Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel
Abstract
1. Introduction
2. Experimental Methods
2.1. Specimen
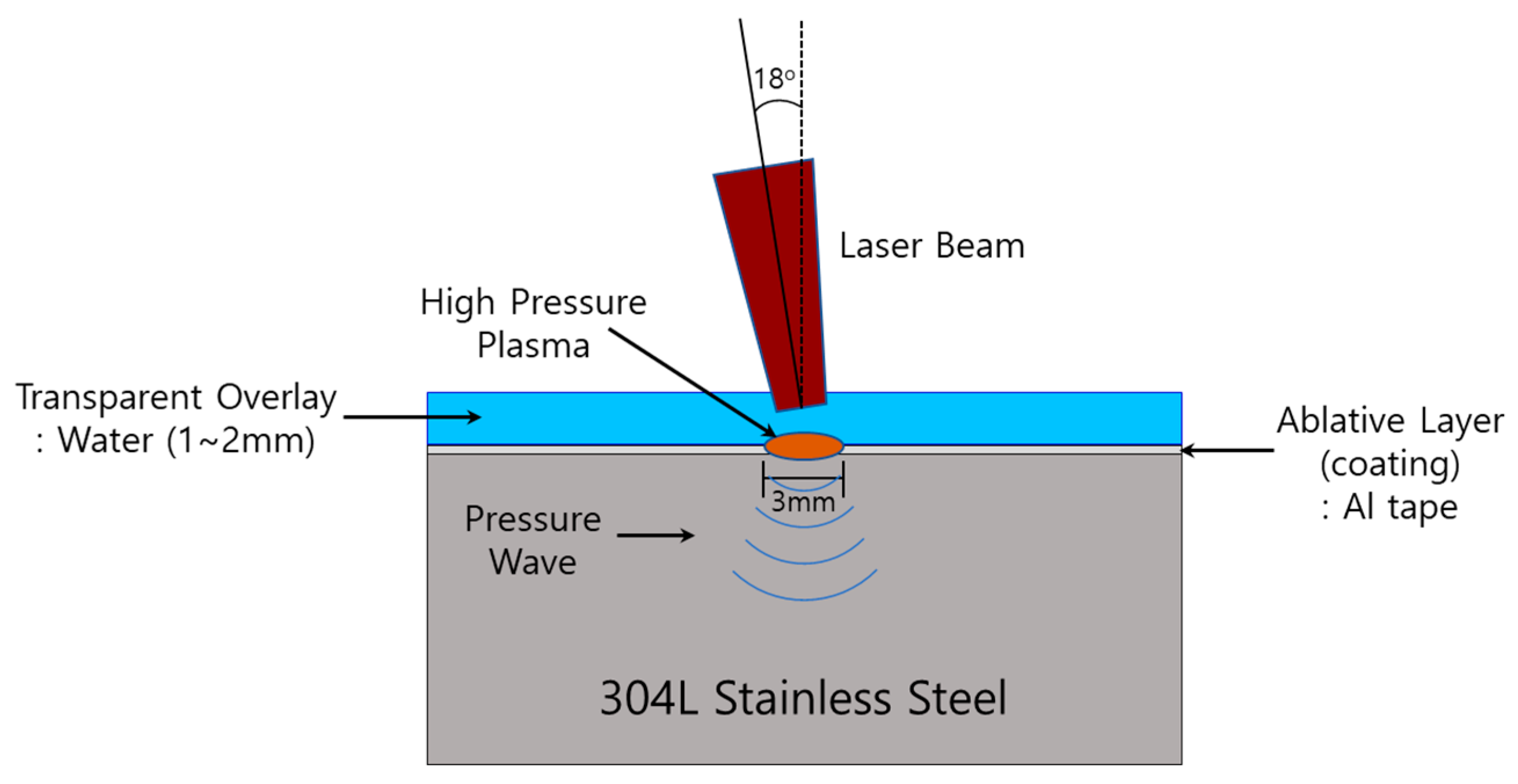
2.2. Laser Peening (LP)
2.3. Qualitative Degree of Sensitization Measurement: ASTM A262 Pr. A
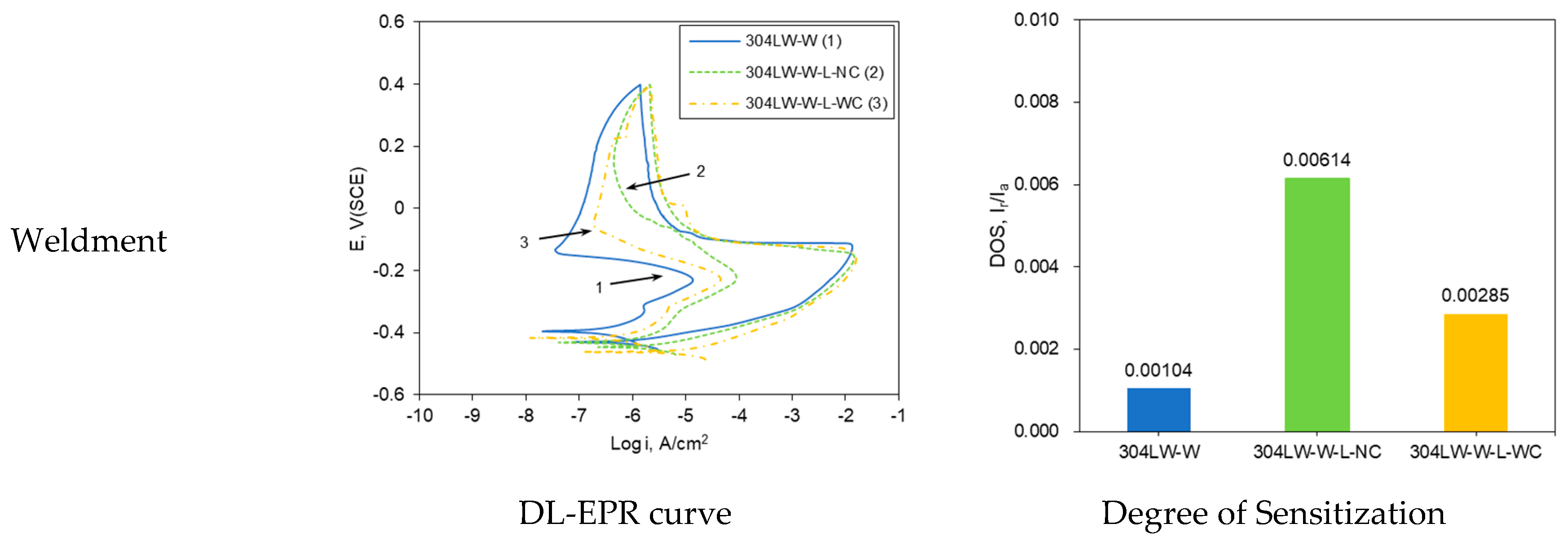
2.4. Quantitative Degree of Sensitization Measurement: DL-EPR Test
2.5. Intergranular Corrosion Rate Measurement: ASTM A262 Pr. C
2.6. Anodic Polarization Test
2.7. AC Impedance Test
2.8. Residual Stress Measurement
3. Results and Discussion
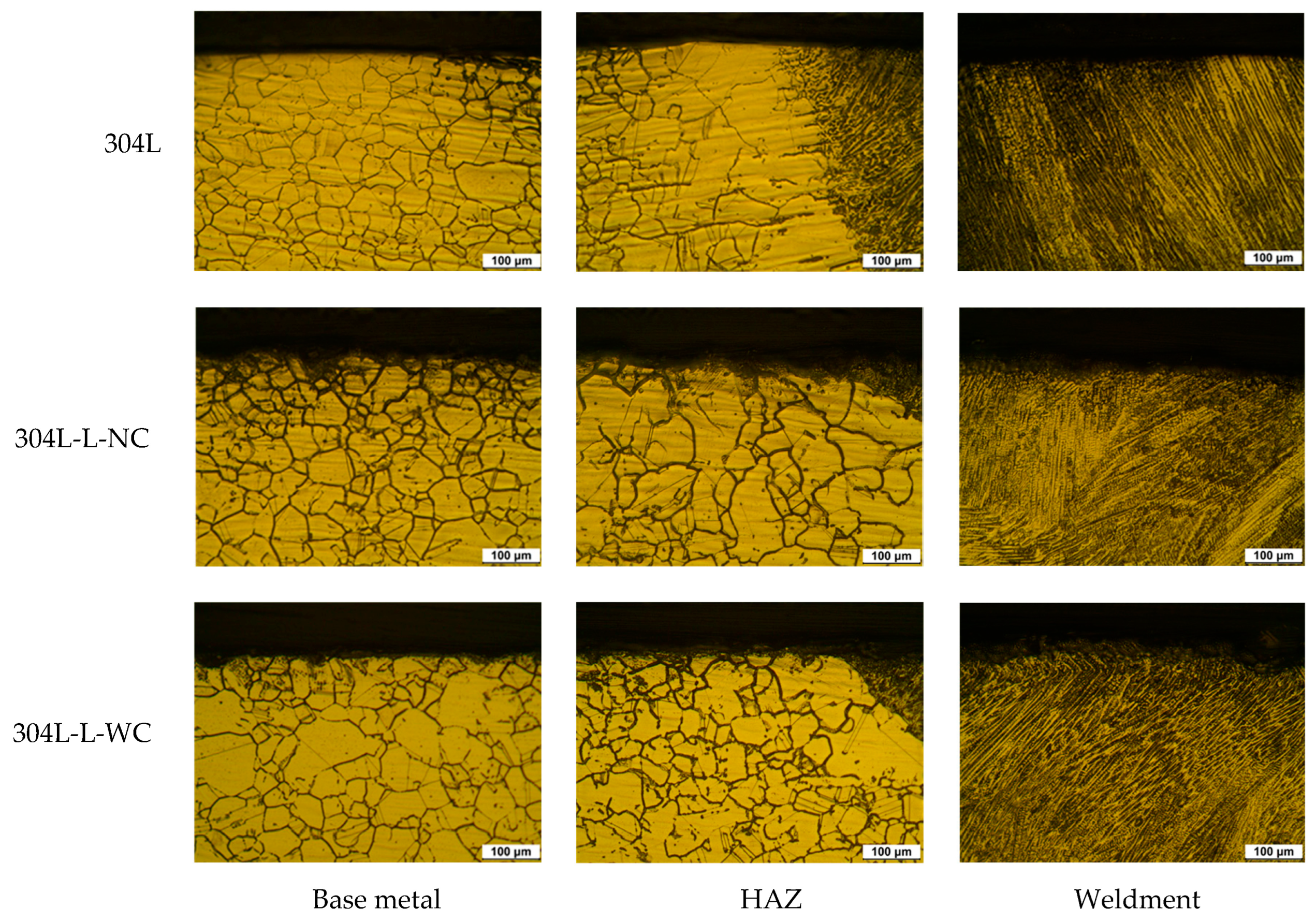
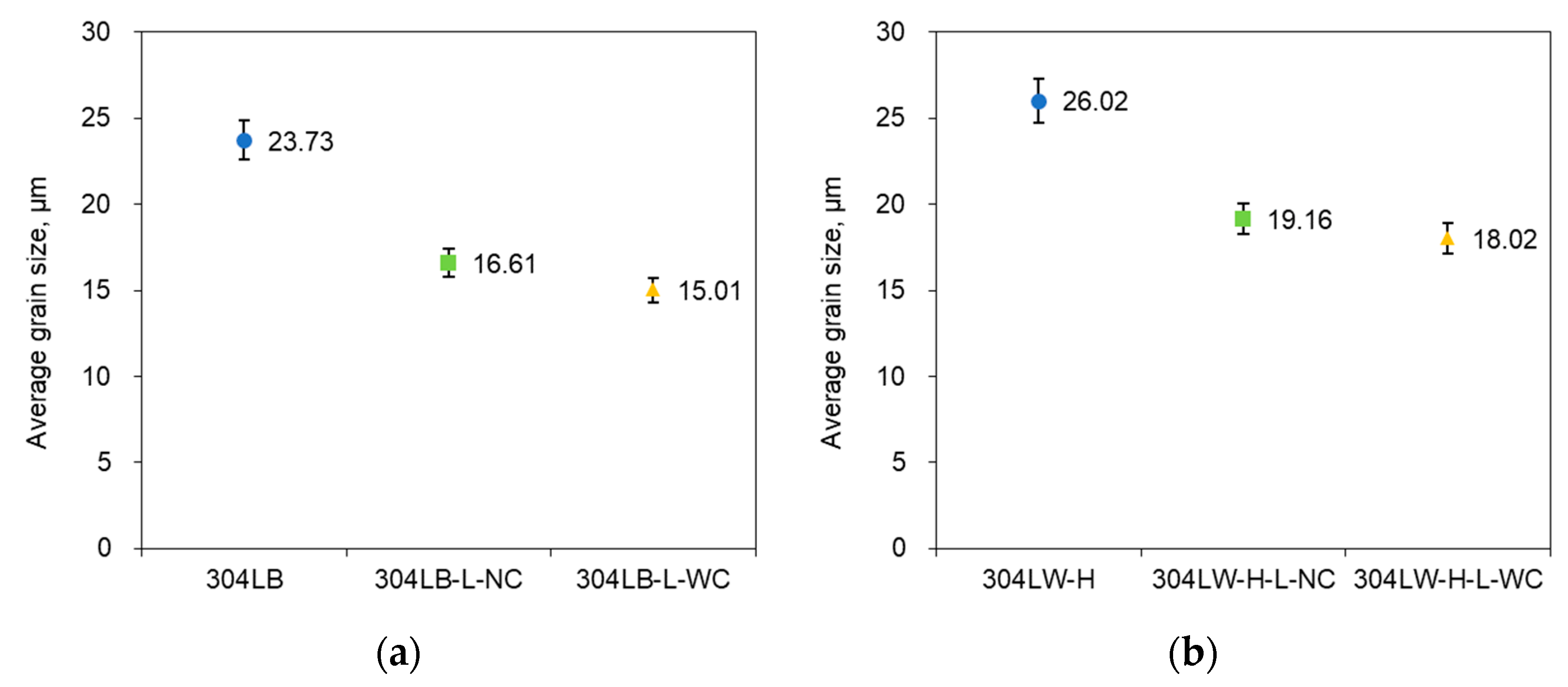
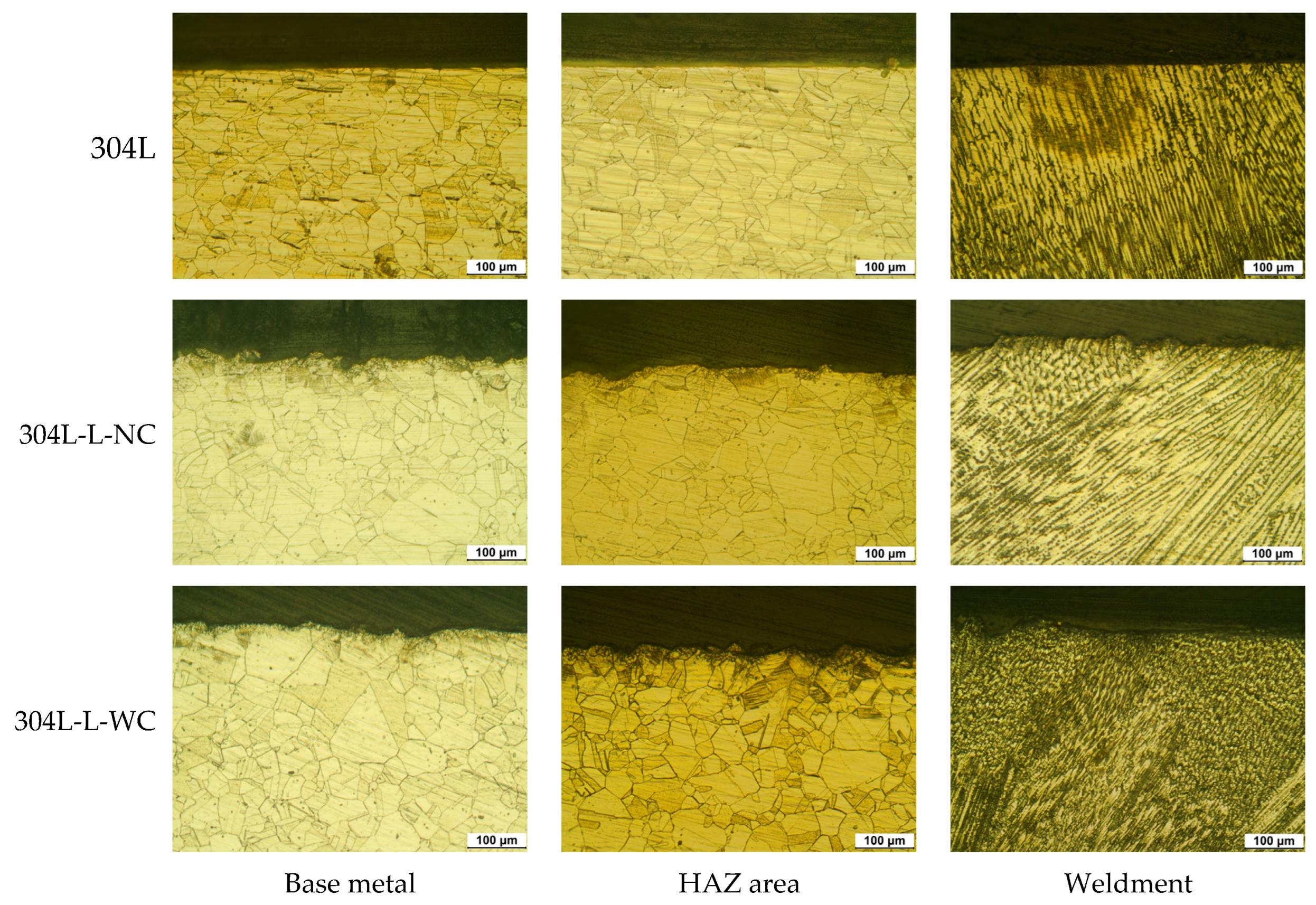
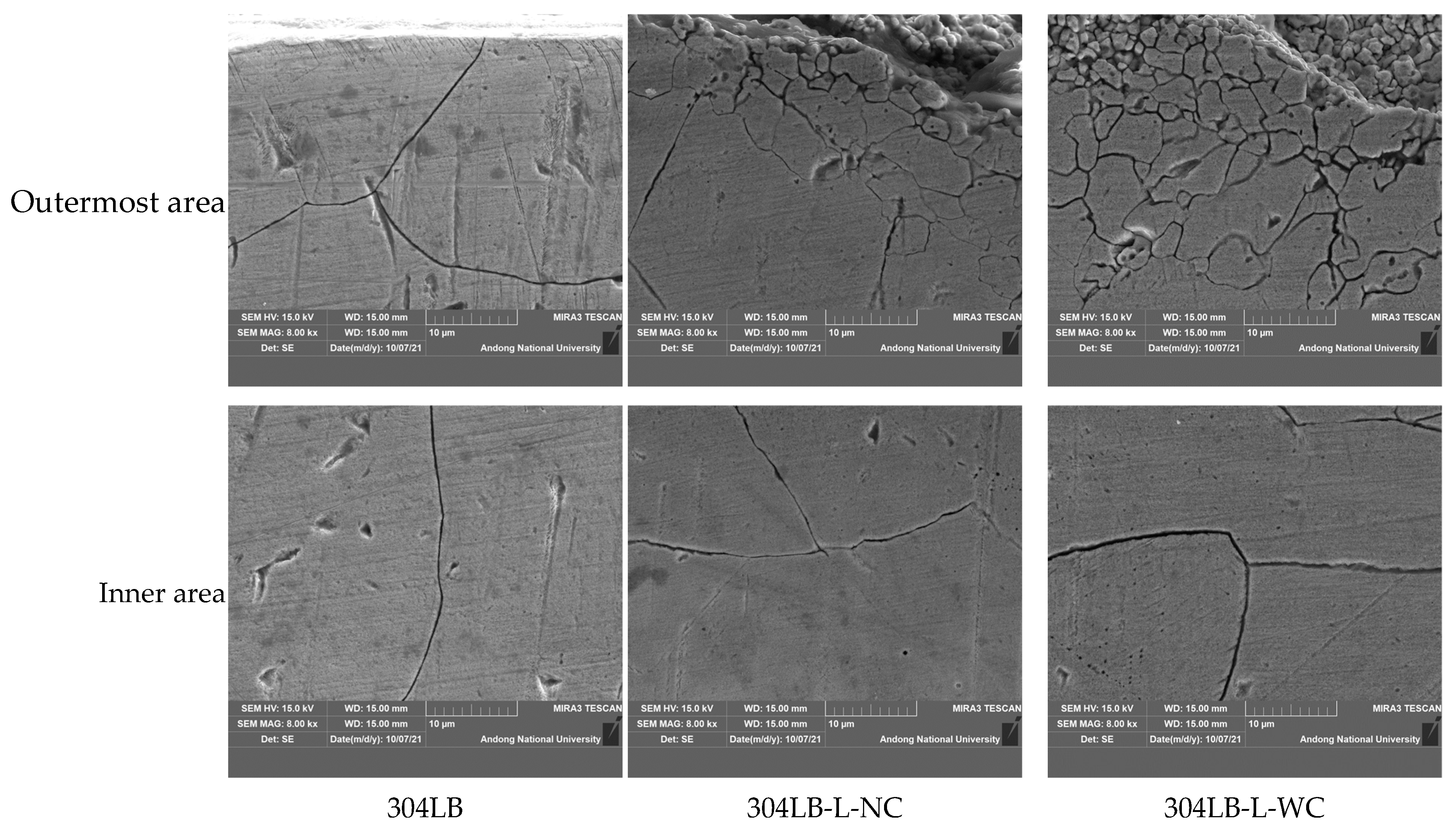
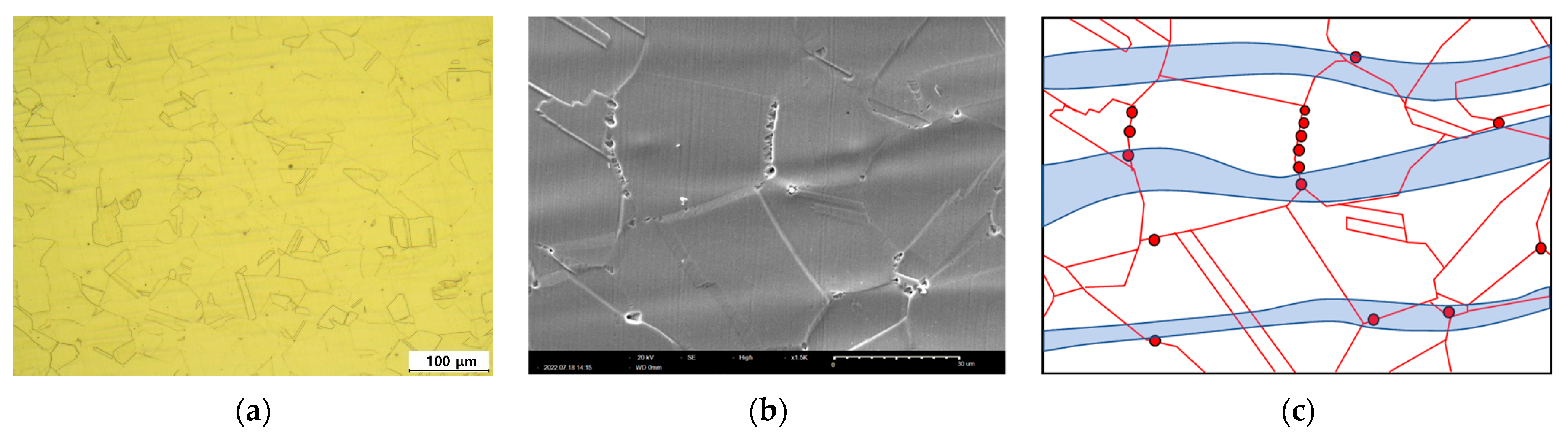
3.1. Effect of Laser Peening on the Intergranular Corrosion of 304L Stainless Steel
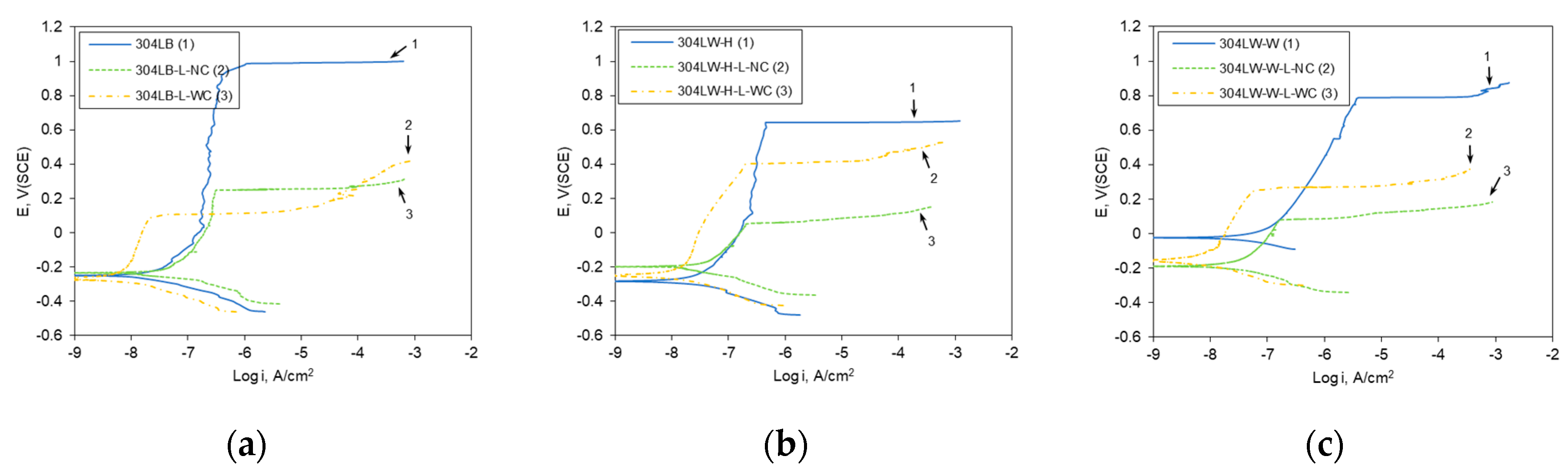
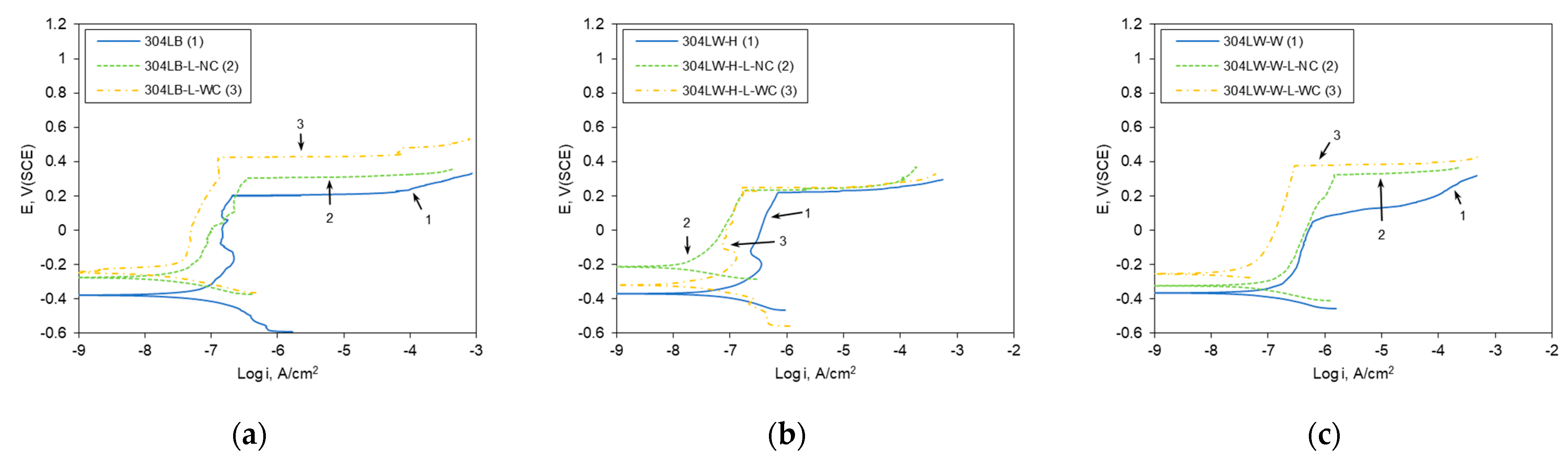
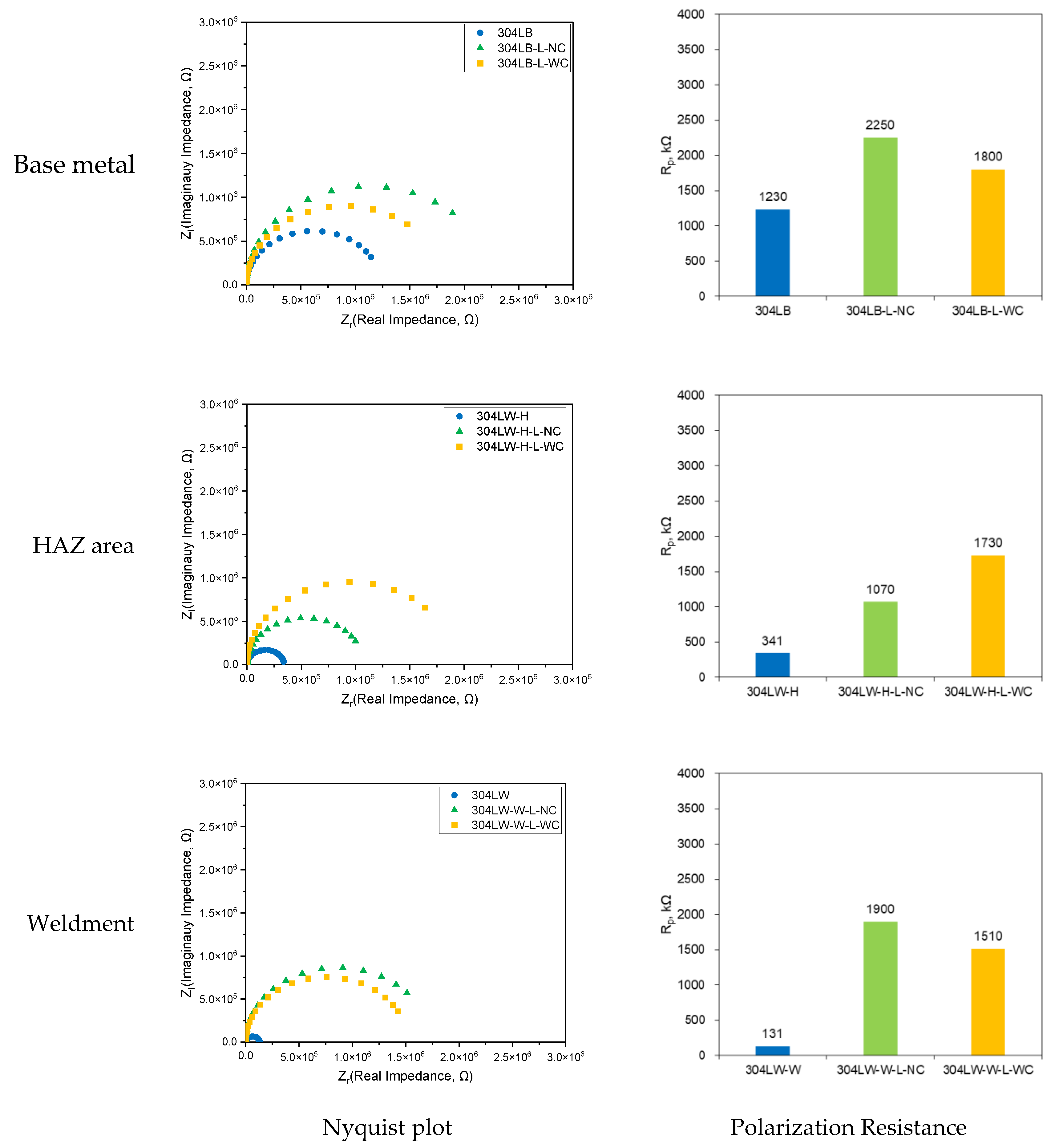
3.2. Effect of Laser Peening on the Polarization Behavior of 304L Stainless Steel
4. Conclusions
- (1)
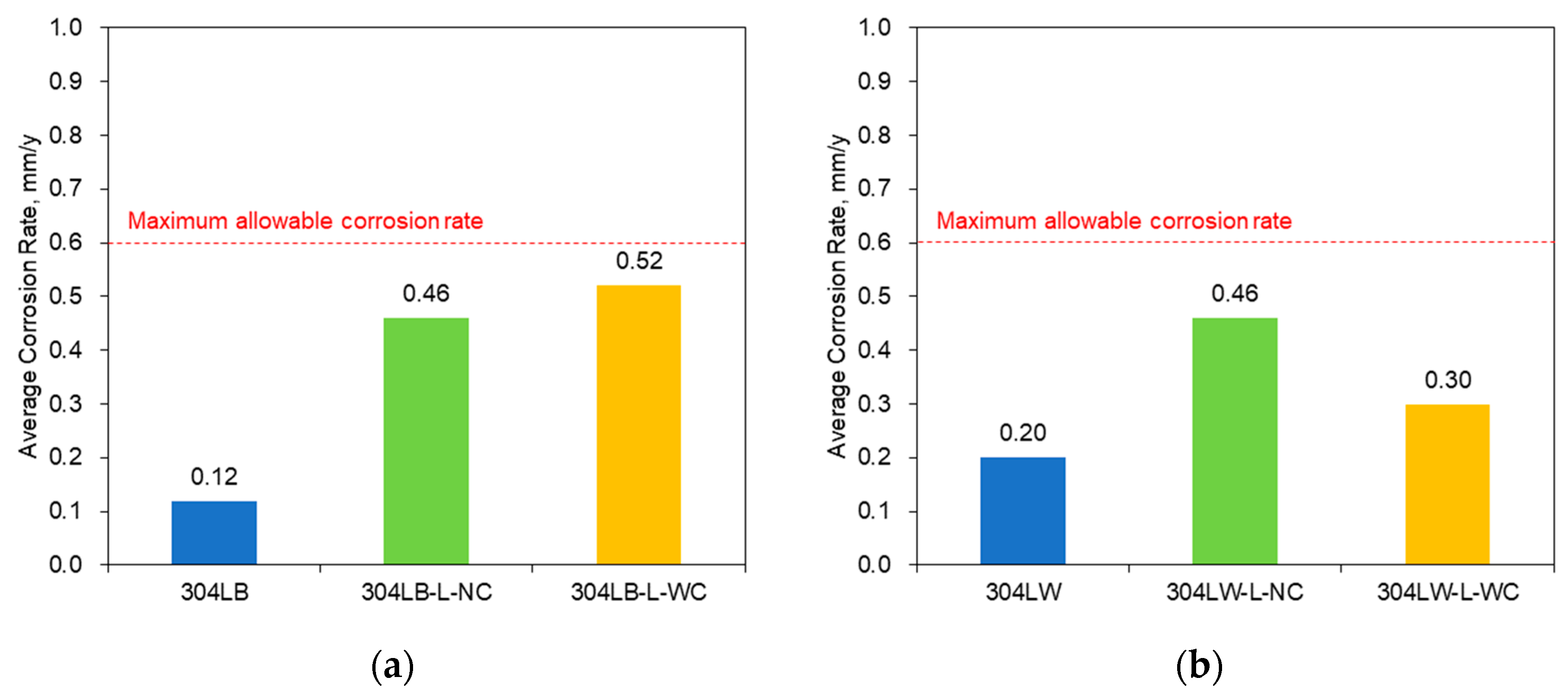
- The intergranular corrosion rate of the laser-peened 304L stainless steel was a little faster than the rate of the non-peened specimen. The increased area of grain boundaries by laser peening reduced the intergranular corrosion resistance, while an Al coating layer did not influence resistance.
- (2)
- Laser peening of 304L stainless steel enhanced the polarization properties of the cross-section. This behavior was related to the grain refinement and compressive residual stress induced by laser peening treatment, irrespective of Al coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larry, J. Welding and Metal Fabrication, 3rd ed.; Delmar Cengage Learning: New York, NY, USA, 2012; pp. 1–15. [Google Scholar]
- Farnsworth, S.R. Welding for Dummies, 3rd ed.; Wiley Publishing: New York, NY, USA, 2010; pp. 7–53. [Google Scholar]
- Kim, J.H.; Lee, W.R.; Kim, T.G.; Cheong, S.K. Micro-shockwave measurement and evaluation of laser shock peening. Trans. Korean Soc. Mech. Eng. B 2011, 35, 1041–1046. [Google Scholar] [CrossRef]
- Amini, S.; Kariman, S.A.; Teimouri, R. The effects of ultrasonic peening on chemical corrosion behavior of aluminum 7075. Int. J. Adv. Manuf. Technol. 2017, 91, 1091–1102. [Google Scholar] [CrossRef]
- Ma, C.; Andani, M.T.; Qin, H.; Moghaddam, N.S.; Ibrahim, H.; Jahadakbar, A.; Amerinatanzi, A.; Ren, Z.; Zhang, H.; Doll, G.L.; et al. Improving surface finish and wear resistance of additive manufactured nickel-titanium by ultrasonic nano-crystal surface modification. J. Mater. Process. Technol. 2017, 249, 433–440. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chang, C.S.; Lee, J.T. Effects of shot peening on corrosion and stress corrosion cracking behaviors of sensitized alloy 600 in thiosulfate solution. Corros. Sci. 1994, 50, 98–105. [Google Scholar] [CrossRef]
- Okimura, K.; Konno, T.; Narita, M.; Ohta, T.; Toyoda, M. Reliability of water jet peening as residual stress improvement method for alloy 600 PWSCC mitigation. In Proceedings of the International Conference on Nuclear Engineering, Olando, FL, USA, 11–15 May 2008. [Google Scholar] [CrossRef]
- Gujba, A.K.; Medraj, M. Laser Peening Process and Its Impact on Materials Properties in Comparison with Shot Peening and Ultrasonic Impact Peening. Materials 2014, 7, 7925–7974. [Google Scholar] [CrossRef] [PubMed]
- Shiganov, I.N.; Misurov, A.I.; Melnikov, D.M. Laser shock peening of welded joints. J. Phys. Conf. Ser. 2018, 1109, 012018. [Google Scholar] [CrossRef]
- Sadeh, S.; Malik, A. Investigation into the effects of laser shock peening as a post treatment to laser impact welding. Mater. Des. 2021, 205, 109701. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Huang, S.; Sheng, J.; Lu, J.Z.; Wang, C.D.; Chen, K.M.; Ruan, H.Y.; Chen, H.S. Effect of repeated impacts on mechanical properties and fatigue fracture morphologies of 6061-T6 aluminum subject to laser peening. Mater. Sci. Eng. A 2012, 539, 360–368. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, J.Z.; Sheng, J.; Lu, J.Z.; Sun, G.F.; Meng, X.K.; Zuo, L.D.; Ruan, H.Y.; Chen, H.S. Effects of laser energy on fatigue crack growth properties of 6061-T6 aluminum alloy subjected to multiple laser peening. Eng. Fract. Mech. 2013, 99, 87–100. [Google Scholar] [CrossRef]
- Yella, P.; Venkateswarlu, P.; Buddu, R.K.; Vidyasagar, D.V.; Rao, K.B.S.; Kiran, P.P.; Rajulapati, K.V. Laser shock peening studies on SS316LN plate with various sacrificial layers. Appl. Surf. Sci. 2018, 435, 271–280. [Google Scholar] [CrossRef]
- Go´rnikowska, M.R.; Kusiński, J.; Cieniek, Ł. Effect of laser shock peening on the microstructure and properties of the inconel 625 surface layer. J. Mater. Eng. Perform. 2020, 29, 1544–1549. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Luo, K. Residual stress distribution and microstructure at a laser spot of AISI 304 stainless steel subjected to different laser shock peening impacts. Metals 2016, 6, 6. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Kalainathan, S. Compound technology of manufacturing and multiple laser peening on microstructure and fatigue life of dual-phase spring steel. Mater. Sci. Eng. A 2016, 674, 634–645. [Google Scholar] [CrossRef]
- Kumagai, M.; Curd, M.E.; Soyama, H.; Ungár, T.; Ribárik, G.; Withers, P.J. Depth-profiling of residual stress and microstructure for austenitic stainless steel surface treated by cavitation, shot and laser peening. Mater. Sci. Eng. A 2021, 813, 141037. [Google Scholar] [CrossRef]
- Yoo, Y.R.; Kim, J.S.; Kim, Y.S. Effect of laser peening on microstructural changes in GTA-welded 304L stainless steel. Materials 2022, 15, 3947. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, D.; Li, C.; Zhang, X.; Yang, Z.; Lei, M. Effect of shot peening and plasma electrolytic oxidation on the intergranular corrosion behavior of 7A85 aluminum alloy. Acta Metall. Sin. 2014, 27, 705–713. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, J.K.; Chattopadhyay, K.; Srinivas, N.C.S.; Singh, V. Influence of ultrasonic shot peening on corrosion behavior of 7075aluminum alloy. J. Alloy. Compd. 2017, 723, 826–840. [Google Scholar] [CrossRef]
- Lu, J.Z.; Qi, H.; Luo, K.Y.; Luo, M.; Cheng, X.N. Corrosion behaviour of AISI 304 stainless steel subjected to massive laser shock peening impacts with different pulse energies. Corros. Sci. 2014, 80, 53–59. [Google Scholar] [CrossRef]
- Wang, T.; Yu, J.; Dong, B. Surface nano crystallization induced by shot peening and its effect on corrosion resistance of 1Cr18Ni9Ti stainless steel. Surf. Coat. Technol. 2006, 200, 4777–4781. [Google Scholar] [CrossRef]
- Telang, A.; Gill, A.S.; Teysseyre, S.; Mannava, S.R.; Qian, D.; Vasudevan, V.K. Effects of laser shock peening on SCC behavior of Alloy 600 in tetrathionate solution. Corros. Sci. 2015, 90, 434–444. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kumar, B.S.; Sundar, R.; Sankar, P.R.; Ganesh, P.; Kaul, R.; Kain, V.; Ranganathan, K.; Bindra, K.S.; Singh, B. Enhancement of intergranular corrosion resistance of type 304 stainless steel through laser shock peening. Corros. Eng. Sci. Technol. 2017, 52, 220–225. [Google Scholar] [CrossRef]
- Karthik, D.; Swaroop, S. Effect of laser peening on electrochemical properties of titanium stabilized 321 steel. Mater. Chem. Phys. 2017, 193, 147–155. [Google Scholar] [CrossRef]
- Liu, D.; Shi, Y.; Liu, J.; Wen, L. Effect of laser shock peening on corrosion resistance of 316L stainless steel laser welded joint. Surf. Coat. Technol. 2019, 378, 124824. [Google Scholar] [CrossRef]
- Kalainathan, S.; Sathyajith, S.; Swaroop, S. Effect of laser shot peening without coating on the surface properties and corrosion behavior of 316L steel. Opt. Lasers Eng. 2012, 50, 1740–1745. [Google Scholar] [CrossRef]
- Lim, H.T.; Kim, P.K.; Jeong, H.M.; Jeong, S.H. Enhancement of abrasion and corrosion resistance of duplex stainless steel by laser shock peening. J. Mater. Process. Technol. 2012, 212, 1347–1354. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Kulkarni, A.; Vasanth, G.; Kalainathan, S.; Shukla, P.; Vasudevan, V.K. Laser shock peening without coating induced residual stress distribution, wettability characteristics and enhanced pitting corrosion resistance of austenitic stainless steel. Appl. Surf. Sci. 2018, 428, 17–30. [Google Scholar] [CrossRef]
- Liu, P.; Sun, S.; Hu, J. Effect of laser shock peening on the microstructure and corrosion resistance in the surface of weld nugget zone and heat-affected zone of FSW joints of 7050 Al alloy. Opt. Laser Technol. 2019, 112, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Tong, Z.; Zhou, W.; Yang, Y.; Jiao, J.; Ren, X. Improving electrochemical corrosion properties of AZ31 magnesium alloy via phosphate conversion with laser shock peening pretreatment. J. Alloy. Compd. 2020, 846, 155837. [Google Scholar] [CrossRef]
- Martin, U.; Ress, J.; Bosch, J.; Bastidas, D.M. Evaluation of the DOS by DL−EPR of UNSM processed inconel 718. Metals 2020, 10, 204. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, Y.S. The Effect of the static load in the UNSM process on the corrosion properties of alloy 600. Materials 2019, 12, 3165. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Kim, Y.S. Effect of the amplitude in ultrasonic nano-crystalline surface modification on the corrosion properties of alloy 600. Corros. Sci. Technol. 2019, 18, 196–205. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S. Intergranular corrosion of 316L stainless steel by aging and UNSM (Ultrasonic Nano-crystal Surface Modification) treatment. Corros. Sci. Technol. 2015, 14, 313–324. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, K.T.; Pyoun, Y.S.; Kim, Y.S. Intergranular corrosion mechanism of slightly-sensitized and UNSM-treated 316L stainless steel. Corros. Sci. Technol. 2016, 15, 226–236. [Google Scholar] [CrossRef]
- Kim, K.T.; Lee, J.H.; Kim, Y.S. Effect of ultrasonic nano-crystal surface modification (UNSM) on the passivation behavior of aged 316L stainless steel. Materials 2017, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Amanov, A. Effect of local treatment temperature of ultrasonic nanocrystalline surface modification on tribological behavior and corrosion resistance of stainless steel 316L produced by selective laser melting. Surf. Coat. Technol. 2020, 398, 126080. [Google Scholar] [CrossRef]
- ASTM. A262-2002; Standard Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM. G108-2004; Standard Test Method for Electrochemical Reactivation (EPR) for Detecting Sensitization of AISI Type 304 and 304L Stainless Steels. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM. G5-2004; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM G3-2004; Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM E1382; Standard Test Method for Determining Average Grain Size Using Semiautomatic and Automatic Image Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- ISO 12732-2006; Electrochemical Potentiokinetic Reactivation Measurement Using the Double Loop Method. ISO: Geneva, Switzerland, 2006.

| C | Cr | Ni | Mn | Si | Cu | Mo | Co | P | N | S | Cb + Ta | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 304L | 0.02 | 18.6 | 9.6 | 1.65 | 0.47 | - | - | 0.03 | 0.022 | 0.07 | 0.03 | - | Bal. |
| ER308L | 0.015 | 19.81 | 9.84 | 1.691 | 0.351 | 0.115 | 0.046 | 0.030 | 0.024 | 0.041 | 0.03 | 0.008 | Bal. |
| Welding Process | Current (A) | Voltage (V) | Speed (cm/min) | Shield Gas (%) | Groove Angle (°) | Welding Electrode |
|---|---|---|---|---|---|---|
| GTAW | 245~250 | 14~15 | 9~10 | Ar. 99.9 | 15 | ER308L (Dia. 0.9 mm wire) |
| Alloy | Non-Peened | Laser Peening | ||
|---|---|---|---|---|
| Non-Coated | With Coating | |||
| 304L | Base metal | 304LB | 304LB-L-NC | 304LB-L-WC |
| HAZ area | 304LW-H | 304LW-H-L-NC | 304LW-H-L-WC | |
| Weldment | 304LW-W | 304LW-W-L-NC | 304LW-W-L-WC | |
| Laser Type | Laser Energy (J) | Laser Spot Diameter (mm) | Laser Overlay (%) | Transparent Overlay | Laser Incident Beam Angle (°) | Coating |
|---|---|---|---|---|---|---|
| Nd-YAG (1064 nm, IR) | 4.4 | 3 | 50 | Water (1~2 mm) | 18 | Al tape |
| 304LB | 304LB-L-NC | 304LB-L-WC | 304LW-W | 304LW-W-L-NC | 304LW-W-L-WC | |
|---|---|---|---|---|---|---|
| DOS, Ir/Ia | 0.00003 | 0.00641 | 0.0002 | 0.00104 | 0.00614 | 0.00396 |
| IGC rate, mm/y | 0.12 | 0.46 | 0.52 | 0.2 | 0.46 | 0.3 |
| Surface Area | Non-Peened 304L | 304L-L-NC | 304L-L-WC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| iR, nA/cm2 | ER, V(SCE) | EP, V(SCE) | iR, nA/cm2 | ER, V(SCE) | EP, V(SCE) | iR, nA/cm2 | ER, V(SCE) | EP, V(SCE) | |
| Base metal | 12.28 | −0.248 | 0.985 | 26.48 | −0.232 | 0.25 | 4.03 | −0.266 | 0.107 |
| HAZ | 11.91 | −0.283 | 0.646 | 23.06 | −0.197 | 0.056 | 5.83 | −0.247 | 0.402 |
| Weldment | 24.34 | −0.020 | 0.795 | 23.99 | −0.188 | 0.086 | 3.29 | −0.155 | 0.268 |
| Cross-Section Area | Non-Peened 304L | 304L-L-NC | 304L-L-WC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| iR, nA/cm2 | ER, V(SCE) | EP, V(SCE) | iR, nA/cm2 | ER, V(SCE) | EP, V(SCE) | iR, nA/cm2 | ER, V(SCE) | EP, V(SCE) | |
| Base metal | 42.97 | −0.378 | 0.212 | 17.94 | −0.275 | 0.23 | 7.89 | −0.245 | 0.428 |
| HAZ | 58.00 | −0.367 | 0.222 | 10.33 | −0.213 | 0.235 | 24.73 | −0.317 | 0.250 |
| Weldment | 46.31 | −0.364 | 0.065 | 44.82 | −0.325 | 0.326 | 16.48 | −0.252 | 0.375 |
| Residual Stress, MPa | Non-Peened 304L | 304L-L-NC | 304L-L-WC | |||
|---|---|---|---|---|---|---|
| Surface | 1 mm-Depth | Surface | 1 mm-Depth | Surface | 1 mm-Depth | |
| Base metal | 92 | 14 | −786 | −128 | −794 | −131 |
| HAZ | 335 | 41 | −497 | −55 | −565 | −56 |
| Weldment | 11 | 119 | −532 | −118 | −629 | −229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.-R.; Choi, S.-H.; Kim, Y.-S. Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel. Materials 2023, 16, 804. https://doi.org/10.3390/ma16020804
Yoo Y-R, Choi S-H, Kim Y-S. Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel. Materials. 2023; 16(2):804. https://doi.org/10.3390/ma16020804
Chicago/Turabian StyleYoo, Young-Ran, Seung-Heon Choi, and Young-Sik Kim. 2023. "Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel" Materials 16, no. 2: 804. https://doi.org/10.3390/ma16020804
APA StyleYoo, Y.-R., Choi, S.-H., & Kim, Y.-S. (2023). Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel. Materials, 16(2), 804. https://doi.org/10.3390/ma16020804








