Carbon in Commercially Pure Titanium
Abstract
1. Introduction
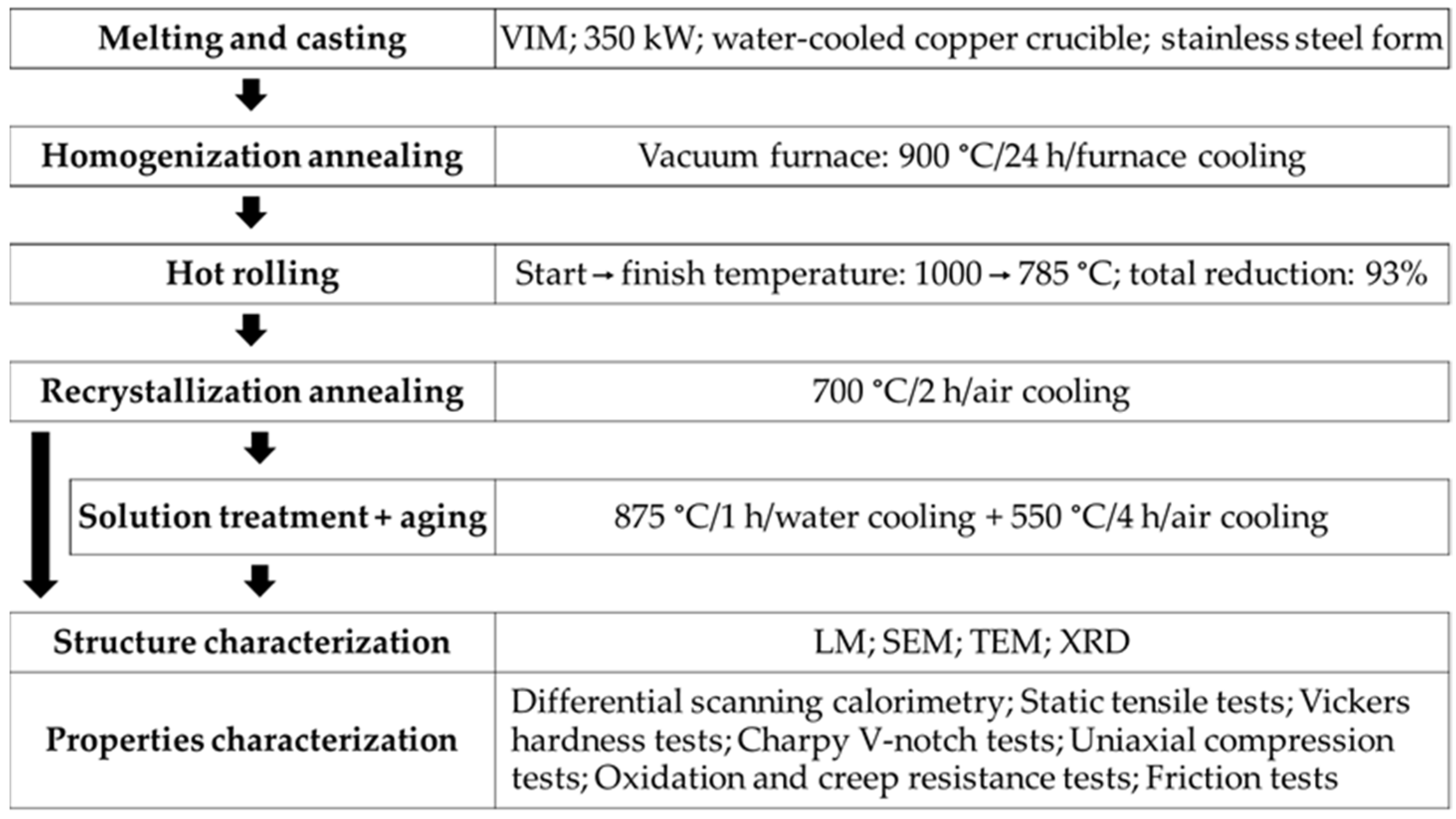
2. Materials and Methods
3. Results and Discussion
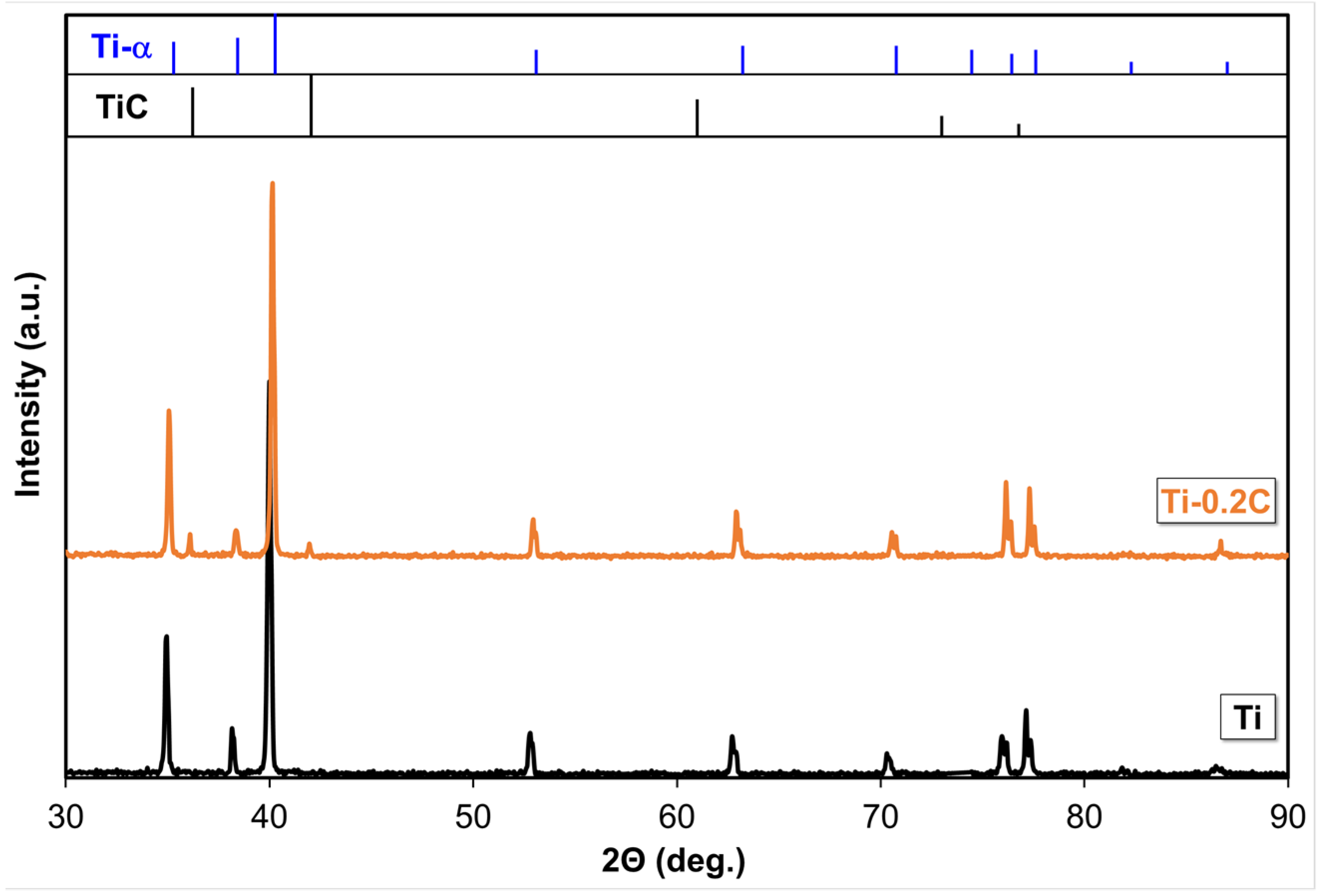
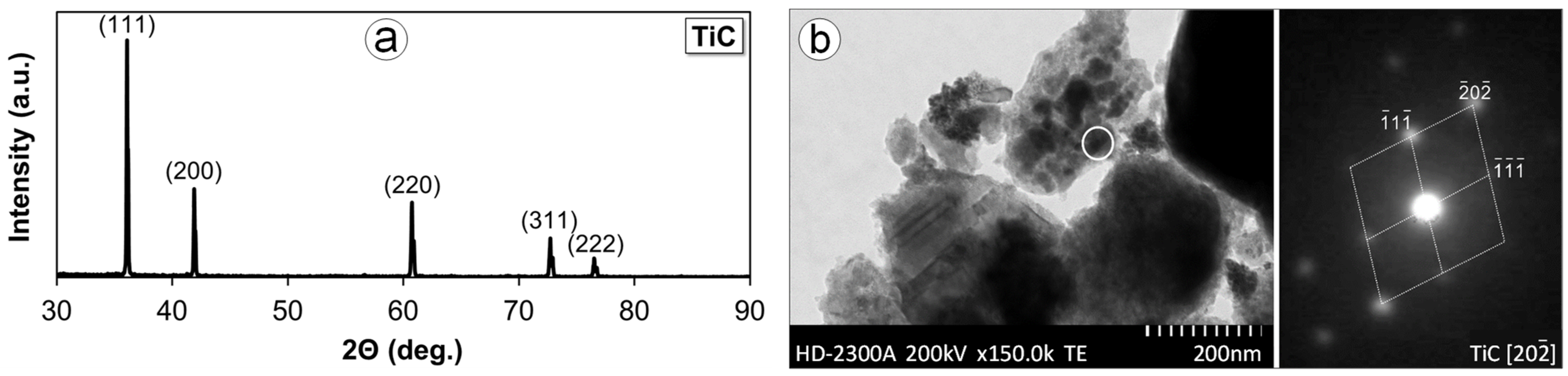
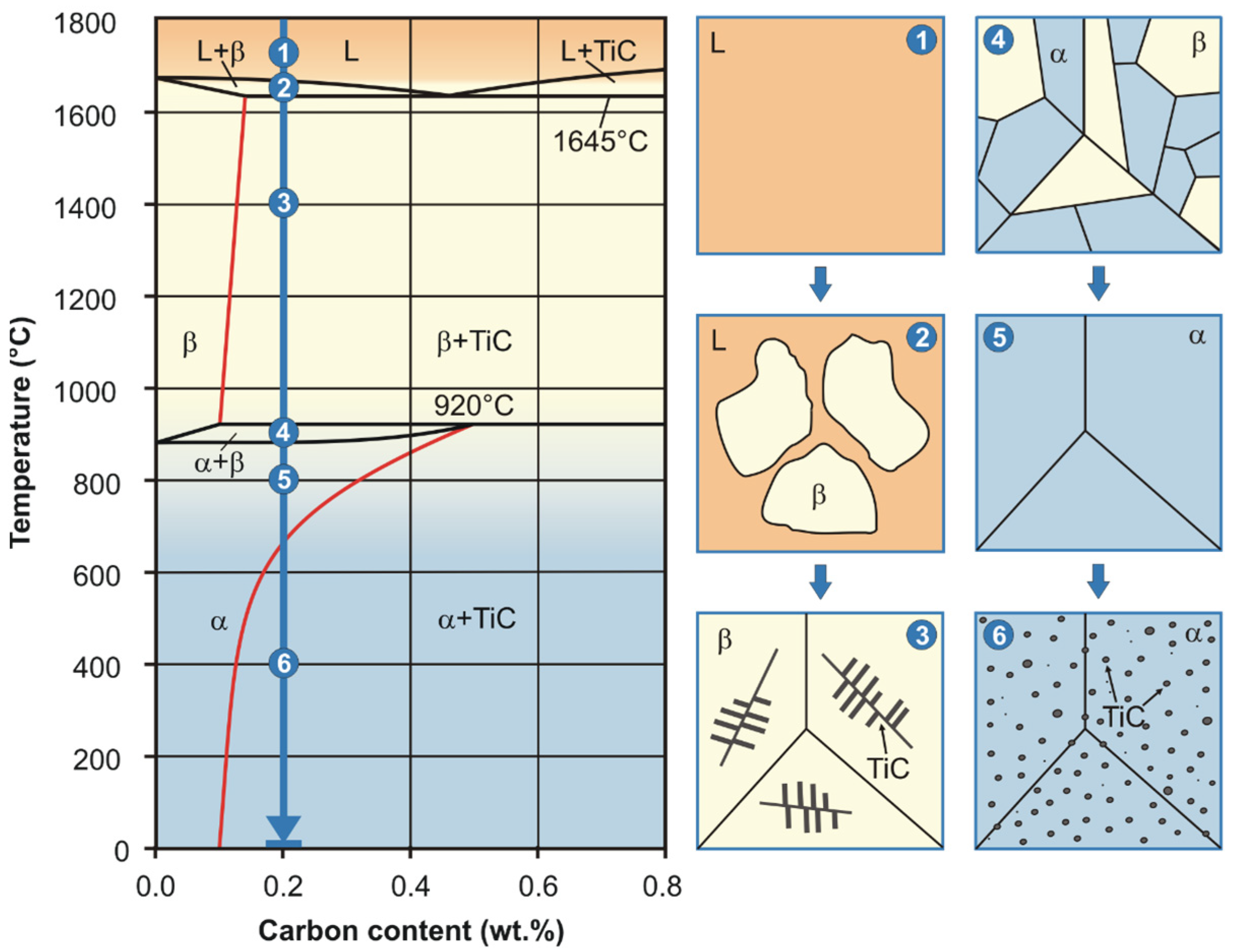
3.1. Alloys Phase Composition
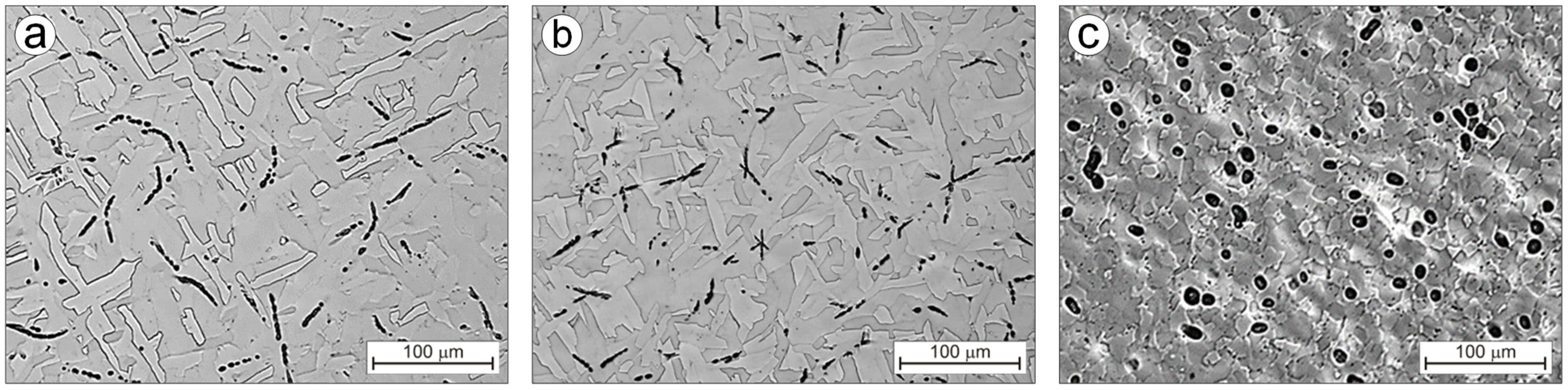
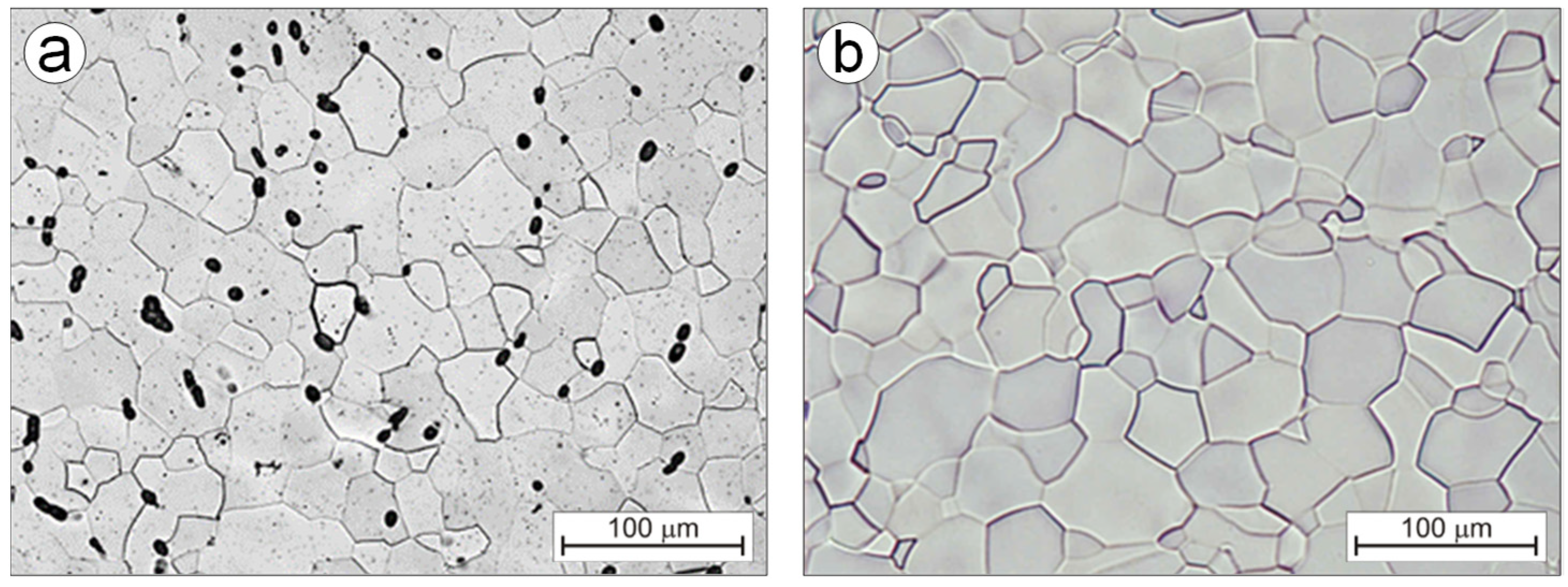
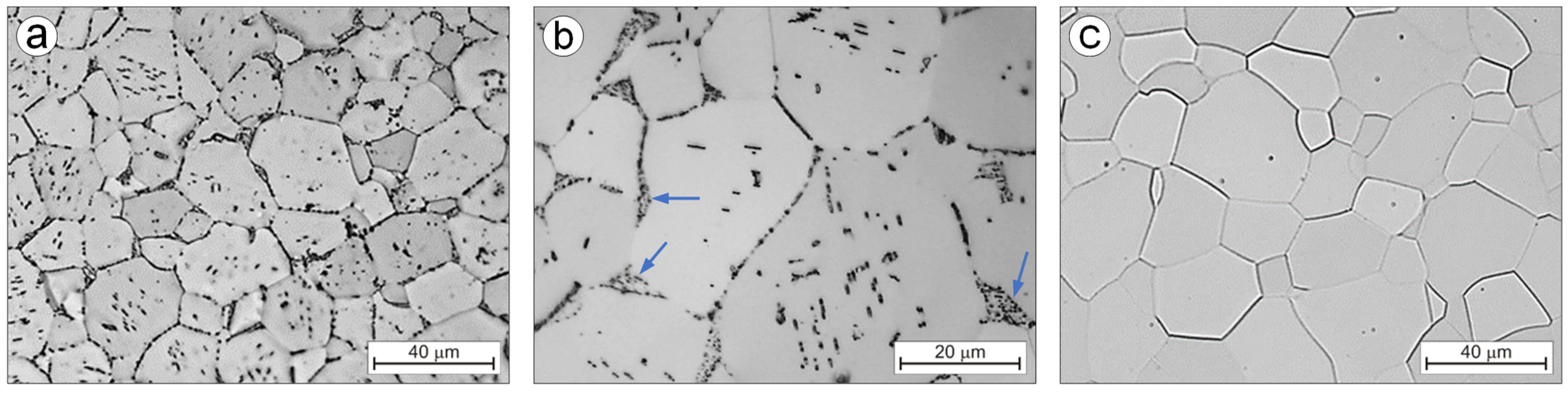
3.2. Alloys Microstructure
3.3. Alloys Properties
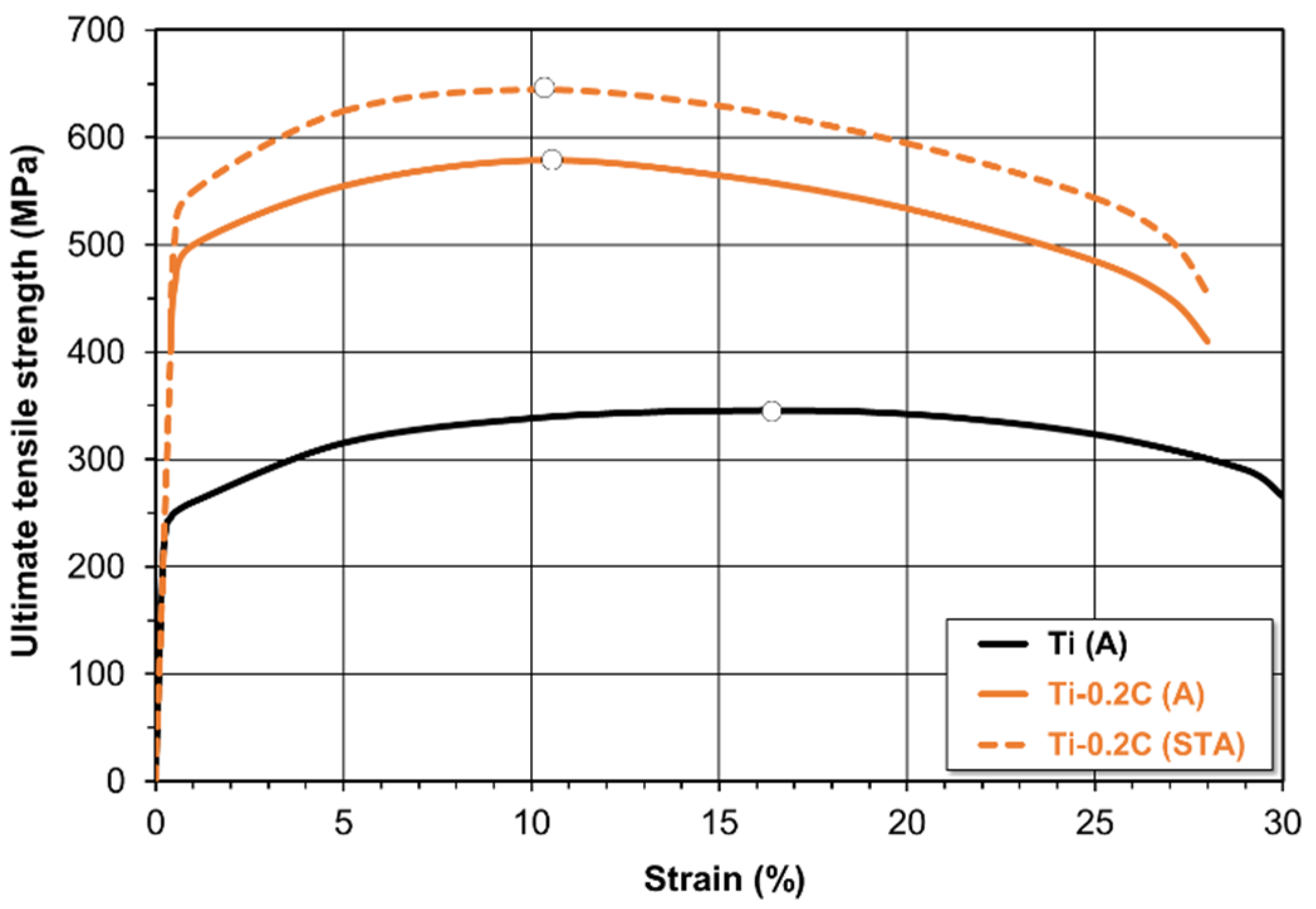
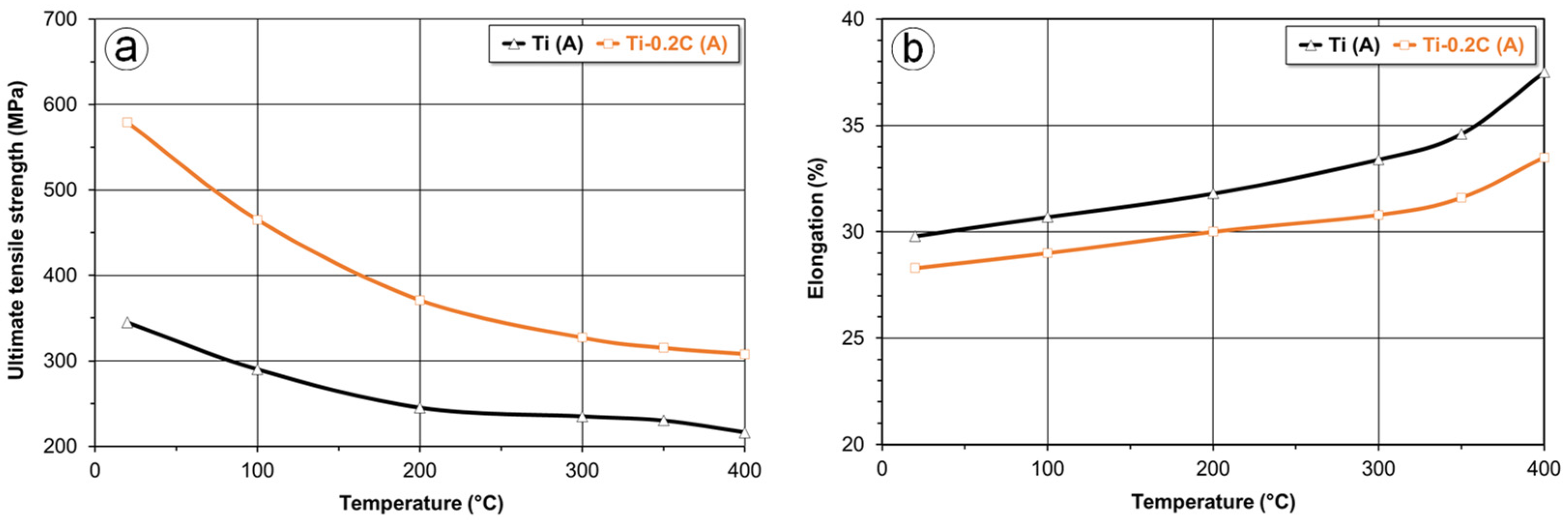
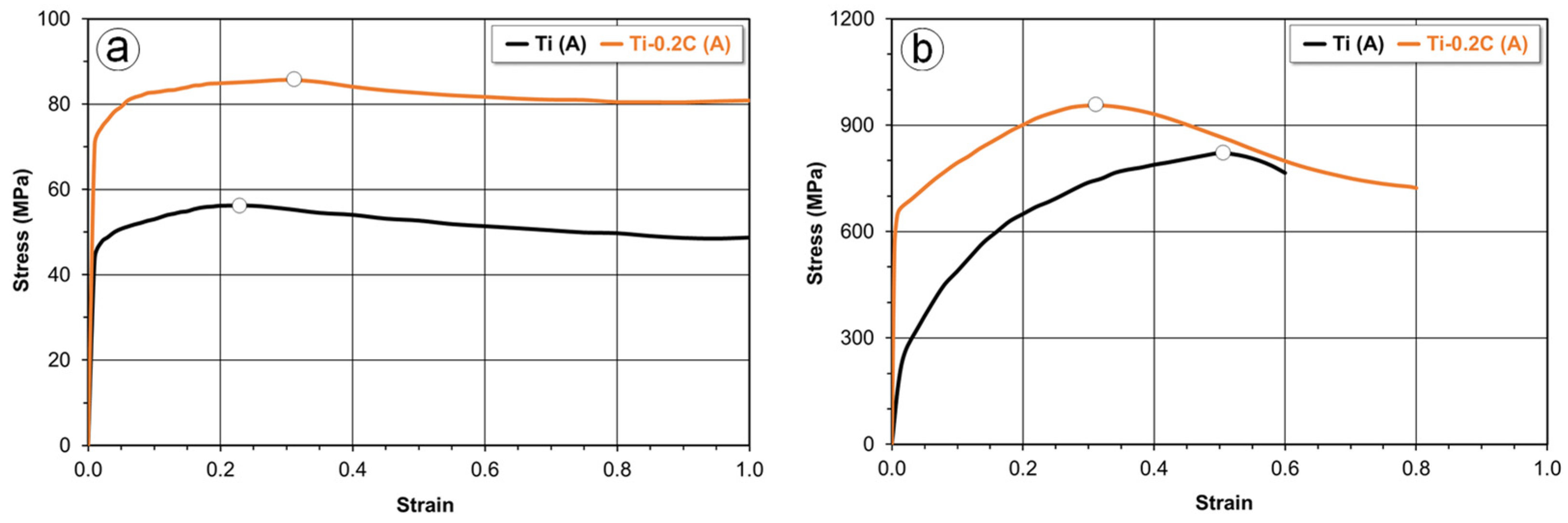
3.3.1. Mechanical Properties
3.3.2. Plastic Formability
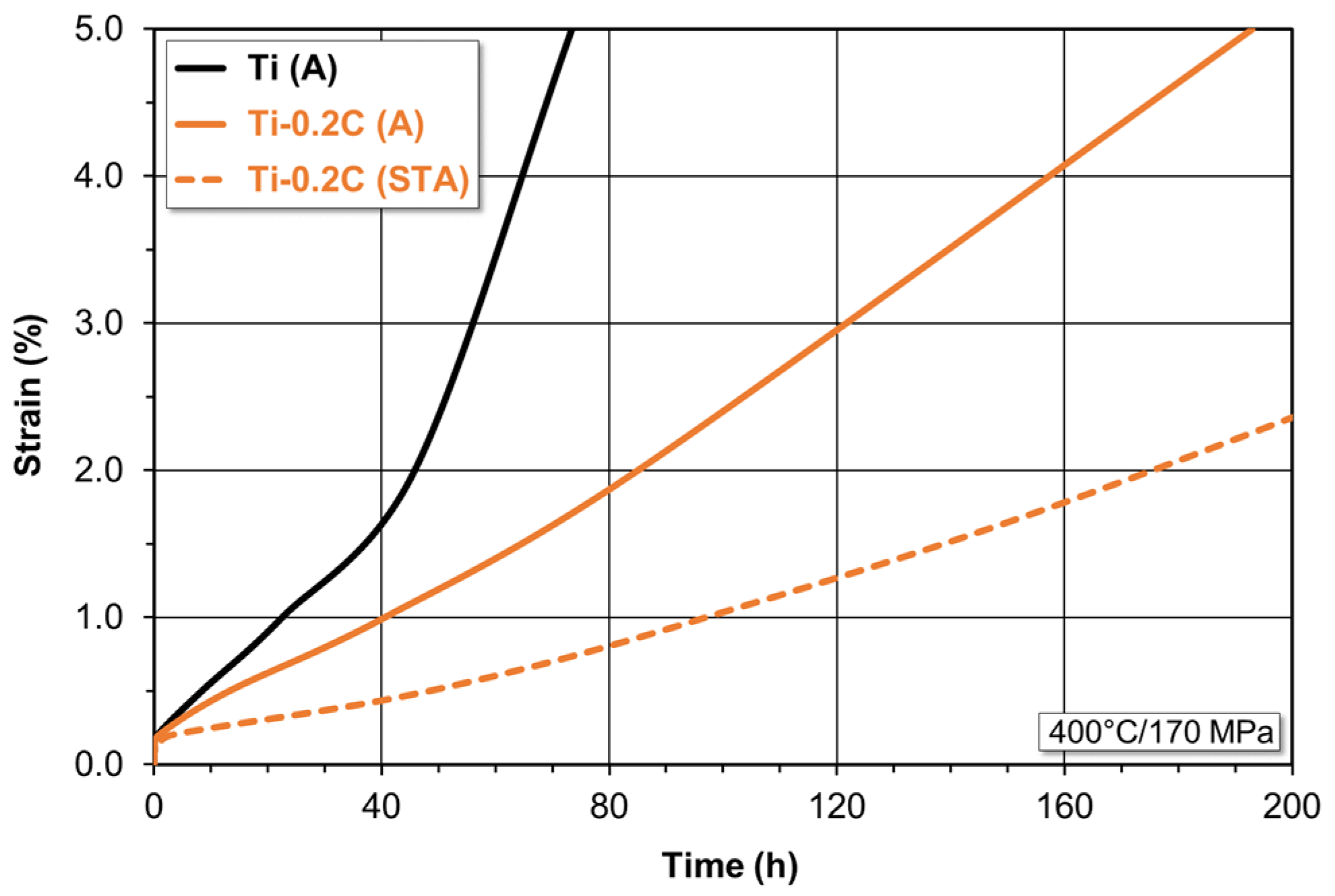
3.3.3. Creep Resistance
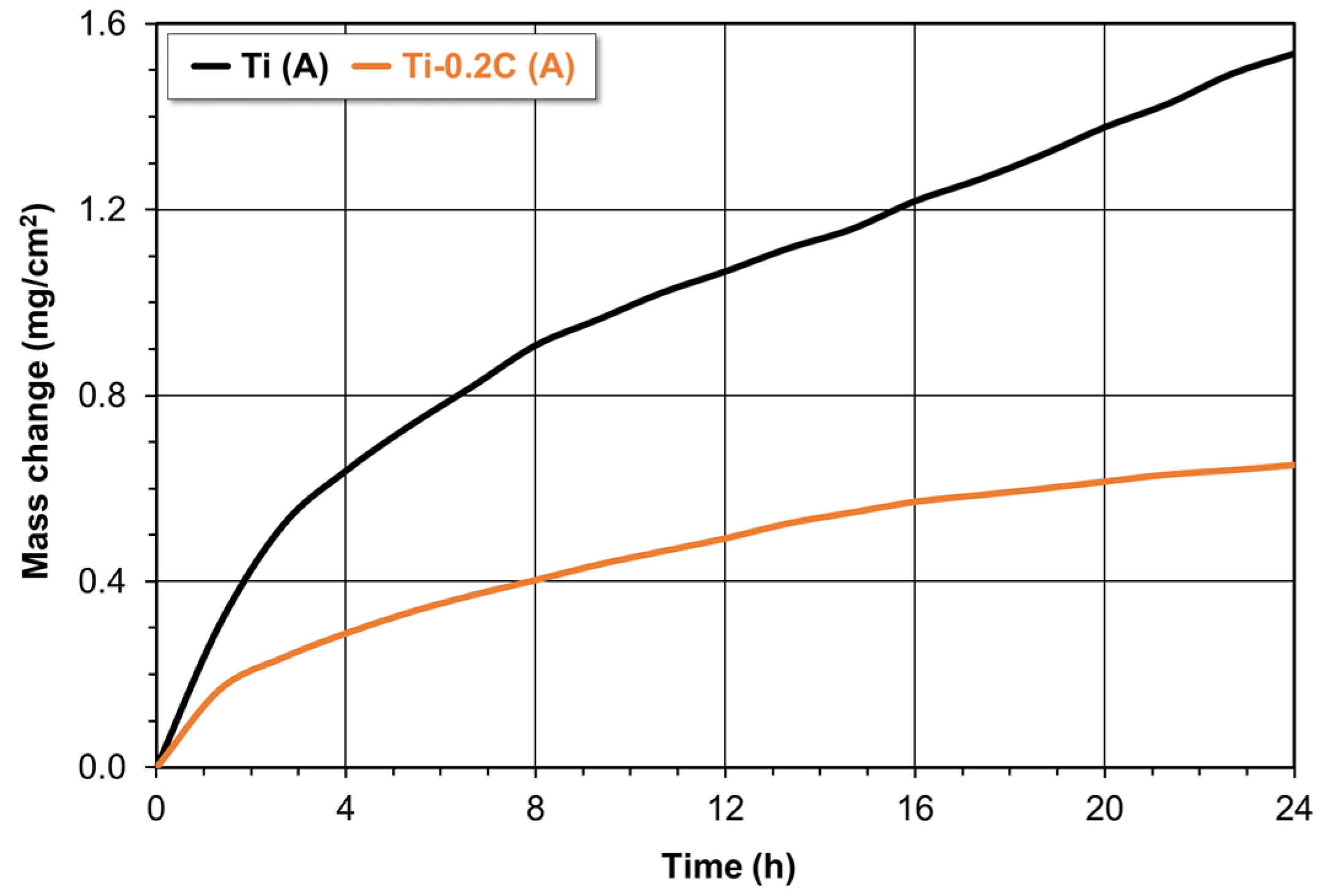
3.3.4. Oxidation Resistance
3.3.5. Abrasive Wear Resistance
4. Conclusions
- Carbon added to commercially pure titanium (CP-Ti) in the amount of 0.2 wt.% is within the maximum solubility limits in the α-Ti-based interstitial solid solution, while in the remaining part of titanium carbides, it increases approximately 0.3% of the values of lattice constants of the phases, and the α→β transformation temperature from 890 to 920 °C reduces the susceptibility to grain growth and creates the possibility for additional hardening of approximately 11.0% in the combined solution treatment and aging processes.
- The addition of 0.2 wt.% carbon to CP-Ti results in the expected improvement in its properties by an increase in the tensile strength by approximately 85%, the yield strength by approximately 125%, and the hardness by approximately 55%, a slight increase in the Young’s modulus from 103 to 105 MPa, and a significant increase in the creep resistance (a 2.5-times decrease in the steady-state creep rate), oxidation resistance (by about 80% reduction in the value of the oxidation rate constant), and abrasive wear resistance (by an approximately 28.5% reduction in the value of the coefficient of friction. At the same time, it does not cause a significant deterioration of plasticity measured during the static tensile test (by approximately 4–5%). The only negative consequences of the presence of 0.2 wt.% C in CP-Ti is a deterioration of the impact strength and hot as well as cold formability to the acceptable level.
- The results of the comparison of the phase composition, structure, and most important properties of CP-Ti and the Ti-0.2C alloy with the maximum permitted carbon content of approximately 0.2 wt.%, according to the recommendations in force, presented in the form of positive, negative, and neutral effects of carbon on the various properties of the test alloys and showed that carbon in titanium alloys, at the controlled content of up to approximately 0.2 wt.%, can perform many useful functions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gusev, A.I. Phase equilibria, phases and compounds in the Ti-C system. Russ. Chem. Rev. 2002, 71, 507–532. [Google Scholar] [CrossRef]
- Predel, B. C-Ti (Carbon-Titanium). In B-Ba … Cu—Zr. Landolt-Börnstein—Group IV Physical Chemistry; Predel, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 12B, pp. 147–149. [Google Scholar]
- Ogden, H.; Jaffee, R. The Effects of Carbon, Oxygen, and Nitrogen on the Mechanical Properties of Titanium and Titanium Alloys. Titanium Metallurgical Laboratory Report No. 20, Ohio. 1955. Available online: https://www.osti.gov/biblio/4370612 (accessed on 17 December 2022).
- Jaffee, R.; Ogden, H.; Maykuth, D. Alloys of titanium with carbon, oxygen, and nitrogen. JOM 1950, 188, 1261–1266. [Google Scholar] [CrossRef]
- Solonina, O.P.; Ulyakova, N.M. Effect of carbon on the mechanical properties and structure of titanium alloys. Mater. Sci. Technol. 1974, 16, 28–30. [Google Scholar] [CrossRef]
- Grauman, J.; Fox, S.; Nyakana, S. Titanium Alloy Having Improved Corrosion Resistance and Strength. United States Patent US20070062614A1, 22 March 2007. [Google Scholar]
- Wain, N.; Hao, X.J.; Ravi, G.A.; Wu, H. The influence of carbon on precipitation of α in Ti-5Al-5Mo-5V-3Cr. Mater. Sci. Eng. A 2010, 527, 7673–7683. [Google Scholar] [CrossRef]
- Song, X.; Chen, L.; Li, K.; Chen, G. Effect of carbon and oxygen additions on microstructure and mechanical properties of Ti-4.3Fe-7.1Cr-3Al alloy. Int. J. Mod. Phys. B 2009, 23, 814–820. [Google Scholar] [CrossRef]
- Yan, M.; Qian, M.; Kong, C.; Dargusch, M.S. Impacts of trace carbon on the microstructure of as-sintered biomedical Ti-15Mo alloy and reassessment of the maximum carbon limit. Acta Biomater. 2014, 10, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Banoth, R.; Sarkar, R.; Bhattacharjee, A.; Nandy, T.K.; Nageswara Rao, G.V.S. Effect of boron and carbon addition on microstructure and mechanical properties of metastable beta titanium alloys. Mater. Des. 2015, 67, 50–63. [Google Scholar] [CrossRef]
- Del Prado, J.; Song, X.; Hu, D.; Wu, X. The influence of oxygen and carbon-content on aging of Ti-15-3. J. Mater. Eng. Perform. 2005, 14, 728–734. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Loretto, M.H. Effect of carbon additions on the microstructure and mechanical properties of Ti-15-3. Mater. Sci. Technol. 2004, 20, 343–349. [Google Scholar] [CrossRef]
- Sun, F.S.; Lavernia, E.J. Creep behavior of nonburning Ti-35V-15Cr-xC alloys. J. Mater. Eng. Perform. 2005, 14, 784–787. [Google Scholar] [CrossRef]
- Wu, X.; del Prado, J.; Li, Q.; Huang, A.; Hu, D.; Loretto, M.H. Analytical electron microscopy of C-free and C-containing Ti-15-3. Acta Mater. 2006, 54, 5433–5448. [Google Scholar] [CrossRef]
- Saskar, R.; Ghosal, P.; Muraleedharan, H.; Nandy, T.K.; Ray, K.K. Effect of boron and carbon addition on Ti-15-3 alloy. Mater. Sci. Eng. A 2011, 528, 4819–4829. [Google Scholar] [CrossRef]
- Zhao, D.; Chang, K.; Ebel, T.; Qian, M.; Willumeit, R.; Yan, M.; Pyczak, F. Microstructure and mechanical behavior of metal injection molded Ti-Nb binary alloys as biomedical material. J. Mech. Behav. Biomed Mater. 2013, 28, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ebel, T.; Yan, M.; Qian, M. Trace carbon in biomedical beta-titanium alloys: Recent progress. JOM 2015, 67, 2236–2243. [Google Scholar] [CrossRef]
- Novovic, D.; Aspinwall, D.K.; Dewes, R.C.; Bowen, P.; Griffiths, B. The effect of surface and subsurface condition on the fatigue life of Ti-25V-15Cr-2Al-0.2C%wt alloy. CIRP Ann. Manuf. Technol. 2016, 65, 523–528. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Li, M.M.; Yang, R. Mechanism and kinetics of carbide dissolution in near alpha Ti-5.6Al-4.8Sn-2Zr-1Mo-0.35Si-0.7Nd titanium alloy. Mater. Charact. 2011, 62, 1151–1155. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Hieda, J.; Cho, K.; Akahori, T.; Hayashi, K.; Itsumi, Y.; Murakami, S.; Oyama, H. Tensile and fatigue properties of carbon-solute-strengthened (α+β)-type titanium alloy. Mater. Trans. 2013, 54, 169–175. [Google Scholar] [CrossRef]
- Chu, M.; Jones, L.P.; Wu, X. Effect of carbon on microstructure and mechanical properties of a eutectoid α titanium alloy. J. Mater. Eng. Perform. 2005, 14, 735–740. [Google Scholar] [CrossRef]
- Szkliniarz, A.; Szkliniarz, W. Effect of Carbon Content on the Microstructure and Properties of Ti-6Al-4V alloy. Arch. Metall. Mater. 2020, 65, 1197–1204. [Google Scholar]
- Wu, Z.; Hu, R.; Zhang, T.; Zhang, F.; Kou, H.; Li, J. Understanding the role of carbon atoms on microstructure and phase transformation of high Nb containing TiAl alloys. Mater. Charact. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Kamyshnykova, K.; Lapin, J.; Pelachová, T.; Cegan, T.; Jurica, J.; Volodarskaja, A. Microstructure and mechanical properties of Ti–45Al–2W–xC alloys. Intermetallics 2022, 148, 107618. [Google Scholar] [CrossRef]
- Wang, S.; Fang, H.; Chen, D.; Chen, R.; Zhou, L.; Yang, X.; Su, Y. A novel method and mechanism for simultaneously refining microstructure and controlling grain orientation in Ti48Al2Cr2Nb2.5C alloy. Appl. Mater. Today 2022, 28, 10151. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Lütjering, G.; Williams, J.C. Titanium; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007. [Google Scholar]
- Froes, F.H. Titanium: Physical Metallurgy, Processing, and Applications; ASM International: Materials Park, OH, USA, 2015. [Google Scholar]
- Szkliniarz, A.; Szkliniarz, W. Effect of solution treatment on the microstructure of Ti-C alloys. Solid State Phenom. 2014, 212, 21–24. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Hu, H.Z.; Li, G.P.; Liu, Y.Y.; Yang, R. Effect of carbon and aging treatment on precipitation of ordered α2 in Ti-5.6Al-4.8Sn-2Zr-1Mo-0.35Si-0.8Nd alloy. Mater. Sci. Eng. A 2005, 408, 290–296. [Google Scholar] [CrossRef]
- Ozerov, M.S.; Klimenko, D.N.; Rtishcheva, L.P.; Kopylov, V.N.; Stepanov, N.D.; Zherebtsov, S.V. Effect of carbon on microstructure and mechanical properties of titanium. Mater. Sci. Eng. 2021, 1014, 012039. [Google Scholar] [CrossRef]
- Mousavi, E.; Aboutalebi, M.R.; Seyedein, S.H.; Abbasi, S.M. Influence of carbon on the aging behaviour of Ti-13V-11Cr-3Al. Iran. J. Mater. Sci. Eng. 2014, 11, 40–47. [Google Scholar]
- Oyama, H.; Kojima, S.; Ono, K.; Ito, Y. A new alpha-beta alloy, Ti-4.5Al-4Cr-0.5Fe-0.2C, solution hardened by ultra-high content of carbon. Mater. Sci. Forum. 2003, 426–432, 713–718. [Google Scholar] [CrossRef]
- Yu, J.; Yin, Z.; Huang, Z.; Zhao, S.; Huang, H.; Yu, K.; Zhou, R.; Xiao, H. Effect of Aging Treatment on Microstructural Evolution and Mechanical Properties of the Electron Beam Cold Hearth Melting Ti-6Al-4V Alloy. Materials 2022, 15, 7122. [Google Scholar] [CrossRef] [PubMed]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- ASTM B348/B348M-21; Standard Specification for Titanium and Titanium Alloy Bars and Billets. ASTM International: West Conshohocken, PA, USA, 2021.
- Venkatesh, V.; Pilchak, A.L.; Allison, J.E.; Ankem, S.; Boyer, R.; Christodoulou, J.; Fraser, H.L.; Imam, M.A.; Kosaka, Y.; Rack, H.J.; et al. (Eds.) Proceedings of the 13th World Conference on Titanium: 2015—San Diego (USA); John Wiley & Sons: San Francisco, CA, USA, 2016. [Google Scholar]
- Boyer, R.R. Attributes, characteristics, and applications of titanium and its alloys. JOM 2010, 62, 21–24. [Google Scholar] [CrossRef]
- Kitashima, T.; Suresh, K.S.; Yamabe-Mitarai, Y. Effect of germanium and silicon additions on the mechanical properties of a near-α titanium alloy. Mater. Sci. Eng. A 2014, 597, 212–218. [Google Scholar] [CrossRef]
- Mishra, H.; Satyanarayana, D.V.V.; Nandy, T.K.; Sagar, P.K. Effect of trace impurities on the creep behaviour of a near α titanium alloy. Scri. Mater. 2008, 59, 591–594. [Google Scholar] [CrossRef]
- Collins, E.W.; Gegel, H.L.; Ho, J.C. Solid Solution Strengthening and Fundamental Design of Titanium Alloys. Air Force Materials Laboratory Technical Report: TR-72-171, Ohio. 1972. Available online: https://apps.dtic.mil/sti/pdfs/AD0754240.pdf (accessed on 17 December 2022).
- Wang, Z.; Huang, Y.; Yang, Y.; Wang, J.; Liu, C.T. Atomic-size effect and solid solubility of multicomponent alloys. Scri. Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- Wierzchoń, T.; Morgiel, J. New estimate of phase sequence in diffusive layer formed on plasma nitrided Ti-6Al-4V alloy. Surf. Coat. Technol. 2014, 259, 473–483. [Google Scholar]
- Niinomi, M.; Gakkai, N.K. (Eds.) Ti-2007 Science and Technology. In Proceedings of the 11th World Conference on Titanium, Kyoto, Japan, 3–7 June 2007; Japan Institute of Metals: Sendai, Japan, 2007. [Google Scholar]
- Zhou, L. (Ed.) Ti-2011. In Proceedings of the 12th World Conference on Titanium, Beijing, China, 19–24 June 2011; Science Press: Beijing, China, 2012. [Google Scholar]
- Borgioli, F.; Galvanetto, E.; Iozzelli, F.; Pradelli, G. Improvement of wear resistance of Ti-6Al-4V alloy by means of thermal oxidation. Mater. Lett. 2005, 59, 2159–2162. [Google Scholar] [CrossRef]
- Obadele, B.A.; Andrews, A.; Mathew, M.T.; Olubambi, P.A.; Pityana, S. Improving the tribocorrosion resistance of Ti6Al4V surface by laser surface cladding with TiNiZrO2 composite coating. Appl. Surf. Sci. 2015, 345, 99–108. [Google Scholar] [CrossRef]
- Hu, C.-J.; Chiu, P.-H. Wear and corrosion resistance of pure titanium subjected to aluminization and coated with a microarc oxidation ceramic coating. Int. J. Electrochem. Sci. 2015, 10, 4290–4302. [Google Scholar]


| Alloy | Elements Content (wt.%) | ||||
|---|---|---|---|---|---|
| C | O | N | H | Ti | |
| CP-Ti | 0.03 | 0.16 | 0.03 | 0.014 | Balance |
| Ti-0.2C | 0.26 | 0.17 | 0.03 | 0.015 | |
| Parameter | Alloy | |
|---|---|---|
| CP-Ti | Ti-0.2C | |
| aα (nm) | 0.2945 | 0.2952 |
| cα (nm) | 0.4672 | 0.4686 |
| cα/aα | 1.5864 | 1.5874 |
| aTiC (nm) | - | 0.4308 |
| Tα→β (°C) | 890 | 920 |
| Alloy | Condition | Hardness HV | Stereological Parameters of Carbides | ||
|---|---|---|---|---|---|
| AA (%) | A (μm2) | ξ | |||
| Ti-0.2C | Casting | 204 ± 6 | 1.51 | 10.45 | 0.62 |
| Homogenization | 230 ± 4 | 1.20 | 8.98 | 0.68 | |
| Hot rolling | 191 ± 7 | 1.36 | 16.21 | 0.93 | |
| Recrystallization annealing | 205 ± 4 | 1.16 | 16.46 | 0.94 | |
| Solution treatment | 212 ± 4 | 0.42 | 5.65 | 0.92 | |
| Solution treatment and aging | 244 ± 6 | 1.08 | 4.25 | 0.90 | |
| Alloy | Condition | UTS (MPa) | YS (MPa) | EL (%) | RA (%) | E (GPa) | HV | CVN (J) |
|---|---|---|---|---|---|---|---|---|
| CP-Ti | A | 345 | 245 | 29.8 | 51.5 | 103 | 157 | 84.5 |
| STA | 355 | 252 | 29.2 | 50.3 | 103 | 158 | 86.0 | |
| Ti-0.2C | A | 579 | 496 | 28.6 | 48.6 | 104 | 205 | 67.0 |
| STA | 643 | 554 | 28.3 | 46.6 | 105 | 244 | 54.5 | |
| Grade 4 | A | 550 | 485 | 15.0 | 30.0 | 104 | 250 | 20.0 |
| Alloy | CP-Ti | Ti-0.2C |
|---|---|---|
| Coefficient of friction change | 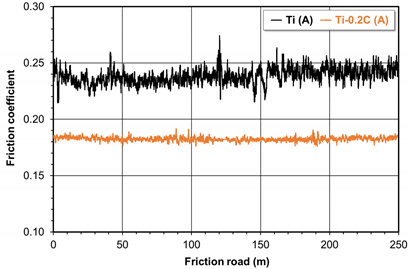 | |
| Coefficient of friction | 0.252 ± 0.006 | 0.180 ± 0.002 |
| Mass loss (g) | 0.020 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkliniarz, A.; Szkliniarz, W. Carbon in Commercially Pure Titanium. Materials 2023, 16, 711. https://doi.org/10.3390/ma16020711
Szkliniarz A, Szkliniarz W. Carbon in Commercially Pure Titanium. Materials. 2023; 16(2):711. https://doi.org/10.3390/ma16020711
Chicago/Turabian StyleSzkliniarz, Agnieszka, and Wojciech Szkliniarz. 2023. "Carbon in Commercially Pure Titanium" Materials 16, no. 2: 711. https://doi.org/10.3390/ma16020711
APA StyleSzkliniarz, A., & Szkliniarz, W. (2023). Carbon in Commercially Pure Titanium. Materials, 16(2), 711. https://doi.org/10.3390/ma16020711








