Alternative to Conventional Solutions in the Development of Membranes and Hydrogen Evolution Electrocatalysts for Application in Proton Exchange Membrane Water Electrolysis: A Review
Abstract
:1. Introduction
2. Hydrogen Economy in Europe
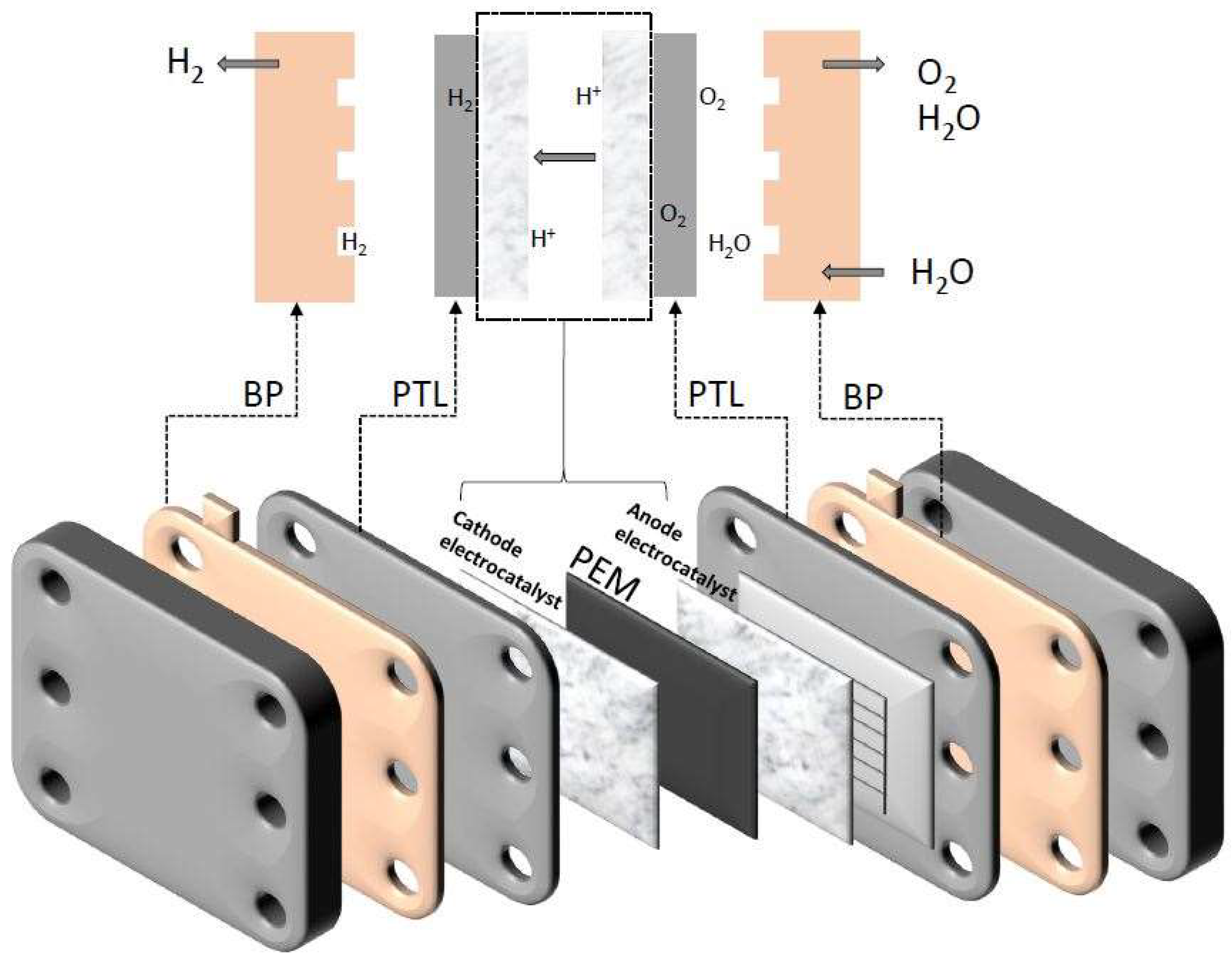
3. Proton Exchange Membrane for Water Electrolysis (PEMWE)
3.1. Components of PEMWE
3.1.1. Porous Transport Layers (PTLs)
3.1.2. Bipolar Plates (BPs)
PFSA–PEM Membranes
Hydrocarbon-Based Membranes
| Membrane | Material | Thickness/μm | Cathode Loading /Cathode Catalyst | IEC | Conductivity/mS cm−1 | T/°C | Current Density | Stability Test | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PFSA | |||||||||
| Fumapem®/graphene | Per-Fluorinated Sulfonic Acid (PFSA)/PTFE copolymer; 0.38 w/v—graphene loading | 112 | - | 0.82 mmol g−1 | 115 | 80 | - | - | [80] |
| (S-TiO2)/Nafion | sulfated titania (S-TiO2)-dopped Nafion | 100–110 | 0.5 ± 0.1 mg cm−2 of Pt Pt/Vulcan XC-72—30% | 0.82 ± 0.01 meq g−1 | ≈70 | 100 | 4 A cm−2 at 2 V | - | [81] |
| biaxially stretched Nafion 117 | PFSA, Nafion series 117 | 28.2 ± 1.7 | 0.4 mg cm−2 of Pt Pt/C—0.5 | 0.92 meq g−1 | σi = 73 σt = 54 | 80 | 3 A cm−2 at 1.9 V. | 0.4 A cm−2 for 50 h | [82] |
| hBN/Nafion | monolayer hexagonal boron nitride/Nafion | - | 0.4 mg cm−2 of Pt | - | 18.7 ± 0.9 | 70 | - | 0.4 A cm−2 for 100 h (50°C) | [83] |
| Aq830-PSU(5 wt %) | electrospun polysulfone fiber web/Aquivion® | 45 ± 2 | 0.5 mg cm−2 of Pt and 33 wt% Nafion® ionomer (5 wt% solution) Pt/C—40 wt% | - | 220 | 80 | 2 A cm−2 at 1.76 V | - | [84] |
| 3M 729/ePTFE (annealed at 180°) AQ 720/ePTFE (annealed at 180°) | ePTFE porous support was impregnated with 3M 729 and AQ 720 and annealed at different temperatures | 55–60 | 0.25–0.30 mg cm−2 of Pt Pt/C—40 wt% | 1.30 meq g−1 1.31 meq g−1 | 106 112 | 80 | - | - | [62] |
| NPP-95 | Nafion/poly(acrylic acid)/poly(vinyl alcohol) 95:2.5:2.5 | 50–60 | 0.1 mg cm−2 of Pt | 0.84 meq g−1 | 189.2 ± 12.1 | 80 | 4.310 A cm−2 at 2.0 V | - | [59] |
| Hydrocarbon membranes | |||||||||
| BPSH50 (random) | hydrocarbon-based sulfonated poly(arylene ether sulfone) | 40–50 | 0.5 mg cm−2 of Pt Pt/C—0.4 | 1.86 meq g−1 | 178 | 80 | 5.3 A cm−2 at 1.9 V | 3 A cm−2 for 90 h | [85] |
| CSPPSU | crosslinked sulfonated polyphenylsulfone | 70–130 | 0.3 mg cm−2 of Pt Pt/C—20 wt% | 1.71 meq g−1 | 30 | 150 | 0.456 A cm−2 at 1.8 V | - | [86] |
| sPPS | sulfonated poly(phenylene sulfone) | 115 ± 12 | 0.5 mg cm−2 of Pt Pt/C—1.6 wt% | 2.78 meq g−1 | - | 80 | 3.48 ± 0.03 A cm−2 at 1.8 V | 1 A cm−2 for 80 h | [87] |
| SPAES50 | Sulfonated poly(arylene ether sulfone) | 20 | 0.4 mg cm−2 of Pt with a 10 wt% P50 content Pt/C –40 wt% | 1.89 meq g−1 | 330.1 ± 6.0 | 90 | 1.069 A cm−2 at 1.6 V | - | [75] |
| 12%MKT-NW/C-sPEEK | MXene/potassium titanate nanowire cross-linked sulfonated polyether ether ketone | - | - | 1.88 meq g−1 | 9.7 | room temperature | - | - | [88] |
| 4%MXene-Cu2O/sPEEK | Titanium carbide-copper oxide cross-linked sulfonated poly ether ether ketone | - | - | 1.66 meq g−1 | 10.5 | 30 | - | - | [89] |
| SPPNBP_3 SPPNBP_5 | multi-block copolymer membranes consisting of sulfonated poly(p-phenylene) and naphthalene containing poly(arylene ether ketone) | 42–57 | 0.5 mg cm−2 of Pt Pt/C—0.4 | 2.05 2.49 meq g−1 | 200 152 | 80 | 4.8 5.5 A cm−2 at 1.9 V | - | [90] |
| G-sPSS-1.95 | grafting a highly sulfonated poly-(phenylene sulfide sulfone) side chain onto a poly(arylene ether sulfone) main chain | 50–60 | 0.4 mg cm−2 of Pt Pt/C—40 wt% | 1.95 meq g−1 | 290 | 90 | 6 A cm−2 at 1.9 V | 1 A cm−2 for 50 h | [91] |
4. Electrocatalysts for Hydrogen Production
4.1. HER Electrocatalysts with Substituted Noble Metals Content
| Cathode Catalyst | Electrochemical Characterization | Membrane | T | Performance | Ref. |
|---|---|---|---|---|---|
| MoO3 nanowires | Three-electrode cell with 1 M H2SO4 electrolyte | - | - | 11.3 and 56.8 mA cm−2 at potential of 0.0 and 0.1 V with a Tafel slope of 116 mV/decade | [115] |
| Co-Cu alloys | PEMWE single cell with Co-Cu deposited on a carbon paper (CP) as a cathode and IrO2 electrodeposited on a CP as an anode | N212 (DuPont) | 90 °C | 1.2 A cm−2 at 2.0 Vcell | [116] |
| CoP | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Current density of 20 mA cm−2 at an overpotential of 85 mV | [117] |
| CoP/CC | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Onset overpotential of 38 mV with a Tafel slope of 51 mV/decade | [118] |
| WC@NC | PEMWE single cell with WC@NC as the cathode and IrO2 (Sunlaite) as an anode | N212 (DuPont) | 80 °C | 0.78 A cm−2 at 2.0 Vcell | [119] |
| OsP2@NPC | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | 10 mA cm−2 at onset overpotential of 46 mV | [120] |
| NiMo/CF/CP | PEMWE single cell with NiMo/CF/CP as the cathode and IrO2/CP as an anode | N212 (DuPont) | 90 °C | ~2.0 A cm−2 at 2.0 Vcell | [121] |
| Ni–Mo–N | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 53 mV at 20 mA cm−2 | [122] |
| NiS2 | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 213 mV at 10 mA cm−2 | [123] |
| NiSe2 | Overpotential of 156 mV at 10 mA cm−2 | ||||
| NiTe2 | Overpotential of 276 mV at 10 mA cm−2 | ||||
| MoP/C (NaCl) | Home-made electrolyzer using MoP/C (NaCl) as cathode and IrO2 (Sunlaite) as an anode | N211 (DuPont) | 80 °C | 0.71 A cm−2 at 2.0 Vcell | [124] |
| MoP@PC | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 258 mV at 10 mA cm−2, with a Tafel slope of 59.3 mV/decade | [125] |
| MoP@PC | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 51 mV at 10 mA cm−2 with a Tafel slope of 45 mV/decade | [126] |
| MoP@PC | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Onset overpotential of 77 mV, overpotential of 153 mV at 10 mA cm−2, with a Tafel slope of 66 mV/decade | [127] |
| MoP/NG | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 94 mV at 10 mA cm−2 with a Tafel slope of 50.1 mV/decade | [128] |
| MoP/NC | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 120 mV at 10 mA cm−2 | [129] |
| MoP|S | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 86 mV at 10 mA cm−2 | [130] |
| N–Mo2C | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Onset overpotential of 78.1 mV for HER and a Tafel slope of 59.6 mV/decade | [131] |
| Mo2C/C | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Tafel slope of 56 mV/decade | [132] |
| Mo2C/C | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 180 mV at 10 mA cm−2 | [133] |
| CuxMo100−x/CP | PEMWE single cell with Cu93.7Mo6.3/CP as the cathode and IrO2/CP as an anode | N212 (DuPont) | 90 °C | 0.50 A cm−2 at 1.9 Vcell | [96] |
| Cu1−xNixWO4 | Three-electrode cell with 1 M H2SO4 electrolyte | - | - | 4.3 mA cm−2 at the anodic peak potential of 0.09 V | [134] |
| Ni–P supported by copper foam (CF) on CP | PEMWE single cell with Ni–P/CF/CP as the cathode and IrO2/CP as an anode | N212 (DuPont) | 90 °C | 0.67 A cm−2 at 2.0 Vcell | [135] |
| NiMo/CF/CP | PEMWE single cell with Ni–Mo/CF/CP as the cathode and IrO2/CP as an anode | N212 (DuPont) | 90 °C | 2.0 A cm−2 at 2.0 Vcell | [121] |
| FeCo/N–G | Three-electrode cell with 1 M H2SO4 electrolyte | - | - | Onset overpotential of 88 mV and overpotential of 262 mV at 10 mA cm−2 | [136] |
| P–Ag@NC | Three-electrode cell with 1 M H2SO4 electrolyte | - | - | Overpotential of 78 mV at 10 mA cm−2 | [137] |
| Co@N–CNTs@RGO | Three-electrode cell with 0.5 M H2SO4 electrolyte | - | - | Overpotential of 87 mV at 10 mA cm−2 | [138] |
4.2. HER Electrocatalysts with Reduced Noble Metals Content
| Cathode Catalyst | Electrolyte | Overpotential@Current Density | Tafel Slope | Stability | Ref. |
|---|---|---|---|---|---|
| Au@AuIr2 (core-shell structure nanoparticles (NPs) with Au core and AuIr2 alloy shell) | 0.5 M H2SO4 | 29 mV@ of 10 mA cm−2 | 15.6 mV/decade | 40 h | [152] |
| PdCu/Ir core shell nanocrystals | 0.5 M H2SO4 | 20 mV@ of 10 mA cm−2
| - | 15 h@20 mA cm−2 | [153] |
| IrPdPtRhRu high entropy alloy (HEA) NPs | 0.05 M H2SO4 | 33 mV@ of 10 mA cm−2
| - | CV for 3000 cycles | [154] |
| PtRu@RFCs (Pt is alloyed with Ru and embedded in porous resorcinol-formaldehyde carbon spheres) Pt loading 99.9% less than commercial Pt-based catalyst | 0.5 M H2SO4 | 19.7 mV@10 mA cm−2 43.1 mV @ 100 mA cm−2 | 27.2 mV/decade for comparison: Pt/C (commercial) = 29.9 mV/decade | CV for 5000 cycles | [155] |
| RuP synthesized by dry chemistry method | 0.1 M HClO4 | 36 mV@10 mA cm−2
| 39.8 0.5 mV/decade | CV for 8000 cycles | [156] |
| Pd4S-SNC (palladium sulfide supported by S, N-doped carbon NPs) | 0.5 M H2SO4 | 32 mV@ of 10 mA cm−2 | 52 mV/decade | CV for 1000 cycles | [157] |
| PtNx cluster loaded on a TiO2 support (PtNx/TiO2) | 0.5 M H2SO4 | 67 mV@ of 10 mA cm−2 | 52 mV/decade | CV for 5000 cycles | [158] |
| Pt nanoclusters (NCs) anchored on porous TiO2 nanosheets with rich oxygen vacancies (Vo-rich Pt/TiO2) | 0.5 M H2SO4 | - | 34 mV/decade
| CV for 1000 cycles | [159] |
Pt/OLC (onion-like nanospheres on carbon (OLC) with atomically dispersed Pt)
| 0.5 M H2SO | 38 mV@10 mA cm−2 | 36 mV/decade
| 100 h@10 mA cm−2 | [160] |
5. Challenges and Insights for Future Clean Hydrogen Production Using PEM Water Electrolyzers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, K.; Tareen, A.K.; Aslam, M.; Zhang, Y.; Wang, R.; Ouyang, Z.; Gou, Z.; Zhang, H. Recent Advances in Two-Dimensional Materials and Their Nanocomposites in Sustainable Energy Conversion Applications. Nanoscale 2019, 11, 21622–21678. [Google Scholar] [CrossRef] [PubMed]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative Assessment of Hydrogen Production Methods from Renewable and Non-Renewable Sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Aricò, A.S.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer Electrolyte Membrane Water Electrolysis: Status of Technologies and Potential Applications in Combination with Renewable Power Sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Directives Directive (Eu) 2018/2001 of The European Parliament and of The Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources (Recast) (Text with EEA Relevance). Official Journal of the European Union, 21 December 2018.
- Eftekhari, A. Electrocatalysts for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- European Commission. A Hydrogen Strategy for a Climate-Neutral Europe; European Commission: Brussels, Belgium, 2020; pp. 1–24. [Google Scholar]
- European Parliament. European Council European Climate Law; European Commission: Brussels, Belgium, 2021; Volume 2021, pp. 1–17. [Google Scholar]
- European Commission. Fit for 55; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. REPowerEU; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- European Clean Hydrogen Alliance. In Proceedings of the European Electrolyser Summit Joint Declaration, Brussels, Belgium, 5 May 2022; pp. 1–8.
- Europen Investment Bank. Available online: https://www.eib.org/en/index (accessed on 5 May 2023).
- Clean Hydrogen Partnership. Strategic Research and Innovation Agenda 2021–2027. Unpublished 2022; p. 179. Available online: https://www.clean-hydrogen.europa.eu/system/files/2022-02/Clean%20Hydrogen%20JU%20SRIA%20-%20approved%20by%20GB%20-%20clean%20for%20publication%20%28ID%2013246486%29.pdf (accessed on 8 May 2023).
- Hydrogen Public Funding Compass. Available online: https://single-market-economy.ec.europa.eu/industry/strategy/hydrogen/funding-guide_en (accessed on 8 May 2023).
- European Commission. Clean Hydrogen Alliance Learbook on Hydrogen Supply Corridors; European Commission: Brussels, Belgium, 2023; pp. 1–54. [Google Scholar]
- European Clean Hydrogen Alliance. Available online: https://single-market-economy.ec.europa.eu/industry/strategy/industrial-alliances/european-clean-hydrogen-alliance_en (accessed on 9 May 2023).
- Hydrogen Europe. Available online: https://hydrogeneurope.eu/ (accessed on 9 May 2023).
- Clean Hydrogen Partnership. Available online: https://www.clean-hydrogen.europa.eu/index_en (accessed on 9 May 2023).
- Hydrogen Valleys. Available online: https://www.clean-hydrogen.europa.eu/hydrogen-valleys-0_en (accessed on 10 May 2023).
- Gospodinova, S.; Miccoli, F. Hydrogen: Commission Supports Industry Commitment to Boost by Tenfold Electrolyser Manufacturing Capacities in the EU; European Commission-Press Release: Brussels, Belgium, 2022; pp. 1–2. [Google Scholar]
- IRENA. Green Hydrogen Cost Reduction. Scaling up Electrolysers to Meet the 1.5 °C Climate Goal (IRENA); International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2020; pp. 1–106. ISBN 978-92-9260-295-6. [Google Scholar]
- Russell, J.H.; Nuttall, L.J.; Fickett, A.P. Hydrogen Generation by Solid Polymer Electrolyte Water Electrolysis. Am. Chem. Soc. Div. Fuel Chem. Prepr. 1973, 18, 24–40. [Google Scholar]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Saruhan, B.; Freitag, O.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Gago, A.S.; Friedrich, K.A. Low-Cost and Durable Bipolar Plates for Proton Exchange Membrane Electrolyzers. Sci. Rep. 2017, 7, srep44035. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current Status of Water Electrolysis for Energy Storage, Grid Balancing and Sector Coupling via Power-to-Gas and Power-to-Liquids: A Review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Marshall, A.; Børresen, B.; Hagen, G.; Tsypkin, M.; Tunold, R. Hydrogen Production by Advanced Proton Exchange Membrane (PEM) Water Electrolysers-Reduced Energy Consumption by Improved Electrocatalysis. Energy 2007, 32, 431–436. [Google Scholar] [CrossRef]
- Badea, G.E.; Hora, C.; Maior, I.; Cojocaru, A.; Secui, C.; Filip, S.M.; Dan, F.C. Sustainable Hydrogen Production from Seawater Electrolysis: Through Fundamental Electrochemical Principles to the Most Recent Development. Energies 2022, 15, 8560. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Moussab, H. Recent Advances on Hydrogen Production through Seawater Electrolysis. Mater. Sci. Energy Technol. 2020, 3, 780–807. [Google Scholar] [CrossRef]
- Sun, X.; Xu, K.; Fleischer, C.; Liu, X.; Grandcolas, M.; Strandbakke, R.; Bjørheim, T.S.; Norby, T.; Chatzitakis, A. Earth-Abundant Electrocatalysts in Proton Exchange Membrane Electrolyzers. Catalysts 2018, 8, 657. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and Perspectives of Key Materials for PEM Electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Xu, J.; Xia, C.; Wang, P.; Xia, B.Y.; Yan, Y.; Wang, X. Key Components and Design Strategy for a Proton Exchange Membrane Water Electrolyzer. Small Struct. 2023, 4, 2200130. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen Storage Technologies for Stationary and Mobile Applications: Review, Analysis and Perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Onda, K.; Kyakuno, T.; Hattori, K.; Ito, K. Prediction of Production Power for High-Pressure Hydrogen by High-Pressure Water Electrolysis. J. Power Sources 2004, 132, 64–70. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Korobtsev, S.V.; Porembskiy, V.I.; Pepic, M.; Etievant, C.; Puyenchet, C.; Fateev, V.N. Hydrogen Safety Aspects Related to High-Pressure Polymer Electrolyte Membrane Water Electrolysis. Int. J. Hydrogen Energy 2009, 34, 5986–5991. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef]
- Mo, J.; Kang, Z.; Yang, G.; Retterer, S.T.; Cullen, D.A.; Toops, T.J.; Green, J.B.; Zhang, F.Y. Thin Liquid/Gas Diffusion Layers for High-Efficiency Hydrogen Production from Water Splitting. Appl. Energy 2016, 177, 817–822. [Google Scholar] [CrossRef]
- Rakousky, C.; Reimer, U.; Wippermann, K.; Kuhri, S.; Carmo, M.; Lueke, W.; Stolten, D. Polymer Electrolyte Membrane Water Electrolysis: Restraining Degradation in the Presence of Fluctuating Power. J. Power Sources 2017, 342, 38–47. [Google Scholar] [CrossRef]
- Kang, Z.; Mo, J.; Yang, G.; Li, Y.; Talley, D.A.; Retterer, S.T.; Cullen, D.A.; Toops, T.J.; Brady, M.P.; Bender, G.; et al. Thin Film Surface Modifications of Thin/Tunable Liquid/Gas Diffusion Layers for High-Efficiency Proton Exchange Membrane Electrolyzer Cells. Appl. Energy 2017, 206, 983–990. [Google Scholar] [CrossRef]
- Liu, C.; Shviro, M.; Gago, A.S.; Zaccarine, S.F.; Bender, G.; Gazdzicki, P.; Morawietz, T.; Biswas, I.; Rasinski, M.; Everwand, A.; et al. Exploring the Interface of Skin-Layered Titanium Fibers for Electrochemical Water Splitting. Adv. Energy Mater. 2021, 11, 2002926. [Google Scholar] [CrossRef]
- Hackemüller, F.J.; Borgardt, E.; Panchenko, O.; Müller, M.; Bram, M. Manufacturing of Large-Scale Titanium-Based Porous Transport Layers for Polymer Electrolyte Membrane Electrolysis by Tape Casting. Adv. Eng. Mater. 2019, 21, 1801201. [Google Scholar] [CrossRef]
- Stiber, S.; Balzer, H.; Wierhake, A.; Wirkert, F.J.; Roth, J.; Rost, U.; Brodmann, M.; Lee, J.K.; Bazylak, A.; Waiblinger, W.; et al. Porous Transport Layers for Proton Exchange Membrane Electrolysis under Extreme Conditions of Current Density, Temperature, and Pressure. Adv. Energy Mater. 2021, 11, 2100630. [Google Scholar] [CrossRef]
- Langemann, M.; Fritz, D.L.; Müller, M.; Stolten, D. Validation and Characterization of Suitable Materials for Bipolar Plates in PEM Water Electrolysis. Int. J. Hydrogen Energy 2015, 40, 11385–11391. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. PEM Water Electrolysis for Hydrogen Production: Fundamentals, Advances, and Prospects. Carbon Neutrality 2022, 1, 1–19. [Google Scholar] [CrossRef]
- Shirvanian, P.; van Berkel, F. Novel Components in Proton Exchange Membrane Water Electrolyzers (PEMWE): Status, Challenges and Future Needs. Electrochem. Commun 2020, 114, 106704. [Google Scholar] [CrossRef]
- Jung, H.Y.; Huang, S.Y.; Ganesan, P.; Popov, B.N. Performance of Gold-Coated Titanium Bipolar Plates in Unitized Regenerative Fuel Cell Operation. J. Power Sources 2009, 194, 972–975. [Google Scholar] [CrossRef]
- Wakayama, H.; Yamazaki, K. Low-Cost Bipolar Plates of Ti4O7-Coated Ti for Water Electrolysis with Polymer Electrolyte Membranes. ACS Omega 2021, 6, 4161–4166. [Google Scholar] [CrossRef]
- Rojas, N.; Sánchez-Molina, M.; Sevilla, G.; Amores, E.; Almandoz, E.; Esparza, J.; Cruz Vivas, M.R.; Colominas, C. Coated Stainless Steels Evaluation for Bipolar Plates in PEM Water Electrolysis Conditions. Int. J. Hydrogen Energy 2021, 46, 25929–25943. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E.; Atyabi, S.A. Thermal and Electrochemical Analysis of Different Flow Field Patterns in a PEM Electrolyzer. Electrochim. Acta 2018, 267, 234–245. [Google Scholar] [CrossRef]
- Goñi-Urtiaga, A.; Presvytes, D.; Scott, K. Solid Acids as Electrolyte Materials for Proton Exchange Membrane (PEM) Electrolysis: Review. Int. J. Hydrogen Energy 2012, 37, 3358–3372. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite Polymers Development and Application for Polymer Electrolyte Membrane Technologies—A Review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Peng, L.; Lai, X.; Ni, M.; Lehnert, W. Mechanical Failure and Mitigation Strategies for the Membrane in a Proton Exchange Membrane Fuel Cell. Renew. Sustain. Energy Rev. 2019, 113, 109289. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Wolska, J.; Koroniak, H. Polymers Application in Proton Exchange Membranes for Fuel Cells (PEMFCs). Phys. Sci. Rev. 2017, 2, 20170018. [Google Scholar]
- Higashihara, T.; Matsumoto, K.; Ueda, M. Sulfonated Aromatic Hydrocarbon Polymers as Proton Exchange Membranes for Fuel Cells. Polymer 2009, 50, 5341–5357. [Google Scholar] [CrossRef]
- Kusoglu, A.; Dursch, T.J.; Weber, A.Z. Nanostructure/Swelling Relationships of Bulk and Thin-Film PFSA Ionomers. Adv. Funct. Mater. 2016, 26, 4961–4975. [Google Scholar] [CrossRef]
- Sunda, A.P.; Venkatnathan, A. Atomistic Simulations of Structure and Dynamics of Hydrated Aciplex Polymer Electrolyte Membrane. Soft Matter 2012, 8, 10827–10836. [Google Scholar] [CrossRef]
- Park, S.; Lee, W.; Na, Y. Performance Comparison of Proton Exchange Membrane Water Electrolysis Cell Using Channel and PTL Flow Fields through Three-Dimensional Two-Phase Flow Simulation. Membranes 2022, 12, 1260. [Google Scholar] [CrossRef] [PubMed]
- Al Munsur, A.Z.; Goo, B.H.; Kim, Y.; Kwon, O.J.; Paek, S.Y.; Lee, S.Y.; Kim, H.J.; Kim, T.H. Nafion-Based Proton-Exchange Membranes Built on Cross-Linked Semi-Interpenetrating Polymer Networks between Poly(Acrylic Acid) and Poly(Vinyl Alcohol). ACS Appl. Mater. Interfaces 2021, 13, 28188–28200. [Google Scholar] [CrossRef] [PubMed]
- Kusoglu, A.; Karlsson, A.M.; Santare, M.H.; Cleghorn, S.; Johnson, W.B. Mechanical Behavior of Fuel Cell Membranes under Humidity Cycles and Effect of Swelling Anisotropy on the Fatigue Stresses. J. Power Sources 2007, 170, 345–358. [Google Scholar] [CrossRef]
- Tang, Y.; Kusoglu, A.; Karlsson, A.M.; Santare, M.H.; Cleghorn, S.; Johnson, W.B. Mechanical Properties of a Reinforced Composite Polymer Electrolyte Membrane and Its Simulated Performance in PEM Fuel Cells. J. Power Sources 2008, 175, 817–825. [Google Scholar] [CrossRef]
- Shin, S.H.; Nur, P.J.; Kodir, A.; Kwak, D.H.; Lee, H.; Shin, D.; Bae, B. Improving the Mechanical Durability of Short-Side-Chain Perfluorinated Polymer Electrolyte Membranes by Annealing and Physical Reinforcement. ACS Omega 2019, 4, 19153–19163. [Google Scholar] [CrossRef]
- Laberty-Robert, C.; Vallé, K.; Pereira, F.; Sanchez, C. Design and Properties of Functional Hybrid Organic–Inorganic Membranes for Fuel Cells. Chem. Soc. Rev. 2011, 40, 961–1005. [Google Scholar] [CrossRef]
- Di Noto, V.; Boaretto, N.; Negro, E.; Stallworth, P.E.; Lavina, S.; Giffin, G.A.; Greenbaum, S.G. Inorganic-Organic Membranes Based on Nafion, [(ZrO2)·(HfO2) 0.25] and [(SiO2)·(HfO2) 0.28] Nanoparticles. Part II: Relaxations and Conductivity Mechanism. Int. J. Hydrogen Energy 2012, 37, 6215–6227. [Google Scholar] [CrossRef]
- Casciola, M.; Bagnasco, G.; Donnadio, A.; Micoli, L.; Pica, M.; Sganappa, M.; Turco, M. Conductivity and Methanol Permeability of Nafion-Zirconiumphosphate Compositemembranes Containing High Aspect Ratio Filler Particles. Fuel Cells 2009, 9, 394–400. [Google Scholar] [CrossRef]
- Slade, S.M.; Smith, J.R.; Campbell, S.A.; Ralph, T.R.; Ponce De León, C.; Walsh, F.C. Characterisation of a Re-Cast Composite Nafion® 1100 Series of Proton Exchange Membranes Incorporating Inert Inorganic Oxide Particles. Electrochim. Acta 2010, 55, 6818–6829. [Google Scholar] [CrossRef]
- Di Noto, V.; Piga, M.; Lavina, S.; Negro, E.; Yoshida, K.; Ito, R.; Furukawa, T. Structure, Properties and Proton Conductivity of Nafion/[(TiO2) ṡ (WO3)0.148 ]ψ TiO2 Nanocomposite Membranes. Electrochim. Acta 2010, 55, 1431–1444. [Google Scholar] [CrossRef]
- Cele, N.P.; Ray, S.S. Effect of Multiwalled Carbon Nanotube Loading on the Properties of Nafion® Membranes. J. Mater. Res. 2014, 34, 66–78. [Google Scholar] [CrossRef]
- Baglio, V.; Ornelas, R.; Matteucci, F.; Martina, F.; Ciccarella, G.; Zama, I.; Arriaga, L.G.; Antonucci, V.; Aricó, A.S. Solid Polymer Electrolyte Water Electrolyser Based on Nafion-TiO2 Composite Membrane for High Temperature Operation. Fuel Cells 2009, 9, 247–252. [Google Scholar] [CrossRef]
- Antonucci, V.; Di Blasi, A.; Baglio, V.; Ornelas, R.; Matteucci, F.; Ledesma-Garcia, J.; Arriaga, L.G.; Aricò, A.S. High Temperature Operation of a Composite Membrane-Based Solid Polymer Electrolyte Water Electrolyser. Electrochim. Acta 2008, 53, 7350–7356. [Google Scholar] [CrossRef]
- Schuster, M.; Kreuer, K.D.; Andersen, H.T.; Maier, J. Sulfonated Poly(Phenylene Sulfone) Polymers as Hydrolytically and Thermooxidatively Stable Proton Conducting Ionomers. Macromolecules 2007, 40, 598–607. [Google Scholar] [CrossRef]
- Yazili, D.; Marini, E.; Saatkamp, T.; Münchinger, A.; de Wild, T.; Gubler, L.; Titvinidze, G.; Schuster, M.; Schare, C.; Jörissen, L.; et al. Sulfonated Poly(Phenylene Sulfone) Blend Membranes Finding Their Way into Proton Exchange Membrane Fuel Cells. J. Power Sources 2023, 563, 232791. [Google Scholar] [CrossRef]
- Wu, G.; Lin, S.J.; Hsu, I.C.; Su, J.Y.; Chen, D.W. Study of High Performance Sulfonated Polyether Ether Ketone Composite Electrolyte Membranes. Polymers 2019, 11, 1177. [Google Scholar] [CrossRef]
- Maria Mahimai, B.; Sivasubramanian, G.; Sekar, K.; Kannaiyan, D.; Deivanayagam, P. Sulfonated Poly(Ether Ether Ketone): Efficient Ion-Exchange Polymer Electrolytes for Fuel Cell Applications-a Versatile Review. Mater. Adv. 2022, 3, 6085–6095. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, J.; Han, J.; Kim, K.; Park, S.B.; Kim, S.; Park, H.S.; Cho, Y.H.; Lee, J.C.; Sung, Y.E. High-Performance Proton-Exchange Membrane Water Electrolysis Using a Sulfonated Poly(Arylene Ether Sulfone) Membrane and Ionomer. J. Memb. Sci. 2021, 620, 118871. [Google Scholar] [CrossRef]
- Energy Transitions Commission. Making the Hydrogen Economy Possible: Accelerating Clean Hydrogen in an Electrified Economy The Making Mission Possible Series; Energy Transitions Commission: London, UK, 2021. [Google Scholar]
- Chatenet, M.; Pollet, B.G.; Dekel, D.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.; Bazant, M.Z.; Eikerling, M.; Staffell, I. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- IEA. Net Zero by 2050—A Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Bender, G.; Carmo, M.; Smolinka, T.; Gago, A.; Danilovic, N.; Mueller, M.; Ganci, F.; Fallisch, A.; Lettenmeier, P.; Friedrich, K.A.; et al. Initial Approaches in Benchmarking and Round Robin Testing for Proton Exchange Membrane Water Electrolyzers. Int. J. Hydrogen Energy 2019, 44, 9174–9187. [Google Scholar] [CrossRef]
- Ion-Ebrasu, D.; Pollet, B.G.; Spinu-Zaulet, A.; Soare, A.; Carcadea, E.; Varlam, M.; Caprarescu, S. Graphene Modified Fluorinated Cation-Exchange Membranes for Proton Exchange Membrane Water Electrolysis. Int. J. Hydrogen Energy 2019, 44, 10190–10196. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Nicotera, I.; Mazzapioda, L.; Aricò, A.S.; Panero, S.; Navarra, M.A. Sulfated Titania as Additive in Nafion Membranes for Water Electrolysis Applications. Int. J. Hydrogen Energy 2017, 42, 27851–27858. [Google Scholar] [CrossRef]
- Lee, C.J.; Song, J.; Yoon, K.S.; Rho, Y.; Yu, D.M.; Oh, K.H.; Lee, J.Y.; Kim, T.H.; Hong, Y.T.; Kim, H.J.; et al. Controlling Hydrophilic Channel Alignment of Perfluorinated Sulfonic Acid Membranes via Biaxial Drawing for High Performance and Durable Polymer Electrolyte Membrane Water Electrolysis. J. Power Sources 2022, 518, 230772. [Google Scholar] [CrossRef]
- Kim, T.; Sihn, Y.; Yoon, I.H.; Yoon, S.J.; Lee, K.; Yang, J.H.; So, S.; Park, C.W. Monolayer Hexagonal Boron Nitride Nanosheets as Proton-Conductive Gas Barriers for Polymer Electrolyte Membrane Water Electrolysis. ACS Appl. Nano Mater. 2021, 4, 9104–9112. [Google Scholar] [CrossRef]
- Giancola, S.; Zatoń, M.; Reyes-Carmona, Á.; Dupont, M.; Donnadio, A.; Cavaliere, S.; Rozière, J.; Jones, D.J. Composite Short Side Chain PFSA Membranes for PEM Water Electrolysis. J. Memb. Sci. 2019, 570–571, 69–76. [Google Scholar] [CrossRef]
- Han, S.Y.; Yu, D.M.; Mo, Y.H.; Ahn, S.M.; Lee, J.Y.; Kim, T.H.; Yoon, S.J.; Hong, S.; Hong, Y.T.; So, S. Ion Exchange Capacity Controlled Biphenol-Based Sulfonated Poly(Arylene Ether Sulfone) for Polymer Electrolyte Membrane Water Electrolyzers: Comparison of Random and Multi-Block Copolymers. J. Memb. Sci. 2021, 634, 119370. [Google Scholar] [CrossRef]
- Kim, J.; Ohira, A. Crosslinked Sulfonated Polyphenylsulfone (Csppsu) Membranes for Elevated-Temperature Pem Water Electrolysis. Membranes 2021, 11, 861. [Google Scholar] [CrossRef]
- Klose, C.; Saatkamp, T.; Münchinger, A.; Bohn, L.; Titvinidze, G.; Breitwieser, M.; Kreuer, K.D.; Vierrath, S. All-Hydrocarbon MEA for PEM Water Electrolysis Combining Low Hydrogen Crossover and High Efficiency. Adv. Energy Mater. 2020, 10, 1903995. [Google Scholar] [CrossRef]
- Waribam, P.; Jaiyen, K.; Samart, C.; Ogawa, M.; Guan, G.; Kongparakul, S. MXene Potassium Titanate Nanowire/Sulfonated Polyether Ether Ketone (SPEEK) Hybrid Composite Proton Exchange Membrane for Photocatalytic Water Splitting. RSC Adv. 2021, 11, 9327–9335. [Google Scholar] [CrossRef]
- Waribam, P.; Jaiyen, K.; Samart, C.; Ogawa, M.; Guan, G.; Kongparakul, S. MXene-Copper Oxide/Sulfonated Polyether Ether Ketone as a Hybrid Composite Proton Exchange Membrane in Electrochemical Water Electrolysis. Catal. Today 2023, 407, 96–106. [Google Scholar] [CrossRef]
- Ko, E.J.; Lee, E.; Lee, J.Y.; Yu, D.M.; Yoon, S.J.; Oh, K.H.; Hong, Y.T.; So, S. Multi-Block Copolymer Membranes Consisting of Sulfonated Poly(p-Phenylene) and Naphthalene Containing Poly(Arylene Ether Ketone) for Proton Exchange Membrane Water Electrolysis. Polymers 2023, 15, 1748. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M.; Park, J.E.; Jang, G.Y.; Jeong, H.Y.; Yu, D.M.; Jang, J.K.; Lee, J.C.; Cho, Y.H.; Kim, T.H. Highly Sulfonated Aromatic Graft Polymer with Very High Proton Conductivity and Low Hydrogen Permeability for Water Electrolysis. ACS Energy Lett. 2022, 7, 4427–4435. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A Review of Proton Exchange Membrane Water Electrolysis on Degradation Mechanisms and Mitigation Strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, S.K.; Ahn, S.H. A Transition Metal Oxysulfide Cathode for the Proton Exchange Membrane Water Electrolyzer. Appl. Catal. B 2018, 232, 93–100. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Ramakrishna, S.U.B.; Rama Devi, B.; Himabindu, V. Phosphorus-Doped Carbon Nanoparticles Supported Palladium Electrocatalyst for the Hydrogen Evolution Reaction (HER) in PEM Water Electrolysis. Ionics 2018, 24, 3113–3121. [Google Scholar] [CrossRef]
- Genova-Koleva, R.V. Electrocatalyst Development for PEM Water Electrolysis and DMFC: Towards the Methanol Economy; Universitat de Barcelona: Barcelona, Spain, 2017. [Google Scholar]
- Kim, H.; Hwang, E.; Park, H.; Lee, B.S.; Jang, J.H.; Kim, H.J.; Ahn, S.H.; Kim, S.K. Non-Precious Metal Electrocatalysts for Hydrogen Production in Proton Exchange Membrane Water Electrolyzer. Appl. Catal. B 2017, 206, 608–616. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Ahn, S.H. Electrodeposited Rhodium Phosphide with High Activity for Hydrogen Evolution Reaction in Acidic Medium. ACS Sustain. Chem. Eng. 2019, 7, 14041–14050. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Kim, D.K.; Choi, I.; Kim, S.K. Pulse-Electrodeposited Nickel Phosphide for High-Performance Proton Exchange Membrane Water Electrolysis. J. Alloys Compd. 2019, 785, 296–304. [Google Scholar] [CrossRef]
- Ng, J.W.D.; Hellstern, T.R.; Kibsgaard, J.; Hinckley, A.C.; Benck, J.D.; Jaramillo, T.F. Polymer Electrolyte Membrane Electrolyzers Utilizing Non-Precious Mo-Based Hydrogen Evolution Catalysts. ChemSusChem 2015, 8, 3512–3519. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science (1979) 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Senthil Kumar, S.M.; Selvakumar, K.; Thangamuthu, R.; Karthigai Selvi, A.; Ravichandran, S.; Sozhan, G.; Rajasekar, K.; Navascues, N.; Irusta, S. Hydrothermal Assisted Morphology Designed MoS2 Material as Alternative Cathode Catalyst for PEM Electrolyser Application. Int. J. Hydrogen Energy 2016, 41, 13331–13340. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Sánchez, T.; Ampurdanés, J.; Urakawa, A. MoS2-Based Materials as Alternative Cathode Catalyst for PEM Electrolysis. Int. J. Hydrogen Energy 2014, 39, 20837–20843. [Google Scholar] [CrossRef]
- Tahira, A.; Ibupoto, Z.H.; Mazzaro, R.; You, S.; Morandi, V.; Natile, M.M.; Vagin, M.; Vomiero, A. Advanced Electrocatalysts for Hydrogen Evolution Reaction Based on Core-Shell MoS2/TiO2 Nanostructures in Acidic and Alkaline Media. ACS Appl. Energy Mater. 2019, 2, 2053–2062. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.; Chen, Y. Chapter 2—Electronic Properties of Sulfide Minerals. In Density Functional Theory and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–81. [Google Scholar]
- Vaughan, D.J. Sulfides. In Encyclopedia of Geology, 2nd ed; Academic Press: Cambridge, MA, USA, 2020; Volume 1–6, pp. 395–412. ISBN 9780081029091. [Google Scholar]
- Di Giovanni, C.; Reyes-Carmona, Á.; Coursier, A.; Nowak, S.; Grenèche, J.M.; Lecoq, H.; Mouton, L.; Rozière, J.; Jones, D.; Peron, J.; et al. Low-Cost Nanostructured Iron Sulfide Electrocatalysts for PEM Water Electrolysis. ACS Catal. 2016, 6, 2626–2631. [Google Scholar] [CrossRef]
- Wang, D.Y.; Gong, M.; Chou, H.L.; Pan, C.J.; Chen, H.A.; Wu, Y.; Lin, M.C.; Guan, M.; Yang, J.; Chen, C.W.; et al. Highly Active and Stable Hybrid Catalyst of Cobalt-Doped FeS2 Nanosheets-Carbon Nanotubes for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Kim, J.; Kim, J.H.; Ahn, S.H. Electrochemical Fabrication of Fe-Based Binary and Ternary Phosphide Cathodes for Proton Exchange Membrane Water Electrolyzer. J. Alloys Compd. 2019, 807, 148813. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, W.; Liu, D.; Liu, Y.; Liu, C. Carbon Nanotubes Decorated with Nickel Phosphide Nanoparticles as Efficient Nanohybrid Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A Mater. 2015, 3, 13087–13094. [Google Scholar] [CrossRef]
- Fan, L.; Liu, P.F.; Yan, X.; Gu, L.; Yang, Z.Z.; Yang, H.G.; Qiu, S.; Yao, X. Atomically Isolated Nickel Species Anchored on Graphitized Carbon for Efficient Hydrogen Evolution Electrocatalysis. Nat. Commun. 2016, 7, 10667. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Wang, C.H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly Active and Durable Nanostructured Molybdenum Carbide Electrocatalysts for Hydrogen Production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, J.; Shi, Y.; Liu, D.; Zhang, B. Metallic WO2-Carbon Mesoporous Nanowires as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 6983–6986. [Google Scholar] [CrossRef] [PubMed]
- Phuruangrat, A.; Ham, D.J.; Thongtem, S.; Lee, J.S. Electrochemical Hydrogen Evolution over MoO3 Nanowires Produced by Microwave-Assisted Hydrothermal Reaction. Electrochem. Commun 2009, 11, 1740–1743. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Oh, S.; Kim, S.K. Facile Electrochemical Preparation of Nonprecious Co-Cu Alloy Catalysts for Hydrogen Production in Proton Exchange Membrane Water Electrolysis. Int. J. Energy Res. 2020, 44, 2833–2844. [Google Scholar] [CrossRef]
- Popczun, E.J.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Highly Active Electrocatalysis of the Hydrogen Evolution Reaction by Cobalt Phosphide Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 5427–5430. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Fen, Q.; Xiong, Y.; Xie, L.; Zhang, Z.; Lu, X.; Wang, Y.; Yuan, X.Z.; Fan, J.; Li, H.; Wang, H. Tungsten Carbide Encapsulated in Graphe-like N-Doped Carbon Nanospheres: One-Step Facile Synthesis for Low-Cost and Highly Active Electrocatalysts in Proton Exchange Membrane Water Electrolyzers. ACS Appl. Mater. Interfaces. 2019, 11, 25123–25132. [Google Scholar]
- Chakrabartty, S.; Barman, B.K.; Raj, C.R. Nitrogen and Phosphorus Co-Doped Graphitic Carbon Encapsulated Ultrafine OsP2 Nanoparticles: A pH Universal Highy Durable Catalyst for Hydrogen Evolution Reaction. Chem. Commun. 2013, 55, 4399–4402. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.; Kim, H.; Kim, J.; Ahn, S.H. Facile Fabrication of Nanostructured NiMo Cathode for High-Performance Proton Exchange Membrane Water Electrolyzer. J. Ind. Eng. Chem. 2019, 79, 255–260. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Liu, Y.; Zheng, J.; Li, X. A Highly Efficient and Stable Biphasic Nanocrystalline Ni-Mo-N Catalyst for Hydrogen Evolution in Both Acidic and Alkaline Electrolytes. Nano Energy 2016, 22, 111–119. [Google Scholar] [CrossRef]
- Ge, Y.; Gao, S.P.; Dong, P.; Baines, R.; Ajayan, P.M.; Ye, M.; Shen, J. Insight into the Hydrogen Evolution Reaction of Nickel Dichalcogenide Nanosheets: Activities Related to Non-Metal Ligands. Nanoscale 2017, 9, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zeng, L.; Xu, J.; Zhang, Z.; Zhao, Z.; Wang, Y.; Yuan, X.Z.; Li, H.; Wang, H. NaCl Template-Directed Approach to Ultrathin Lamellar Molybdenum Phosphide-Carbon Hybrids for Efficient Hydrogen Production. J. Power Sources 2019, 438. [Google Scholar] [CrossRef]
- Li, J.S.; Zhang, S.; Sha, J.Q.; Wang, H.; Liu, M.Z.; Kong, L.X.; Liu, G.D. Confined Molybdenum Phosphide in P-Doped Porous Carbon as Efficient Electrocatalysts for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 17140–17146. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Feng, Y.; Zhang, F.; Yang, C.; Yao, Z.; Zhao, W.; Qiu, F.; Yang, L.; Yao, Y.; Zhuang, X.; et al. Metal-Phosphide-Containing Porous Carbons Derived from an Ionic-Polymer Framework and Applied as Highly Efficient Electrochemical Catalysts for Water Splitting. Adv. Funct. Mater. 2015, 25, 3899–3906. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Wang, X.; He, D.; Wu, G.; Yang, Q.; Hong, X.; Wu, Y.; Li, Y. Porous Molybdenum Phosphide Nano-Octahedrons Derived from Confined Phosphorization in UIO-66 for Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 12854–12858. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Pi, C.; Zhang, X.; Ding, K.; Qin, P.; Fu, J.; Peng, X.; Gao, B.; Chu, P.K.; Huo, K. In Situ Synthesis of MoP Nanoflakes Intercalated N-Doped Graphene Nanobelts from MoO3–Amine Hybrid for High-Efficient Hydrogen Evolution Reaction. Small 2018, 14, e1800667. [Google Scholar] [CrossRef]
- Huang, Y.; Song, X.; Deng, J.; Zha, C.; Huang, W.; Wu, Y.; Li, Y. Ultra-Dispersed Molybdenum Phosphide and Phosphosulfide Nanoparticles on Hierarchical Carbonaceous Scaffolds for Hydrogen Evolution Electrocatalysis. Appl. Catal. B 2019, 245, 656–661. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F. Molybdenum Phosphosulfide: An Active, Acid-Stable, Earth-Abundant Catalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 14433–14437. [Google Scholar] [CrossRef]
- Jiang, R.; Fan, J.; Hu, L.; Dou, Y.; Mao, X.; Wang, D. Electrochemically Synthesized N-Doped Molybdenum Carbide Nanoparticles for Efficient Catalysis of Hydrogen Evolution Reaction. Electrochim. Acta 2018, 261, 578–587. [Google Scholar] [CrossRef]
- Wang, D.; Guo, T.; Wu, Z. Hierarchical Mo2C/C Scaffolds Organized by Nanosheets as Highly Efficient Electrocatalysts for Hydrogen Production. ACS Sustain. Chem. Eng. 2018, 6, 13995–14003. [Google Scholar] [CrossRef]
- Wu, C.; Li, J. Unique Hierarchical Mo2C/C Nanosheet Hybrids as Active Electrocatalyst for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 41314–41322. [Google Scholar] [CrossRef]
- Selvan, R.K.; Gedanken, A. The Sonochemical Synthesis and Characterization of Cu1-XNi XWO4 Nanoparticles/Nanorods and Their Application in Electrocatalytic Hydrogen Evolution. Nanotechnology 2009, 20, 105602. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.; Kim, J.; Kim, H.; Guo, W.; Han, G.H.; Hong, S.; Ahn, S.H. Electrodeposited Nickel Phosphide Supported by Copper Foam for Proton Exchange Membrane Water Electrolyzer. Korean J. Chem. Eng. 2020, 37, 1379–1386. [Google Scholar] [CrossRef]
- Yang, Y.; Lun, Z.; Xia, G.; Zheng, F.; He, M.; Chen, Q. Non-Precious Alloy Encapsulated in Nitrogen-Doped Graphene Layers Derived from MOFs as an Active and Durable Hydrogen Evolution Reaction Catalyst. Energy Environ. Sci. 2015, 8, 3563–3571. [Google Scholar] [CrossRef]
- Ji, X.; Liu, B.; Ren, X.; Shi, X.; Asiri, A.M.; Sun, X. P-Doped Ag Nanoparticles Embedded in N-Doped Carbon Nanoflake: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 4499–4503. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Liu, Y.; Ha, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co Nanoparticles Encapsulated in Carbon-Nanotubes-Grafted Graphene Sheets as Advanced Electrocatalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, e1802011. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Jang, H.; Chen, L.; Nam, G.; Liu, X.; Cho, J. Low Loading of RhxP and RuP on N, P Codoped Carbon as Two Trifunctional Electrocatalysts for the Oxygen and Hydrogen Electrode Reactions. Adv. Energy Mater. 2018, 8, 1801478. [Google Scholar] [CrossRef]
- Ayers, K.E.; Renner, J.N.; Danilovic, N.; Wang, J.X.; Zhang, Y.; Maric, R.; Yu, H. Pathways to Ultra-Low Platinum Group Metal Catalyst Loading in Proton Exchange Membrane Electrolyzers. Catal. Today 2016, 262, 121–132. [Google Scholar] [CrossRef]
- Ayers, K.E.; Capuano, C.; Carter, B.; Dalton, L.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research Advances Towards Low Cost, High Efficiency PEM Electrolysis. ECS Meet. Abstr. 2010, 33, 3–15. [Google Scholar]
- Kang, Z.; Yang, G.; Mo, J.; Li, Y.; Yu, S.; Cullen, D.A.; Retterer, S.T.; Toops, T.J.; Bender, G.; Pivovar, B.S.; et al. Novel Thin/Tunable Gas Diffusion Electrodes with Ultra-Low Catalyst Loading for Hydrogen Evolution Reactions in Proton Exchange Membrane Electrolyzer Cells. Nano Energy 2018, 47, 434–441. [Google Scholar] [CrossRef]
- Mirshekari, G.; Ouimet, R.; Zeng, Z.; Yu, H.; Bliznakov, S.; Bonville, L.; Niedzwiecki, A.; Capuano, C.; Ayers, K.; Maric, R. High-Performance and Cost-Effective Membrane Electrode Assemblies for Advanced Proton Exchange Membrane Water Electrolyzers: Long-Term Durability Assessment. Int. J. Hydrog. Energy 2021, 6, 1526–1539. [Google Scholar] [CrossRef]
- Ravichandran, S.; Venkatkarthick, R.; Sankari, A.; Vasudevan, S.; Jonas Davidson, D.; Sozhan, G. Platinum Deposition on the Nafion Membrane by Impregnation Reduction Using Nonionic Surfactant for Water Electrolysis—An Alternate Approach. Energy 2014, 68, 148–151. [Google Scholar] [CrossRef]
- Wang, X.; Hsing, I.M. Surfactant Stabilized Pt and Pt Alloy Electrocatalyst for Polymer Electrolyte Fuel Cells. Electrochim. Acta 2002, 47, 2981–2987. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Ryu, H.; Dhathathreyan, K.S. Platinum Catalysed Membranes for Proton Exchange Membrane Fuel Cells—Higher Performance. Chem. Eng. J. 2004, 102, 241–247. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Y.; Du, Y.; Chen, Y.; Cheng, G.; Chen, S.; Luo, W. A Monodisperse Rh2P-Based Electrocatalyst for Highly Efficient and PH-Universal Hydrogen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703489. [Google Scholar] [CrossRef]
- Duan, H.; Li, D.; Tang, Y.; He, Y.; Ji, S.; Wang, R.; Lv, H.; Lopes, P.P.; Paulikas, A.P.; Li, H.; et al. High-Performance Rh2P Electrocatalyst for Efficient Water Splitting. J. Am. Chem. Soc. 2017, 139, 5494–5502. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, B.; Lin, F.; Lv, F.; Luo, M.; Zhou, P.; Liu, Q.; Zhang, W.; Yang, C.; Tang, Y.; et al. Wrinkled Rh2P Nanosheets as Superior PH-Universal Electrocatalysts for Hydrogen Evolution Catalysis. Adv. Energy Mater. 2018, 8, 1801891. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; He, D.; Wang, M.; Li, G.; Mu, S. Activating Rhodium Phosphide-Based Catalysts for the PH-Universal Hydrogen Evolution Reaction. Nanoscale 2018, 10, 12407–12412. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and Hydrogen Evolution Reactions on Ru, RuO2, Ir, and IrO2 Thin Film Electrodes in Acidic and Alkaline Electrolytes: A Comparative Study on Activity and Stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.-N.; Wu, D.; Cao, M.; Sun, F.; Zhang, H.; You, H.; Zhuang, W.; Cao, R. Significantly Enhanced Overall Water Splitting Performance by Partial Oxidation of Ir through Au Modification in Core–Shell Alloy Structure. J. Am. Chem. Soc. 2021, 143, 4639–4645. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Z.; Xia, Z.; Luo, M.; Zhang, Q.; Qin, Y.; Tao, L.; Yin, K.; Chao, Y.; Gu, L.; et al. Exclusive Strain Effect Boosts Overall Water Splitting in PdCu/Ir Core/Shell Nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 8243–8250. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Gueye, I.; Seo, O.; Kim, J.; Hiroi, S.; Sakata, O.; et al. On the Electronic Structure and Hydrogen Evolution Reaction Activity of Platinum Group Metal-Based High-Entropy-Alloy Nanoparticles. Chem. Sci. 2020, 11, 12731–12736. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Wang, Y.; Ge, J.; Liu, C.; Xing, W. Enhanced Electrocatalytic Performance for Hydrogen Evolution Reaction through Surface Enrichment of Platinum Nanocluster Alloying with Ruthenium In-Situ Embedded in Carbon. Energy Environ. Sci. 2018, 11, 1232–1239. [Google Scholar] [CrossRef]
- Galyamin, D.; Torrero, J.; Elliott, J.D.; Rodríguez-García, I.; Sánchez, D.G.; Salam, M.A.; Gago, A.S.; Mokhtar, M.; de la Fuente, J.L.G.; Bueno, S.V.; et al. Insights into the High Activity of Ruthenium Phosphide for the Production of Hydrogen in Proton Exchange Membrane Water Electrolyzers. Adv. Energy Sustain. Res. 2023, 2300059. [Google Scholar] [CrossRef]
- Huang, Y.; Seo, K.-D.; Park, D.-S.; Park, H.; Shim, Y.-B. Hydrogen Evolution and Oxygen Reduction Reactions in Acidic Media Catalyzed by Pd4S Decorated N/S Doped Carbon Derived from Pd Coordination Polymer. Small 2021, 17, 104739. [Google Scholar] [CrossRef]
- Cheng, X.; Lu, Y.; Zheng, L.; Cui, Y.; Niibe, M.; Tokushima, T.; Li, H.; Zhang, Y.; Chen, G.; Sun, S.; et al. Charge Redistribution within Platinum–Nitrogen Coordination Structure to Boost Hydrogen Evolution. Nano Energy 2020, 73, 104739. [Google Scholar] [CrossRef]
- Wei, Z.W.; Wang, H.J.; Zhang, C.; Xu, K.; Lu, X.L.; Lu, T.B. Reversed Charge Transfer and Enhanced Hydrogen Spillover in Platinum Nanoclusters Anchored on Titanium Oxide with Rich Oxygen Vacancies Boost Hydrogen Evolution Reaction. Angew. Chem.—Int. Ed. 2021, 60, 16622–16627. [Google Scholar] [CrossRef]
- Koca, A.; Ozkaya, A.R.; Akyuz, D. Atomically Dispersed Platinum Supported on Curved Carbon Supports for Efficient Electrocatalytic Hydrogen Evolution. ECS Meet. Abstr. 2016, MA2016-01, 1728. [Google Scholar] [CrossRef]

| No. | Parameter | Unit | SoA | Targets | |
|---|---|---|---|---|---|
| 2020 | 2024 | 2030 | |||
| 1 | Electricity consumption @ nominal capacity | kWh/kg | 55 | 52 | 48 |
| 2 | Capital cost | EUR/(kg/d) EUR/kW | 2100 900 | 1550 700 | 1000 500 |
| 3 | O&M cost | EUR/(kg/d)/y | 41 | 30 | 21 |
| 4 | Hot idle ramp time | s | 2 | 1 | 1 |
| 5 | Cold start ramp time | s | 30 | 10 | 10 |
| 6 | Degradation | %/1000 h | 0.19 | 0.15 | 0.12 |
| 7 | Current density | A/cm2 | 2.2 | 2.4 | 3 |
| 8 | Use of critical raw materials as catalysts | mg/W | 2.5 | 1.25 | 0.25 |
| Company | Manufacturing Site | Electrolyzer Type |
|---|---|---|
| AREVA H2 | France, Germany | PEM |
| CarboTech | Germany | PEM |
| Cummins—Hydrogenics | Belgium, Canada, Germany | PEM and ALKALINE |
| DeNora | Italy, Japan, USA | PEM and ALKALINE |
| iGas | Germany | PEM |
| ITM | UK | PEM |
| Nel Hydrogen | Denmark, Norway, USA | PEM and ALKALINE |
| Siemens Energy | Germany | PEM |
| Comparison between Technologies | |||
|---|---|---|---|
| AWE | PEMWE | SOWE | |
| Operating temperature | 70–90 °C | 50–80 °C | 700–850 °C |
| Operating pressure | 1–30 bar | <70 bar | 1 bar |
| Electrolyte | Potassium hydroxide (KOH) 5–7 mol L−1 | PFSA membranes | Yttria-stabilized zirconia (YSZ) |
| Separator | ZrO2 stabilized with PPS mesh | Solid electrolyte (above) | Solid electrolyte (above) |
| Electrode/catalyst (oxygen side) | Nickel-coated perforated stainless steel | Iridium oxide | Perovskite-type (e.g., LSCF, LSM) |
| Electrode/catalyst (hydrogen side) | Nickel-coated perforated stainless steel | Platinum nanoparticles on carbon black | Ni/YSZ |
| Porous transport layer anode | Nickel mesh (not always present) | Platinum-coated sintered porous titanium | Coarse nickel mesh or foam |
| Porous transport layer cathode | Nickel mesh | Sintered porous titanium or carbon cloth | None |
| Bipolar plate anode | Nickel-coated stainless steel | Platinum-coated titanium | None |
| Bipolar plate cathode | Nickel-coated stainless steel | Gold-coated titanium | Cobalt-coated stainless steel |
| Frames and sealing | PSU, PTFE, EPDM | PTFE, PSU, ETFE | PTFE, silicon |
| PEMWE vs. AWE | |||
| Advantages | Disadvantages | ||
Compact system design
| Acidic electrolyte
| ||
| Manufacturer | Structure | Parameters | Ref. | |
|---|---|---|---|---|
| Nafion® | DuPont (Wilmington, DE, USA) | 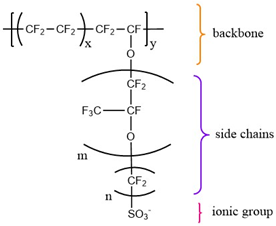 | m = 1; n = 2; x = 5–13.5; y = 1000 e.g., EW * = 1000; x = ~5.5 | [50,53] |
| Flemion® | Asahi Glass (Tokyo, Japan) | m = 0 or 1; n = 1–5 | [53,54,55] | |
| 3M® | 3M™ Corporation (Maplewood, MN, USA) | m = 0; n = 4; x = ~3–5 for EW = 660–825; e.g., for EW = 1000; x = ~6.5 or EW = 700; x = ~3 | [50,53,56] | |
| Aciplex® | Asahi Kasei (Tokyo, Japan) | M = 0–3; n = 2–5; x = 1.5–14 e.g., for EW = 1130; x = ~7 | [53,54,55,57] | |
| Aquivion®/Dow SSC® | Solvay Specialty Polymers (Brussels, Belgium) | m = 0; n = 2; x = 3.6–10 e.g., for EW = 1000; x = ~7 | [50,53] |
| 2022 | Target 2050 | Research and Development | |
|---|---|---|---|
| Nominal current density | 1–3 A cm−2 | 4–6 A cm−2 | Membranes |
| Voltage | 1.4–2.3 V | <1.7 V | Catalysts, membranes |
| Operating temperature | 50–80 °C | 80 °C | Durability of the membranes |
| Cell pressure | ≤50 bar | >70 bar | Membranes, catalysts |
| Load Range | 5–130% | 5–300% | Membranes |
| H2 purity | 99.9–99.9999% | 99.9–99.9999% | Membranes |
| Voltage efficiency (LHV) | 50–68% | >80% | Catalysts |
| Electrical efficiency (stack) | 44–66 kWh/kg H2 | <42 kWh/kg H2 | Catalysts, membranes |
| Lifetime (stack) | 50,000–80,000 h | 100,000–120,000 h | Catalysts, membranes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perović, K.; Morović, S.; Jukić, A.; Košutić, K. Alternative to Conventional Solutions in the Development of Membranes and Hydrogen Evolution Electrocatalysts for Application in Proton Exchange Membrane Water Electrolysis: A Review. Materials 2023, 16, 6319. https://doi.org/10.3390/ma16186319
Perović K, Morović S, Jukić A, Košutić K. Alternative to Conventional Solutions in the Development of Membranes and Hydrogen Evolution Electrocatalysts for Application in Proton Exchange Membrane Water Electrolysis: A Review. Materials. 2023; 16(18):6319. https://doi.org/10.3390/ma16186319
Chicago/Turabian StylePerović, Klara, Silvia Morović, Ante Jukić, and Krešimir Košutić. 2023. "Alternative to Conventional Solutions in the Development of Membranes and Hydrogen Evolution Electrocatalysts for Application in Proton Exchange Membrane Water Electrolysis: A Review" Materials 16, no. 18: 6319. https://doi.org/10.3390/ma16186319
APA StylePerović, K., Morović, S., Jukić, A., & Košutić, K. (2023). Alternative to Conventional Solutions in the Development of Membranes and Hydrogen Evolution Electrocatalysts for Application in Proton Exchange Membrane Water Electrolysis: A Review. Materials, 16(18), 6319. https://doi.org/10.3390/ma16186319






