Abstract
The performance of iron-rich calcium sulfoaluminate (IR-CSA) cement is greatly affected by mineral composition and mineral activity in the clinker. This study aims to identify the effect of CaO sources, either CaCO3 or CaSO4, on the phase formation and mineral composition of the IR-CSA clinker. Targeted samples were prepared with different proportions of CaCO3 and CaSO4 as CaO sources at 1300 °C for 45 min. Multiple methods were used to identify the mineralogical conditions. The results indicate that the mineral composition and performance of the IR-CSA clinker could be optimized by adjusting the CaO source. Both Al2O3 and Fe2O3 tend to incorporate into C4A3−xFx with an increase in CaSO4 as a CaO source, which leads to an increased content of C4A3−xFx but a decreased ferrite phase. In addition, clinkers prepared with CaSO4 as a CaO source showed much higher x value in C4A3−xFx and higher compressive strength than clinker prepared with CaCO3 as the sole CaO source. The crystal types of both C4A3−xFx and C2S were also affected, but showed different trends with the transition of the CaO source. The findings provide a possible method to produce IR-CSA cement at a low cost through cooperative utilization of waste gypsum and iron-bearing industrial solid wastes.
1. Introduction
Calcium sulfoaluminate (CSA) cement, which was first developed in China 40 years ago, has attracted increasing attention due to its lower energy consumption and CO2 emissions than Portland cement (PC) [1,2,3]. CSA clinker consists mainly of calcium sulfoaluminate (C4A3), dicalcium silicate (C2S), and ferrite phases (C2F~C6A2F). It can be produced at 1200 °C–1300 °C, which is about 150 °C lower than PC clinker production [4]. CSA cement exhibits excellent performance in engineering construction, such as precast products, 3D printing, marine-engineering concrete and cold environments, due to its characteristics of high early-age strength, rapid setting, low permeability, and low alkalinity [5,6,7,8]. Therefore, CSA cement will be a promising alternative to PC in future.
The high cost of raw meal has limited the massive scale application of CSA cement in engineering construction, especially expensive bauxite. To reduce production costs, CSA has been produced by using industrial solid wastes (ISWs) containing aluminum, such as aluminum ash and red mud, instead of high-grade bauxite [4,7,8,9]. However, aluminum-bearing ISWs such as red mud, steel slag, and tailings are rich in iron. The presence of iron has an impact on the formation, composition, and hydration activity of the key mineral phases in CSA clinker [10,11,12,13]. Iron is reported mainly to transform to the ferrite phase and ye’elimite [14] and that will make difference to the cement performance. The ferrite phase is beneficial to the early strength, but the ye’elimite is beneficial to both the early and late compressive strength [15,16]. More importantly, the incorporation of iron into ye’elimite makes it possible to decrease the aluminum content in raw materials and expand the aluminum sources. Therefore, researchers have tried to introduce more Fe2O3 into C4A3−xFx in iron-rich calcium sulfoaluminate (IR-CSA) clinker.
Several researchers produced IR-CSA clinker in CaO-SiO2-Al2O3-Fe2O3-SO3-based systems and found that the x value in C4A3−xFx is greatly affected by the Fe2O3, SO3, and CaO proportions in the raw mixture [14,17,18,19]. Studies have confirmed that the x value in C4A3−xFx presents a rising trend with the increase in Fe2O3 content in the raw material; however, not all Fe2O3 can be substituted for Al2O3 to participate in the formation of C4A3−xFx [18]. Low CaO and rich SO3 content in raw mixtures have also proven effective in promoting the incorporation of Fe2O3 into C4A3−xFx in calcium sulpholuminate ferrite–based systems [17,19]. It is obvious that both CaO and SO3 could be provided by CaSO4. Thus, it is feasible to ensure sufficient SO3 by batching excessive CaSO4 as CaO sources in the raw material.
CaO is derived from both CaCO3 and CaSO4 during the sintering process. Some researchers have indicated that the content of CaSO4 as a CaO source has an influence on the mineral composition of the clinker and further affects the performance of the cement. They have reported that a small amount of CaSO4 as a CaO source makes no obvious difference to the mineral composition of the clinker and that the loss of SO3 was compensated to promote adequate formation of ye’elimite; however, excess CaSO4 as a CaO source was reported to lead to the formation of gehlenite, thereby decreasing the compressive strength of the cement [20,21]. Others have indicated that CSA clinker could be obtained with CaO as provided by the partial decomposition of CaSO4, with both the key mineral phase composition of the clinker and the properties of the cement being similar to that produced by traditional methods (CaO source from CaCO3) [22]. In particular, CSA clinker with CaSO4 as the entire CaO source was produced with adequate formation of key mineral phases [23]. In our previous study, we found that batching the slight excess of CaSO4 in the raw mixture is an effective method of promoting the utilization rate of Al2O3 (to form C4A3−xFx) [17,20]. In addition, the crystal structure of ye’elimite transformed from orthorhombic symmetry to cubic symmetry because of the incorporation of Fe2O3, and the x value of C4A3−xFx increased with the increase in CaSO4 content. We also prepared IR-CSA with gypsum as the entire CaO source and found that the added iron did not form the ferrite phase first but was incorporated into other phases such as ye’elimite [14]. From the above studies, we found that the phase formation, transformation, and composition of IR-CSA clinker is greatly affected by CaO sources. However, the conclusions of the above studies are slightly biased due to the different preparation conditions. Systematic research into the effect of CaO sources on the phase formation and mineralogic conditions of IR-CSA clinker are still limited, and the mechanism remains unclear.
To make clear the effect of the CaO source, as either CaCO3/limestone or CaSO4/gypsum, on the mineralogic conditions of IR-CSA clinker, the targeted clinker was prepared with the mineral proportion of C4A3:C2S:C4AF as 50:30:20 by mass. Five groups of raw materials with increasing proportions of CaSO4 as a CaO source were designed; namely, whole CaCO3 (the CaSO4 was assumed to be undecomposed), 90 at.% CaCO3 + 10 at.% CaSO4 (at.% is the abbreviation form of the atomic ratio in the text), 80 at.% CaCO3 + 20 at.% CaSO4, 50 at.% CaCO3 + 50 at.% CaSO4, 20 at.% CaCO3 + 80 at.% CaSO4 and 100 at.% CaSO4 were designed to produce the clinkers. To ensure that the mineral formation was complete, all clinker was prepared at 1300 °C for 45 min. The mineralogic conditions as well as the microstructure and the chemical composition of key minerals, which would be influenced by the CaO source, were identified by multiple methods. Finally, the compressive strength was tested as a supplemental validation to verify the feasibility of optimizing the mineralogic conditions of the IR-CSA clinker and further the performance of the IR-CSA cement by adjusting the content of CaSO4 as the CaO source.
2. Materials and Methods
2.1. Raw Mixture
The raw materials (CaCO3, Al2O3, CaSO4·2H2O, Fe2O3, and SiO2) for preparing the IR clinker were analytical reagents provided by the Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China. CaCO3 was used as the CaO source. CaSO4 provided both CaO and SO3 during the calcination process of the IR-CSA clinker.
The targeted mineral composition of all clinker and the contents of the raw materials in the preparation of 100 g clinker with chemical reagents are shown in Table 1. The Bogue method was used to obtain the corresponding raw mixture [17]. The clinkers were named as S00, S10, S20, S50, S80, and S100, based on the content of CaO in the form of CaSO4. Cm indicates the degree of CaO in the raw material in order to satisfy that required for the formation of useful minerals in the corresponding clinker. The formula of Cm is defined as in Equation (13), and the value is fixed as 1.00 in this study. The Fe2O3/(Al2O3 + Fe2O3) ratio was the same for all the clinker in this study.

Table 1.
Target mineral composition of the clinker and chemical reagent proportions in the raw materials to prepare 100 g clinker.
2.2. Preparation Method
The reagents were dried at 110 °C for 2 h in a muffle furnace (LYL-16MA, LUO YANG LIYU KILN CO., LTD, Luoyang, China) and were then ground together in an agate bowl for 1 h to ensure the homogenization of the raw mixture; alcohol was added as a dispersant in the grind progress. After homogenization, 50 g per sample of raw mixture was added to the stainless steel mold (Tianjin Jingtuo Instrument Technology Co., LTD, Tianjin, China); a columnlike raw mixture with a 35 mm diameter was obtained under a pressure of 20 MPa. Thereafter, the obtained columnlike raw mixtureswere fired in an elevator-hearth furnace (LYL-17SJ, LUOYANG LIYU KILN CO., LTD, Luoyang, China) at 1300 ℃ for 45 min and then rapidly cooled at room temperature. Finally, the IR-CSA cement was obtained by milling the clinkers and gypsum. The gypsum added to the clinkers to react with ye’elimite would promote the formation of C6A3H32 (AFt) according to Equation (14), but samples with higher M ratios hydrated too fast to form cracks between AFt [24] and thus decreased the mechanical properties of the cement. The optimal gypsum content mixed with clinkers were samples with 0.8 m ratios of gypsum- (the anhydrate in clinkers was involved) to-ye’elimite (M) suggested by [10] and [25]. The content of ye’elimite and anhydrate were determined by the Rietveld refinement method [26]. Subsequently, the cement was prepared as cubic specimens (20 mm × 20 mm × 20 mm) with the water-to-cement ratio of 0.28.
where C4A3, CHx and C6A3H32 are the abbreviated form of 3CaO Al2O3 CaSO4, CaSO4 x H2O and 6CaO Al2O3 SO3 32H2O, respectively, and the superscript on S represents sulfate in the compound.
2.3. Testing Methods
The phenyl formic acid–ethyl alcohol titration method was adopted to determine the f-CaO content of the IR-CSA clinker according to the Chinese standard GB/T 176–2017.
The sulfate loss was calculated according to the difference in the SO3 amount between the raw materials and the clinkers. The SO3 contents were determined according to the barium sulfate precipitation in accordance with the Chinese standard GB/T176–2008 [23]. The gypsum decomposition rate is expressed as follows:
where M1 and S1 represent the mass and SO3 content of a raw sample, respectively; M2 and S2 are the mass and SO3 content of a clinker sample, respectively.
The mineral phase assemblages of all clinkers were determined by an X-ray diffractometer (Aeris, Malvern Panalytical, Malverin, UK) with Cu-Kα radiation. The voltage and the current were set to 40 KV and 15 mA, respectively. The detection range of the X-ray diffraction spectra was set to 10–60° with a scanning speed of 0.0027° per step. The mineral composition was quantified by Rietveld refinement using Topas-Academic V6 software. The crystallographic structures of the phases involved are listed in Table 2. The refined parameters included the background coefficients, instrument parameters, zero-shift error and unit cell parameters [14].

Table 2.
Crystallographic structures and ICSD codes of phases involved in the Rietveld refinement.
The microstructure of the pattern was acquired by scanning electron microscopy (SEM, Quattro S, Thermo Fisher Scientific, Waltham, MA, USA) with an acceleration voltage of 5 kV.
The chemical composition of clinkers was obtained by a field emission electron probe (FE-EPMA, JXA-8530F-Plus, Tokyo, Japan), and the backscattered electron (BSE) images and elemental distribution images of the patterns were detected with an acceleration voltage of 15 KV and an electron beam current of 5 × 10−8 A. The samples were first completely covered with epoxy resin and polished to a mirror state; the prepared patterns were then sprayed with carbon to improve their electrical conductivity for microanalysis.
The cement slurry was fully stirred with a water-to-cement ratio of 0.28 and was then poured into a 20 mm × 20 mm × 20 mm mold. The cement slurry specimens were demolded after curing with a temperature of 20 ± 1 ℃ and 99% relative humidity after 24 h. They were then cured for a standard time of 1, 3 and 28d to test the compressive strength [27].
3. Results and Discussion
3.1. Decomposition Rate of CaSO4 to Free-CaO
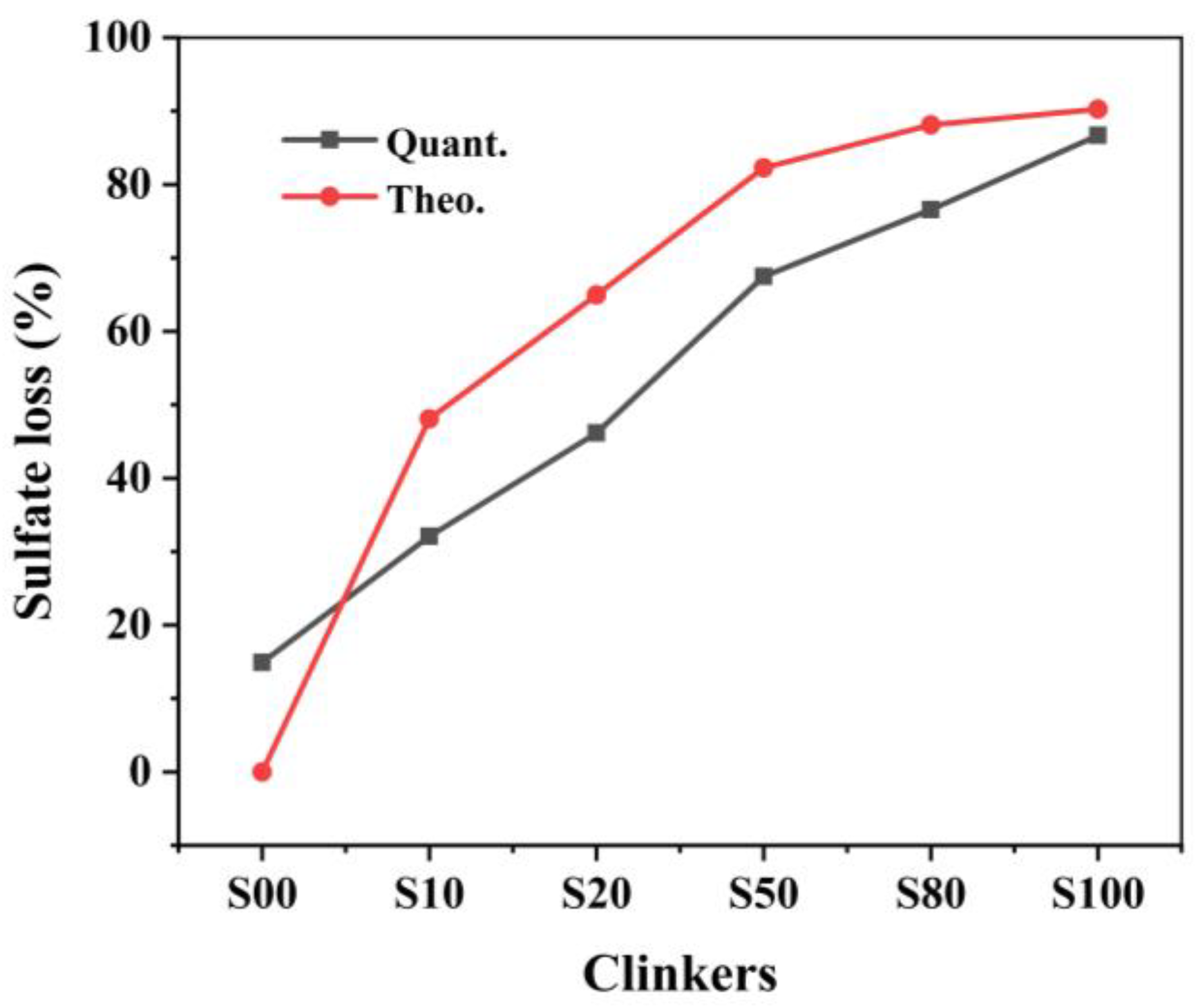
The decomposition of CaSO4 determines the actual CaO content participating in the reactions. In addition, the decomposition of CaSO4 has a great influence on the phase formation, phase composition, and even the chemical composition of the mineral phases according to the study of [14,17]. However, the decomposition of CaSO4 to CaO is affected by its proportion in the raw mixture [20]. Thus, it is necessary to confirm the decomposition rate after the calcination. Figure 1 shows the quantitative and theoretical sulfate loss of the clinkers with different CaO sources produced at 1300 ℃. As shown in Figure 1, although the same ye’elimite content was designed in clinkers, the actual SO3 content differs in all clinkers. The SO3 content increased gradually from clinkers S00 to S80, but then decreased in S100.
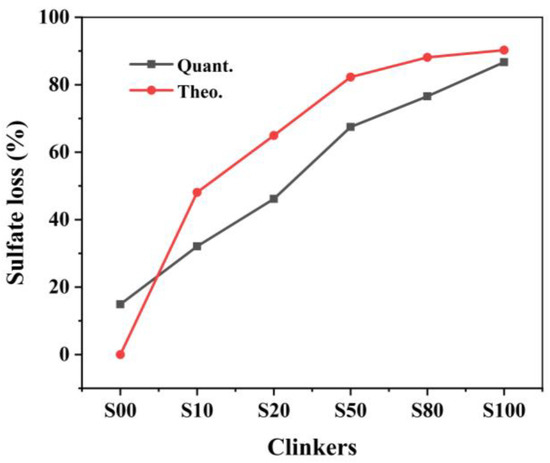
Figure 1.
Quantitative and theoretical sulfate loss in the clinker with different CaO sources.
The gypsum decomposition rates of all clinker were lower than the theoretical value except for S00. In S00, all CaSO4 was assumed to participate in the reactions to form ye’elimite. In fact, there was 14.7 wt.% sulfate loss in the clinker, which led to a higher CaO than the stoichiometric value. However, there was no f-CaO observed in the sample, which means that the extra CaO was involved in the reactions. Extra CaO induces phase equilibrium with a greater ferrite phase (with the CaO/(Al2O3 + Fe2O3) ratio of 2.0) formed rather than ye’elimite (with the CaO/(Al2O3 + Fe2O3) ratio of 1.3), because of the higher CaO/(Al2O3 + Fe2O3) ratio [28]. In clinkers S10−S100, the sulfate loss was less than the designed value and resulted in less CaO participating in the reactions, which led to more Fe2O3 being incorporated into the ye’elimite [17]. The CaO and SO3 content of the clinkers and the differences between the quantitative and theoretical values are listed in Table 3. The differences caused by different proportions of CaCO3/CaSO4 affected the formation of the mineral phases.

Table 3.
CaO and SO3 content of clinkers and their differences between quantitative and theoretical values.
The actual CaO content involved in the reactions is calculated as in Equation (16). CaCO3 was assumed to decompose completely above 900 °C and to participate in all reactions. CaO from CaSO4 was calculated due to the SO3 content.
3.2. Mineralogical Characterization
3.2.1. Qualitative Phase Analysis
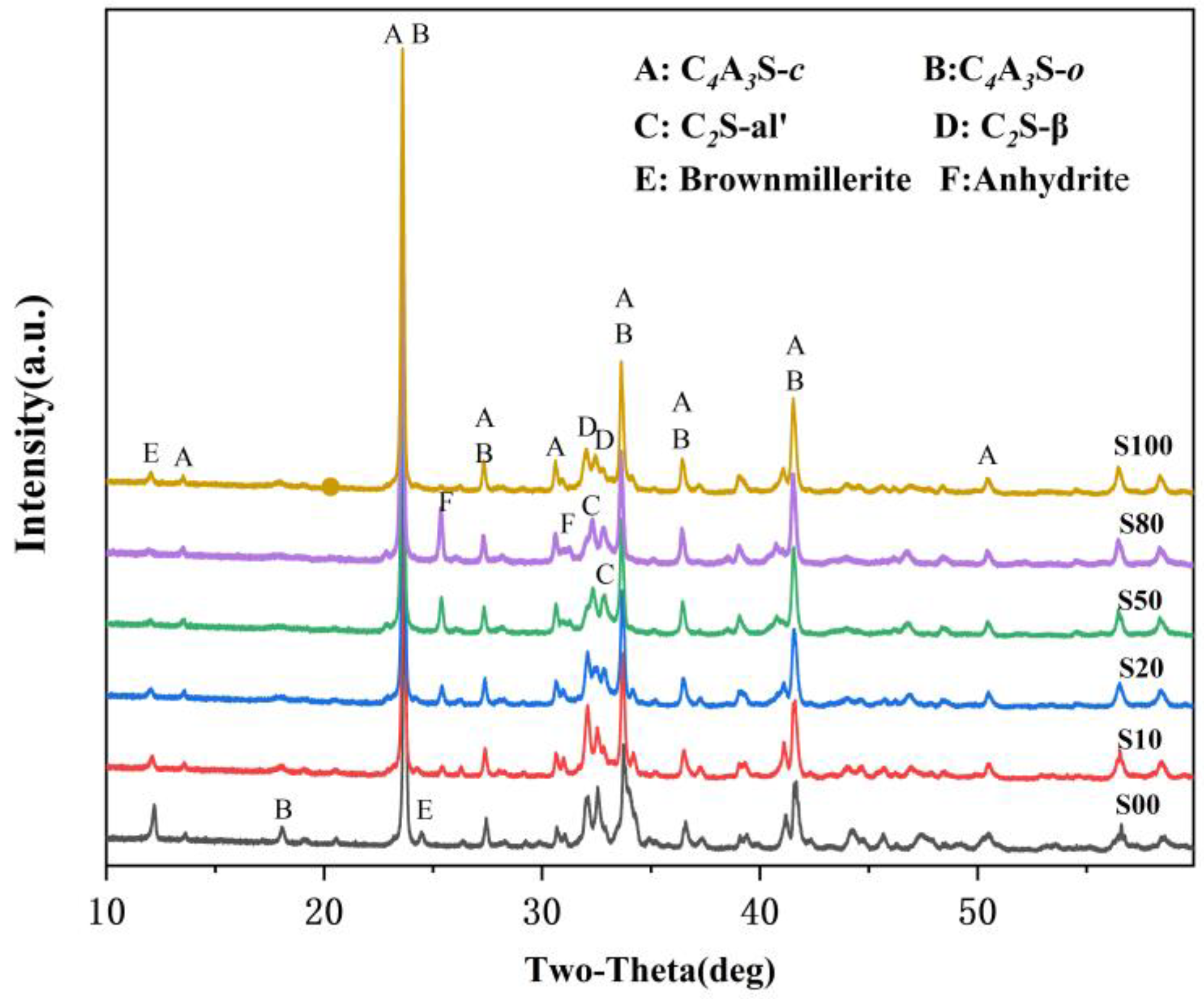
The XRD patterns of the IR-CSA clinker are shown in Figure 2. The key mineral phases, such as ye’elimite and belite existed in all the clinkers, while anhydrite and brownmillerite were partially present. The characteristic peak of anhydrate only presented in clinker S10–S80 and became stronger gradually, which is consistent with the SO3 content as tested in Section 3.1. The characteristic peak of brownmillerite (around 12.1°) in S00 is the strongest and becomes weaker gradually with the increase in CaSO4 as a CaO source. The characteristic peak of brownmillerite (around 12.1°) vanished in S50 and S80, but its intensity increased slightly in S100. On one hand, more CaSO4 batching in the raw material is beneficial for Al2O3 to form ye’elimite rather than brownmillerite. With the increase in CaSO4 as the CaO source, more CaO or CA is wrapped by CaSO4 so that the formation paths of ye’elimite expressed as Equations (1)–(3) are prior to that of the ferrite phase expressed as Equations (7)–(11). On the other hand, the residual CaSO4 in the clinker means the low CaO content participates in the reactions, and this induces more Fe2O3 to be incorporated into C4A3-xFx than to the form ferrite phase [17].
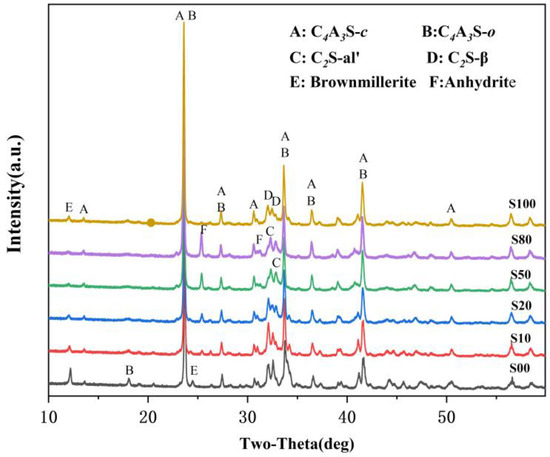
Figure 2.
XRD patterns of clinkers S00−S100.
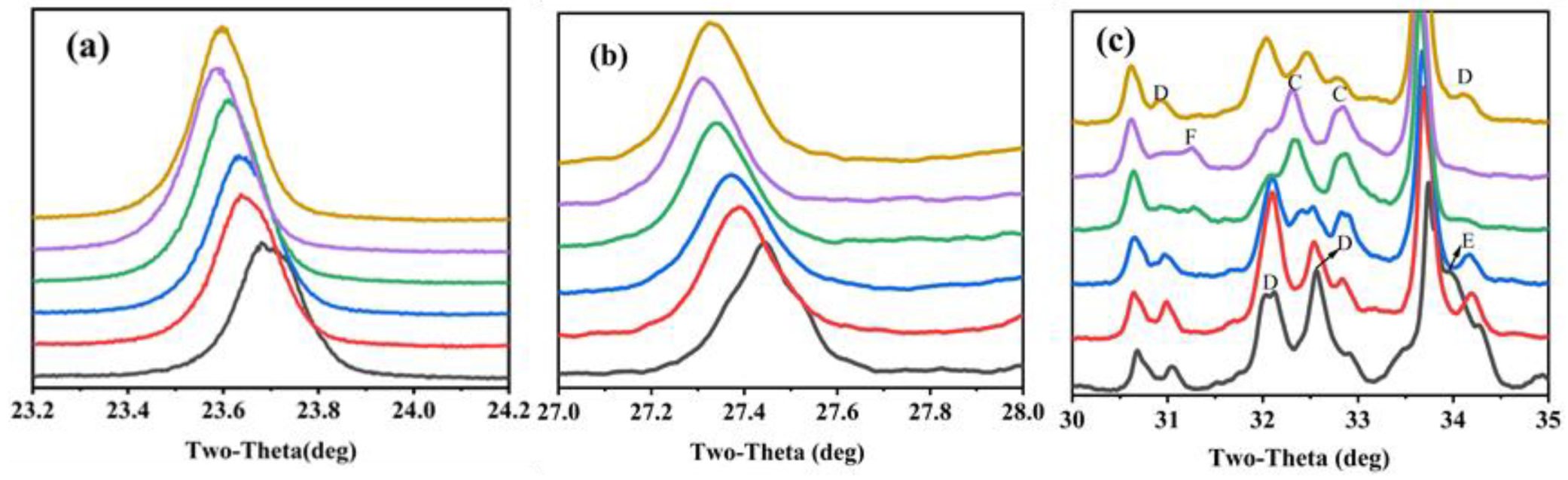
The characteristic peaks of C4A3 and C2S were also different between the clinkers. The characteristic peak of C4A3-o (at about 18.1°) was obvious in S00 and became weaker, gradually vanishing when CaSO4 provided more than 50% CaO. Moreover, the main characteristic peaks of ye’elimite (at approximately 23.7° and 27.4°) gradually shifted to lower angles with the increase in the CaO derived from CaSO4 (Figure 3a,b). This was mainly because the interplanar spaces of ye’elimite (with the crystal plane exponent of (022) in C4A3-o and (211) in C4A3-c, respectively) were increased with the amount of Al3+ (0.535 Å) substituted by Fe3+(0.645 Å) [17]. Similarly, the crystal structure of belite was additionally affected by CaO sources and the crystal structure of α’-C2S was more likely to occur when the CaO source of CaSO4 was increased (Figure 3c). The most convenient condition for the formation of α’-C2S is that CaSO4 provides CaO with the proportion of 50–80 at.% and with anhydrite residue in the clinker.
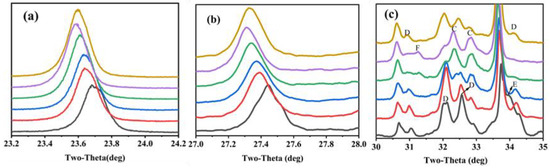
Figure 3.
Zoomed section of characteristic peaks migrate with variation proportion of CaSO4 as a CaO source (a) ye’elimite at about 23.7°, (b) ye’elimite at about 27.4° and (c) belite-al’(C), belite-beta (D), brownmillerite (E) and anhydrate (F).
3.2.2. Quantitative Phase Analysis
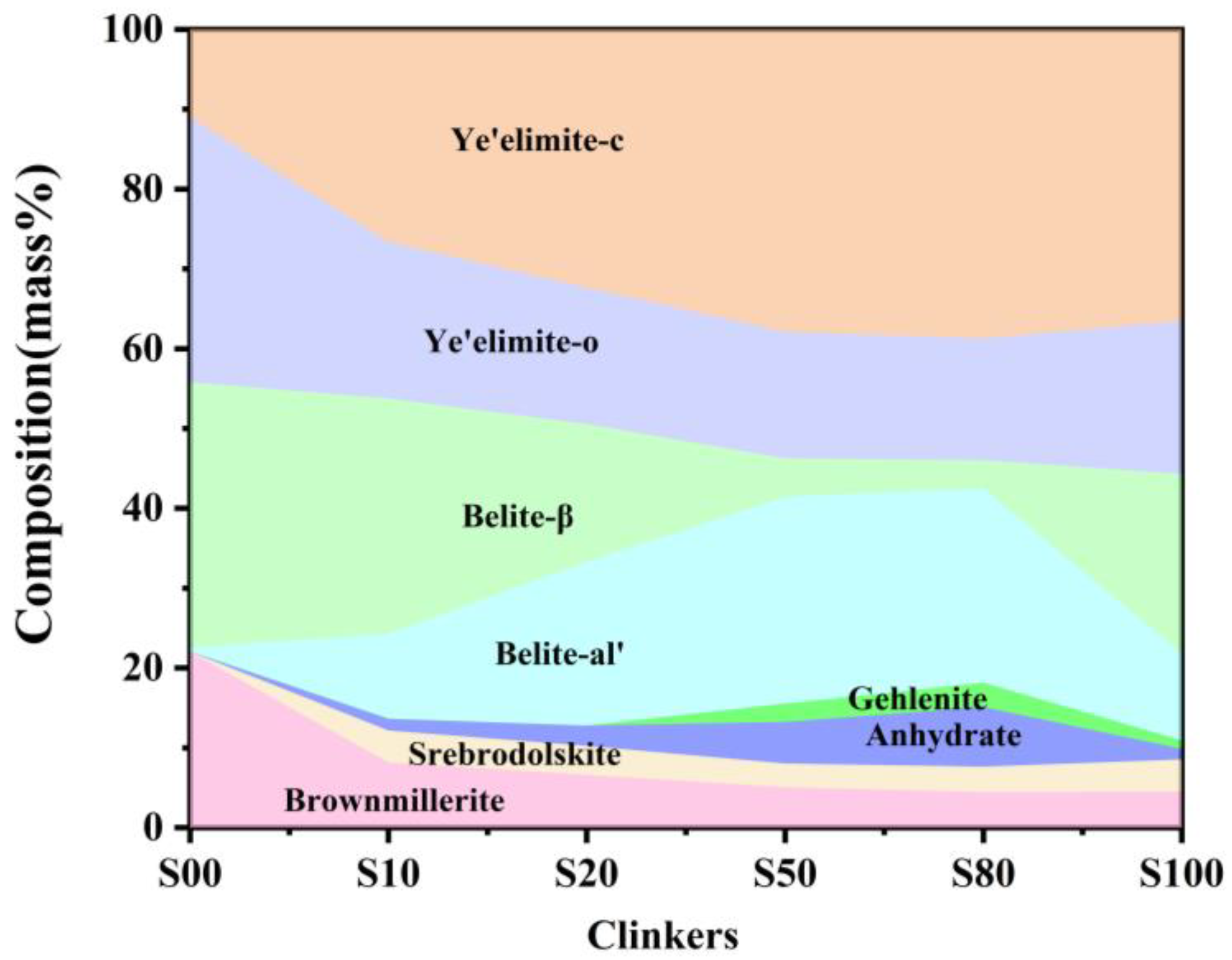
Quantitative analysis was conducted using the Rietveld refinement to better understand the formation and transformation of the key mineral phases affected by CaO sources. The results (Figure 4) indicate that the C4A3-o decreased while the C4A3-c increased with the increase in CaSO4 as a CaO source, which indicates that more Fe2O3 is incorporated into C4A3-xFx to stabilize C4A3 as a pseudocubic crystal during the cooling process [29,30]. The crystal types of belite were also affected by CaO sources. The results indicate that C2S only existed as a beta crystal type in S00; β-C2S subsequently decreased gradually from S00 to S80, but increased in S100. However, the α’-C2S phase showed the opposite trend. The literature has reported that the crystal structure of C2S is correlated to its formation paths and that C2S as obtained from Equations (5) and (6) exists as β-C2S, while from Equation (10) as α’-C2S [15]. It is clear that the increasing content of CaSO4 induces a greater formation as the transition phase and finally decomposes as α’-C2S.
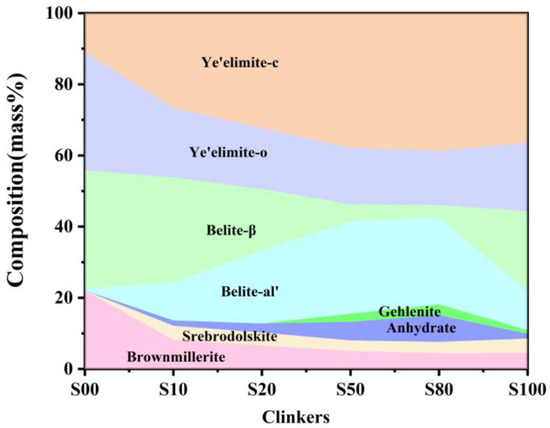
Figure 4.
Phase evolution of clinkers S00−S100.
The ferrite phase usually refers to a solid solution with the chemical composition of the formula Ca2(AlyFe2-y)O5, where y can vary from 0 to . The ferrite phase, with a chemical composition of y = 0, is specified as srebrodolskite (C2F), and the set of assemblages with 0 < y < usually have a chemical composition approximating to brownmillerite (C4AF) [17]. The formation of brownmillerite is considered to comprise a continuous solid solution of calcium aluminate with srebrodolskite (C2F) [31]. In this study, both srebrodolskite and brownmillerite existed in all clinker except for S00. There was no srebrodolskite in clinker S00, which shows that srebrodolskite reacts with calcium aluminate to form brownmillerite. In addition, the content of brownmillerite is slightly higher than the designed value; the main reason for this is that the CaSO4 is insufficient to react with CA to form C4A3 due to the partial decomposition, and more CA will be a solid soluble in brownmillerite according to Equations (11) and (12). The content of brownmillerite decreases gradually with the increase in CaSO4. as the CaO source. In addition, the brownmillerite is much lower than the designed value in the clinker when CaSO4 provides the CaO. Small amounts of srebrodolskite were present in clinker S10–S100, which shows that CA is more likely to act with CaSO4 than C2F when CaSO4 batches in raw material as the CaO source.
3.3. Chemical Composition of Key Mineral Phases in Iron-Rich Sulfoaluminate Clinker
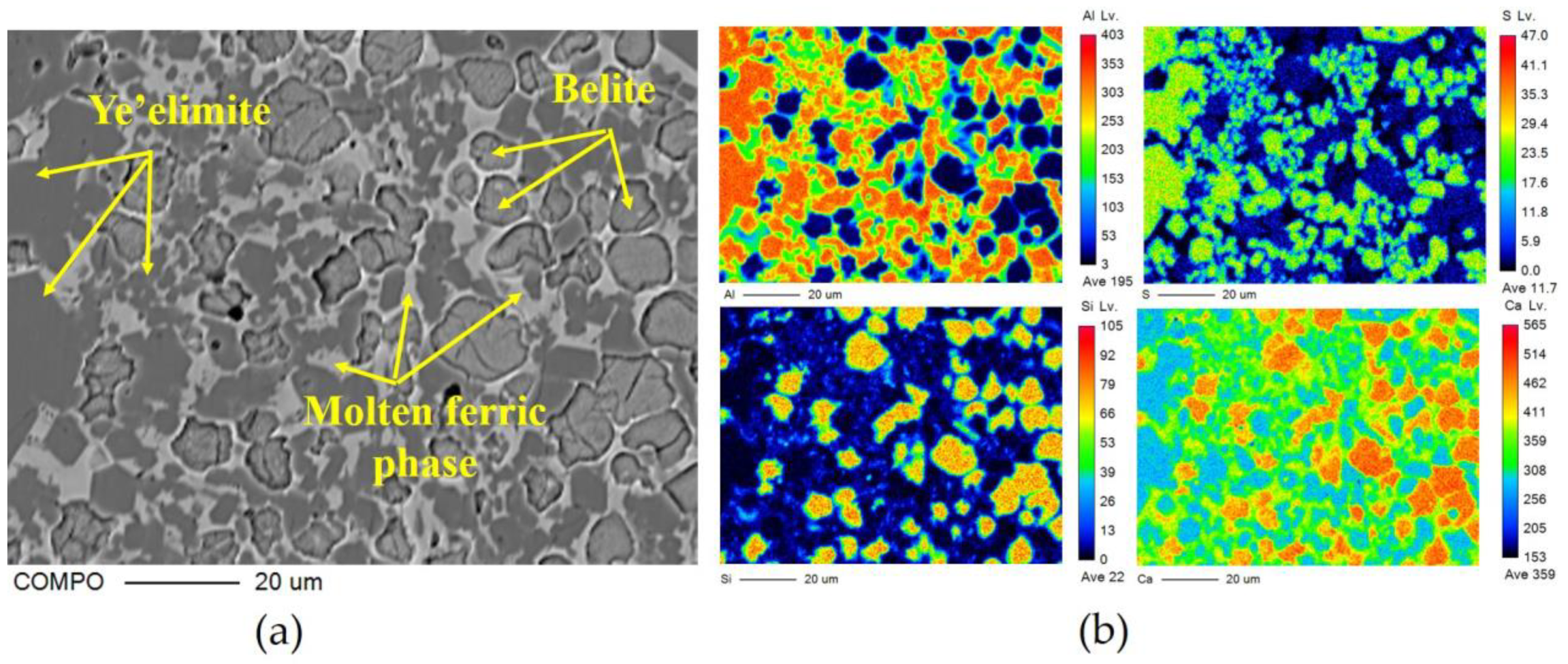
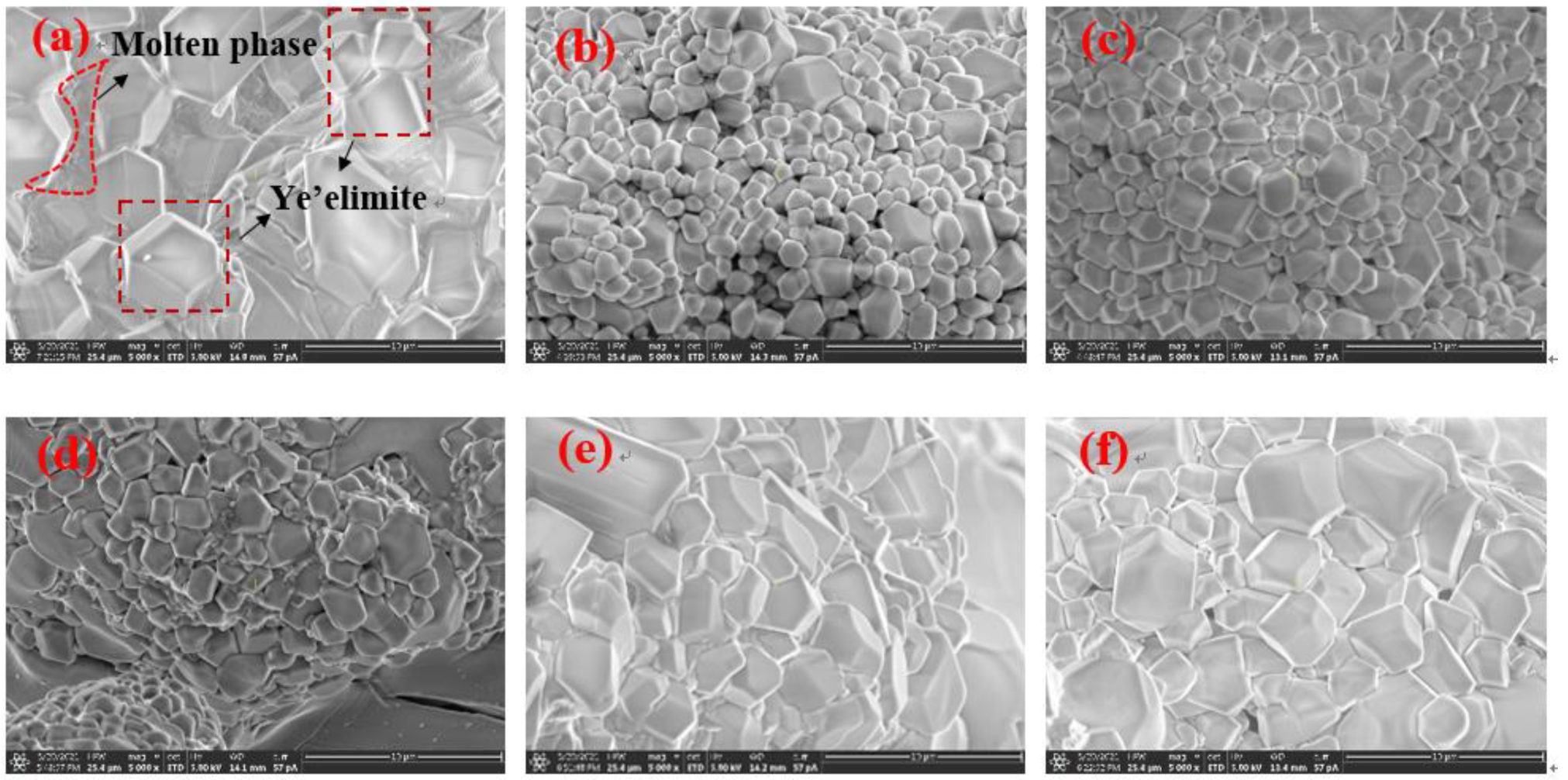
The crystal type of ye’elimite is determined by the incorporation level of Fe2O3, and be’lite is affected by the incorporation of SO3 and Fe2O3, to inhibit crystal transformation during cooling. It is clear that the chemical composition of the mineral phase is affected by the CaO source, in accordance with the results specified in Section 3.2, and this could be determined by the elemental distribution obtained from the polished sections of the sample slices by EPMA; the results are shown in Figure 5 (back-scattered electron (BSE) micrograph Figure 5a—S00, Figure 5b—S50, Figure 5c–S80, Figure 5d—S100) and Figure 6 (a: with the back-scattered electron (BSE) micrograph; b element distribution map). There are 300 × 225 quantitative points of the polished area with elements as shown on the map.
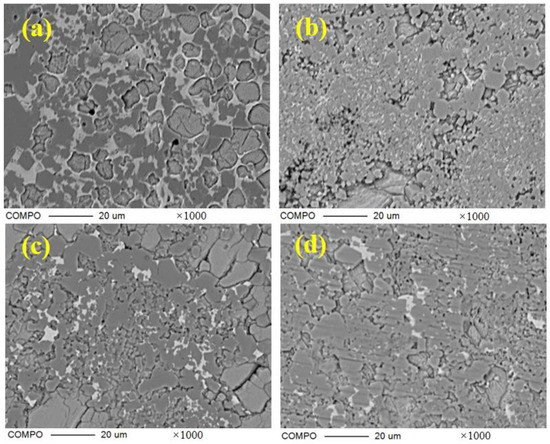
Figure 5.
BSE micrograph of polished section of samples with 1000 times magnification; (a) S00; (b) S50; (c) S80; (d) S100.
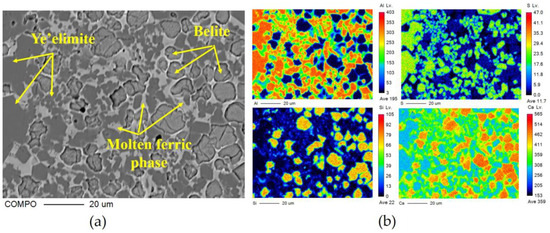
Figure 6.
EPMA results of clinker S00: (a) BSE micrograph of polished section; (b) distribution of all measured elements (Al, S, Si and Ca).
3.3.1. Chemical Composition of Ye’Elimite
To obtain the chemical composition of ye’elimite, element contents were sorted and calculated from the ye’elimite phase area. The elemental composition of oxygen atoms was normalized to 16 for comparison. The results are presented in Table 4. The Al3+ is substituted by Fe3+ in all clinkers; however, the substitution rate was influenced by the CaO source. The content of Al3+ substituted by Fe3+ was much lower in clinker S00 than the other three. The maximum substitution rate of Fe3+ for Al3+ reached 17.34 wt.% in clinker S80, and the maximum proportion of Fe2O3 in the ye’elimite phase was 6.89 wt.%, expressed as C4A2.71F0.29.

Table 4.
Mineralogical composition of ye’elimite phase of samples S00, S50, S80 and S100.
3.3.2. Chemical Composition of Belite
The same method was used to study the chemical composition of the belite phase as was used for ye’elemite. The elemental composition of the belite phase area was calculated and oxygen atoms were normalized to four for comparison. The chemical composition of the belite phase is presented in Table 5. The result indicates that the amount of Fe2O3 solidified into belite was much lower than that in C4A3; the maximum value reached 1.86 wt.% in clinker S00. The main reason may be that Fe3+ is easier to substitute than Al3+ in C4A3 due to the same valence state and similar ionic radius rather than entering the lattice gap of C2S.

Table 5.
Mineralogical composition of belite phase of samples S00, S50, S80 and S100.
SO3 was also incorporated into C2S, and the incorporated amount increased with the increasing content of CaSO4 as the CaO sources in the raw mixture until 80%, and then decreased slightly when CaO was entirely sourced from CaSO4. The maximum incorporation content of SO3 reached 5.58 wt.% in clinker S80 but was 4.65 wt.% in clinker S100. It is worth noting that the crystal type of belite is closely related to the SO3 content incorporated into the C2S. Usually, belite exists as α’-C2S at 830–1470 °C and transforms to β-C2S at temperatures between 520 °C and 670 °C. Finally, it transforms to γ-C2S below 520 °C. However, the crystal transformation of C2S can slow down or even not occur under the influence of CaSO4. In addition, C2S obtained from Equations (5) and (6) exists as β-C2S but as α’-C2S from Equation (10) according to [15]. In clinker S00, the CaSO4 is exhausted after reacting with calcium aluminate or gehlenite to form ye’elimite that no more CaSO4 exist and the reactions of Equations (9) and (10) will not happen. Thus, the belite in clinker S00 exists as β-C2S. It is obvious that the increasing CaSO4 content induces more formed as a transition phase and finally decomposed as α’-C2S [15]. Therefore, more α’-C2S is formed at the expense of β-C2S with the increase in CaSO4 as a CaO source. However, the decomposition of CaSO4 in S100 may be faster than in the others and CaSO4 rarely exists in the clinker. The reactions of Equations (9) and (10) are hampered, and the proportion of α’-C2S decreases with the increased CaSO4 in the raw mixture.
3.3.3. Chemical Composition of Ferrite Phase
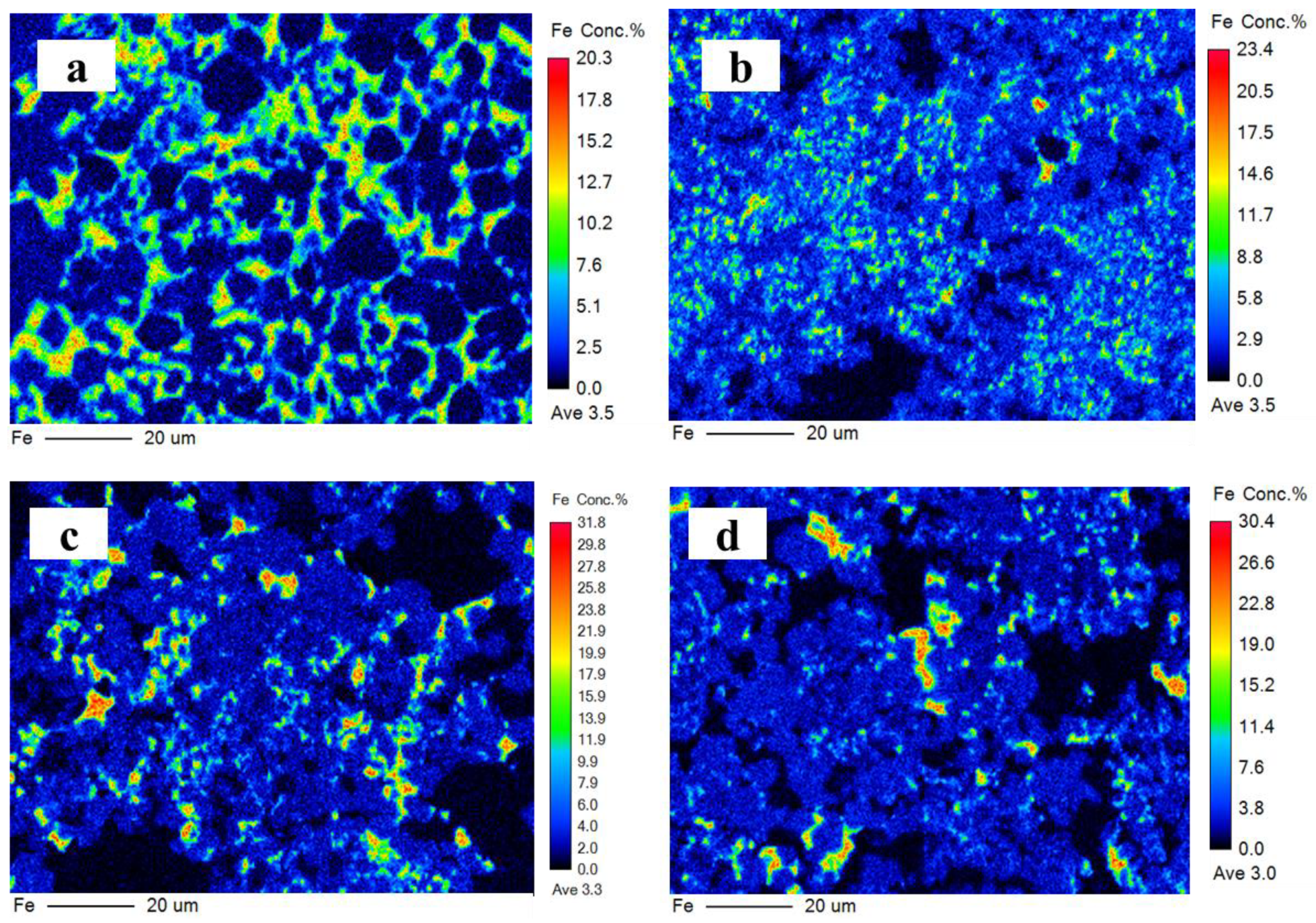
The ferrite phase is a solid solution that mainly contains C2F, C6AF2, C4AF, and C6A2F. It is generally believed that iron is distributed in the CSA clinker in the form of C4AF but mainly exists as C6AF2 in the IR-CSA clinker. Iron distributed in the ferrite phase is presented in Figure 7 and the EPMA confirms its inhomogeneous distribution. Iron decreased from the center to the edge in all clinkers, which is consistent with the results of [14]. The ferrite phase is uniformly dispersed around the ye’elimite in clinker S00 and S50, but is concentrated in several areas as small clumps in S80 and S100. The content of the ferrite phase decreased with the increase in CaSO4 as the CaO source. To obtain the chemical composition of the ferrite phase, oxygen atoms were normalized to 15 for comparison. The results (presented in Table 6) indicate that the chemical composition in the ferrite phase is close to C4AF in S00 and S50 but close to C6AF2 in clinker S80 and S100. Although there is less iron in the form of the ferrite phase because of the incorporation into ye’elimite in clinkers S80 and S100 than in clinkers S00 and S50, CA is more likely to combine with CaSO4 Equation (3) than C2F Equation (8) or C6AF2 Equation (11) with the increasing CaSO4 content in the raw mixture. Thus, the ferrite phase in clinkers S80 and S100 has a chemical composition with lower aluminum but higher iron content than in clinkers S00 and S50.
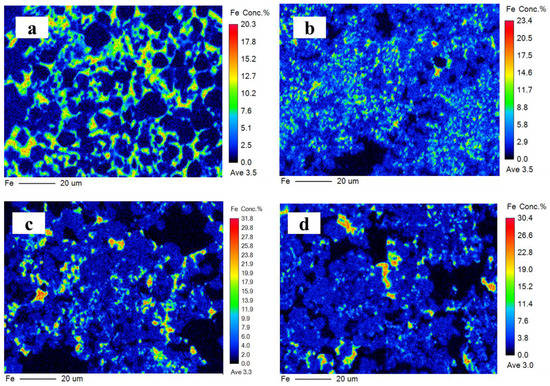
Figure 7.
Iron distribution on polished surface of clinker S00 (a), S50 (b), S80 (c), and S100 (d).

Table 6.
Chemical composition of ferrite phase of samples S00, S50, S80, and S100.
3.4. Microstructural Characterization
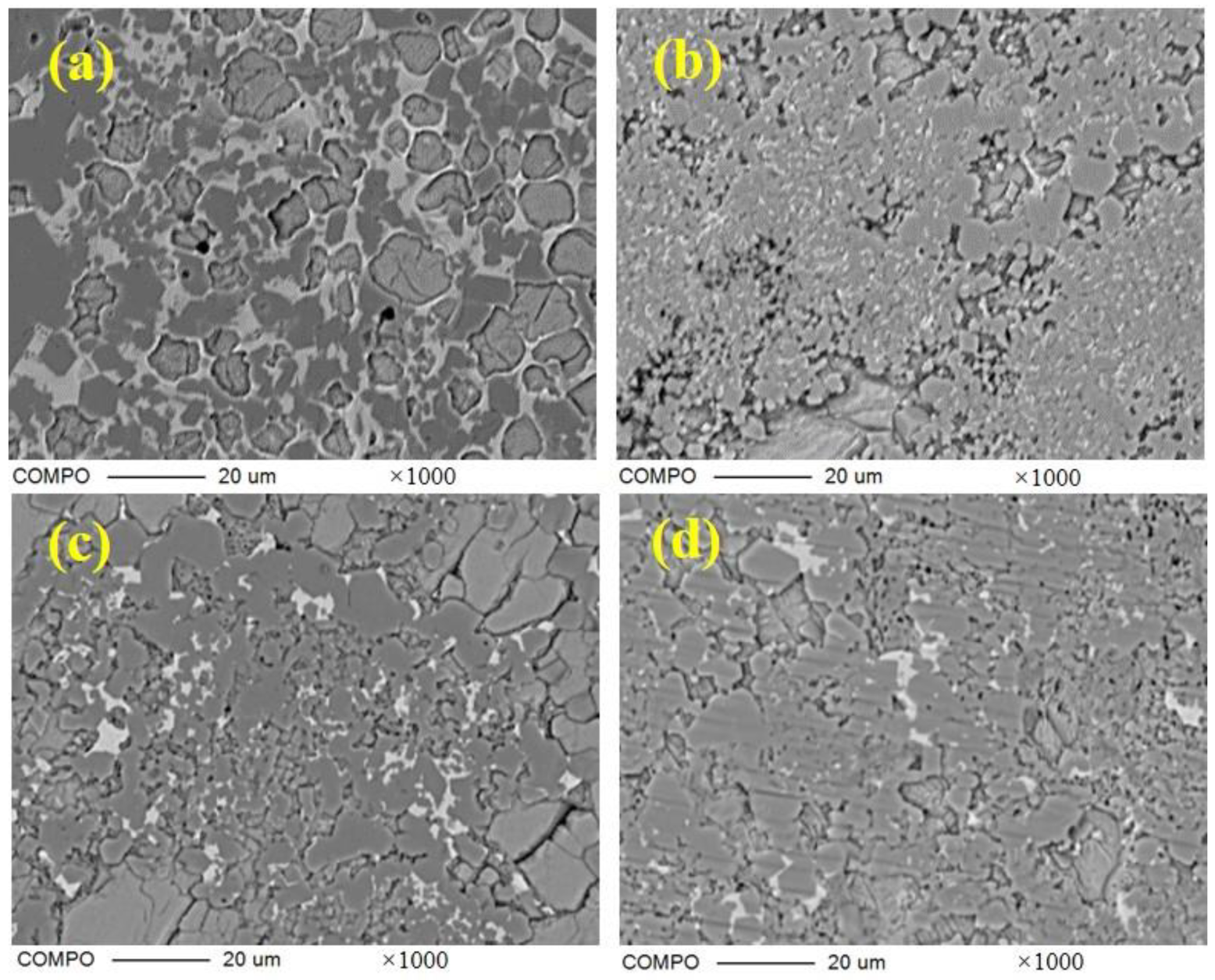
Figure 8 shows the scanning electron micrograph of the section of the clinker sintered at 1300 ℃ for 45 min. All samples are magnified 5000 times. The hexagonal platy structure or quadrilateral columnar structure can be seen, and the particle size of C4A3-xFx in S00 is about 10 μm, decreasing drastically in clinker S10 and then increasing gradually in S100. The iron-rich molten phase can be seen clearly around the ye’elimite in S00, which indicates a large amount of liquid ferrite phase during the process. However, the molten phase could hardly be observed in any other clinker with CaSO4 as the CaO source.
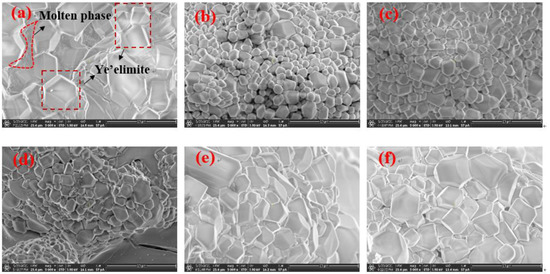
Figure 8.
Scanning electron micrograph of section of clinkers S00 (a), S10 (b), S20 (c), S50 (d), S80 (e) and S100 (f).
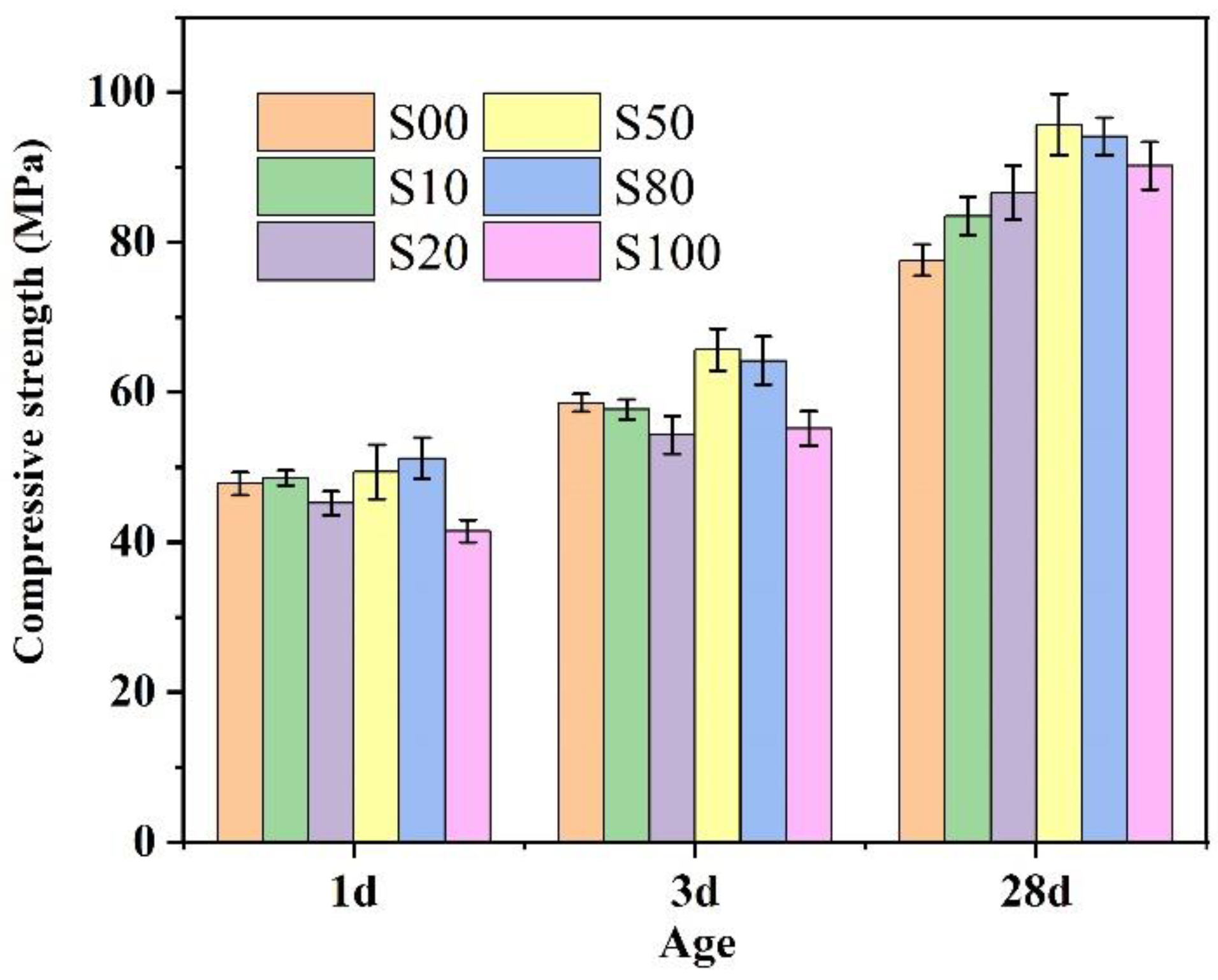
3.5. Compressive Strength of the IR-CSA Cement
The compressive strength of IR-CSA cement clinkers S00–S100 after 1 d, 3 d, and 28 d of curing is shown in Figure 9. With the increase in CaO derived from CaSO4, the 1 d and 3 d compressive strength exhibited slight strength advantages in cements S50 and S80, but disadvantages in S100. The 28d compressive strength was higher for cement S50–S100 than for S00–S20. To be specific, the cement in S50 showed the best early and late compressive strength. The main reasons may be as follows: (ⅰ) The anhydrate in cements S50 and S80 consumes more water for ye’elimite hydration than gypsum according to Equation (14); thus, the actual water/cement ratios of cements S50 and S80 were lower than other samples, affecting their mechanical properties; (ⅱ) The Al/Fe ratio of the ferrite phase of S50 (1:1.12) was higher than S80 (1:1.96) and S100 (1:2.12), which resulted in higher hydration activity of the ferrite phase and early compressive strength of the S50 than the S80 and S100. Furthermore, the C4A3-xFx particle size of S50 was smaller than S80 and S100, which was beneficial for the hydration rate and early compressive strength; (ⅲ) The late compressive strength was related to the hydration of C4A3-xFx and C2S. With the increase in CaSO4 as a CaO source, the content of C4A3-xFx in clinkers S50–S100 was higher than in S00–S20. In addition, the C2S hydration activity in S50 and S80 is higher than in the other cements because of the crystal type (the crystal type of α’-C2S exhibits higher hydration activity than β-C2S at room temperature).
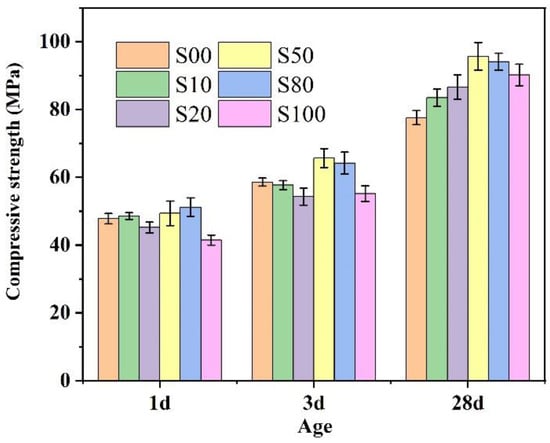
Figure 9.
Compressive strength of cements S00–S100.
4. Conclusions
The effect of CaO sources, from either CaCO3 or CaSO4, on phase formation and mineral composition of iron-rich clinker was investigated by varying their proportions in raw materials. Compared with CaCO3, CaO derived from CaSO4 was more conducive for Al2O3 and Fe2O3 to form C4A3-xFx rather than the ferrite phase which resulted in an increased content of ye’elimite but a decreased ferrite phase. More c-C4A3-xFx formed at the expense of o-C4A3-xFx in the clinker because of the incorporation of Fe2O3. In addition, the belite phase was more inclined to exhibit as α’-C2S instead of β-C2S when taking CaSO4 as a CaO source, which is conducive to the hydration activity of the clinker. Finally, the crystal grain sizes decreased dramatically and then increased gradually with the increase in the proportion of CaO derived from CaSO4. In view of the influence of the mineral composition, crystal structure and crystal grain sizes, cement with optimal mechanical properties was obtained with the proportion of 1:1 of CaCO3 and CaSO4 as a CaO source.
Under the specified conditions, the substitution of Fe3+ for Al3+ reached a maximum value of 17.34 wt.%, and the maximum proportion of Fe2O3 in the ye’elimite phase was 6.89 wt.%, expressed as C4A2.71F0.29. However, the incorporation amount of Fe2O3 into C2S was no more than 1.86 wt.% and showed an irregular change trend with CaO sources. The chemical formula of the ferrite phase was calculated and found to be similar to C4AF when the amount of CaO derived from CaSO4 was less than 50% but similar to C6AF2 when the amount of CaO derived from CaSO4 was more than 80%, which also indicates that Al2O3 is more inclined to be involved in C4A3-xFx with the increase in CaSO4 as a CaO source.
The findings provide a possible method to optimize the mineral composition of IR-CSA clinker by adjusting the content of CaSO4 as a CaO source and to produce high-performance IR-CSA cement at a low cost through cooperative utilization of waste gypsum and iron-bearing industrial solid wastes.
Author Contributions
Methodology, Writing-original draft, Data Curation, W.J.; writing—review and editing, C.W. and C.Z.; Project administration, X.W. and J.L.; Investigation, S.W. and Y.Y.; Formal analysis, Y.L. and W.W.; Conceptualization, resources, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (No.2020YFC191000) and the Key Research and Development Program of Shandong Province-Major Scientific and Technological Innovation Project (No.2020 CXGC011403).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
No conflict of interest exits in the submission of this manuscript, and the manuscript was approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and is not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
References
- Sharp, J.H. Calcium sulfoaluminate cements—Low-energy cements, special cements or what? Adv. Cem. Res. 1999, 11, 3–13. [Google Scholar] [CrossRef]
- Zhang, L.; Su, M.; Wang, Y. Development of the use of sulfo- and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Haha, M.B.; Winnefeld, F.; Pisch, A. Advances in understanding ye’elimite-rich cements. Cem. Concr. Res. 2019, 123, 105778. [Google Scholar] [CrossRef]
- Singh, M.; Upadhayhay, S.; Prasad, P. Preparation of iron rich cements using red mud. Cem. Concr. Res. 1997, 27, 1037–1046. [Google Scholar] [CrossRef]
- Khalil, N.; Aouad, G.; El Cheikh, K.; Rémond, S. Use of calcium sulfoaluminate cements for setting control of 3D-printing mortars. Constr. Build. Mater. 2017, 157, 382–391. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2004, 34, 671–676. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, H.; Wang, W.; Jia, M.; Che, X. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material. J. Hazard Mater. 2020, 385, 121580. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Wu, C.; Li, J.; Wu, S.; Jiang, W.; Wang, X.; Wang, W.; Feng, M. Investigation of hierarchical porous cold bonded lightweight aggregates produced from red mud and solid-waste-based cementitious material. Constr. Build. Mater. 2021, 308, 124990. [Google Scholar] [CrossRef]
- Ge, Z.; Yuan, H.; Sun, R.; Zhang, H.; Wang, W.; Qi, H. Use of green calcium sulphoaluminate cement to prepare foamed concrete for road embankment: A feasibility study. Constr. Build. Mater. 2020, 237, 117791. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; León-Reina, L.; Aranda, M.A.G.; De la Torre, A.G. Hydration Reactions and Mechanical Strength Developments of Iron-Rich Sulfobelite Eco-cements. Ind. Eng. Chem. Res. 2013, 52, 16606–16614. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Scholten, T.; Scrivener, K.L.; Ben Haha, M.; Wolter, A. Formation, composition and stability of ye’elimite and iron-bearing solid solutions. Cem. Concr. Res. 2020, 131, 106009. [Google Scholar] [CrossRef]
- Idrissi, M.; Diouri, A.; Damidot, D.; Greneche, J.M.; Talbi, M.A.; Taibi, M. Characterisation of iron inclusion during the formation of calcium sulfoaluminate phase. Cem. Concr. Res. 2010, 40, 1314–1319. [Google Scholar] [CrossRef]
- Guo, Y.; Su, M.; Deng, J.; Wang, Y. A study on hydration characteristics of ferrite phase in ferro aluminate cement. J. Chin. CreamSoc. 1989, 17, 296–301. [Google Scholar]
- Wu, S.; Yao, X.; Ren, C.; Yao, Y.; Zhang, C.; Wu, C.; Wang, W. Effect of iron on the preparation of iron-rich calcium sulfoatablluminate cement using gypsum as the sole calcium oxide source and its incorporation into mineral phases. Constr. Build. Mater. 2021, 290, 123214. [Google Scholar] [CrossRef]
- Wang, Y.; Su, M.; Zhang, L. Sulphoaluminate Cement; Beijing University of Technology Press: Beijing, China, 1999. [Google Scholar]
- Chen, D.; Feng, X.; Long, S. The influence of ferric oxide on the properties of 3CaO · 3Al2O3 · CaSO4. Thermochim. Acta 1993, 215, 157–169. [Google Scholar] [CrossRef]
- Yao, X.; Yang, S.; Dong, H.; Wu, S.; Liang, X.; Wang, W. Effect of CaO content in raw material on the mineral composition of ferric-rich 1 sulfoaluminate clinker. Constr. Build. Mater. 2020, 263, 120431. [Google Scholar] [CrossRef]
- Huang, Y.; Pei, Y.; Qian, J.; Gao, X.; Liang, J.; Duan, G.; Zhao, P.; Lu, L.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties. Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Yao, X.; Yang, S.; Huang, Y.; Wu, S.; Yao, Y.; Wang, W. Effect of CaSO4 batching in raw material on the iron-bearing mineral transition of ferric-rich sulfoaluminate cement. Constr. Build. Mater. 2020, 250, 118783. [Google Scholar] [CrossRef]
- Ren, C.; Wang, W.; Li, G. Preparation of high-performance cementitious materials from industrial solid waste. Constr. Build. Mater. 2017, 152, 39–47. [Google Scholar] [CrossRef]
- Liu, N.; Chen, Q.; Dang, Y.; Li, F.; Zhang, J.; Li, X.; Qian, J. Partial Decomposition of Phosphogypsum for the Preparation of Belite Calcium Sulphoaluminate Cement. Bull. Chin. Ceram. Soc. 2016, 35, 3763–3769. [Google Scholar]
- Wu, S.; Yao, X.; Ren, C.; Li, J.; Xu, D.; Wang, W. Co-preparation of calcium sulfoaluminate cement and sulfuric acid through mass utilization of industrial by-product gypsum. J. Clean. Prod. 2020, 265, 121801. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.; Chun, S.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, W.; Ge, Z.; Ren, C.; Yao, X.; Wu, S. Hydration study and characteristic analysis of a sulfoaluminate high-performance cementitious material made with industrial solid wastes. Cem. Concr. Compos. 2020, 112, 103687. [Google Scholar] [CrossRef]
- Touzo, B.; Scrivener, K.L.; Glasser, F.P. Phase compositions and equilibria in the CaO–Al2O3–Fe2O3–SO3 system, for assemblages containing ye’elimite and ferrite Ca2(Al,Fe)O5. Cem. Concr. Res. 2013, 54, 77–86. [Google Scholar] [CrossRef]
- Ndzila, J.S.; Liu, S.; Jing, G.; Wang, S.; Ye, Z. The effect of Fe3+ ion substitution on the crystal structure of ye’elimite. Ceram. Silik. 2020, 64, 18–28. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, X.; Ma, S.; Chen, L.; Zhong, B. Effect of Fe2O3 on the formation of calcium sulpholuminate mineral. J. Chin. Ceram. Soc. 2007, 35, 485–488. [Google Scholar]
- Guo, Y.; Deng, J.; Su, M.; Wang, Y. A study on formation mechanism of ferrite phase in ferroaluminate cement. J. Chin. CreamSoc. 1988, 16, 481–488. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).