Electrodeposition of Stable Noble-Metal-Free Co-P Electrocatalysts for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
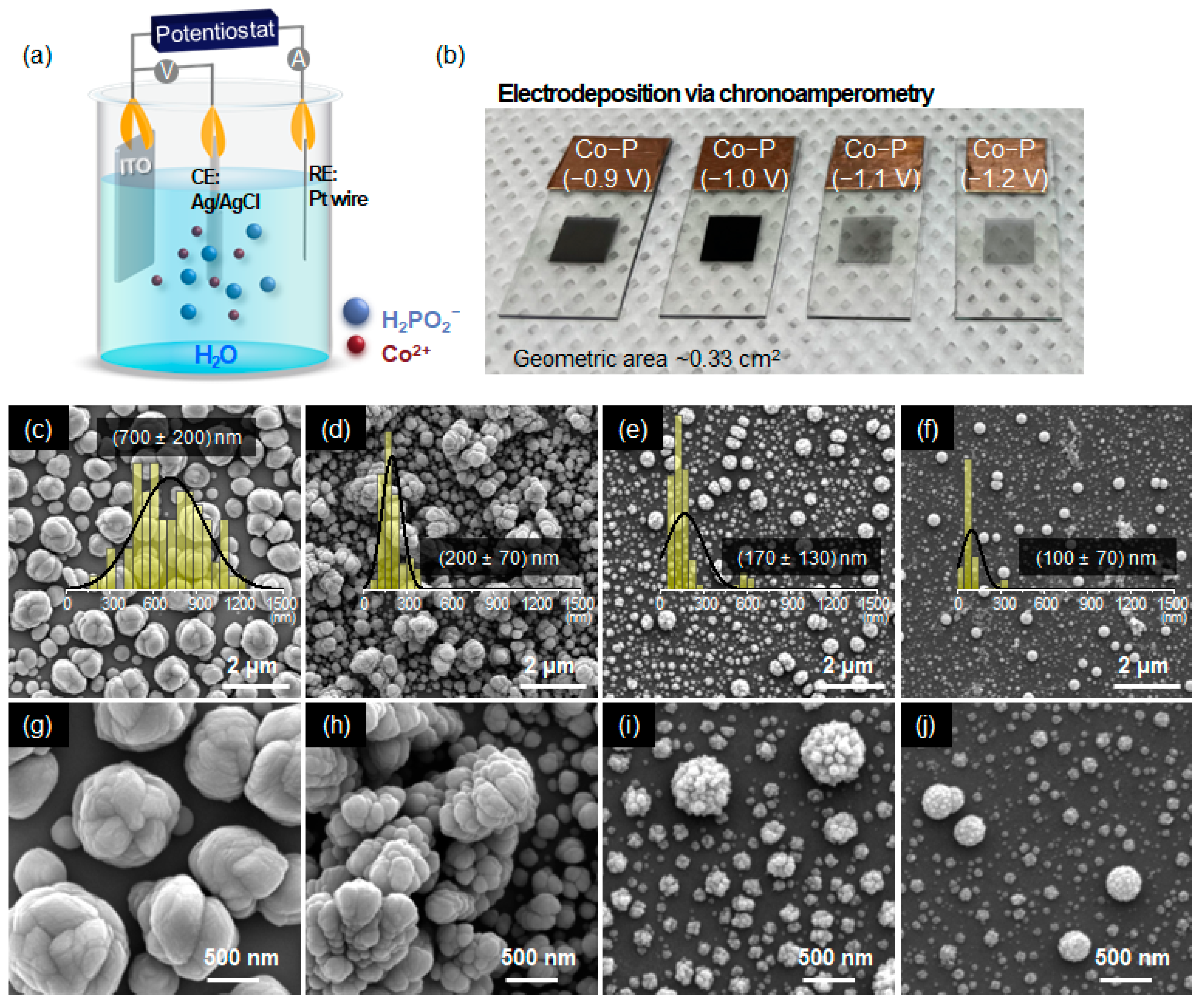
2.1. Electrochemical Deposition of Electrocatalysts
2.2. Material Characterizations
2.3. Electrochemical Measurement
3. Results and Discussion
3.1. Structural Evolution of Co-P Nanostructures
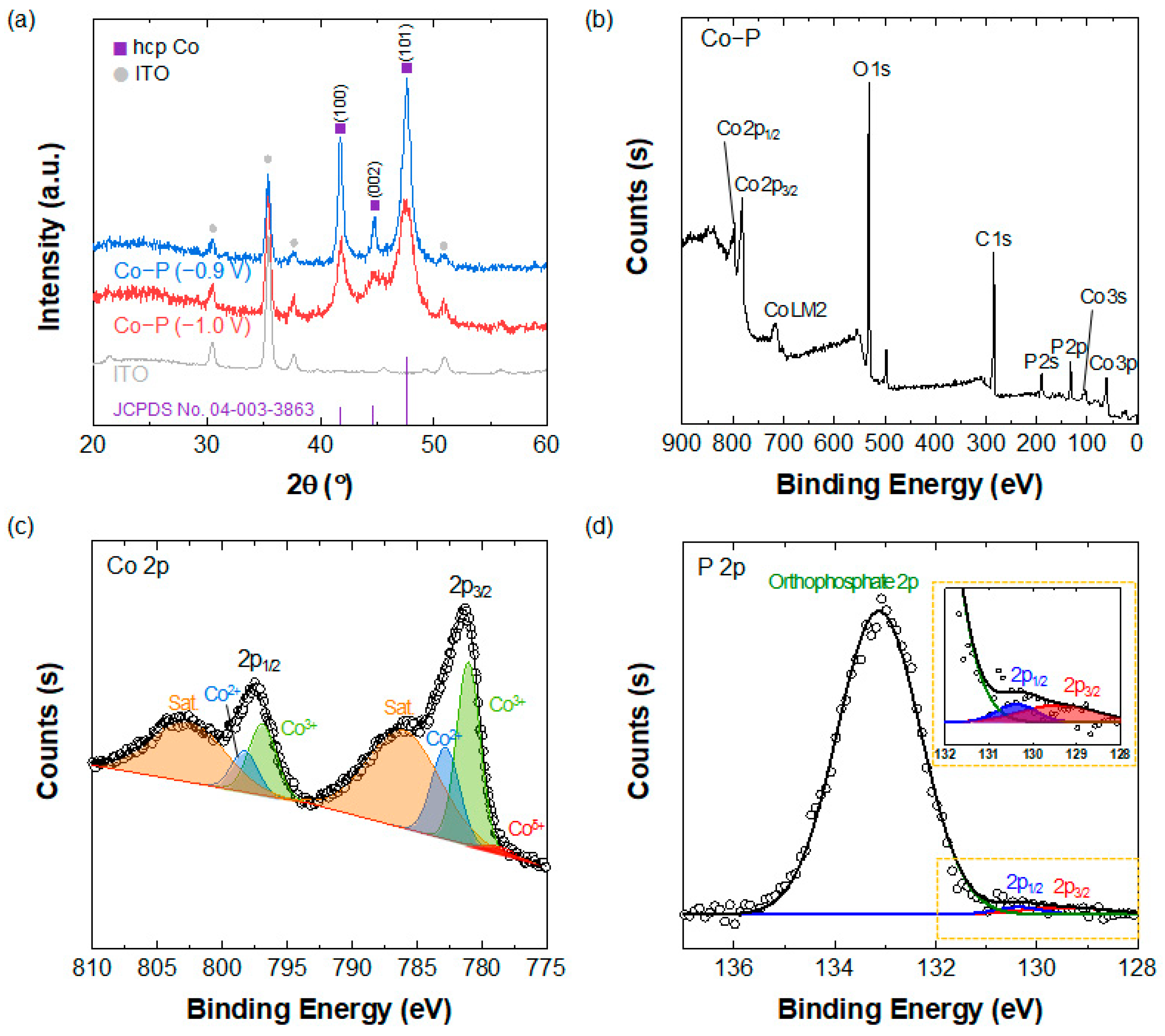
3.2. Analysis of the Chemical Composition of Co-P Nanostructures
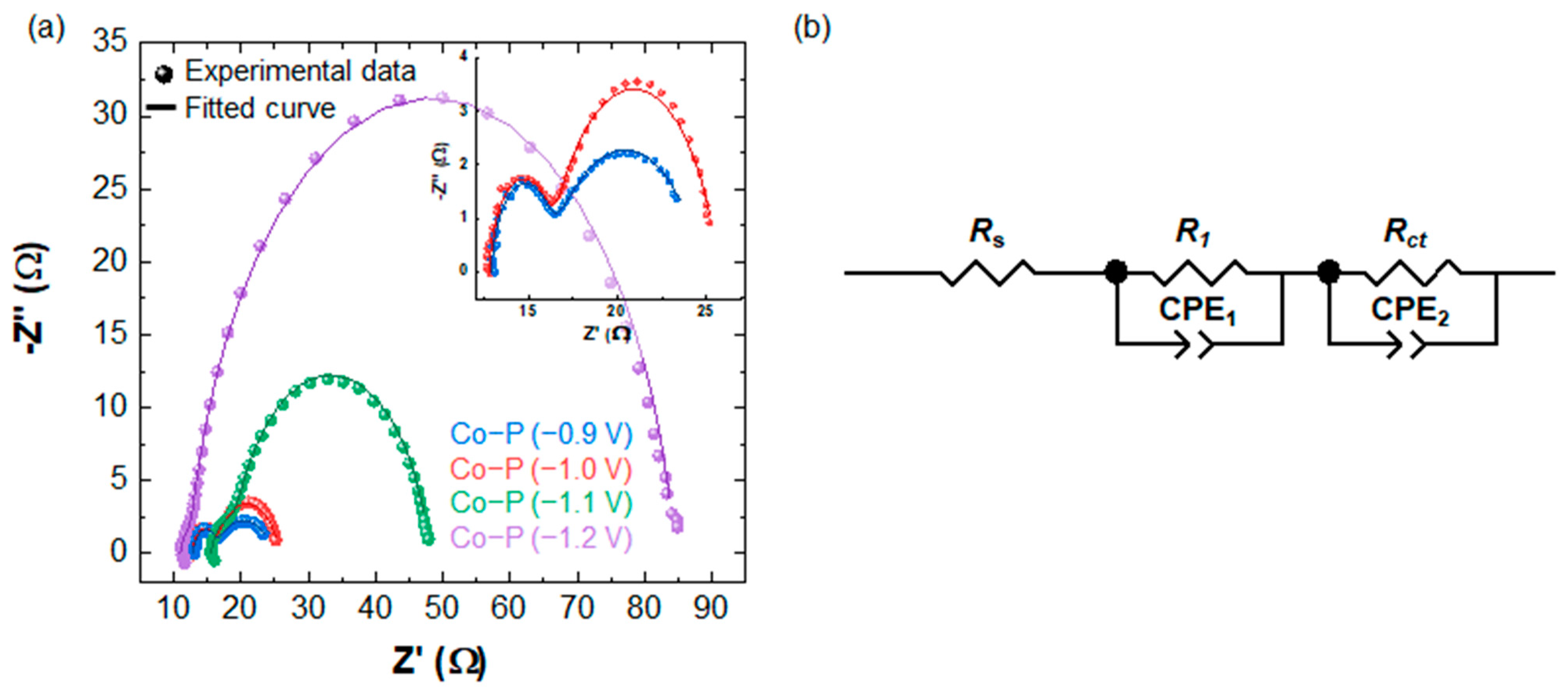
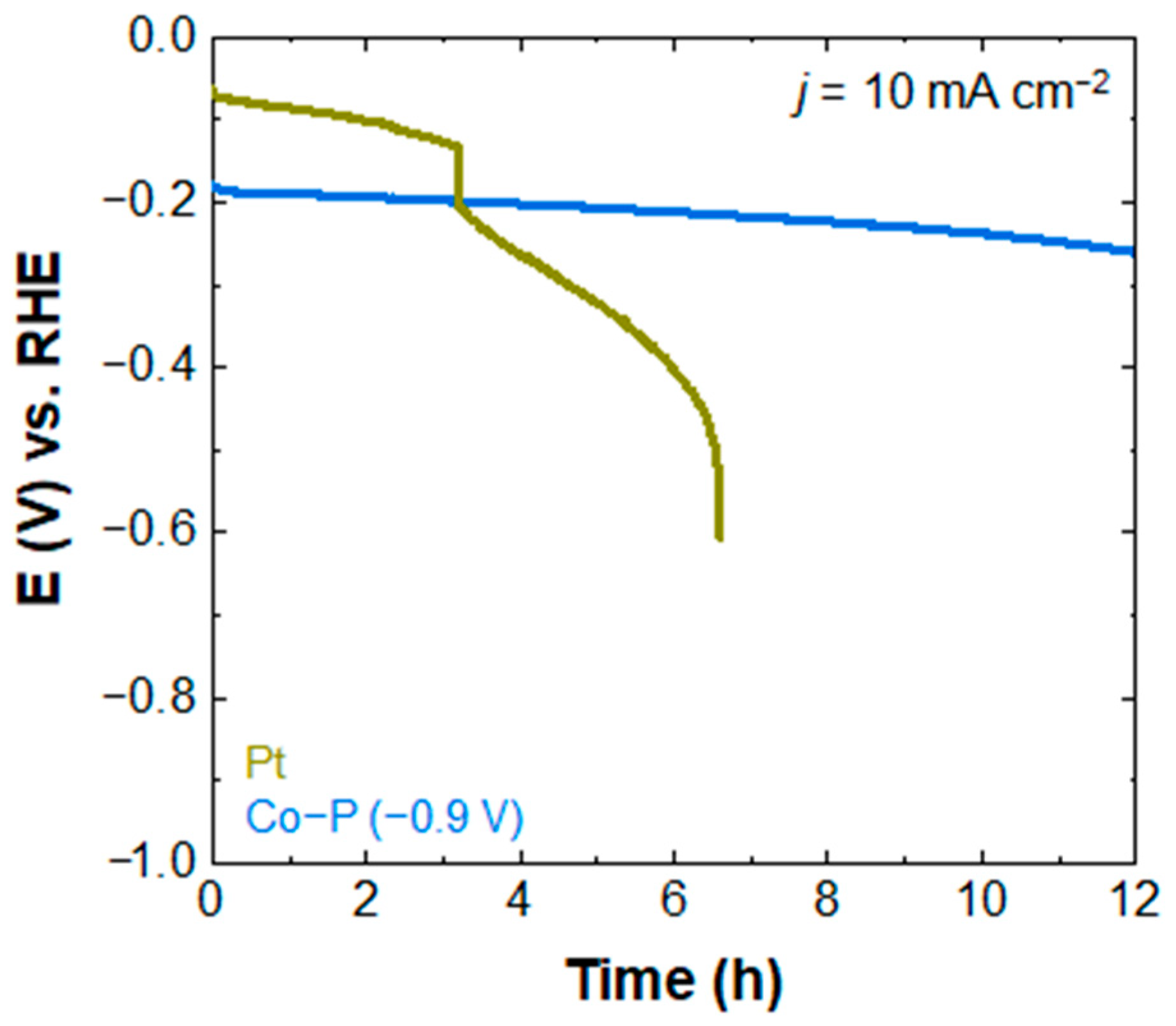
3.3. HER Performance of Co-P Electrocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-hydrogen energy conversion based on water splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef]
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking natural photosynthesis: Solar to renewable H2 fuel synthesis by Z-scheme water splitting systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Zhao, Y.; Hoivik, N.; Wang, K. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Chen, P.; Ye, J.; Wang, H.; Ouyang, L.; Zhu, M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J. Alloys Compd. 2021, 883, 160833. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, L.; Shi, J. Anion-containing noble-metal-free bifunctional electrocatalysts for overall water splitting. ACS Catal. 2018, 8, 3688–3707. [Google Scholar] [CrossRef]
- Li, Z.; Feng, H.; Song, M.; He, C.; Zhuang, W.; Tian, L. Advances in CoP electrocatalysts for water splitting. Mater. Today Energy 2021, 20, 100698. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon nanotubes decorated with CoP nanocrystals: A highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. 2014, 53, 6710–6714. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.J.; Chen, Z. CoP nanoframes as bifunctional electrocatalysts for efficient overall water splitting. ACS Catal. 2020, 10, 412–419. [Google Scholar] [CrossRef]
- Hu, G.; Tang, Q.; Jiang, D.-e. CoP for hydrogen evolution: Implications from hydrogen adsorption. Phys. Chem. Chem. Phys. 2016, 18, 23864–23871. [Google Scholar] [CrossRef]
- Cao, E.; Chen, Z.; Wu, H.; Yu, P.; Wang, Y.; Xiao, F.; Chen, S.; Du, S.; Xie, Y.; Wu, Y.; et al. Boron-induced electronic-structure reformation of CoP nanoparticles drives enhanced pH-universal hydrogen evolution. Angew. Chem. Int. Ed. 2020, 59, 4154–4160. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M.; Li, Y.; Dong, H.; Sun, D.; Liu, X.; Xu, L.; Tian, Z.; Tang, Y. Regulating the electronic structure of CoP nanosheets by O incorporation for high-efficiency electrochemical overall water splitting. Adv. Funct. Mater. 2020, 30, 1905252. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.; He, L.; Jiang, X.; Asiri, A.M.; Sun, X. Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation. Adv. Mater. 2017, 29, 1602441. [Google Scholar] [CrossRef]
- Liu, T.; Ma, X.; Liu, D.; Hao, S.; Du, G.; Ma, Y.; Asiri, A.M.; Sun, X.; Chen, L. Mn doped of CoP Nanosheets array: An efficient electrocatalyst for hydrogen evolution reaction with enhanced activity at all pH values. ACS Catal. 2017, 1, 98–102. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, M.; Song, S.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. Hierarchical Mo-doped CoP3 interconnected nanosheet arrays on carbon cloth as an efficient bifunctional electrocatalyst for water splitting in an alkaline electrolyte. Dalton Trans. 2020, 49, 5563–5572. [Google Scholar] [CrossRef]
- Song, S.; Guo, M.; Zhang, S.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B.; Xu, M. Plasma-assisted synthesis of hierarchical NiCoxPy nanosheets as robust and stable electrocatalyst for hydrogen evolution reaction in both acidic and alkaline media. Electrochim. Acta 2020, 331, 135431. [Google Scholar] [CrossRef]
- Guo, M.; Song, S.; Zhang, S.; Yan, Y.; Zhan, K.; Yang, J.; Zhao, B. Fe-doped Ni-Co phosphide nanoplates with planar defects as an efficient bifunctional electrocatalyst for overall water splitting. ACS Sustain. Chem. Eng. 2020, 8, 7436–7444. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Pu, J.; Shen, Z.; Zhong, C.; Zhou, Q.; Liu, J.; Zhu, J.; Zhang, H. Electrodeposition technologies for Li-based batteries: New frontiers of energy storage. Adv. Mater. 2020, 32, 1903808. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Bi, J.; Zhu, Q.; Han, B. Design and preparation of electrocatalysts by electrodeposition for CO2 reduction. Chem. Eur. J. 2022, 28, e202200242. [Google Scholar]
- Han, G.-Q.; Li, X.; Liu, Y.-R.; Dong, B.; Hu, W.-H.; Shang, X.; Zhao, X.; Chai, Y.-M.; Liu, Y.-Q.; Liu, C.-G. Controllable synthesis of three dimensional electrodeposited Co–P nanosphere arrays as efficient electrocatalysts for overall water splitting. RSC Adv. 2016, 6, 52761–52771. [Google Scholar] [CrossRef]
- Saadi, F.H.; Carim, A.I.; Verlage, E.; Hemminger, J.C.; Lewis, N.S.; Soriaga, M.P. CoP as acid-stable active electrocatalyst for the hydrogen-evolution reaction: Electrochemical synthesis, interfacial characterization and performance evaluation. J. Phys. Chem. C 2014, 118, 29294–29300. [Google Scholar] [CrossRef]
- Purtov, J.; Verch, A.; Rogin, P.; Hensel, R. Improved development procedure to enhance the stability of microstructures created by two-photon polymerization. Microelectron. Eng. 2018, 194, 45–50. [Google Scholar] [CrossRef]
- Purtov, J.; Rogin, P.; Verch, A.; Johansen, V.E.; Hensel, R. Nanopillar diffraction gratings by two-photon lithography. Nanomaterials 2019, 9, 1495. [Google Scholar] [CrossRef]
- Wei, N.; Tian, Y.; Liao, Y.; Komatsu, N.; Gao, W.; Lyuleeva-Husemann, A.; Zhang, Q.; Hussain, A.; Ding, E.-X.; Yao, F.; et al. Colors of single-wall carbon nanotubes. Adv. Mater. 2021, 33, 2006395. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Liu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Self-Supported Cobalt Phosphide Mesoporous Nanorod Arrays: A Flexible and Bifunctional Electrode for Highly Active Electrocatalytic Water Reduction and Oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347. [Google Scholar] [CrossRef]
- Huang, J.W.; Li, Y.R.; Xia, Y.F.; Zhu, J.T.; Yi, Q.H.; Wang, H.; Xiong, J.; Sun, Y.H.; Zou, G.F. Flexible cobalt phosphide network electrocatalyst for hydrogen evolution at all pH values. Nano Res. 2017, 10, 1010–1020. [Google Scholar] [CrossRef]
- Liang, H.F.; Gandi, A.N.; Anjum, D.H.; Wang, X.B.; Schwingenschlögl, U.; Alshareef, H.N. Plasma-assisted synthesis of NiCoP for efficient overall water splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; Xu, Y.; Duan, F.; Yu, Q.; Wang, J.; Chen, L.; Dan, Y.; Cheng, X. Electrodeposited cobalt phosphides with hierarchical nanostructure on biomass carbon for bifunctional water splitting in alkaline solution. J. Alloys Compd. 2020, 829, 154535. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Hou, Y.; Xie, Q.; Wang, L.; Geng, H.; Peng, D. Magnetically separable and recyclable urchin-like Co–P hollow nanocomposites for catalytic hydrogen generation. J. Power Sources 2014, 260, 100–108. [Google Scholar] [CrossRef]
- Carenco, S.; Hu, Y.; Florea, I.; Ersen, O.; Boissiere, C.; Mezailles, N.; Sanchez, C. Metal-dependent interplay between crystallization and phosphorus diffusion during the synthesis of metal phosphide nanoparticles. Chem. Mater. 2012, 24, 4134–4145. [Google Scholar] [CrossRef]
- Liu, T.; Li, P.; Yao, N.; Cheng, G.; Chen, S.; Luo, W.; Yin, Y. CoP-doped MOF-based electrocatalyst for pH-universal hydrogen evolution reaction. Angew. Chem. Int. Ed. 2019, 58, 4679–4684. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef]
- Merki, D.; Vrubel, H.; Rovelli, L.; Fierro, S.; Hu, X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012, 3, 2515–2525. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, C.; Xie, Y.; Tumlin, T.; Giri, L.; Karna, S.P.; Lin, J. Laser induced MoS2/carbon hybrids for hydrogen evolution reaction catalysts. J. Mater. Chem. A 2016, 4, 6824–6830. [Google Scholar] [CrossRef]
- Yoon, H.; Song, H.J.; Ju, B.; Kim, D.-W. Cobalt phosphide nanoarrays with crystalline-amorphous hybrid phase for hydrogen production in universal-pH. Nano Res. 2020, 13, 2469–2477. [Google Scholar] [CrossRef]
- Ali, M.; Wahid, M.; Majid, K. Mixed NiCo-phosphate/sulphide heterostructure as an efficient electrocatalyst for hydrogen evolution reaction. J. Appl. Electrochem. 2022; in press. [Google Scholar]
- Bockris, J.O.’M.; Potter, E.C. The mechanism of the cathodic hydrogen evolution reaction. J. Electochem. Soc. 1952, 99, 169–186. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acidic vs alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Oh, S.; Kim, H.; Kwon, Y.; Kim, M.; Cho, E.; Kwon, H. Porous Co-P foam as an efficient bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 18272–18277. [Google Scholar] [CrossRef]
- Kornienko, N.; Heidary, N.; Cibin, G.; Reisner, E. Catalysis by design: Development of a bifunctional water splitting catalyst through an operando measurement directed optimization cycle. Chem. Sci. 2019, 9, 5322–5333. [Google Scholar] [CrossRef]
- Anumol, E.A.; Kundu, P.; Deshpande, P.A.; Madras, G.; Ravishankar, N. New insights into selective heterogeneous nucleation of metal nanoparticles on oxides by microwave-assisted reduction: Rapid synthesis of high-activity supported catalysts. ACS Nano 2011, 5, 8049–8061. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jang, Y.J.; Jang, Y.H. Electrodeposition of Stable Noble-Metal-Free Co-P Electrocatalysts for Hydrogen Evolution Reaction. Materials 2023, 16, 593. https://doi.org/10.3390/ma16020593
Kim J, Jang YJ, Jang YH. Electrodeposition of Stable Noble-Metal-Free Co-P Electrocatalysts for Hydrogen Evolution Reaction. Materials. 2023; 16(2):593. https://doi.org/10.3390/ma16020593
Chicago/Turabian StyleKim, Jeongwon, Yu Jin Jang, and Yoon Hee Jang. 2023. "Electrodeposition of Stable Noble-Metal-Free Co-P Electrocatalysts for Hydrogen Evolution Reaction" Materials 16, no. 2: 593. https://doi.org/10.3390/ma16020593
APA StyleKim, J., Jang, Y. J., & Jang, Y. H. (2023). Electrodeposition of Stable Noble-Metal-Free Co-P Electrocatalysts for Hydrogen Evolution Reaction. Materials, 16(2), 593. https://doi.org/10.3390/ma16020593






