Evaluating the Impact of Concrete Design on the Effectiveness of the Electrochemical Chloride Extraction Process
Abstract
1. Introduction
2. Materials
3. Test Methods
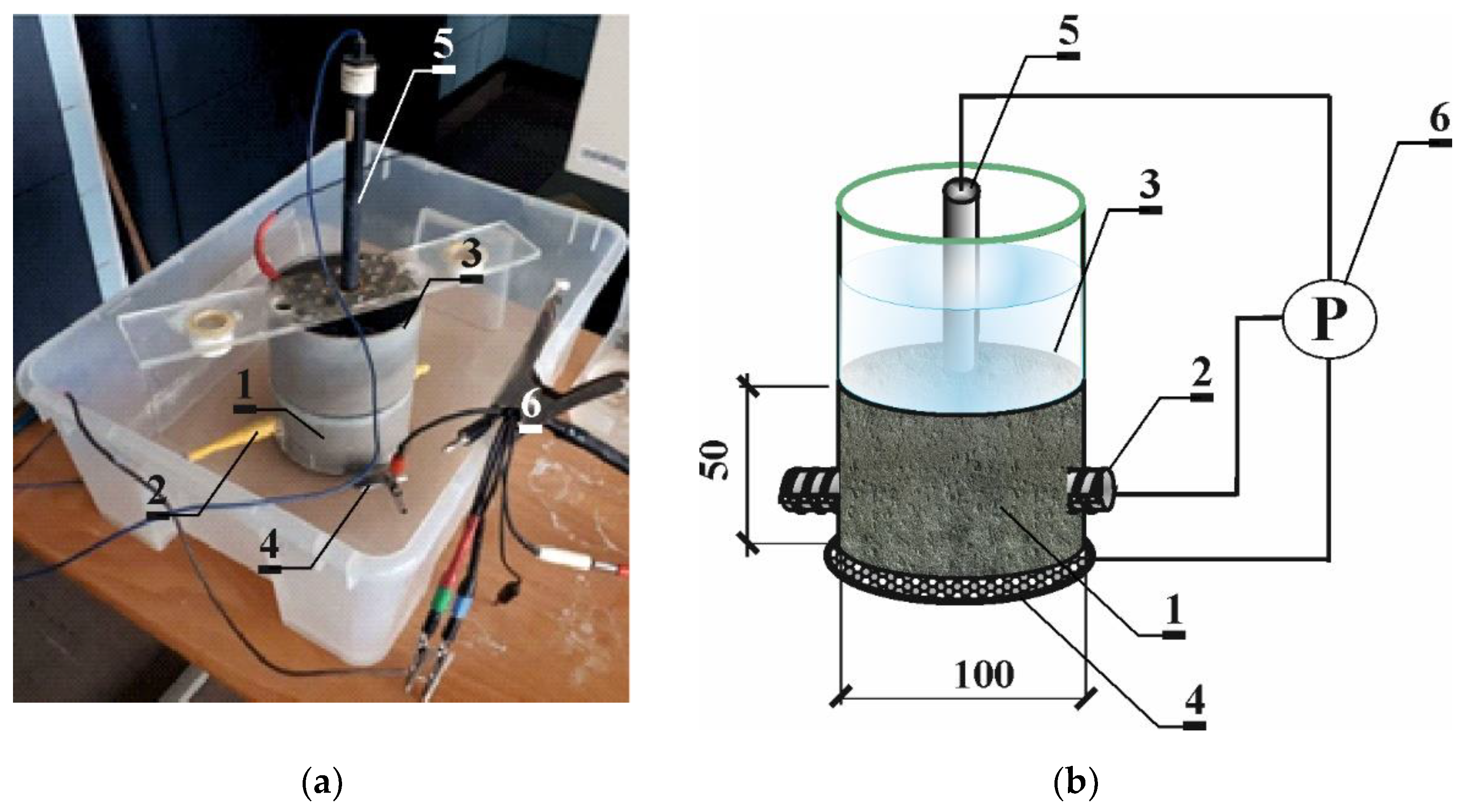
3.1. Migration of Chloride Ions Accelerated with the LPR Method with Simultaneous Control over Corrosion
3.2. Material Tests—Determination of Distribution of Chloride and Hydroxide Ion Concentration at the Depth of Concrete Cover
3.3. Electrochemical Chloride Extraction (ECE) with Simultaneous Control over Corrosion Processes with the Electrochemical Method
3.4. Determination of Coefficients of Migration and Extraction in Concrete
4. Discussion of the Results Obtained
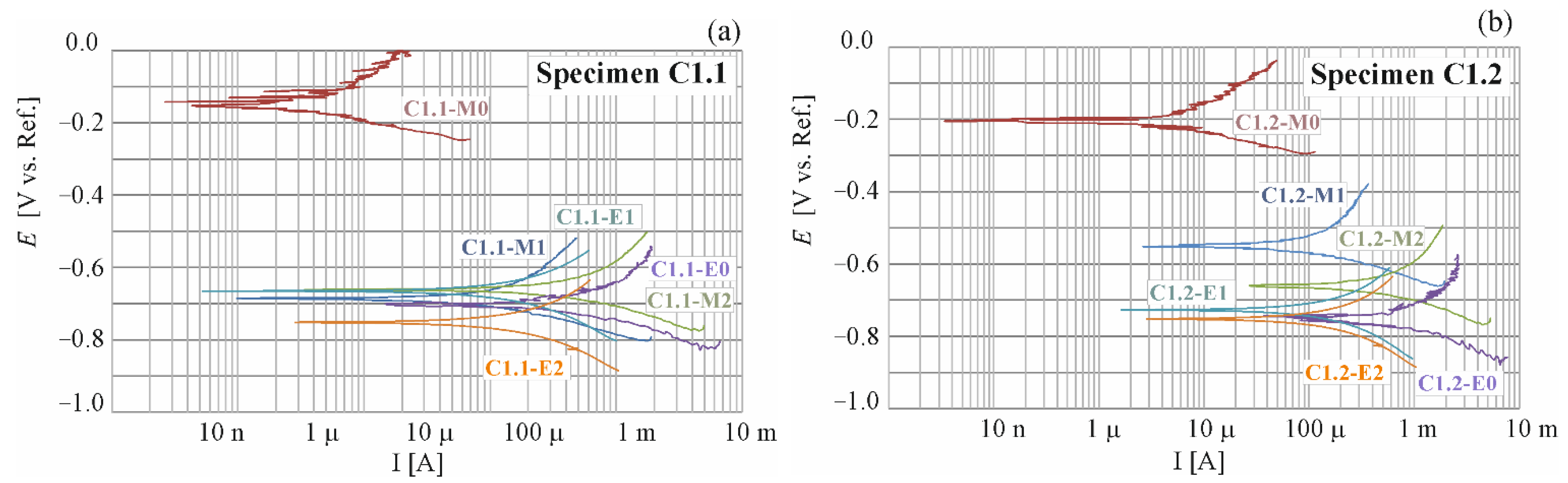
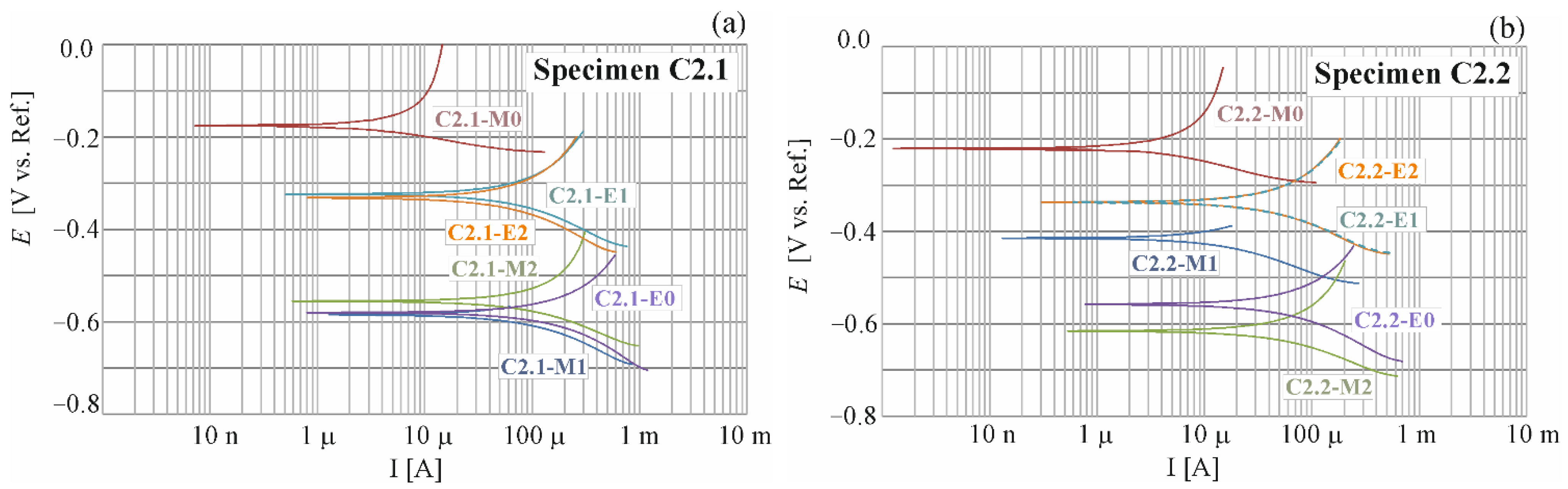
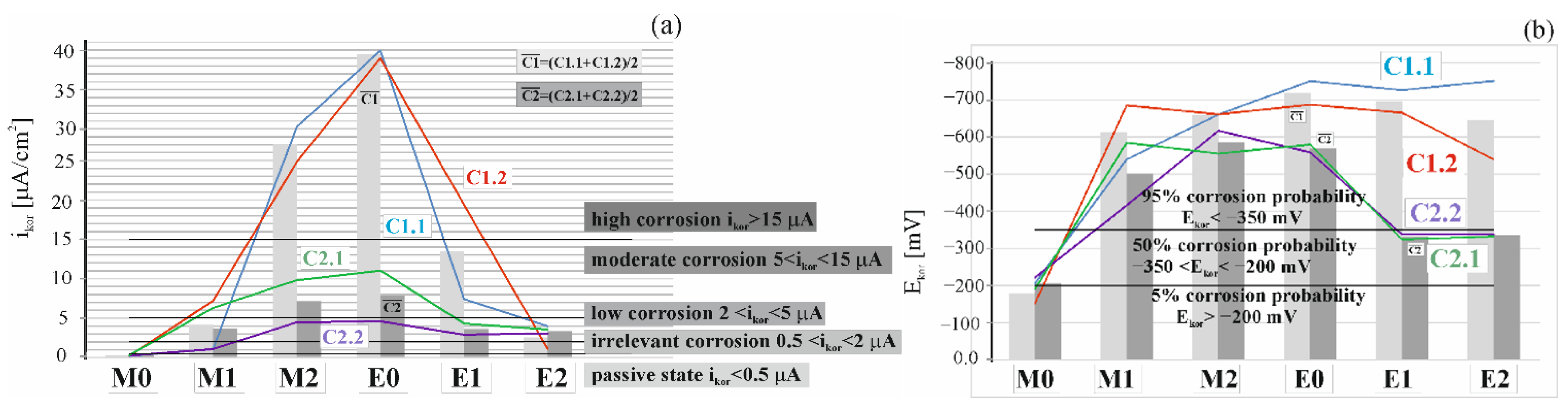
4.1. Results of Polarization Measurements for Reinforcement
4.2. Results from Material Tests on Concentration of Chloride and Hydroxide Ions in Concrete
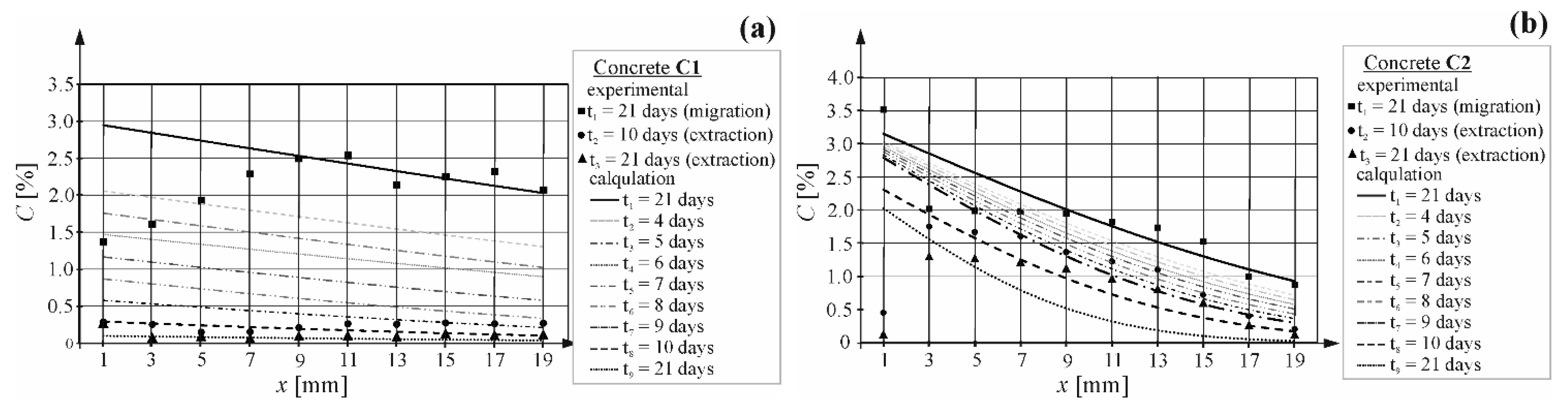
4.3. Results of Chloride Extraction from Concretes
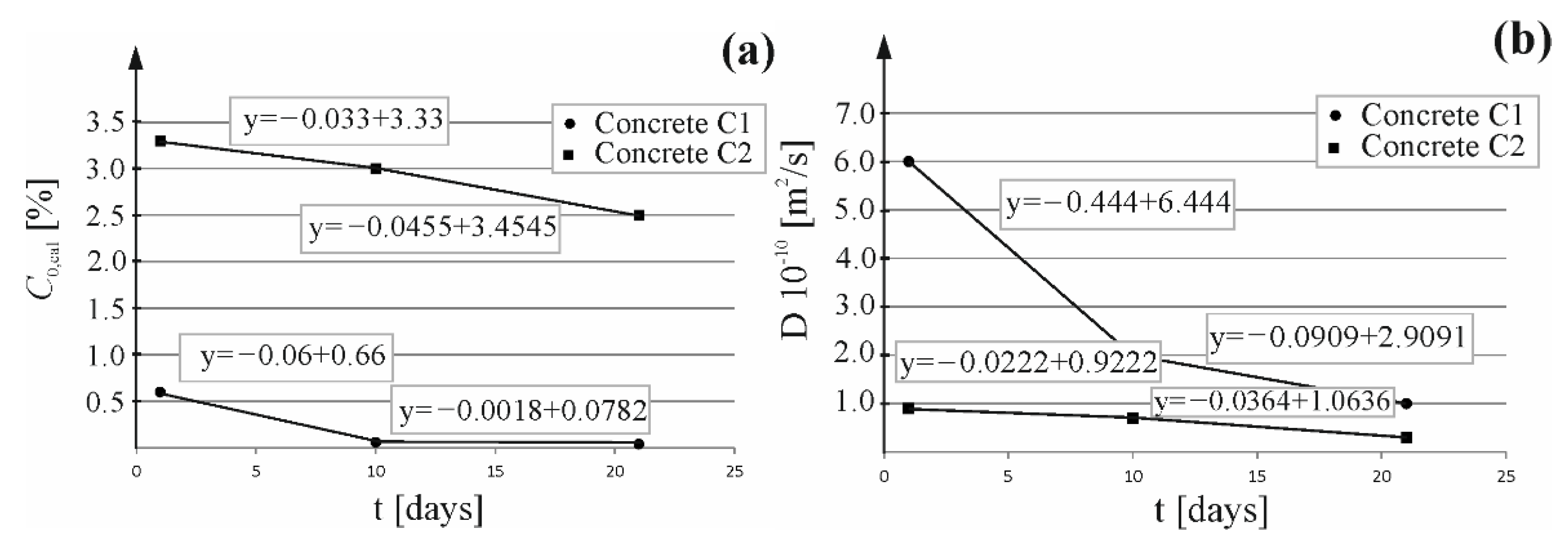
4.4. Predicting Duration of Extraction Using the Coefficient of Chloride Migration and Extraction
5. Conclusions and Recommendations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Measure No. | Time Days | Ecorr mV | ba mV | bc mV | Rp kΩ | RpA kΩcm2 | Icorr μA/cm2 | Vr μm/year |
|---|---|---|---|---|---|---|---|---|
| C1.1-M0 | 0 | −205 | 169 | 78 | 4.90 | 111.6 | 0.21 | 0.2 |
| C1.2-M0 | 0 | −151 | 204 | 82 | 6.32 | 143.44 | 0.18 | 0.2 |
| C1.1-M1 | 7 | −540 | 369 | 88 | 1.30 | 29.58 | 1.04 | 1.1 |
| C1.2-M1 | 7 | −686 | 343 | 119 | 0.24 | 5.36 | 7.16 | 7.9 |
| C1.1-M2 | 14 | −661 | 374 | 101 | 0.05 | 1.18 | 29.25 | 32.1 |
| C1.2-M2 | 14 | −662 | 382 | 104 | 0.06 | 1.43 | 24.82 | 27.3 |
| C1.1-E0 | 21 | −751 | 230 | 91 | 0.03 | 0.77 | 38.94 | 42.8 |
| C1.2-E0 | 21 | −688 | 480 | 162 | 0.06 | 1.38 | 37.98 | 41.8 |
| C1.1-E1 | 10 | −751 | 461 | 81 | 0.18 | 4.04 | 7.40 | 8.1 |
| C1.2-E1 | 10 | −666 | 420 | 323 | 0.18 | 4.09 | 19.40 | 21.3 |
| C1.1-E2 | 21 | −752 | 118 | 141 | 0.31 | 7.04 | 3.96 | 4.4 |
| C1.2-E2 | 21 | −540 | 415 | 93 | 1.31 | 29.74 | 1.11 | 1.2 |
| Measure No. | Time Days | Ecorr mV | ba mV/dec | bc mV/dec | Rp kΩ | RpA kΩ cm2 | icorr μA/cm2 | Vr μm/year |
|---|---|---|---|---|---|---|---|---|
| C2.1-M0 | 0 | −191 | 513 | 54 | 3.39 | 76.95 | 0.28 | 0.3 |
| C2.2-M0 | 0 | −221 | 414 | 69 | 4.93 | 111.91 | 0.23 | 0.3 |
| C2.1-M1 | 7 | −585 | 332 | 115 | 0.26 | 5.90 | 6.28 | 6.9 |
| C2.2-M1 | 7 | −416 | 255 | 104 | 1.35 | 30.65 | 1.05 | 1.2 |
| C2.1-M2 | 14 | −556 | 784 | 149 | 0.25 | 5.56 | 9.78 | 10.8 |
| C2.2-M2 | 14 | −617 | 451 | 133 | 0.44 | 10.03 | 4.45 | 4.9 |
| C2.1-E0 | 21 | −580 | 317 | 148 | 0.18 | 3.97 | 11.03 | 12.1 |
| C2.2-E0 | 21 | −559 | 289 | 158 | 0.43 | 9.67 | 4.59 | 5.0 |
| C2.1-E1 | 10 | −324 | 343 | 104 | 0.36 | 8.08 | 4.29 | 4.7 |
| C2.2-E1 | 10 | −338 | 272 | 131 | 0.58 | 13.21 | 2.91 | 3.2 |
| C2.1-E2 | 21 | −332 | 203 | 120 | 0.41 | 9.19 | 3.56 | 3.9 |
| C2.2-E2 | 21 | −337 | 290 | 140 | 0.59 | 13.35 | 3.07 | 3.4 |
References
- Quraishi, M.; Nayak, D.; Kumar, R.; Kumar, V. Corrosion of Reinforced Steel in Concrete and Its Control: An overview. J. Steel Struct. Constr. 2017, 3, 1000124. [Google Scholar] [CrossRef]
- PN-EN 206+A1:2016-12; Concrete Specification, Performance, Production and Conformity. PKN/KT 274: Warszawa, Poland, 2016.
- BS 8110: Part 1; Structural Use of Concrete–Code of Practice for Design and Construction. Br Stand Institute: London, UK, 1985; p. 38.
- ACI 201.2R-08; Guide to Durable Concrete. American Concrete Institute: Farmington Hills, MI, USA, 2008.
- Revisions to: Guide for the Design and Construction of Fixed Offshore Concrete Structures. ACI J. Proc. 1984, 81, 632–639. [CrossRef]
- ACI 222R-01; Protection of Metals in Concrete against Corrosion. ACI Committee: Farmington Hills, MI, USA, 2001; pp. 1–41.
- Hausmann, D.A. Steel corrosion in concrete--How does it occur? Mater. Prot. 1967, 6, 19–23. [Google Scholar]
- Yonezawa, T.; Ashworth, V.; Procter, R.P.M. Pore Solution Composition and Chloride Effects on the Corrosion of Steel in Concrete. Corrosion 1988, 44, 489–499. [Google Scholar] [CrossRef]
- Kayyali, O.A.; Haque, M.N. The C1−/OH− ratio in chloride-contaminated concrete—A most important criterion. Mag. Concr. Res. 1995, 47, 235–242. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, C. Accelerated simultaneous determination of the chloride depassivation threshold and of the non-stationary diffusion coefficient values. Corros. Sci. 2002, 44, 2409–2424. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.-W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Morris, W.; Vico, A.; Vazquez, M.; de Sanchez, S. Corrosion of reinforcing steel evaluated by means of concrete resistivity measurements. Corros. Sci. 2002, 44, 81–99. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.; Chen, Z. Probabilistic evaluation method for corrosion risk of steel reinforcement based on concrete resistivity. Constr. Build. Mater. 2017, 138, 101–113. [Google Scholar] [CrossRef]
- Bamforth, P.B. The derivation of input data for modelling chloride ingress from eight-year UK coastal exposure trials. Mag. Concr. Res. 1999, 51, 87–96. [Google Scholar] [CrossRef]
- EN 14038-2; Electrochemical Realkalization and Chloride Extraction Treatments for Reinforced Concrete—Part 2: Chloride Extraction. European Standard: Brussels, Belgium, 2020.
- Chang, C.; Yeih, W.; Chang, J.; Huang, R. Effects of stirrups on electrochemical chloride removal efficiency. Constr. Build. Mater. 2014, 68, 692–700. [Google Scholar] [CrossRef]
- Yeih, W.; Chang, J.J.; Hung, C.C. Selecting an adequate procedure for the electrochemical chloride removal. Cem. Concr. Res. 2006, 36, 562–570. [Google Scholar] [CrossRef]
- Ihekwaba, N.M.; Hope, B.B.; Hansson, C.M. Structural shape effect on rehabilitation of vertical concrete structures by ECE technique. Cem. Concr. Res. 1996, 26, 165–175. [Google Scholar] [CrossRef]
- Szweda, Z.; Jaśniok, T.; Jaśniok, M. Evaluation of the effectiveness of electrochemical chloride extraction from concrete on the basis of testing reinforcement polarization and chloride concentration. Ochr. Przed Korozja 2018, 61, 3–9. [Google Scholar] [CrossRef]
- Toumi, A.; Francois, R.; Alvarado, O. Experimental and numerical study of electrochemical chloride removal from brick and concrete specimens. Cem. Concr. Res. 2007, 37, 54–62. [Google Scholar] [CrossRef]
- Andrade, C.; Diez, J.; Alamán, A.; Alonso, C. Mathematical modelling of electrochemical chloride extraction from concrete. Cem. Concr. Res. 1995, 25, 727–740. [Google Scholar] [CrossRef]
- Chang, J. Bond degradation due to the desalination process. Constr. Build. Mater. 2003, 17, 281–287. [Google Scholar] [CrossRef]
- Carmona, J.; Climent, M.; Antón, C.; De Vera, G.; Garcés, P. Shape Effect of Electrochemical Chloride Extraction in Structural Reinforced Concrete Elements Using a New Cement-Based Anodic System. Materials 2015, 8, 2901–2917. [Google Scholar] [CrossRef]
- Bouteiller, V.; Tissier, Y.; Marie-Victoire, E.; Chaussadent, T.; Joiret, S. The application of electrochemical chloride extraction to reinforced concrete—A review. Constr. Build. Mater. 2022, 351, 128931. [Google Scholar] [CrossRef]
- Perkowski, Z.; Szweda, Z. The “Skin Effect” Assessment of Chloride Ingress into Concrete Based on the Identification of Effective and Apparent Diffusivity. Appl. Sci. 2022, 12, 1730. [Google Scholar] [CrossRef]
- Herrera, J.O.; Escadeillas, G.; Arliguie, G. Electro-chemical chloride extraction: Influence of C3A of the cement on treatment efficiency. Cem. Concr. Res. 2006, 36, 1939–1946. [Google Scholar] [CrossRef]
- Zofia, S.; Adam, Z. Theoretical Model and Experimental Tests on Chloride Diffusion and Migration Processes in Concrete. Procedia Eng. 2013, 57, 1121–1130. [Google Scholar] [CrossRef]
- Stern, M. Closure to “Discussion of ‘Electrochemical Polarization: 1. A Theoretical Analysis of the Shape of Polarization Curves’ [M. Stern and A. L. Geary (pp. 56–63, Vol. 104)]”. J. Electrochem. Soc. 1957, 104, 751–752. [Google Scholar] [CrossRef]
- Raczkiewicz, W. Use of polypropylene fibres to increase the resistance of reinforcement to chloride corrosion in concretes. Sci. Eng. Compos. Mater. 2021, 28, 555–567. [Google Scholar] [CrossRef]
- Raczkiewicz, W.; Koteš, P.; Konečný, P. Influence of the Type of Cement and the Addition of an Air-Entraining Agent on the Effectiveness of Concrete Cover in the Protection of Reinforcement against Corrosion. Materials 2021, 14, 4657. [Google Scholar] [CrossRef]
- Green, W.; Lyon, S.; Scantlebury, J. Electrochemical changes in chloride-contaminated reinforced concrete following cathodic polarisation. Corros. Sci. 1993, 35, 1627–1631. [Google Scholar] [CrossRef]
- Elsener, B.; Molina, M.; Böhni, H. The electrochemical removal of chlorides from reinforced concrete. Corros. Sci. 1993, 35, 1563–1570. [Google Scholar] [CrossRef]
- Kim, K.B.; Hwang, J.P.; Ann, K.Y. Influence of cementitious binder on chloride removal under electrochemical treatment in concrete. Constr. Build. Mater. 2016, 104, 191–197. [Google Scholar] [CrossRef]
- Szweda, Z. Estimating coefficient of chloride extraction from concrete. Ochr. Przed Koroz 2019, 62, 393–398. [Google Scholar] [CrossRef]



| Constituent | C1 | C2 |
|---|---|---|
| CEM I 42.5 R | CEM III/A 42.5 N-LH/HSR/NA | |
| Cement | 324 | |
| Sand (0–2 mm) | 722 | |
| Gravel (2–8 mm) | 512 | |
| Gravel (8–16 mm) | 681 | |
| Water | 162 | |
| w/c | 0.5 | |
| Compressive strength fcm MP | 54.2 | 49.5 |
| Volume weight kg/m3 | 2271 | 2269 |
| Time of Extraction t (Hour (days)) | 24 (1) | 96 (4) | 120 (5) | 144 (6) | 192 (8) | 216 (9) | 240 (10) | 288 (12) | 384 (16) | 504 (21) | 650 (27) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| initial concentration (%) | C1 | 0.6 | 0.42 | 0.36 | 0.3 | 0.18 | 0.12 | 0.06 | 0.06 | 0.04 | 0.04 | - |
| C2 | 3.3 | 3.2 | 3.17 | 3.13 | 3.07 | 3.03 | 3.00 | 2.91 | 2.73 | 2.50 | 2.27 | |
| coefficient of extraction (10−10 m2/s) | C1 | 5 | 5 | 4 | 4 | 3 | 2 | 2 | 2 | 1 | 1 | - |
| C2 | 0.9 | 0.83 | 0.81 | 0.79 | 0.74 | 0.72 | 0.70 | 0.63 | 0.48 | 0.3 | 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szweda, Z. Evaluating the Impact of Concrete Design on the Effectiveness of the Electrochemical Chloride Extraction Process. Materials 2023, 16, 666. https://doi.org/10.3390/ma16020666
Szweda Z. Evaluating the Impact of Concrete Design on the Effectiveness of the Electrochemical Chloride Extraction Process. Materials. 2023; 16(2):666. https://doi.org/10.3390/ma16020666
Chicago/Turabian StyleSzweda, Zofia. 2023. "Evaluating the Impact of Concrete Design on the Effectiveness of the Electrochemical Chloride Extraction Process" Materials 16, no. 2: 666. https://doi.org/10.3390/ma16020666
APA StyleSzweda, Z. (2023). Evaluating the Impact of Concrete Design on the Effectiveness of the Electrochemical Chloride Extraction Process. Materials, 16(2), 666. https://doi.org/10.3390/ma16020666






