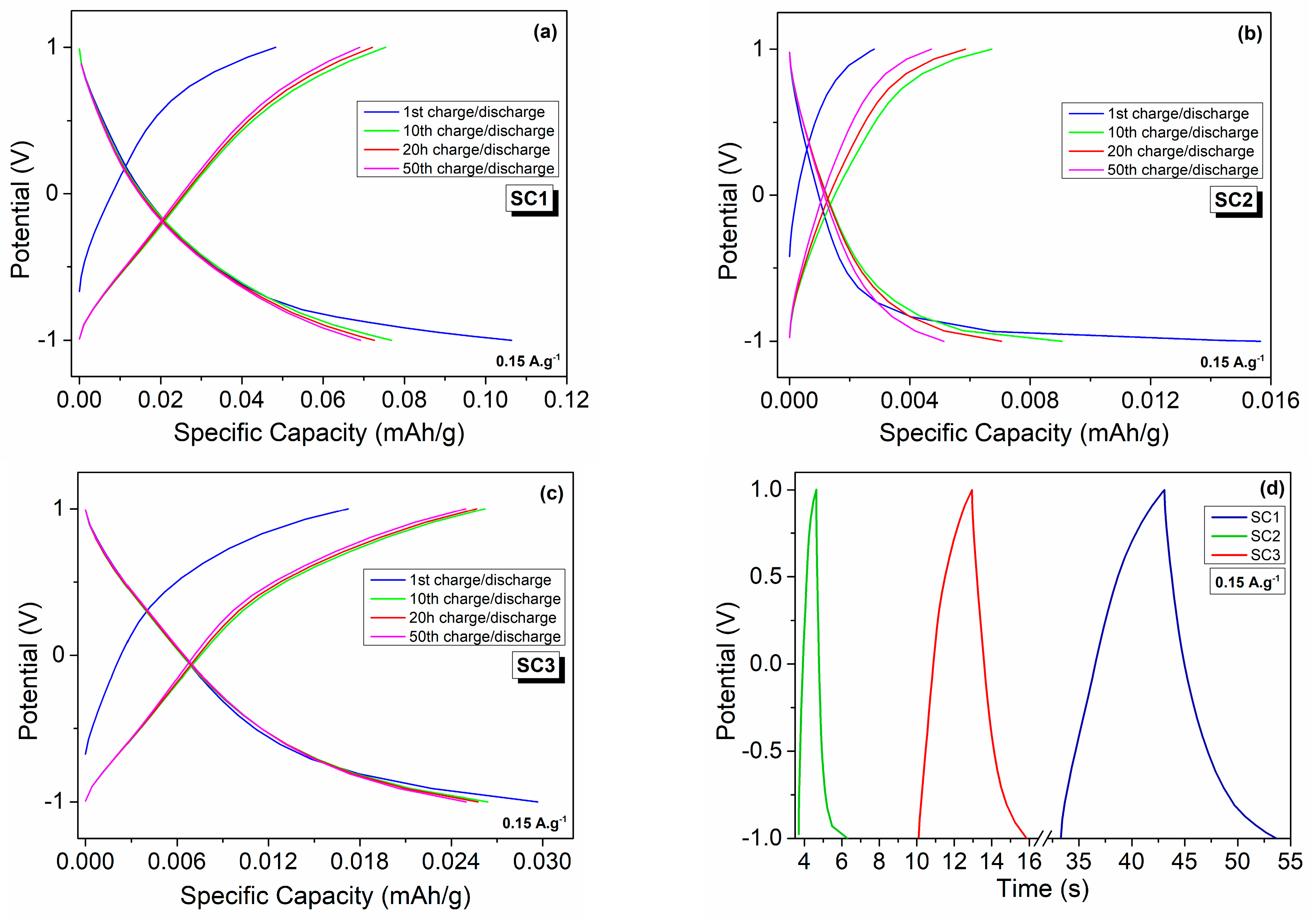
| Device Code | Electrode Material 1 (E1) | Electrode Material 2 (E2) | Electrolyte (El) | Separator (S) | Type |
|---|---|---|---|---|---|
| SC1 | 1Y (polyhedral-equiaxed) | 1Y (polyhedral-equiaxed) | 6 M KOH | Glass fiber | Symmetric |
| SC2 | 2Y (fiber) | 2Y (fiber) | 6 M KOH | Glass fiber | Symmetric |
| SC3 | 1Y (polyhedral-equiaxed) | 2Y (fiber) | 6 M KOH | Glass fiber | Asymmetric |
| Device Code | Energy Density (Wh/kg) | Power Density (W/kg) | Cycling Stability (%) | Specific Capacitance (F/g) | Specific Capacity (mAh/g) |
|---|---|---|---|---|---|
| SC1 | 0.045 | 30 | 84.3 | 0.125 | 0.070 |
| SC2 | 0.009 | 27 | 66.2 | 0.009 | 0.005 |
| SC3 | 0.013 | 35 | 90.4 | 0.045 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avcıoğlu, S.; Buldu-Akturk, M.; Erdem, E.; Kaya, F.; Kaya, C. Boron Carbide as an Electrode Material: Tailoring Particle Morphology to Control Capacitive Behaviour. Materials 2023, 16, 861. https://doi.org/10.3390/ma16020861
Avcıoğlu S, Buldu-Akturk M, Erdem E, Kaya F, Kaya C. Boron Carbide as an Electrode Material: Tailoring Particle Morphology to Control Capacitive Behaviour. Materials. 2023; 16(2):861. https://doi.org/10.3390/ma16020861
Chicago/Turabian StyleAvcıoğlu, Suna, Merve Buldu-Akturk, Emre Erdem, Figen Kaya, and Cengiz Kaya. 2023. "Boron Carbide as an Electrode Material: Tailoring Particle Morphology to Control Capacitive Behaviour" Materials 16, no. 2: 861. https://doi.org/10.3390/ma16020861
APA StyleAvcıoğlu, S., Buldu-Akturk, M., Erdem, E., Kaya, F., & Kaya, C. (2023). Boron Carbide as an Electrode Material: Tailoring Particle Morphology to Control Capacitive Behaviour. Materials, 16(2), 861. https://doi.org/10.3390/ma16020861