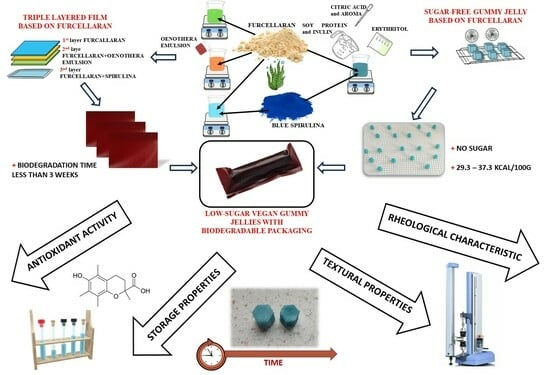
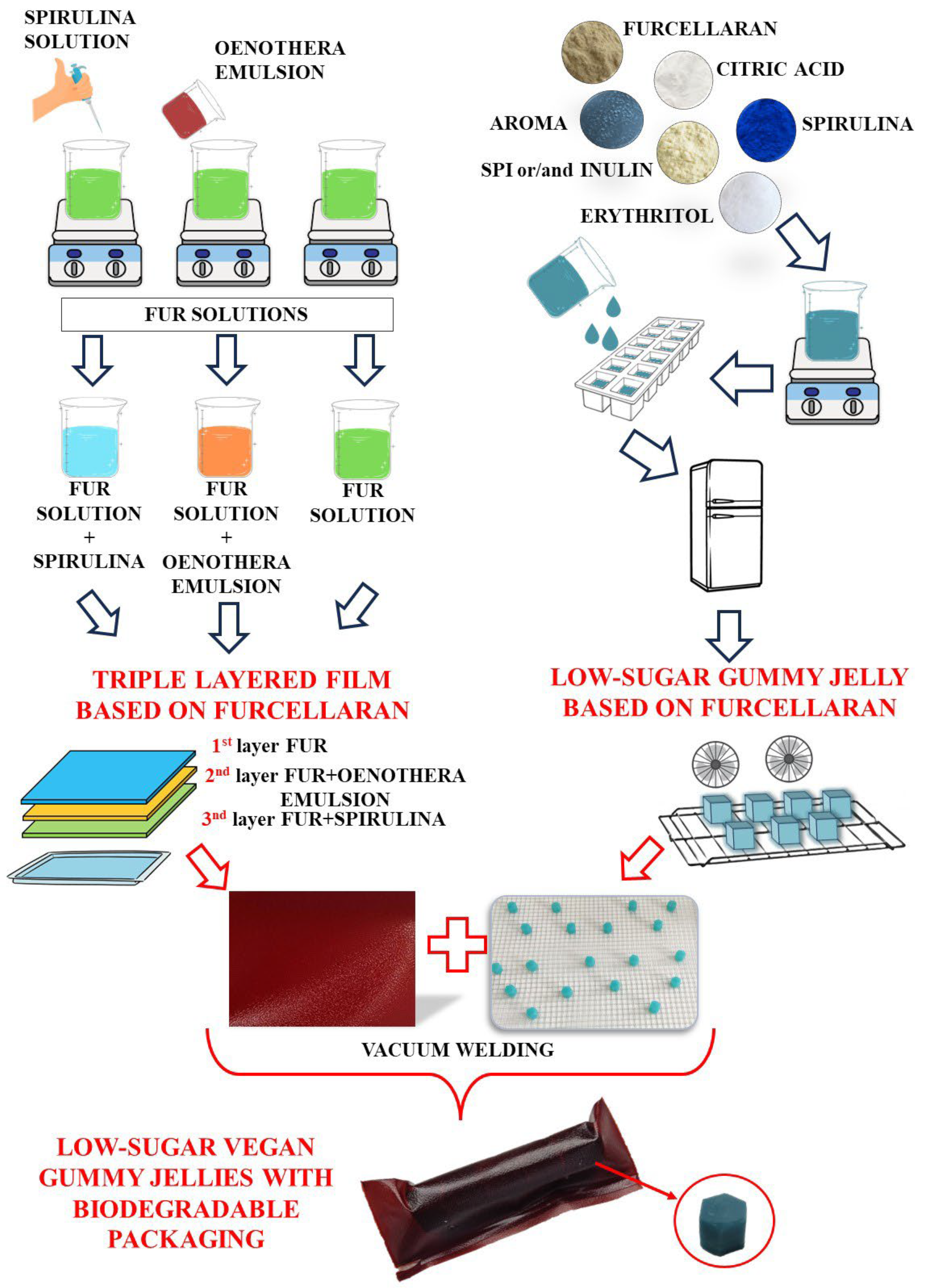
Sugar-Free, Vegan, Furcellaran Gummy Jellies with Plant-Based Triple-Layer Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
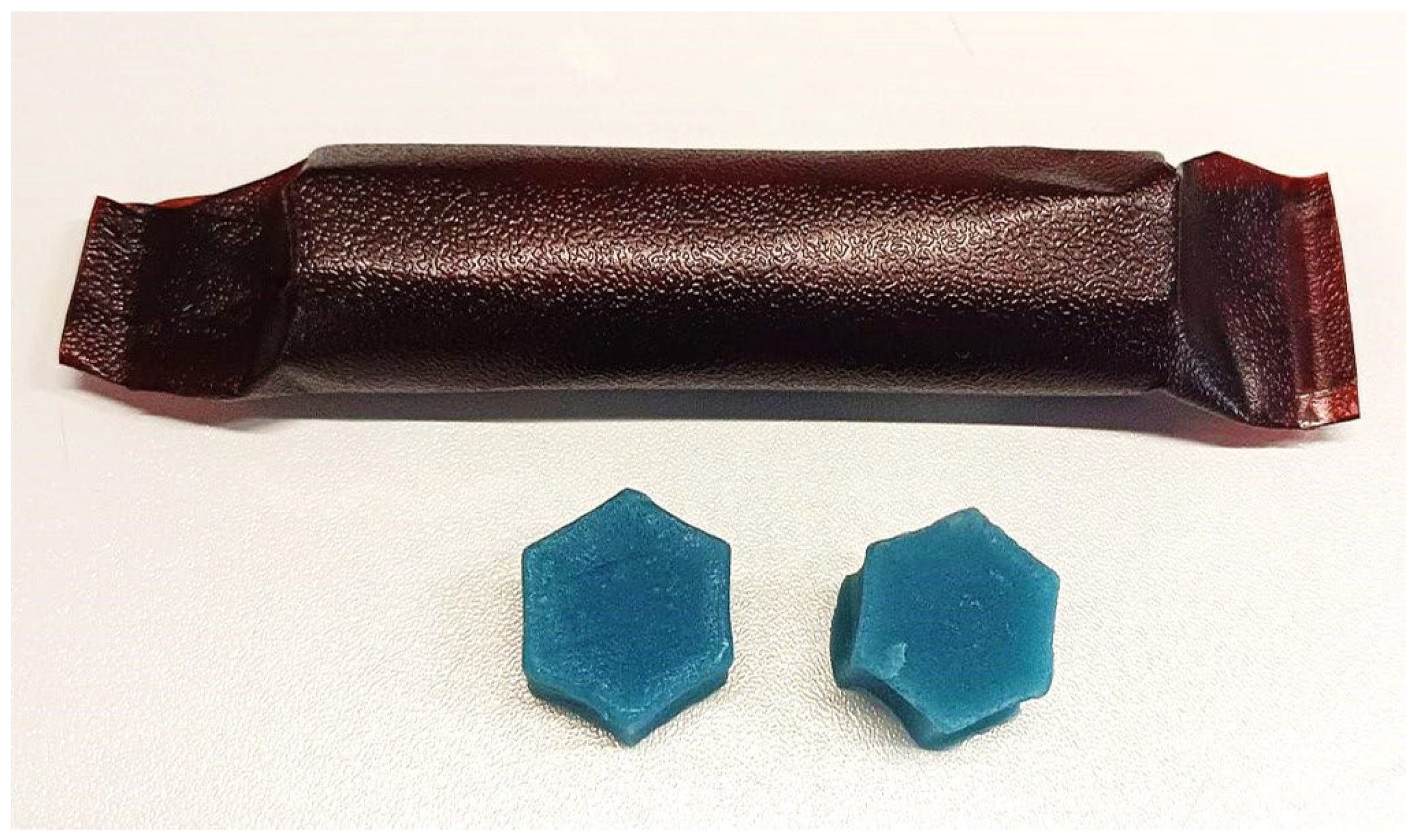
2.2. Preparations of Furcellaran Gummy Jellies
2.3. Rheological Characteristics of the Gels
2.4. Physicochemical Properties and Nutritional Labeling of Gummy Jellies
2.5. Antioxidant Activity
2.6. Texture Profile Analysis
2.7. Film Preparations, Packing and Storage Analysis
2.7.1. Isolation of Protein from Post-Production Oenothera Pomace
2.7.2. Preparation of the Emulsion and Spirulina Solutions
2.7.3. Preparation of the Multilayer Film
2.7.4. Packing and Storage
2.8. Statistical Analyses
3. Results and Discussion
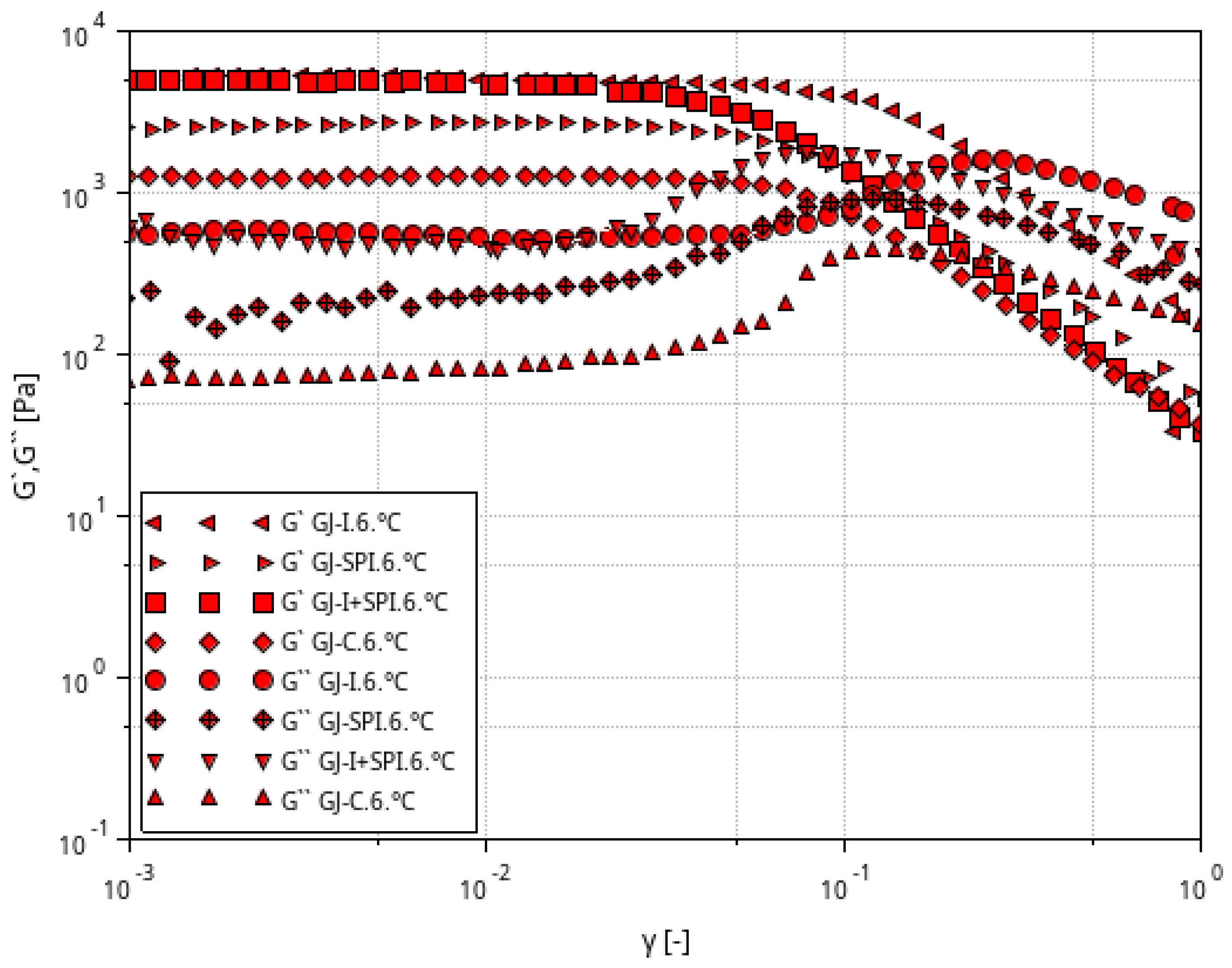
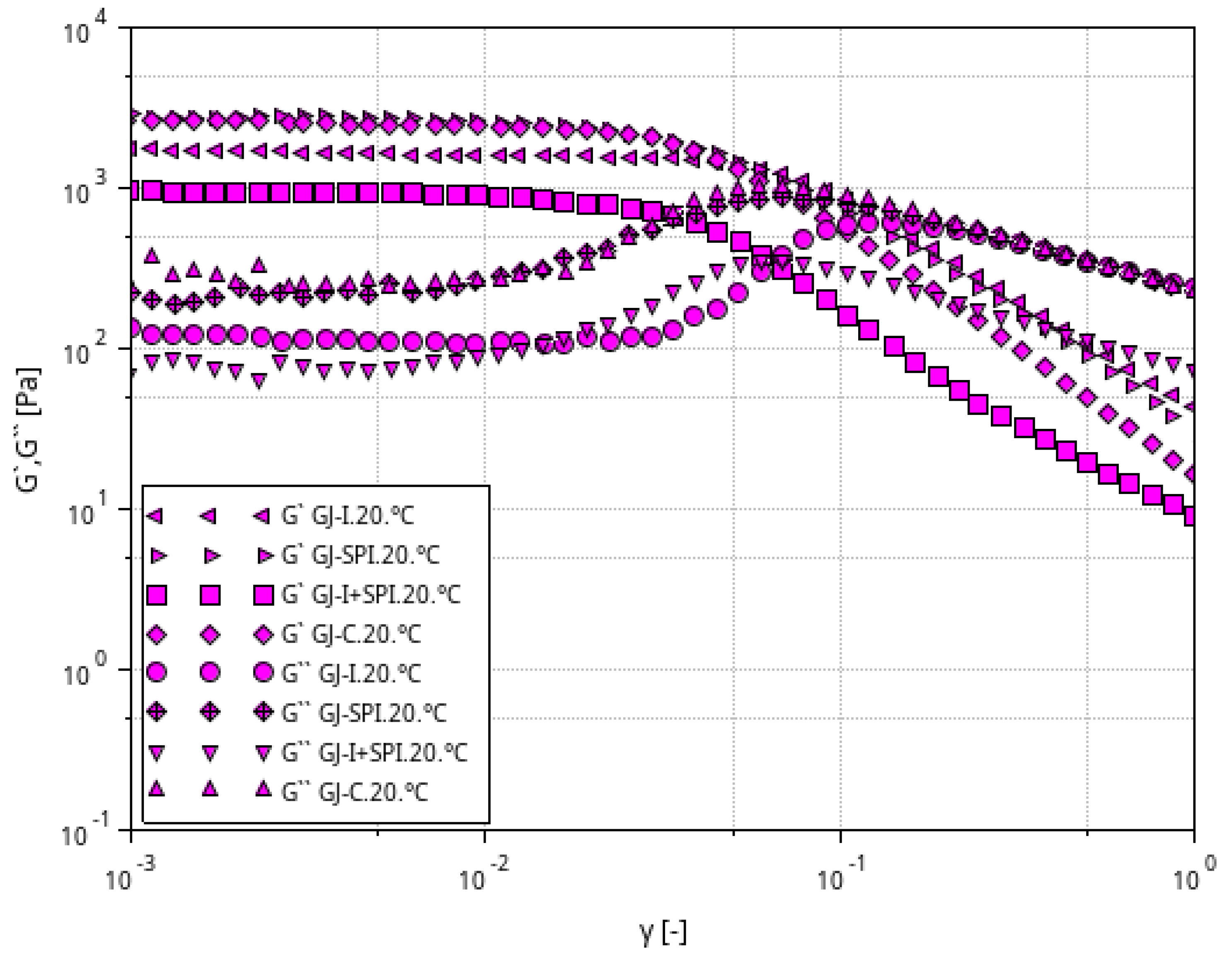
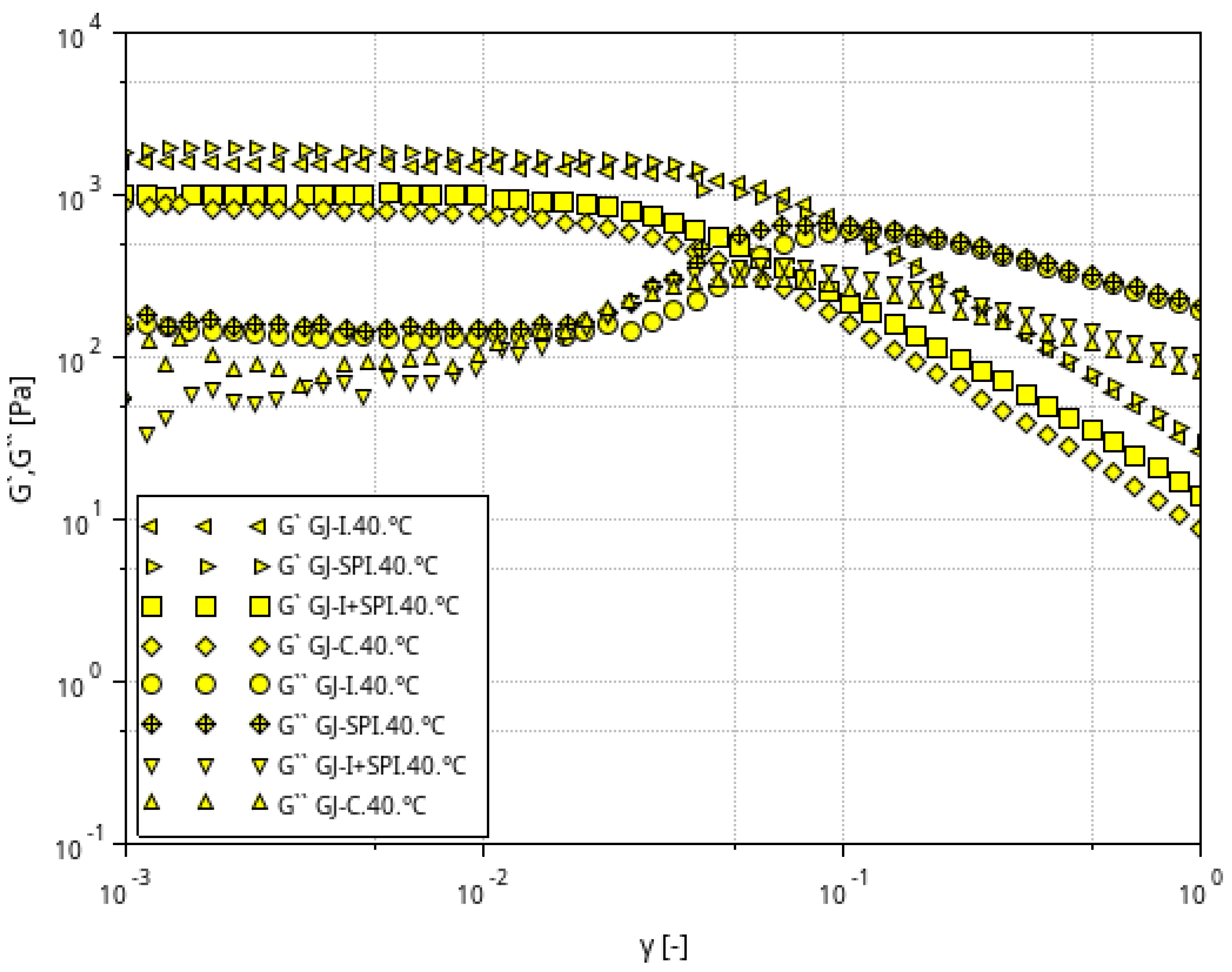
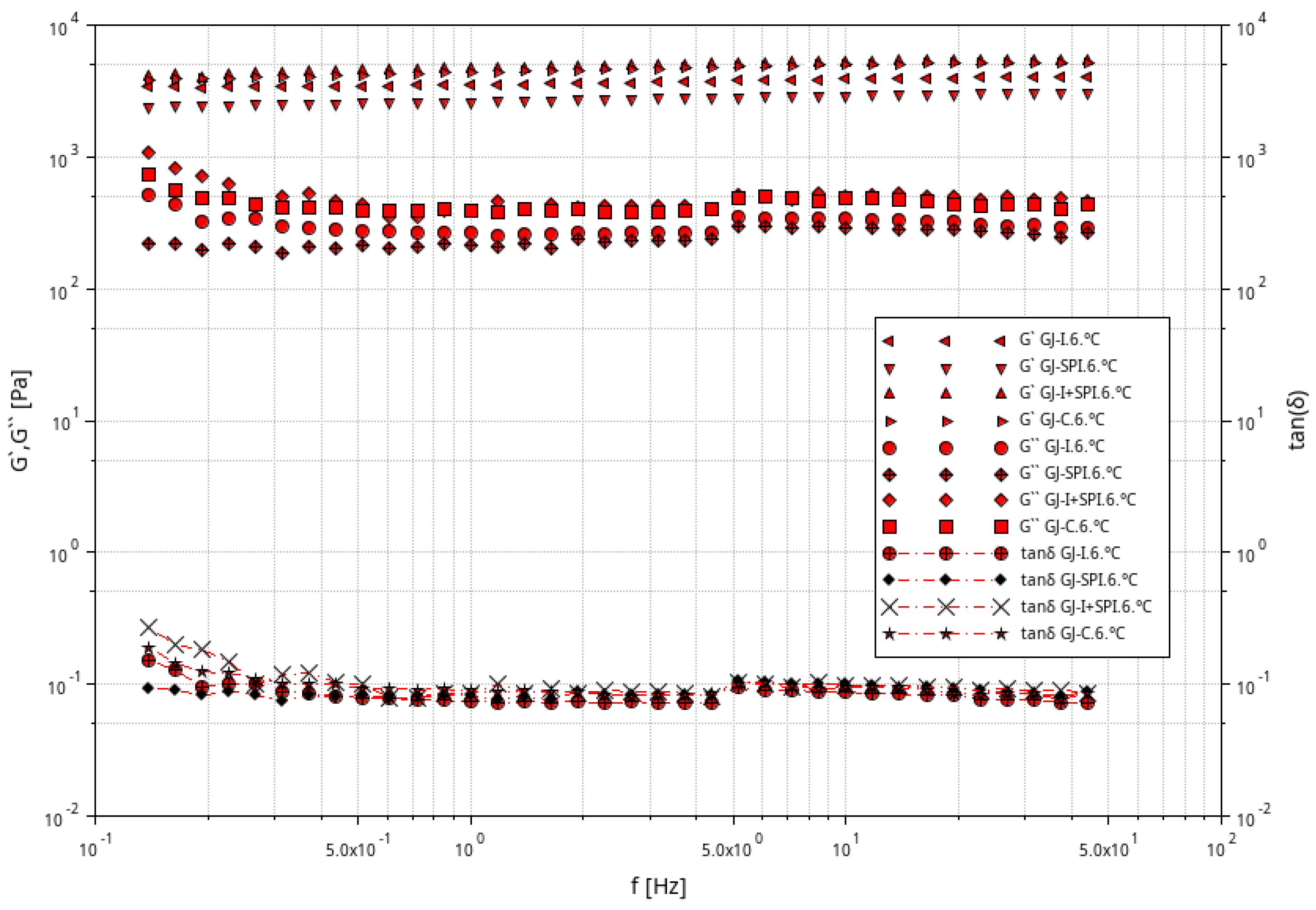
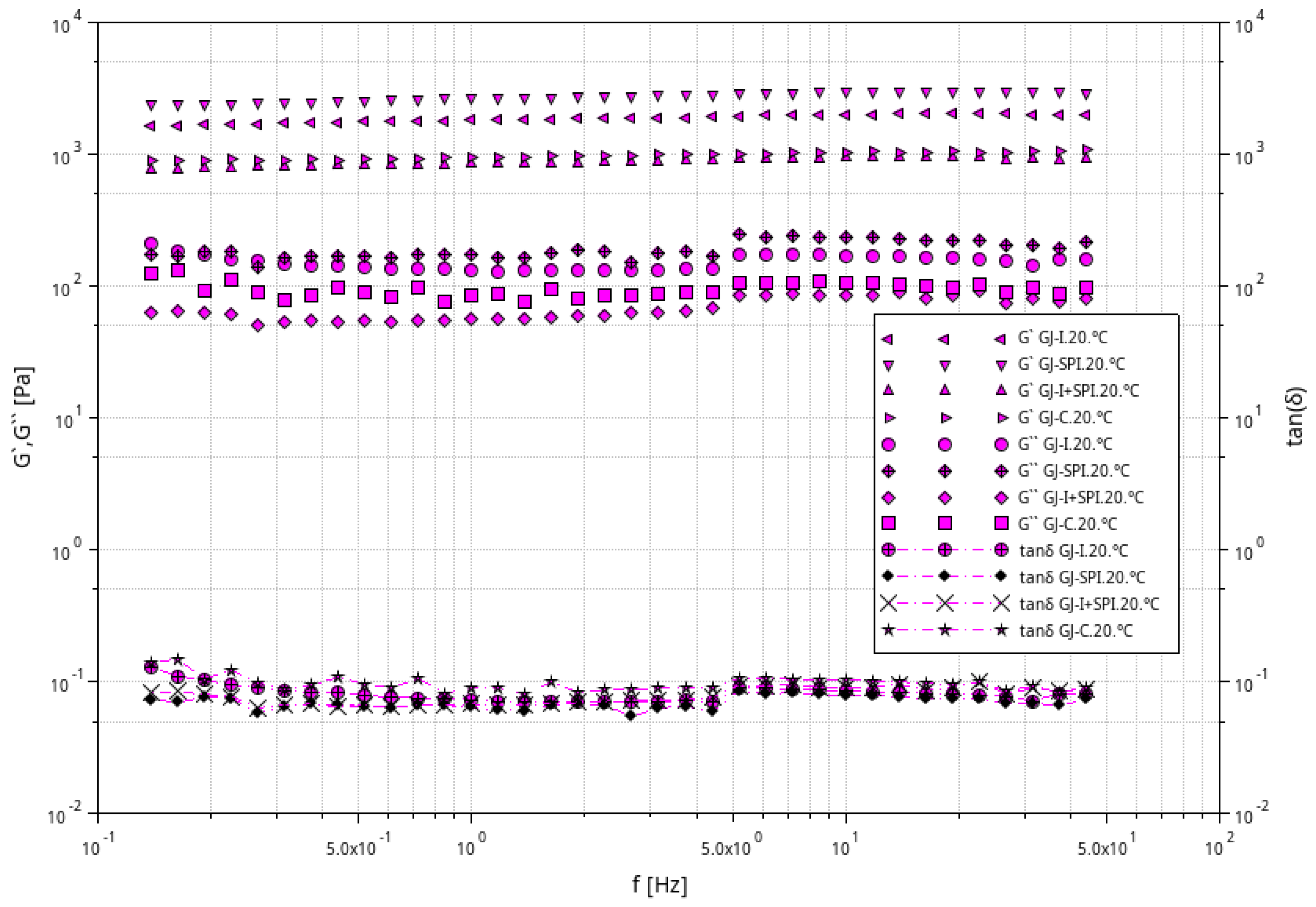
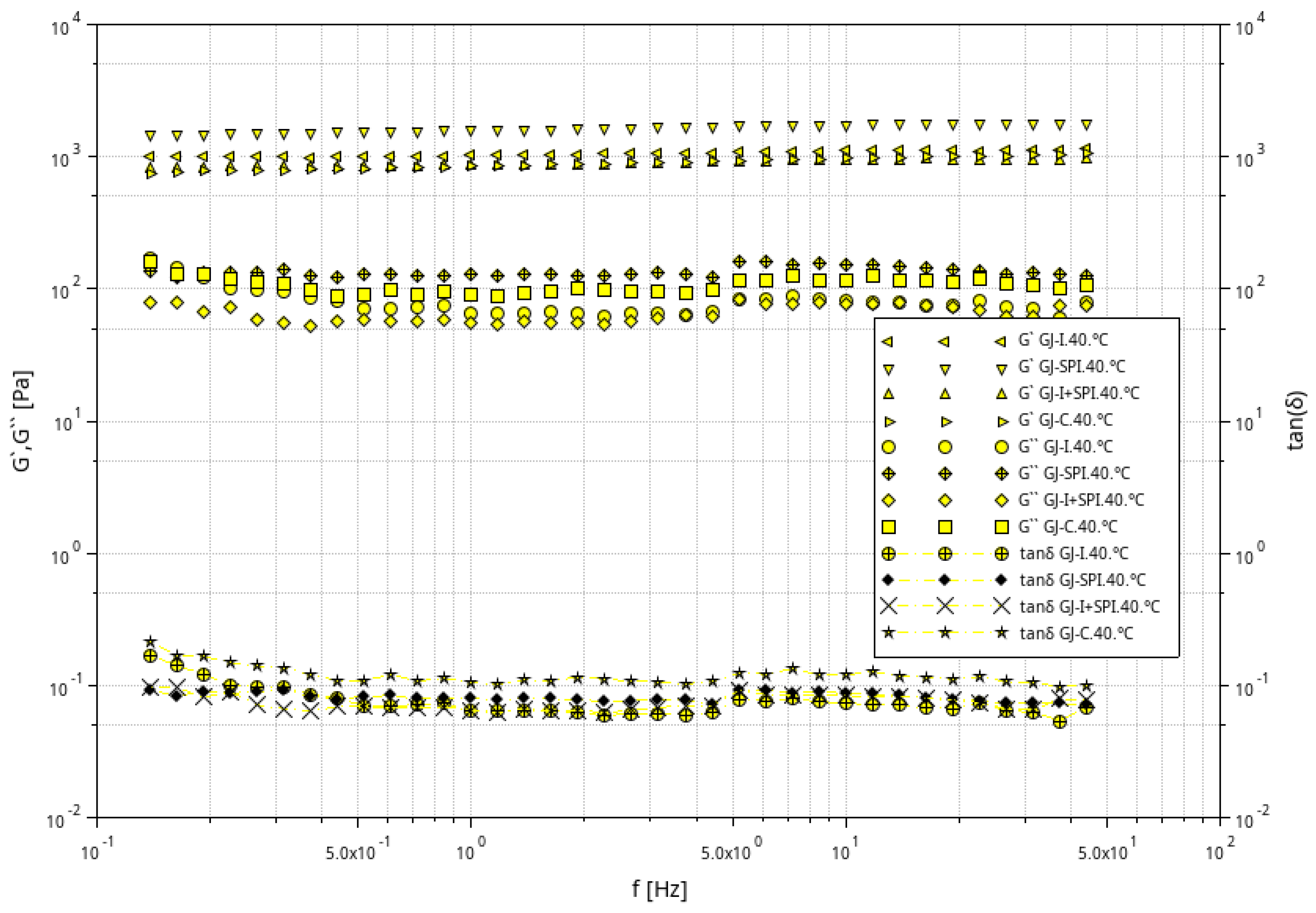
3.1. Rheological Characteristic of Furcellaran Gel Systems
3.2. Physicochemical Properties and Nutritional Labeling of Gummy Candies
3.3. Antioxidant Activity
3.4. Textural Properties of Gummy Jelly
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laos, K.; Ring, S.G. Note: Characterisation of Furcellaran Samples from Estonian Furcellaria Lumbricalis (Rhodophyta). J. Appl. Phycol. 2005, 17, 461–464. [Google Scholar] [CrossRef]
- Imeson, A.P. Carrageenan and Furcellaran. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 164–185. ISBN 978-1-84569-414-2. [Google Scholar]
- Marangoni, L., Jr.; Vieira, R.P.; Jamróz, E.; Anjos, C.A.R. Furcellaran: An Innovative Biopolymer in the Production of Films and Coatings. Carbohydr. Polym. 2021, 252, 117221. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.J.F. Evening Primrose (Oenothera Spp.) Oil and Seed. J. Am. Oil Chem. Soc. 1984, 61, 540–543. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pouramin, S. Optimization of Extraction and Deamidation of Edible Protein from Evening Primrose (Oenothera Biennis L.) Oil Processing by-Products and Its Effect on Structural and Techno-Functional Properties. Food Chem. 2021, 334, 127613. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chiou, B.; Xia, Y.; Chen, M.; Liu, F.; Zhong, F. The Improvement of Texture Properties and Storage Stability for Kappa Carrageenan in Developing Vegan Gummy Candies. J. Sci. Food Agric. 2022, 102, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Utomo, B.S.B.; Darmawan, M.; Hakim, A.R.; Ardi, D.T. Physicochemical properties and sensory evaluation of jelly candy made from different ratio of k-carrageenan and konjac. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2014, 9, 25. [Google Scholar] [CrossRef][Green Version]
- De Avelar, M.H.M.; Efraim, P. Alginate/Pectin Cold-Set Gelation as a Potential Sustainable Method for Jelly Candy Production. LWT 2020, 123, 109119. [Google Scholar] [CrossRef]
- Ghiraldi, M.; Franco, B.G.; Moraes, I.C.F.; Pinho, S.C. Emulsion-Filled Pectin Gels for Vehiculation of Vitamins D 3 and B 12: From Structuring to the Development of Enriched Vegan Gummy Candies. ACS Food Sci. Technol. 2021, 1, 1945–1952. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J. Confectionery Gels: A Review on Formulation, Rheological and Structural Aspects. Int. J. Food Prop. 2009, 12, 176–210. [Google Scholar] [CrossRef]
- Ergun, R.; Lietha, R.; Hartel, R.W. Moisture and Shelf Life in Sugar Confections. Crit. Rev. Food Sci. Nutr. 2010, 50, 162–192. [Google Scholar] [CrossRef]
- Rippe, J.; Angelopoulos, T. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Lekahena, V.N.J.; Boboleha, M.R. The Effects of Sucrose Substitution with Sorbitol on Physicochemical Properties and Sensory Evaluation of Seaweed Jelly Candy. In Proceedings of the 5th International Conference on Food, Agriculture and Natural Resources (FANRes 2019); Atlantis Press: Ternate, Indonesia, 2020. [Google Scholar]
- Riedel, R.; Böhme, B.; Rohm, H. Development of Formulations for Reduced-Sugar and Sugar-Free Agar-Based Fruit Jellies. Int. J. Food Sci. Technol. 2015, 50, 1338–1344. [Google Scholar] [CrossRef]
- Gok, S.; Toker, O.S.; Palabiyik, I.; Konar, N. Usage Possibility of Mannitol and Soluble Wheat Fiber in Low Calorie Gummy Candies. LWT 2020, 128, 109531. [Google Scholar] [CrossRef]
- Bartkiene, E.; Sakiene, V.; Bartkevics, V.; Wiacek, C.; Rusko, J.; Lele, V.; Ruzauskas, M.; Juodeikiene, G.; Klupsaite, D.; Bernatoniene, J.; et al. Nutraceuticals in Gummy Candies Form Prepared from Lacto-Fermented Lupine Protein Concentrates, as High-Quality Protein Source, Incorporated with Citrus Paradise L. Essential Oil and Xylitol. Int. J. Food Sci. Technol. 2018, 53, 2015–2025. [Google Scholar] [CrossRef]
- Lele, V.; Ruzauskas, M.; Zavistanaviciute, P.; Laurusiene, R.; Rimene, G.; Kiudulaite, D.; Tomkeviciute, J.; Nemeikstyte, J.; Stankevicius, R.; Bartkiene, E. Development and Characterization of the Gummy–Supplements, Enriched with Probiotics and Prebiotics. CyTA—J. Food 2018, 16, 580–587. [Google Scholar] [CrossRef]
- Silva, J.R.; Silva, J.B.D.; Costa, G.N.; Santos, J.S.D.; Castro-Gomez, R.J.H. Probiotic Gummy Candy with Xylitol: Development and Potential Inhibition of Streptococcus Mutans UA 159. RSD 2020, 9, e7369108942. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, J.; Yu, H. Effect of Gelatin Content and Oral Processing Ability on Vitamin C Release in Gummy Jelly. J. Food Sci. Technol. 2022, 59, 677–685. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus Officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Gramza-Michalowska, A.; Regula, J. Use of Tea Extracts (Camelia Sinensis) in Jelly Candies as Polyphenols Sources in Human Diet. Asia Pac. J. Clin. Nutr. 2007, 16, 43–46. [Google Scholar]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-Use Plastics: Production, Usage, Disposal, and Adverse Impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-Based Nanocomposite Films and Coatings: Recent Advances in Shelf-Life Improvement of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2022, 62, 1912–1935. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-Based Functional Films for Packaging Applications: A Review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in Polysaccharide and Protein Films and Coatings for Food Contact—A Review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Tkaczewska, J.; Juszczak, L.; Krzyściak, P.; Cholewa-Wójcik, A.; Nowak, N.; Guzik, P.; Szuwarzyński, M.; Mazur, T.; Jamróz, E. Improving the Quality of Multi-Layer Films Based on Furcellaran by Immobilising Active Ingredients and Impact Assessment of the Use of a New Packaging Material. Food Chem. 2023, 428, 136759. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The Verification of Intelligent Properties of Furcellaran Films with Plant Extracts on the Stored Fresh Atlantic Mackerel during Storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Jancikova, S.; Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Dordevic, D. Furcellaran/Gelatin Hydrolysate/Rosemary Extract Composite Films as Active and Intelligent Packaging Materials. Int. J. Biol. Macromol. 2019, 131, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Dziubiński, M.; Kiljański, T.; Sęk, J. Podstawy Teoretyczne i Metody Pomiarowe Reologii; Wydawnictwo Politechniki Łódzkiej: Łódź, Poland, 2014; ISBN 83-7283-641-8. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Zając, M.; Jamróz, E.; Derbew, H. Utilising Waste from Soybean Processing as Raw Materials for the Production of Preparations with Antioxidant Properties, Serving as Natural Food Preservatives—A Pilot Study. LWT 2022, 160, 113282. [Google Scholar] [CrossRef]
- Trigui, I.; Yaich, H.; Sila, A.; Cheikh-Rouhou, S.; Krichen, F.; Bougatef, A.; Attia, H.; Ayadi, M.A. Physical, Techno-Functional and Antioxidant Properties of Black Cumin Seeds Protein Isolate and Hydrolysates. Food Meas. 2021, 15, 3491–3500. [Google Scholar] [CrossRef]
- Ahmed, J.; Basu, S. Advances in Food Rheology and Its Applications; Woodhead Publishing: Thorston, UK, 2016; ISBN 0-08-100432-X. [Google Scholar]
- Eshtiaghi, N.; Markis, F.; Baudez, J.-C.; Slatter, P. Proxy Model Materials to Simulate the Elastic Properties of Digested Municipal Sludge. Water Res. 2013, 47, 5557–5563. [Google Scholar] [CrossRef]
- Pfaff, N.M.; Dijksman, J.A.; Kemperman, A.J.B.; Van Loosdrecht, M.C.M.; Kleijn, J.M. Rheological Characterisation of Alginate-like Exopolymer Gels Crosslinked with Calcium. Water Res. 2021, 207, 117835. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, H.; Yang, H. Effects of Sucrose Addition on the Rheology and Microstructure of κ-Carrageenan Gel. Food Hydrocoll. 2018, 75, 164–173. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Yang, H. Characterisation of Rheology and Microstructures of κ-Carrageenan in Ethanol-Water Mixtures. Food Res. Int. 2018, 107, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Owczarz, P.; Rył, A.; Modrzejewska, Z.; Dziubiński, M. The Influence of The Addition of Collagen on The Rheological Properties of Chitosan Chloride Solutions. PCACD 2017, XXII, 176–189. [Google Scholar] [CrossRef]
- Lapasin, R. Rheology of Industrial Polysaccharides: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 1-4615-2185-8. [Google Scholar]
- Bot, A.; Erle, U.; Vreeker, R.; Agterof, W.G.M. Influence of Crystallisation Conditions on the Large Deformation Rheology of Inulin Gels. Food Hydrocoll. 2004, 18, 547–556. [Google Scholar] [CrossRef]
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M.L. Optical, Mechanical and Sensory Properties of Based-Isomaltulose Gummy Confections. Food Biosci. 2014, 7, 37–44. [Google Scholar] [CrossRef]
- Jamróz, E.; Tkaczewska, J.; Zając, M.; Guzik, P.; Juszczak, L.; Kawecka, A.; Turek, K.; Zimowska, M.; Wojdyło, A. Utilisation of Soybean Post-Production Waste in Single- and Double-Layered Films Based on Furcellaran to Obtain Packaging Materials for Food Products Prone to Oxidation. Food Chem. 2022, 387, 132883. [Google Scholar] [CrossRef]
- Jamróz, E.; Cabaj, A.; Tkaczewska, J.; Kawecka, A.; Krzyściak, P.; Szuwarzyński, M.; Mazur, T.; Juszczak, L. Incorporation of Curcumin Extract with Lemongrass Essential Oil into the Middle Layer of Triple-Layered Films Based on Furcellaran/Chitosan/Gelatin Hydrolysates—In Vitro and in Vivo Studies on Active and Intelligent Properties. Food Chem. 2023, 402, 134476. [Google Scholar] [CrossRef]
- Delgado, P.; Bañón, S. Determining the Minimum Drying Time of Gummy Confections Based on Their Mechanical Properties. CyTA—J. Food 2015, 13, 329–335. [Google Scholar] [CrossRef]
- EU. Regulation (EC) No 1924/2006—Nutrition and Health Claims on Foods; European Union: Maastricht, The Netherlands, 2006. [Google Scholar]
- Rachman, A.; Brennan, M.A.; Morton, J.; Torrico, D.; Brennan, C.S. In-Vitro Digestibility, Protein Digestibility Corrected Amino Acid, and Sensory Properties of Banana-Cassava Gluten-Free Pasta with Soy Protein Isolate and Egg White Protein Addition. Food Sci. Hum. Wellness 2023, 12, 520–527. [Google Scholar] [CrossRef]
- Penta-Ramos, E.A.; Xiong, Y.L. Antioxidant Activity of Soy Protein Hydrolysates in a Liposomal System. J. Food Sci. 2002, 67, 2952–2956. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhou, K. Chelating and Radical Scavenging Activities of Soy Protein Hydrolysates Prepared from Microbial Proteases and Their Effect on Meat Lipid Peroxidation. Bioresour. Technol. 2010, 101, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Kim, S.-J.; You, Y.-S.; Lacroix, M.; Han, J. Inhibitory Effect of Soy Protein Coating Formulations on Walnut (Juglans Regia L.) Kernels against Lipid Oxidation. LWT—Food Sci. Technol. 2013, 51, 393–396. [Google Scholar] [CrossRef]
- Synge, R.L.M. Interactions of Polyphenols with Proteins in Plants and Plant Products. Plant Foods Hum. Nutr. 1975, 24, 337–350. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, L.; Su, Y.; Chang, C.; Yang, Y.; Li, J. Development of Soy Protein Isolate/κ-Carrageenan Composite Hydrogels as a Delivery System for Hydrophilic Compounds: Monascus Yellow. Int. J. Biol. Macromol. 2021, 172, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Baeza, R.I.; Carp, D.J.; Pérez, O.E.; Pilosof, A.M.R. κ -Carrageenan—Protein Interactions: Effect of Proteins on Polysaccharide Gelling and Textural Properties. LWT 2002, 35, 741–747. [Google Scholar] [CrossRef]
| Component (g/100 g) | Sample Code | |||
|---|---|---|---|---|
| GJ-I | GJ-SPI | GJ-I+SPI | GJ-C | |
| Furcellaran | 4.00 | 4.00 | 4.00 | 4.00 |
| Erythritol | 10.00 | 10.00 | 10.00 | 10.00 |
| Soy protein isolate | 0.00 | 2.00 | 1.00 | 0.00 |
| Inulin | 2.00 | 0.00 | 1.00 | 0.00 |
| Citric acid | 0.20 | 0.20 | 0.20 | 0.20 |
| Spirulina | 0.25 | 0.25 | 0.25 | 0.25 |
| Aroma | 0.01 | 0.01 | 0.01 | 0.01 |
| Water | 83.55 | 83.55 | 83.55 | 85.55 |
| Weight (g) | Water Activity | pH | ||
|---|---|---|---|---|
| after gelling | GJ-I | 10.03 ± 0.19 f | 0.982 ± 0.001 b | 3.94 ± 0.09 b |
| GJ-SPI | 9.54 ± 0.14 f | 0.980 ± 0.001 b | 4.24 ± 0.12 cd | |
| GJ-I+SPI | 9.55 ± 0.09 f | 0.980 ± 0.002 b | 4.02 ± 0.06 b | |
| GJ-C | 9.75 ± 0.20 f | 0.981 ± 0.001 b | 4.13 ± 0.12 cd | |
| after drying (4 h/45 °C) | GJ-I | 4.80 ± 0.20 e | 0.819 ± 0.004 a | 3.29 ± 0.11 a |
| GJ-SPI | 4.45 ± 0.18 de | 0.837 ± 0.004 a | 3.71 ± 0.15 e | |
| GJ-I+SPI | 4.53 ± 0.24 de | 0.843 ± 0.006 a | 3.34 ± 0.12 a | |
| GJ-C | 4.26 ± 0.26 d | 0.842 ± 0.003 a | 3.41 ± 0.02 a | |
| after storage without packing | GJ-I | 3.15 ± 0.17 a | 0.816 ± 0.008 a | |
| GJ-SPI | 3.63 ± 0.16 b | 0.828 ± 0.008 a | ||
| GJ-I+SPI | 3.61 ± 0.21 bc | 0.841 ± 0.012 a | ||
| GJ-C | 3.49 ± 0.22 bc | 0.837 ± 0.036 a | ||
| after storage with packing | GJ-I | 4.17 ± 0.21 d | 0.815 ± 0.004 a | |
| GJ-SPI | 4.22 ± 0.10 d | 0.822 ± 0.003 a | ||
| GJ-I+SPI | 4.38 ± 0.31 d | 0.837 ± 0.004 a | ||
| GJ-C | 3.83 ± 0.22 c | 0.836 ± 0.007 a |
| GJ-I (100 g) | GJ-SPI (100 g) | GJ-I+SPI (100 g) | GJ-C (100 g) | |
|---|---|---|---|---|
| Energy (kcal/kJ) | 33.0/138.95 | 33.2/138.95 | 37.3/156.06 | 29.3/122.63 |
| Protein (g) | 0.21 | 4.07 | 2.11 | 0.23 |
| Total carbohydrate (g) | 30.27 | 27.21 | 28.66 | 29.08 |
| Dietary fiber (g) | 8.77 | 5.14 | 6.95 | 5.50 |
| Sugars (g) | 0.00 | 0.00 | 0.00 | 0.00 |
| Fat (g) | 0.00 | 0.21 | 0.21 | 0.00 |
| Sample | FRAP uM Trolox Equivalent/mg | Metal-Chelating Activity (%) | |
|---|---|---|---|
| after drying (2 h/35 °C) | GJ-I | 0.56 ± 0.04 ad | 0 ± 0.00 a |
| GJ-SPI | 0.86 ± 0.06 b | 20.19 ± 2.47 c | |
| GJ-I+SPI | 0.58 ± 0.04 abd | 7.51 ± 1.08 b | |
| GJ-C | 0.50 ± 0.10 ac | 0 ± 0.00 a | |
| after storage without packing | GJ-I | 0.60 ± 0.04 abd | 0 ± 0.00 a |
| GJ-SPI | 0.69 ± 0.05 b | 29.11 ± 1.47 c | |
| GJ-I+SPI | 0.60 ± 0.02 abd | 8.69 ± 2.03 b | |
| GJ-C | 0.44 ± 0.01 c | 0 ± 0.00 a | |
| after storage with packing | GJ-I | 0.52 ± 0.11 ac | 0 ± 0.00 a |
| GJ-SPI | 0.66 ± 0.04 d | 29.11 ± 5.65 c | |
| GJ-I+SPI | 0.52 ± 0.03 ac | 6.34 ± 2.54 b | |
| GJ-C | 0.42 ± 0.05 c | 0 ± 0.00 a |
| After Gelling | ||||
|---|---|---|---|---|
| GJ-I | GJ-SPI | GJ-I+SPI | GJ-C | |
| Hardness (N) | 3.582 ± 0.30 a | 8.827 ± 0.56 c | 8.264 ± 0.57 bc | 2.743 ± 0.27 a |
| Cohesiveness (N) | 0.148 ± 0.021 ac | 0.346 ± 0.026 d | 0.227 ± 0.016 bc | 0.139 ± 0.029 a |
| Springiness | 0.079 ± 0.014 a | 0.103 ± 0.004 a | 0.094 ± 0.006 a | 0.058 ± 0.007 a |
| Gumminess (N) | 3.241 ± 0.633 a | 5.723 ± 0.278 b | 4.424 ± 0.844 ab | 3.537 ± 0.191 a |
| After drying [4 h/45 °C] | ||||
| GJ-I | GJ-SPI | GJ-I+SPI | GJ-C | |
| Hardness (N) | 8.495 ± 0.81 bc | 16.483 ± 0.88 e | 15.138 ± 0.81 d | 7.313 ± 0.30 b |
| Cohesiveness (N) | 0.251 ± 0.025 b | 0.489 ± 0.079 e | 0.444 ± 0.054 e | 0.198 ± 0.010 ab |
| Springiness | 0.482 ± 0.057 c | 0.607 ± 0.040 ef | 0.508 ± 0.075 cd | 0.236 ± 0.031 b |
| Gumminess (N) | 11.734 ± 0.971 d | 16.321 ± 1.010 f | 15.681 ± 0.852 f | 7.681 ± 0.852 c |
| After 7 days without packing | ||||
| GJ-I | GJ-SPI | GJ-I+SPI | GJ-C | |
| Hardness (N) | 16.476 ± 0.84 e | 20.680 ± 0.93 g | 19.073 ± 1.41 f | 15.144 ± 0.82 d |
| Cohesiveness (N) | 0.383 ± 0.012 bc | 0.625 ± 0.083 f | 0.437 ± 0.029 e | 0.286 ± 0.027 cd |
| Springiness | 0.533 ± 0.017 cde | 0.626 ± 0.069 f | 0.570 ± 0.024 def | 0.273 ± 0.019 b |
| Gumminess (N) | 12.863 ± 0.861 de | 21.087 ± 0.995 h | 19.265 ± 0.935 g | 8.392 ± 0.950 c |
| After 7 days with packing | ||||
| GJ-I | GJ-SPI | GJ-I+SPI | GJ-C | |
| Hardness (N) | 26.114 ± 0.98 g | 33.546 ± 0.60 i | 30.566 ± 0.77 h | 18.286 ± 1.16 f |
| Cohesiveness (N) | 0.584 ± 0.060 f | 0.914 ± 0.051 g | 0.850 ± 0.053 g | 0.348 ± 0.021 d |
| Springiness | 0.730 ± 0.090 g | 0.965 ± 0.089 g | 0.825 ± 0.088 h | 0.557 ± 0.053 cdef |
| Gumminess (N) | 13.980 ± 0.779 e | 28.170 ± 1.275 i | 20.642 ± 1.023 h | 8.506 ± 0.779 c |
| ANOVA (F-ratio) | ||||
| Hardness | Cohesiveness | Springiness | Gumminess | |
| Formulation | 1.378 | 9.992 * | 2.324 | 19.161 * |
| Process step | 22.117 * | 24.221 * | 20.916 * | 51.126 * |
| Interaction | 2.207 * | 4.030 * | 10.286 * | 1.894 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, A.; Tkaczewska, J.; Nowak, N.; Grzebieniarz, W.; Goik, U.; Żmudziński, D.; Jamróz, E. Sugar-Free, Vegan, Furcellaran Gummy Jellies with Plant-Based Triple-Layer Films. Materials 2023, 16, 6443. https://doi.org/10.3390/ma16196443
Stępień A, Tkaczewska J, Nowak N, Grzebieniarz W, Goik U, Żmudziński D, Jamróz E. Sugar-Free, Vegan, Furcellaran Gummy Jellies with Plant-Based Triple-Layer Films. Materials. 2023; 16(19):6443. https://doi.org/10.3390/ma16196443
Chicago/Turabian StyleStępień, Anna, Joanna Tkaczewska, Nikola Nowak, Wiktoria Grzebieniarz, Urszula Goik, Daniel Żmudziński, and Ewelina Jamróz. 2023. "Sugar-Free, Vegan, Furcellaran Gummy Jellies with Plant-Based Triple-Layer Films" Materials 16, no. 19: 6443. https://doi.org/10.3390/ma16196443
APA StyleStępień, A., Tkaczewska, J., Nowak, N., Grzebieniarz, W., Goik, U., Żmudziński, D., & Jamróz, E. (2023). Sugar-Free, Vegan, Furcellaran Gummy Jellies with Plant-Based Triple-Layer Films. Materials, 16(19), 6443. https://doi.org/10.3390/ma16196443