Composites and Materials Prepared from Boron Cluster Anions and Carboranes
Abstract
:1. Introduction
2. Decaborane as Boron Source for Boron-Containing Materials
3. Carborane as a Boron Source for Boron-Containing Materials
4. Boron Cluster Anions as Boron Source for Boron-Containing Materials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goodger, E. Unconventional fuels. New Sci. 1957, 27–29. [Google Scholar]
- Martin, D.R. The development of borane fuels. J. Chem. Ed. 1959, 36, 208–214. [Google Scholar] [CrossRef]
- United States Government. Boron High Energy Fuels: Hearings Before the Committee on Science and Astronautics, U.S. House of Representatives. Eighty-Six Congress, First Session; United States Government Printing Office: Washington, DC, USA, 1959.
- Sivaev, I.B. Nitrogen heterocyclic salts of polyhedral borane anions: From ionic liquids to energetic materials. Chem. Heterocycl. Comp. 2017, 53, 638–658. [Google Scholar] [CrossRef]
- Shan, Z.; Sheng, L.; Yang, R. Research progress of icosahedral polyhydroborate B12H122− anion compounds and their application in propellants and explosives. Chin. J. Explos. Propellants 2017, 40, 1–16. [Google Scholar] [CrossRef]
- Jiao, N.; Zhang, Y.; Liu, L.; Shreeve, J.M.; Zhang, S. Hypergolic fuels based on water-stable borohydride cluster anions with ultralow ignition delay times. J. Mater. Chem. A 2017, 5, 13341–13346. [Google Scholar] [CrossRef]
- Rachiero, G.P.; Titi, H.M.; Rogers, R.D. Versatility and remarkable hypergolicity of exo-6, exo-9 imidazole-substituted nido-decaborane. Chem. Commun. 2017, 53, 7736–7739. [Google Scholar] [CrossRef]
- Sheng, L.; Shan, Z.; Guo, X.; Yang, R. Synthesis, characterization and thermal behavior of bis(dialkyl-5-aminotetrazolium) dodecadodecaborates. Chin. J. Org. Chem. 2018, 38, 2093–2100. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, Z.; Jiao, N.; Liu, L.; Zhang, S. B12H122–-Based metal (Cu2+, Ni2+, Zn2+) complexes as hypergolic fuels with superior hypergolicity. Eur. J. Inorg. Chem. 2018, 2018, 981–986. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liu, L.; Jiao, N.; Meng, X.; Zhang, S. Amino functionalized [B12H12]2– salts as hypergolic fuels. New J. Chem. 2018, 42, 3568–3573. [Google Scholar] [CrossRef]
- Derdziuk, J.; Malinowski, P.J.; Jaroń, T. Synthesis, structural characterization and thermal decomposition studies of (N2H5)2B12H12 and its solvates. Int. J. Hydrogen Energy 2019, 44, 27030–27038. [Google Scholar] [CrossRef]
- Sharon, P.; Afri, M.; Mitlin, S.; Gottlieb, L.; Schmerling, B.; Grinstein, D.; Welner, S.; Frimer, A.A. Preparation and characterization of bis(guanidinium) and bis(aminotetrazolium)dodecahydroborate salts: Green high energy nitrogen and boron rich compounds. Polyhedron 2019, 157, 71–89. [Google Scholar] [CrossRef]
- Li, S.; Pan, X.; Jiang, Y.; Chang, S.; Jin, X.; Yang, Y.; Huang, X.; Guo, Y. Ignition and combustion behaviors of high energetic polyhedral boron cluster. Propellants Explos. Pyrotech. 2019, 44, 1319–1326. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Wang, J.; Wang, S.; Wang, Z.-Y.; Cao, M.; He, C.-L.; Yang, J.-Q.; Zang, S.-Q.; Mak, T.C.W. o-Carborane-based and atomically precise metal clusters as hypergolic materials. J. Am. Chem. Soc. 2020, 142, 12010–12014. [Google Scholar] [CrossRef]
- Hawthorne, M.F. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew. Chem. Int. Ed. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The chemistry of neutron capture therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 2009, 1433–1450. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Kugler, M.; Nekvinda, J.; Holub, J.; El Anwar, S.; Das, V.; Šícha, V.; Pospíšilová, K.; Fábry, M.; Král, V.; Brynda, J.; et al. Inhibitors of CA IX enzyme based on polyhedral boron compounds. ChemBioChem 2021, 22, 2741–2761. [Google Scholar] [CrossRef] [PubMed]
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. The medicinal chemistry of carboranes. Coord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Zargham, E.O.; Mason, C.A.; Lee, M.W. The use of carboranes in cancer drug development. Int. J. Cancer Clin. Res. 2019, 6, 110. [Google Scholar] [CrossRef]
- Chen, Y.; Du, F.; Tang, L.; Xu, J.; Zhao, Y.; Wu, X.; Li, M.; Shen, J.; Wen, Q.; Cho, C.H.; et al. Carboranes as unique pharmacophores in antitumor medicinal chemistry. Mol. Ther. Oncolytics 2022, 24, 400–416. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Garaev, T.M.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Physiologically active compounds based on membranotropic cage carriers—Derivatives of adamantane and polyhedral boron clusters (Review). Russ. J. Inorg. Chem. 2022, 67, 28–47. [Google Scholar] [CrossRef]
- Marforio, T.D.; Mattioli, E.J.; Zerbetto, F.; Calvaresi, M. Exploiting blood transport proteins as carborane supramolecular vehicles for boron neutron capture therapy. Nanomaterials 2023, 13, 1770. [Google Scholar] [CrossRef]
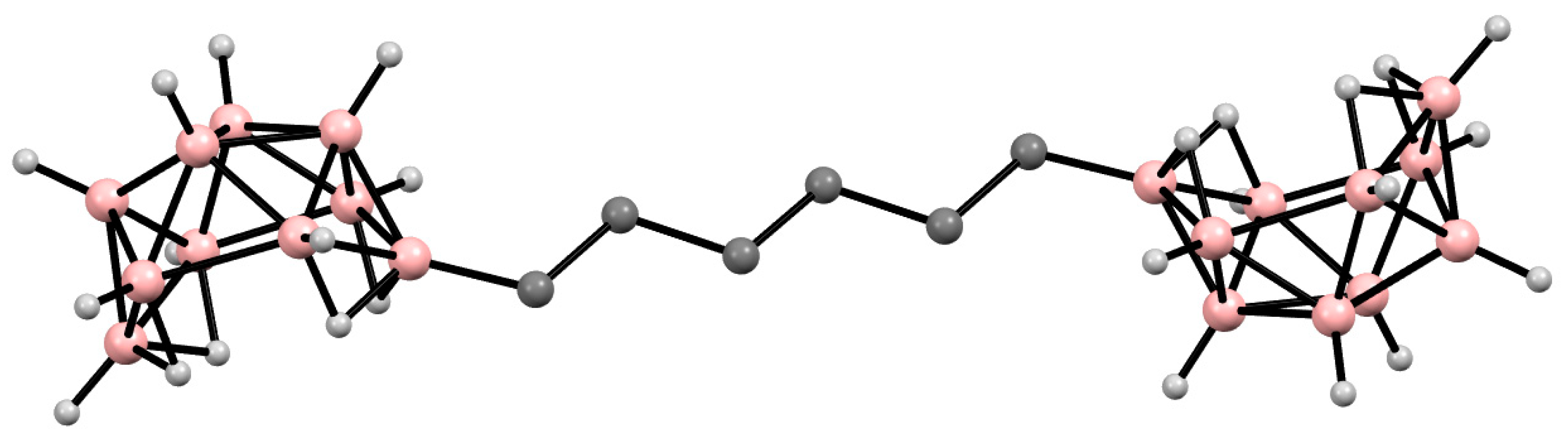
- Morin, J.F.; Sasaki, T.; Shirai, Y.; Guerrero, J.M.; Tour, J.M. Synthetic routes toward carborane-wheeled nanocars. J. Org. Chem. 2007, 72, 9481–9490. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Guerrero, J.M.; Leonard, A.D.; Tour, J.M. Nanotrains and self-assembled two-dimensional arrays built from carboranes linked by hydrogen bonding of dipyridones. Nano Res. 2008, 1, 412–419. [Google Scholar] [CrossRef]
- Chiang, P.-T.; Mielke, J.; Godoy, J.; Guerrero, J.M.; Alemany, L.B.; Villagómez, C.J.; Saywell, A.; Grill, G.; Tour, J.M. Toward a light-driven motorized nanocar: Synthesis and initial imaging of single molecules. ACS Nano 2012, 6, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Lavasani, S.M.; Pishkenari, H.N.; Meghdari, A. How chassis structure and substrate crystalline direction affect the mobility of thermally driven p-carborane-wheeled nanocars. J. Phys. Chem. C 2019, 123, 4805–4824. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Zink, J.I.; Skelton, J.M.; Bayer, M.B.; Liu, C.; Livshits, E.; Baer, R.; Neuhauser, D. Electrical or photocontrol of the rotary motion of a metallacarborane. Science 2004, 303, 1849–1852. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Ramachandran, B.M.; Kennedy, R.D.; Knobler, C.B. Approaches to rotary molecular motors. Pure Appl. Chem. 2006, 78, 1299–1304. [Google Scholar] [CrossRef]
- Shlyakhtina, N.I.; Safronov, A.V.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Synthesis, characterization, and preliminary fluorescence study of a mixed ligand bis(dicarbollyl)nickel complex bearing a tryptophan-BODIPY FRET couple. J. Organomet. Chem. 2015, 798, 234–244. [Google Scholar] [CrossRef]
- Sivaev, I.B. Ferrocene and transition metal bis(dicarbollides) as platform for design of rotatory molecular switches. Molecules 2017, 22, 2201. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Timofeev, S.V.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B. Bis(dicarbollide) complexes of transition metals as a platform for molecular switches. Study of complexation of 8,8’-bis(methylsulfanyl) derivatives of cobalt and iron bis(dicarbollides). Molecules 2020, 25, 5745. [Google Scholar] [CrossRef]
- Bae, Y.S.; Farha, O.K.; Spokoyny, A.M.; Mirkin, C.A.; Hupp, J.T.; Snurr, R.Q. Carborane-based metal–organic frameworks as highly selective sorbents for CO2 over methane. Chem. Commun. 2008, 35, 4135–4137. [Google Scholar] [CrossRef]
- Spokoyny, A.M.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T.; Mirkin, C.A. Porosity tuning of carborane-based metal–organic frameworks (MOFs) via coordination chemistry and ligand design. Inorg. Chim. Acta 2010, 364, 266–271. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Krungleviciute, V.; Clingerman, D.J.; Mondloch, J.E.; Peng, Y.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; et al. Carborane-based metal-organic framework with high methane and hydrogen storage capacities. Chem. Mater. 2013, 25, 3539–3543. [Google Scholar] [CrossRef]
- Kennedy, R.D.; Clingerman, D.J.; Morris, W.; Wilmer, C.E.; Sarjeant, A.A.; Stern, C.L.; O’Keeffe, M.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K.; et al. Metallacarborane-based metal–organic framework with a complex topology. Cryst. Growth Des. 2014, 14, 1324–1330. [Google Scholar] [CrossRef]
- Clingerman, D.J.; Morris, W.; Mondloch, J.E.; Kennedy, R.D.; Sarjeant, A.A.; Stern, C.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Stabilization of a highly porous metal–organic framework utilizing a carborane-based linker. Chem. Commun. 2015, 51, 6521–6523. [Google Scholar] [CrossRef]
- Boldog, I.; Bereciartua, P.J.; Bulánek, R.; Kučeráková, M.; Tomandlová, M.; Dušek, M.; Macháček, J.; De Vos, D.; Baše, T. 10-Vertex closo-carborane: A unique ligand platform for porous coordination polymers. CrystEngComm 2016, 18, 2036–2040. [Google Scholar] [CrossRef]
- Boldog, I.; Dušek, M.; Jelínek, T.; Švec, P.; de Ramos, O.F.S.; Růžička, A.; Bulánek, R. Porous 10- and 12-vertex (bi)-p-dicarba-closo-boranedicarboxylates of cobalt and their gas adsorptive properties. Microporous Mesoporous Mater. 2018, 271, 284–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Krishna, R.; Wang, L.; Yang, L.; Cui, X.; Duttwyler, S.; Xing, H. Rational design of microporous MOFs with anionic boron cluster functionality and cooperative dihydrogen binding sites for highly selective capture of acetylene. Angew. Chem. Int. Ed. 2020, 59, 17664–17669. [Google Scholar] [CrossRef]
- Sun, W.; Hu, J.; Duttwyler, S.; Wang, L.; Krishna, R.; Zhang, Y. Highly selective gas separation by two isostructural boron cluster pillared MOFs. Sep. Purif. Technol. 2022, 283, 120220. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.-B.; Light, M.E.; Carrillo, A.E.; Arauzo, A.; Valvidares, M.; Roscini, C.; Teixidor, F.; Viñas, C.; Gándara, F.; et al. A metal-organic framework incorporating eight different size rare-earth metal elements: Toward multifunctionality À La Carte. Adv. Funct. Mater. 2023, 33, 2307369. [Google Scholar] [CrossRef]
- Sivaev, I.B. Decaborane: From Alfred Stock and rocket fuel projects to nowadays. Molecules 2023, 28, 6287. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Naoufal, D. Fifty years of the closo-decaborate anion chemistry. Collect. Czechoslov. Chem. Commun. 2010, 75, 1149–1199. [Google Scholar] [CrossRef]
- Zhizhin, K.Y.; Zhdanov, A.P.; Kuznetsov, N.T. Derivatives of closo-decaborate anion [B10H10]2− with exo-polyhedral substituents. Russ. J. Inorg. Chem. 2010, 55, 2089–2127. [Google Scholar] [CrossRef]
- Mahfouz, N.; Abi Ghaida, F.; El Hajj, Z.; Diab, M.; Floquet, S.; Mehdi, A.; Naoufal, D. Recent achievements on functionalization within closo-decahydrodecaborate [B10H10]2− clusters. ChemistrySelect 2022, 7, e202200770. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Shelly, K.; Li, F. The versatile chemistry of the [B20H18]2− ions: Novel reactions and structural motifs. Chem. Commun. 2002, 6, 547–554. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Zhizhin, K.Y.; Bernhardt, E.; Kuznetsov, N.T. Structural diversity of dimer clusters based on the octadecahydro-eicosaborate anion. J. Struct. Chem. 2019, 60, 692–712. [Google Scholar] [CrossRef]
- Thompson, A.G. MOCVD technology for semiconductors. Mater. Lett. 1997, 30, 255–263. [Google Scholar] [CrossRef]
- Jones, A.C. Recent developments in the MOCVD of electronic materials. Adv. Mater. Opt. Electron. 2000, 10, 91–92. [Google Scholar] [CrossRef]
- Chow, L.A. Equipment and manufacturability issues in chemical vapor deposition processes. In Handbook of Thin Film Deposition, 4th ed.; Seshan, K., Schepis, D., Eds.; William Andrew: Oxford, UK, 2018; pp. 269–316. [Google Scholar] [CrossRef]
- Global Metal Organic Chemical Vapor Deposition Market: Analysis by Application, by Category, by Region Size and Trends with Impact of COVID-19 and Forecast up to 2026. Available online: https://www.researchandmarkets.com/report/mocvd (accessed on 1 June 2023).
- Roush, G. The Toxicology of the Boranes. J. Occup. Med. 1959, 1, 46–52. Available online: https://www.jstor.org/stable/44999044 (accessed on 1 June 2023). [CrossRef]
- Naeger, L.L.; Leibman, K.C. Mechanisms of decaborane toxicity. Toxicol. Appl. Pharmacol. 1972, 22, 517–527. [Google Scholar] [CrossRef]
- Nakamura, K. Preparation and properties of amorphous boron films deposited by pyrolysis of decaborane in the molecular flow region. J. Electrochem. Soc. 1984, 131, 2691. [Google Scholar] [CrossRef]
- Kamimura, K.; Yoshimura, T.; Nagaoka, T.; Nakao, M.; Onuma, Y.; Makimura, M. Preparation and thermoelectric property of boron thin film. J. Solid State Chem. 2000, 154, 153–156. [Google Scholar] [CrossRef]
- Natsir, M.; Sagara, A.; Motojima, O. Reduction of hydrogen content in boron film by controlling glow discharge conditions. J. Nucl. Mater. 1995, 220–222, 865–868. [Google Scholar] [CrossRef]
- Kodama, H.; Oyaidzu, M.; Sasaki, M.; Kimura, H.; Morimoto, Y.; Oya, Y.; Matsuyama, M.; Sagara, A.; Noda, N.; Okuno, K. Studies on structural and chemical characterization for boron coating films deposited by PCVD. J. Nucl. Mater. 2004, 329–333, 889–893. [Google Scholar] [CrossRef]
- Saidoh, M.; Ogiwara, N.; Shimada, M.; Arai, T.; Hiratsuka, H.; Koike, T.; Shimizu, M.; Ninomiya, H.; Nakamura, H.; Jimbou, R. Initial boronization of JT-60U tokamak using decaborane. Jpn. J. Appl. Phys. 1993, 32, 3276–3281. [Google Scholar] [CrossRef]
- Perkins, F.K.; Rosenberg, R.A.; Lee, S.; Dowben, P.A. Synchrotron-radiation-induced deposition of boron and boron carbide films from boranes and carboranes: Decaborane. J. Appl. Phys. 1991, 69, 4103–4109. [Google Scholar] [CrossRef]
- Kim, Y.G.; Dowben, P.A.; Spencer, J.T.; Ramseyer, G.O. Chemical vapor deposition of boron and boron nitride from decaborane(14). J. Vac. Sci. Technol. A 1989, 7, 2796–2799. [Google Scholar] [CrossRef]
- Chatterjee, S.; Luo, Z.; Acerce, M.; Yates, D.M.; Johnson, A.T.C.; Sneddon, L.G. Chemical vapor deposition of boron nitride nanosheets on metallic substrates via decaborane/ammonia reactions. Chem. Mater. 2011, 23, 4414–4416. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kim, M.J.; Zakharov, D.N.; Kim, S.M.; Stach, E.A.; Maruyama, B.; Sneddon, L.G. Syntheses of boron nitride nanotubes from borazine and decaborane molecular precursors by catalytic chemical vapor deposition with a floating nickel catalyst. Chem. Mater. 2012, 24, 2872–2879. [Google Scholar] [CrossRef]
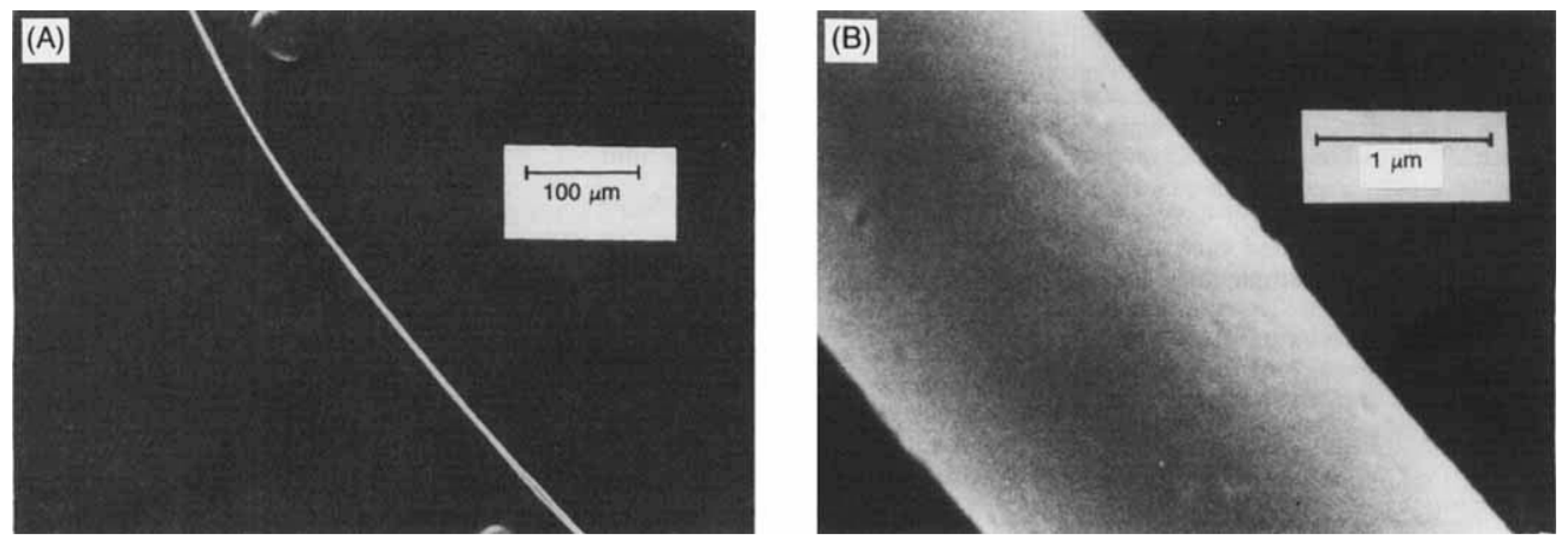
- Bellott, B.J.; Noh, W.; Nuzzo, R.G.; Girolami, G.S. Nanoenergetic materials: Boron nanoparticles from the pyrolysis of decaborane and their functionalisation. Chem. Commun. 2009, 22, 3214–3215. [Google Scholar] [CrossRef] [PubMed]
- Ekimov, E.A.; Zibrov, I.P.; Zoteev, A.V. Preparation of boron microcrystals via high-pressure, high-temperature pyrolysis of decaborane, B10H14. Inorg. Mater. 2011, 47, 1194–1198. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Lebed, J.B.; Sidorov, V.A.; Lyapin, S.G. High-pressure synthesis of crystalline boron in B-H system. Sci. Technol. Adv. Mater. 2011, 12, 055009. [Google Scholar] [CrossRef]
- Kher, S.S.; Spencer, J.T. Chemical vapor deposition precursor chemistry. 3. Formation and characterization of crystalline nickel boride thin films from the cluster-assisted deposition of polyhedral borane compounds. Chem. Mater. 1992, 4, 538–544. [Google Scholar] [CrossRef]
- Tynell, T.; Aizawa, T.; Ohkubo, I.; Nakamura, K.; Mori, T. Deposition of thermoelectric strontium hexaboride thin films by a low pressure CVD method. J. Cryst. Growth 2016, 449, 10–14. [Google Scholar] [CrossRef]
- Kher, S.S.; Tan, Y.; Spencer, J.T. Chemical vapor deposition of metal borides. 4. The application of polyhedral boron clusters to the chemical vapor deposition formation of gadolinium boride thin-film materials. Appl. Organomet. Chem. 1996, 10, 297–304. [Google Scholar] [CrossRef]
- Kher, S.S.; Romero, J.V.; Caruso, J.D.; Spencer, J.T. Chemical vapor deposition of metal borides. 6. The formation of neodymium boride thin film materials from polyhedral boron clusters and metal halides by chemical vapor deposition. Appl. Organomet. Chem. 2008, 22, 300–307. [Google Scholar] [CrossRef]
- Guélou, G.; Martirossyan, M.; Ogata, K.; Ohkubo, I.; Kakefuda, Y.; Kawamoto, N.; Kitagawa, Y.; Ueda, J.; Tanabe, S.; Maeda, K.; et al. Rapid deposition and thermoelectric properties of ytterbium boride thin films using hybrid physical chemical vapor deposition. Materialia 2018, 1, 244–248. [Google Scholar] [CrossRef]
- Ohkubo, I.; Aizawa, T.; Nakamura, K.; Mori, T. Control of competing thermodynamics and kinetics in vapor phase thin-film growth of nitrides and borides. Front. Chem. 2021, 9, 642388. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Sneddon, L.G. A polymer precursor route to metal borides. Chem. Mater. 1993, 5, 1659–1668. [Google Scholar] [CrossRef]
- Pender, M.J.; Sneddon, L.G. An efficient template synthesis of aligned boron carbide nanofibers using a single-source molecular precursor. Chem. Mater. 2000, 12, 280–283. [Google Scholar] [CrossRef]
- Borchardt, L.; Kockrick, E.; Wollmann, P.; Kaskel, S.; Guron, M.M.; Sneddon, L.G.; Geiger, D. Ordered mesoporous boron carbide based materials via precursor nanocasting. Chem. Mater. 2010, 22, 4660–4668. [Google Scholar] [CrossRef]
- Wei, X.; Carroll, P.J.; Sneddon, L.G. Ruthenium-catalyzed ring-opening polymerization syntheses of poly(organodecaboranes): New single-source boron-carbide precursors. Chem. Mater. 2006, 18, 1113–1123. [Google Scholar] [CrossRef]
- Seyferth, D.; Rees, W.S.; Haggerty, J.S.; Lightfoot, A. Preparation of boron-containing ceramic materials by pyrolysis of the decaborane(14)-derived [-B10H12·Ph2POPPh2]x- polymer. Chem. Mater. 1989, 1, 45–52. [Google Scholar] [CrossRef]
- Yu, X.-H.; Cao, K.; Huang, Y.; Yang, J.; Li, J.; Chang, G. Platinum catalyzed sequential hydroboration of decaborane: A facile approach to poly(alkenyldecaborane) with decaborane in the main chain. Chem. Commun. 2014, 50, 4585–4587. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gou, Y.; Jian, K.; Huang, J.; Wang, H. Boron carbide ceramic hollow microspheres prepared from poly(6-CH2=CH(CH2)4-B10H13) precursor. Mater. Des. 2016, 109, 408–414. [Google Scholar] [CrossRef]
- Welna, D.T.; Bender, J.D.; Wei, X.; Sneddon, L.G.; Allcock, H.R. Preparation of boron-carbide/carbon nanofibers from a poly(norbornenyldecaborane) single-source precursor via electrostatic spinning. Adv. Mater. 2005, 17, 859–862. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Liang, Y.; Chen, J.; Xie, M.; Zhou, L.; Xiong, Q.; Li, W. Ruthenium-catalyzed cascade ring-opening polymerization/hydroboration for the synthesis of low cross-linking poly(6-norbornenyldecaborane) and its thermal property. Adv. Mater. Res. 2014, 1035, 288–291. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Cao, K.; Yi, Y.; Yang, J.; Li, B. Synthesis and characterization of B-C polymer hollow microspheres from a new organodecaborane preceramic polymer. RSC Adv. 2015, 5, 86214–86218. [Google Scholar] [CrossRef]
- Wang, J.; Gou, Y.; Zhang, Q.; Jian, K.; Chen, Z.; Wang, H. Linear organodecaborane block copolymer as a single-sourceprecursor for porous boron carbide ceramics. J. Eur. Ceram. Soc. 2017, 37, 1937–1943. [Google Scholar] [CrossRef]
- Li, J.; Cao, K.; Li, J.; Liu, M.; Zhang, S.; Yang, J.; Zhang, Z.; Li, B. Synthesis and ceramic conversion of a new organodecaborane preceramic polymer with high-ceramic-yield. Molecules 2018, 23, 2461. [Google Scholar] [CrossRef]
- Pender, M.J.; Wideman, T.; Carroll, P.J.; Sneddon, L.G. Transition metal promoted reactions of boron hydrides. 15. Titanium-catalyzed decaborane−olefin hydroborations: One-step, high-yield syntheses of monoalkyldecaboranes. J. Am. Chem. Soc. 1998, 120, 9108–9109. [Google Scholar] [CrossRef]
- Mazurowski, J.; Lee, S.; Ramseyer, G.; Dowben, P.A. Characterization of boron carbide films formed by PECVD. MRS Online Proc. Libr. 1992, 242, 637–642. [Google Scholar] [CrossRef]
- Lee, S.; Mazurowski, J.; Ramseyer, G.; Dowben, P.A. Characterization of boron carbide thin films fabricated by plasma enhanced chemical vapor deposition from boranes. J. Appl. Phys. 1992, 72, 4925–4933. [Google Scholar] [CrossRef]
- Lee, S.; Mazurowski, J.; O’Brien, W.L.; Dong, Q.Y.; Jia, J.J.; Callcott, T.A.; Tan, Y.; Miyano, K.E.; Ederer, D.L.; Mueller, D.R.; et al. The structural homogeneity of boron carbide thin films fabricated using plasma enhanced chemical vapor deposition from B5H9+CH4. J. Appl. Phys. 1993, 74, 6919–6924. [Google Scholar] [CrossRef]
- Gold, J.W.; Militscher, C.; Slauson, D.D. Pentaborane Disposal: Taming the Dragon. Available online: https://dokumen.tips/documents/pentaborane-taming-the-dragonpdf.html (accessed on 1 June 2023).
- Rees, W.S.; Seyferth, D. High-yield synthesis of B4C/BN ceramic materials by pyrolysis of polymeric Lewis base adducts of decaborane(14). J. Am. Ceram. Soc. 1988, 71, C194–C196. [Google Scholar] [CrossRef]
- Rees, W.S.; Seyferth, D. Preparation, characterization, and pyrolysis of decaborane (14)—Based polymers: B4C/BN and BN procedures. In A Collection of Papers Presented at the 13th Annual Conference on Composites and Advanced Ceramic Materials: Ceramic Engineering and Science Proceedings; The American Ceramic Society: Westerville, OH, USA, 1989; Volume 10, pp. 837–845. [Google Scholar] [CrossRef]
- Yogo, T.; Naka, S. Synthesis of boron nitride from triammoniadecaborane and hydrazine under pressure. J. Mater. Sci. 1990, 25, 374–378. [Google Scholar] [CrossRef]
- Yogo, T.; Naka, S.; Iwahara, H. Synthesis of cubic boron nitride with boron nitride powder formed from triammoniadecaborane. J. Mater. Sci. 1991, 26, 3758–3762. [Google Scholar] [CrossRef]
- Robertson, B.W.; Adenwalla, S.; Harken, A.; Welsch, P.; Brand, J.I.; Dowben, P.A.; Claassen, J.P. A class of boron-rich solid-state neutron detectors. Appl. Phys. Lett. 2002, 80, 3644–3646. [Google Scholar] [CrossRef]
- Harken, A.D.; Day, E.E.; Robertson, B.W.; Adenwalla, S. Boron-rich semiconducting boron carbide neutron detector. Jpn. J. Appl. Phys. 2005, 44, 444–445. [Google Scholar] [CrossRef]
- Day, E.; Diaz, M.J.; Adenwalla, S. Effect of bias on neutron detection in thin semiconducting boron carbide films. J. Phys. D 2006, 39, 2920–2924. [Google Scholar] [CrossRef]
- Caruso, A.N.; Dowben, P.A.; Balkir, S.; Schemm, N.; Osberg, K.; Fairchild, R.W.; Flores, O.B.; Balaz, S.; Harken, A.D.; Robertson, B.W.; et al. The all boron carbide diode neutron detector: Comparison with theory. Mater. Sci. Eng. B 2006, 135, 129–133. [Google Scholar] [CrossRef]
- Hong, N.; Crow, L.; Adenwalla, S. Time-of-flight neutron detection using PECVD grown boron carbide diode detector. Nucl. Instrum. Methods Phys. Res. Sect. A 2013, 708, 19–23. [Google Scholar] [CrossRef]
- Peterson, G.; Su, Q.; Wang, Y.; Dowben, P.A.; Nastasi, M. Improved p–n heterojunction device performance induced by irradiation in amorphous boron carbide films. Mater. Sci. Eng. B 2015, 202, 25–30. [Google Scholar] [CrossRef]
- Nastasi, M.; Peterson, G.; Su, Q.; Wang, Y.; Ianno, N.J.; Benker, N.; Echeverría, E.; Yost, J.A.; Kelber, A.J.; Dong, B.; et al. Electrical and structural characterization of neutron irradiated amorphous boron carbide/silicon p-n heterojunctions. Nucl. Inst Methods Phys. Res. B 2018, 432, 48–54. [Google Scholar] [CrossRef]
- Byun, D.; Spady, B.R.; Ianno, N.J.; Dowben, P.A. Comparison of different chemical vapor deposition methodologies for fabrication of hetero junction boron-carbide diodes. Nanostruct. Mater. 1995, 5, 465–471. [Google Scholar] [CrossRef]
- Zhang, D.; Mcilroy, D.N.; O’Brien, W.L.; De Stasio, G. The chemical and morphological properties of boron-carbon alloys grown by plasma-enhanced chemical vapour deposition. J. Mater. Sci. 1998, 33, 4911–4915. [Google Scholar] [CrossRef]
- Nordell, B.J.; Karki, S.; Nguyen, T.D.; Rulis, P.; Caruso, A.N.; Purohit, S.S.; Li, H.; King, S.W.; Dutta, D.; Gidley, D.; et al. The influence of hydrogen on the chemical, mechanical, optical/electronic, and electrical transport properties of amorphous hydrogenated boron carbide. J. Appl. Phys. 2015, 118, 035703. [Google Scholar] [CrossRef]
- Bute, A.; Jagannath; Kar, R.; Chopade, S.S.; Desai, S.S.; Deo, M.N.; Rao, P.; Chand, N.; Kumar, S.; Singh, K.; et al. Effect of self-bias on the elemental composition and neutron absorption of boron carbide films deposited by RF plasma enhanced CVD. Mater. Chem. Phys. 2016, 182, 62–71. [Google Scholar] [CrossRef]
- Nordell, B.J.; Keck, C.L.; Nguyen, T.D.; Caruso, A.N.; Purohit, S.S.; Lanford, W.A.; Dutta, D.; Gidley, D.; Henry, P.; King, S.W.; et al. Tuning the properties of a complex disordered material: Full factorial investigation of PECVD-grown amorphous hydrogenated boron carbide. Mater. Chem. Phys. 2016, 173, 268–284. [Google Scholar] [CrossRef]
- Bute, A.; Jena, S.; Bhattacharya, D.; Kumar, S.; Chand, N.; Keskar, N.; Sinha, S. Composition dependent microstructure and optical properties of boron carbide (BxC) thin films deposited by radio frequency-plasma enhanced chemical vapour deposition technique. Mater. Res. Bull. 2019, 109, 175–182. [Google Scholar] [CrossRef]
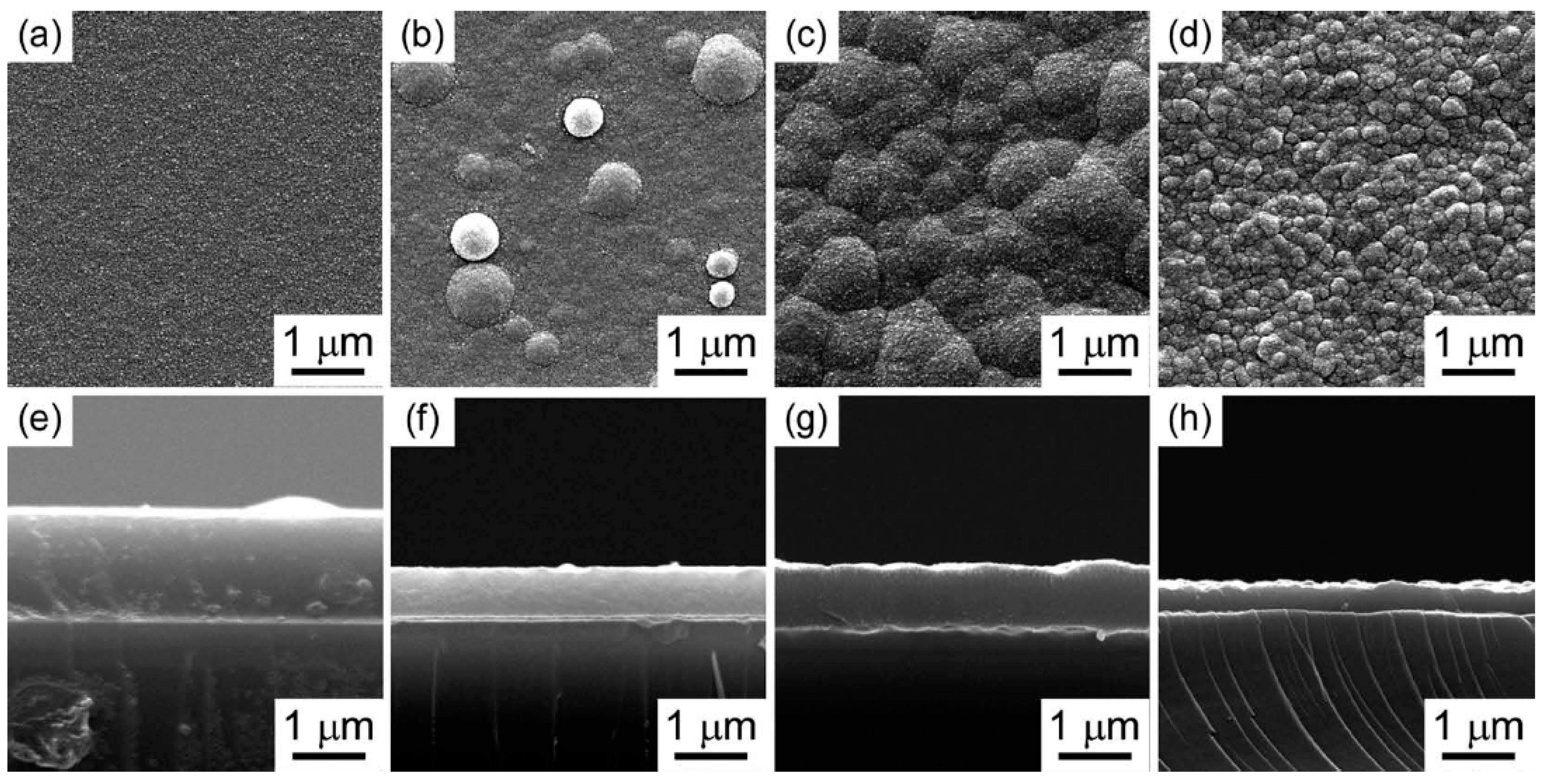
- Tu, R.; Hu, X.; Li, J.; Yang, M.; Li, Q.; Shi, J.; Li, H.; Ohmori, H.; Goto, T.; Zhang, S. Fabrication of (a-nc) boron carbide thin films via chemical vapor deposition using ortho-carborane. J. Asian Ceram. Soc. 2020, 8, 327–335. [Google Scholar] [CrossRef]
- Bute, A.; Jena, S.; Kedia, S.; Udupa, D.V.; Singh, K.; Bhattacharya, D.; Modi, M.H.; Chand, N.; Sinha, S. Boron carbide thin films deposited by RF-PECVD and PLD technique: A comparative study based on structure, optical properties, and residual stress. Mater. Chem. Phys. 2021, 258, 123860. [Google Scholar] [CrossRef]
- Bute, A.; Jena, S.; Sharma, R.K.; Jagannath, U.D.V.; Maiti, N. Linear and non-linear optical properties of boron carbide thin films. Appl. Surf. Sci. 2023, 608, 155101. [Google Scholar] [CrossRef]
- Caruso, A.N.; Billa, R.B.; Balaz, S.; Brand, J.I.; Dowben, P.A. The heteroisomeric diode. J. Phys. Condens. Matter. 2004, 16, L139–L146. [Google Scholar] [CrossRef]
- Caruso, A.N.; Balaz, S.; Xu, B.; Dowben, P.A.; McMullen-Gunn, A.S.; Brand, J.I.; Losovyj, Y.B.; McIlroy, D.N. Surface photovoltage effects on the isomeric semiconductors of boron-carbide. Appl. Phys. Lett. 2004, 84, 1302–1304. [Google Scholar] [CrossRef]
- Echeverría, E.; Dong, B.; Liu, A.; Wilson, E.R.; Peterson, G.; Nastasi, M.; Dowben, P.A.; Kelber, J.A. Strong binding at the gold (Au) boron carbide interface. Surf. Coat. Technol. 2017, 314, 51–54. [Google Scholar] [CrossRef]
- Caruso, A.N. The physics of solid-state neutron detector materials and geometries. J. Phys. Condens. Matter 2010, 22, 443201. [Google Scholar] [CrossRef]
- Peterson, G.G.; Echeverria, E.; Dong, B.; Silva, J.P.; Wilson, E.R.; Kelber, J.A.; Nastasi, M.; Dowben, P.A. Increased drift carrier lifetime in semiconducting boron carbides deposited by plasma enhanced chemical vapor deposition from carboranes and benzene. J. Vac. Sci. Technol. A 2017, 35, 03E101. [Google Scholar] [CrossRef]
- Oyelade, A.; Yost, A.J.; Benker, N.; Dong, B.; Knight, S.; Schubert, M.; Dowben, P.A.; Kelber, J.A. Composition-dependent charge transport in boron carbides alloyed with aromatics: Plasma enhanced chemical vapor deposition aniline/ortho-carborane films. Langmuir 2018, 34, 12007–12016. [Google Scholar] [CrossRef]
- Dong, B.; James, R.; Kelber, J.A. PECVD of boron carbide/aromatic composite films: Precursor stability and resonance stabilization energy. Surf. Coat. Technol. 2016, 290, 94–99. [Google Scholar] [CrossRef]
- Dong, B.; Oyelade, A.; Nandagopal, N.; Kelber, J.A. Aromatic-doped boron carbide films formed by PECVD of metacarborane and aniline or pyridine: Chemical and electronic structure. Surf. Coat. Technol. 2017, 314, 45–50. [Google Scholar] [CrossRef]
- Echeverría, E.; James, R.; Chiluwal, U.; Pasquale, F.L.; Colón Santana, J.A.; Gapfizi, R.; Tae, J.-D.; Driver, M.S.; Enders, A.; Kelber, J.A.; et al. Novel semiconducting boron carbide/pyridine polymers for neutron detection at zero bias. Appl. Phys. A 2015, 118, 113–118. [Google Scholar] [CrossRef]
- Echeverria, E.; Dong, B.; Peterson, G.; Silva, J.P.; Wilson, E.R.; Driver, M.S.; Jun, Y.-S.; Stucky, G.D.; Knight, S.; Hofmann, T.; et al. Semiconducting boron carbides with better charge extraction through the addition of pyridine moieties. J. Phys. D 2016, 49, 355302. [Google Scholar] [CrossRef]
- Oyelade, A.; Osonkie, A.; Yost, A.J.; Benker, N.; Dowben, P.A.; Kelber, J.A. Optical, electronic and visible-range photo-electronic properties of boron carbide-indole films. J. Phys. D 2020, 53, 355101. [Google Scholar] [CrossRef]
- Hwang, S.-D.; Remmes, N.B.; Dowben, P.A.; McIlroy, D.N. Nickel doping of boron carbide grown by plasma enhanced chemical vapor deposition. J. Vac. Sci. Technol. B 1996, 14, 2957–2960. [Google Scholar] [CrossRef]
- Hwang, S.-D.; Remmes, N.; Dowben, P.A.; McIlroy, D.N. Nickel doping of boron-carbon alloy films and corresponding Fermi level shifts. J. Vac. Sci. Technol. B 1997, 15, 854–859. [Google Scholar] [CrossRef]
- Hwang, S.-D.; Yang, K.; Dowben, P.A.; Ahmad, A.A.; Ianno, N.J.; Li, J.Z.; Lin, J.Y.; Jiang, H.X.; McIlroy, D.N. Fabrication of n-type nickel doped B5C1+δ homojunction and heterojunction diodes. Appl. Phys. Lett. 1997, 70, 1028–1030. [Google Scholar] [CrossRef]
- Carlson, L.; LaGraffe, D.; Balaz, S.; Ignatov, A.; Losovyj, Y.B.; Choi, J.; Dowben, P.A.; Brand, J.I. Doping of boron carbides with cobalt, using cobaltocene. Appl. Phys. A 2007, 89, 195–201. [Google Scholar] [CrossRef]
- Ignatov, A.Y.; Losovyj, Y.B.; Carlson, L.; LaGraffe, D.; Brand, J.I.; Dowben, P.A. Pairwise cobalt doping of boron carbides with cobaltocene. J. Appl. Phys. 2007, 102, 083520. [Google Scholar] [CrossRef]
- Dowben, P.A.; Kizilkaya, O.; Liu, J.; Montag, B.; Nelson, K.; Sabirianov, I.; Brand, J.I. 3d transition metal doping of semiconducting boron carbides. Mater. Lett. 2009, 63, 72–74. [Google Scholar] [CrossRef]
- Sharapov, V.M.; Mirnov, S.V.; Grashin, S.A.; Lebedev, S.V.; Kovan, I.A.; Krasilnikov, A.V.; Krupin, V.A.; Levin, L.S.; Romannikov, A.N.; Zakharov, A.P. Boronization of Russian tokamaks from carborane precursors. J. Nucl. Mater. 1995, 220–222, 730–735. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, K.S.; Kim, K.M.; Kim, H.T.; Kim, G.P.; Sun, J.H.; Woo, H.J.; Park, J.M.; Kim, W.C.; Kim, H.K.; et al. KSTAR team. First boronization in KSTAR. Fusion Eng. Des. 2010, 85, 946–949. [Google Scholar] [CrossRef]
- Hong, S.-H.; Lee, K.-S.; Kim, K.-P.; Kim, K.-M.; Kim, H.-T.; Sun, J.-H.; Woo, H.-J.; Park, J.-M.; Park, F.-K.; Kim, W.-C.; et al. First boronization in KSTAR: Experiences on carborane. J. Nucl. Mater. 2011, 415, S1050–S1053. [Google Scholar] [CrossRef]
- Ko, J.; Den Hartog, D.J.; Goetz, J.A.; Weix, P.J.; Limbach, S.T. First gaseous boronization during pulsed discharge cleaning. J. Nucl. Mater. 2013, 432, 146–151. [Google Scholar] [CrossRef]
- Sharapov, V.M. Discharge chamber plasma-chemical conditioning in magnetic confinement fusion devices (Review). Phys. Atom. Nucl. 2021, 84, 1266–1271. [Google Scholar] [CrossRef]
- Zheng, H.; Thorne, K.; Mackenzie, J.D.; Yang, X.; Hawthorne, M.F. Boron carbide-based ceramics via polymer route synthesis. MRS Online Proc. Libr. 1991, 249, 15–23. [Google Scholar] [CrossRef]
- Johnson, S.E.; Yang, X.; Hawthorne, M.F.; Thorne, K.J.; Zheng, H.; Mackenzie, J.D. Alkynyl substituted carboranes as precursors to boron carbide thin films, fibers and composites. MRS Online Proc. Libr. 1992, 271, 833–838. [Google Scholar] [CrossRef]
- Cheng, S.; Yuan, K.; Wang, X.; Han, J.; Jian, X.; Wang, J. Poly(phenylene-carborane) for boron-carbide/carbon ceramic precursor synthesized via nickel catalysis. Polymer 2017, 115, 224–231. [Google Scholar] [CrossRef]
- Yan, D.; Chen, J.; Zhang, Y.; Gou, Y. Synthesis and characterization of a carborane-containing precursor for B4C ceramics. Sci. Discov. 2021, 9, 128–132. [Google Scholar] [CrossRef]
- Yan, D.; Chen, J.; Zhang, Y.; Gou, Y. Preparation of novel carborane-containing boron carbide precursor and its derived ceramic hollow microsphere. Ceram. Int. 2022, 48, 18392–18400. [Google Scholar] [CrossRef]
- Filonenko, V.P.; Zinin, P.V.; Zibrov, I.P.; Anokhin, A.S.; Kukueva, E.V.; Lyapin, S.G.; Fominski, V.Y. Synthesis of star-shaped boron carbide micro-crystallites under high pressure and high temperatures. Crystals 2018, 8, 448. [Google Scholar] [CrossRef]
- Pavlov, I.S.; Ivanova, A.G.; Filonenko, V.P.; Zibrov, I.P.; Voloshin, A.E.; Zinin, P.V.; Vasiliev, A.L. The rhombic hexecontahedronboron carbide microcrystals—Crystal structure analysis. Scr. Mater. 2023, 222, 115023. [Google Scholar] [CrossRef]
- Bagramov, R.H.; Filonenko, V.P.; Zibrov, I.P.; Skryleva, E.A.; Nikolaev, A.V.; Pasternak, D.G.; Vlasov, I.I. Highly boron-doped graphite and diamond synthesized from adamantane and ortho-carborane under high pressure. Materialia 2022, 21, 101274. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Boron cluster anions and their derivatives in complexation reactions. Coord. Chem. Rev. 2022, 469, 214636. [Google Scholar] [CrossRef]
- Matveev, E.Y.; Avdeeva, V.V.; Zhizhin, K.Y.; Malinina, E.A.; Kuznetsov, N.T. Effect of nature of substituents on coordination properties of mono- and disubstituted derivatives of boron cluster anions [BnHn]2– (n = 10, 12) and carboranes with exo-polyhedral B-X Bonds (X = N, O, S, Hal). Inorganics 2022, 10, 238. [Google Scholar] [CrossRef]
- Paskevicius, M.; Hansen, B.R.S.; Jørgensen, M.; Richter, B.; Jensenet, T.R. Multifunctionality of silver closo-boranes. Nat. Commun. 2017, 8, 15136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in three-dimensional aromatic-like closo-dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Isomerism in salts and complexes with boron cluster anions [B10H10]2– and [B20H18]2–. Russ. J. Inorg. Chem. 2020, 65, 335–358. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Isomerism in complexes with the decahydro-closo-decaborate anion. Polyhedron 2016, 105, 205–221. [Google Scholar] [CrossRef]
- Kravchenko, E.A.; Gippius, A.A.; Tkachev, A.V.; Zhurenko, S.V.; Golubev, A.V.; Kubasov, A.S.; Selivanov, N.A.; Buzanov, G.A.; Bykov, A.Y.; Zhizhin, K.Y.; et al. Non-covalent Cl⋯X interactions in several silver compounds based on [B12Cl12]2− clusters: 35Cl NQR and X-ray diffraction. Inorg. Chim. Acta 2023, 544, 121231. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Kravchenko, E.A.; Gippius, A.A.; Vologzhanina, A.V.; Malinina, E.A.; Zhurenko, S.V.; Buzanov, G.A.; Kuznetsov, N.T. Decachloro-closo-decaborate anion in copper(II) complexation reactions with N-donor ligands: 35Cl NQR and X-ray studies. Polyhedron 2017, 127, 238–247. [Google Scholar] [CrossRef]
- Albert, B.; Hofmann, K. Metal Borides: Versatile Structures and Properties. In Handbook of Solid State Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Buzin, M.I.; Dmitrienko, A.O.; Dorovatovskii, P.V.; Malinina, E.A.; Kuznetsov, N.T.; Voronova, E.D.; Zubavichus, Y.V.; Vologzhanina, A.V. Solid-state reactions of eicosaborate [B20H18]2− salts and complexes. Chem. Eur. J. 2017, 23, 16819–16828. [Google Scholar] [CrossRef]
- Eleazer, B.J.; Smith, M.D.; Peryshkov, D.V. Reaction of a ruthenium B-carboranyl hydride complex and BH3(SMe2): Selective formation of a pincer-supported metallaborane LRu(B3H8). Tetrahedron 2019, 75, 1471–1474. [Google Scholar] [CrossRef]
- Korolenko, S.E.; Zhuravlev, K.P.; Tsaryuk, V.I.; Kubasov, A.S.; Avdeeva, V.V.; Malinina, E.A.; Burlov, A.S.; Divaeva, L.N.; Zhizhin, K.Y.; Kuznetsov, N.T. Crystal structures, luminescence, and DFT study of mixed-ligand Zn(II) and Cd(II) complexes with phenyl-containing benzimidazole derivatives with linker CN or NN group. J. Lumin. 2021, 237, 118156. [Google Scholar] [CrossRef]
- Malinina, E.A.; Vologzhanina, A.V.; Avdeeva, V.V.; Goeva, L.V.; Efimov, N.N.; Ugolkova, E.A.; Minin, V.V.; Kuznetsov, N.T. Structures, magnetic properties, and EPR studies of tetranuclear copper(II) complexes [Cu4(OH)4L4]4+ (L = bpa, bipy) stabilized by anions containing decahydro-closo-decaborate anion. Polyhedron 2020, 183, 114540. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Weinberger, M.B.; Levine, J.B.; Kavner, A.; Yang, J.-M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef]
- Latini, A.; Rau, J.V.; Teghil, R.; Generosi, A.; Albertini, V.R. Superhard properties of rhodium and iridium boride films. ACS Appl. Mater. Interfaces 2010, 2, 581–587. [Google Scholar] [CrossRef]
- Xie, M.; Mohammadi, R.; Turner, C.L.; Kaner, R.B.; Kavner, A.; Tolbert, S.H. Lattice stress states of superhard tungsten tetraboride from radial X-ray diffraction under nonhydrostatic compression. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 90, 104104. [Google Scholar] [CrossRef]
- Bazhin, P.; Chizhikov, A.; Bazhina, A.; Konstantinov, A.; Avdeeva, V. Titanium-titanium boride matrix composites prepared in-situ under conditions combining combustion processes and high-temperature shear deformation. Mater. Sci. Eng. A 2023, 874, 145093. [Google Scholar] [CrossRef]
- Prokopets, A.D.; Bazhin, P.M.; Konstantinov, A.S.; Antipov, M.S.; Avdeeva, V.V. Structural features of layered composite material TiB2/TiAl/Ti6Al4 obtained by unrestricted SHS-compression. Mater. Lett. 2021, 300, 130165. [Google Scholar] [CrossRef]
- Petrichko, M.I.; Karavaev, I.A.; Savinkina, E.V.; Grigoriev, M.S.; Buzanov, G.A.; Retivov, V.M. Rare-earth nitrate complexes with dimethylformamide. Russ. J. Inorg. Chem. 2023, 68, 415–423. [Google Scholar] [CrossRef]
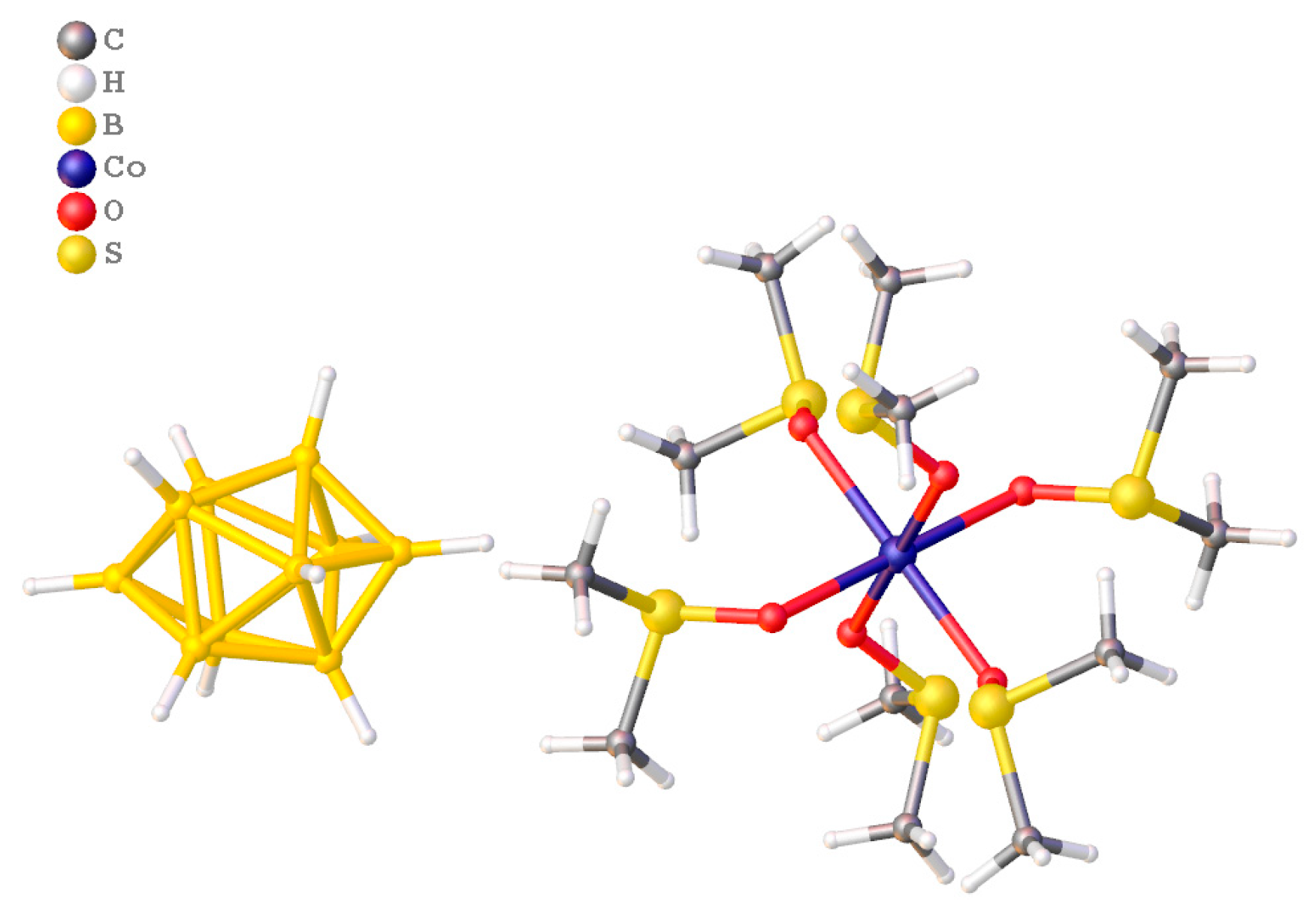
- Avdeeva, V.V.; Polyakova, I.N.; Vologzhanina, A.V.; Goeva, L.V.; Buzanov, G.A.; Generalova, N.B.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. [Co(solv)6][B10H10] (solv = DMF and DMSO) for low-temperature synthesis of borides. Russ. J. Inorg. Chem. 2016, 61, 1125–1134. [Google Scholar] [CrossRef]
- Malinina, E.A.; Myshletsov, I.I.; Buzanov, G.A.; Kubasov, A.S.; Kozerozhets, I.V.; Goeva, L.V.; Nikiforova, S.E.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. A New approach to the synthesis of nanocrystalline cobalt boride in the course of the thermal decomposition of cobalt complexes [Co(DMF)6]2+ with boron cluster anions. Molecules 2023, 28, 453. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Vologzhanina, A.V.; Ugolkova, E.A.; Minin, V.V.; Malinina, E.A.; Kuznetsov, N.T. Synthesis and structures of compounds [ML6][B10Cl10] (M = Co, Ni; L = CH3CN, DMF, DMSO) as precursors for synthesis of cobalt(II) and nickel(II) complexes with organic L ligands. J. Solid State Chem. 2021, 296, 121989. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Avdeeva, V.V.; Efimov, N.N.; Kozerozhets, I.V.; Kuznetsov, N.T. Synthesis and physicochemical properties of binary cobalt(II) borides. Thermal reduction of precursor complexes [CoLn][B10H10] (L = H2O, n = 6; N2H4, n = 3). Russ. J. Inorg. Chem. 2019, 64, 1325–1334. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Avdeeva, V.V.; Efimov, N.N.; Kuznetsov, N.T. A new method for synthesis of binary borides with desired properties. Dokl. Chem. 2019, 487, 180–183. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Retivov, V.M.; Avdeeva, V.V.; Kuznetsov, N.T. Synthesis and thermal reduction of complexes [NiLn][B10H10] (L = DMF, H2O, n = 6; L = N2H4, n = 3): Formation of solid solutions Ni3C1 –xBx. Russ. J. Inorg. Chem. 2020, 65, 126–132. [Google Scholar] [CrossRef]
- Itoh, H.; Tsuzuki, Y.; Yogo, T.; Naka, S. Synthesis of cerium and gadolinium borides using boron cage compounds as a boron source. Mater. Res. Bull. 1987, 22, 1259–1266. [Google Scholar] [CrossRef]
- White III, J.P.; Shore, S.G. Complexes of divalent lanthanides (ytterbium(II), europium(II), and samarium(II)) with decaborates. Inorg. Chem. 1992, 31, 2756–2761. [Google Scholar] [CrossRef]
- Volkov, V.V.; Yur’ev, G.S.; Solomatina, L.Y.; Voronina, G.S.; Myakishev, K.G. Thermal changes in decahydro-closo-decaborate(2-)copper(I) Cu2B10H10 and the nature of copper borides. Bull. Acad. Sci. USSR Div. Chem. Sci. 1990, 39, 435–438. [Google Scholar] [CrossRef]
- Malinina, E.A.; Myshletsov, I.I.; Buzanov, G.A.; Kozerozhets, I.V.; Simonenko, N.P.; Simonenko, T.L.; Nikiforova, S.E.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Physicochemical fundamentals of the synthesis of a Cu@BN composite consisting of nanosized copper enclosed in a boron nitride matrix. Inorganics 2023, 11, 345. [Google Scholar] [CrossRef]
- Skachkova, V.K.; Grachev, A.V.; Shaulov, A.Y.; Berlin, A.A. Inorganic polymers using sodium silicate liquid glass. Features of silicate polycondensation. Russ. J. Phys. Chem. B 2019, 13, 849–852. [Google Scholar] [CrossRef]
- Skachkova, V.K.; Goeva, L.V.; Grachev, A.V.; Avdeeva, V.V.; Malinina, E.A.; Shaulov, A.Y.; Berlin, A.A.; Kuznetsov, N.T. Thermal and thermomechanical properties of trialkylammonium dodecahydro-closo-dodecaborates (R3NH)2[B12H12] (R = Et, Bu). Russ. J. Inorg. Chem. 2017, 62, 84–89. [Google Scholar] [CrossRef]
- Goeva, L.V.; Skachkova, V.K.; Avdeeva, V.V.; Malinina, E.A.; Grachev, A.V.; Shaulov, A.Y.; Berlin, A.A.; Kuznetsov, N.T. Interactions of sodium liquid glass with triethylammonium decahydro-closo-decaborate (Et3NH)2B10H10. Russ. J. Inorg. Chem. 2014, 59, 107–110. [Google Scholar] [CrossRef]
- Skachkova, V.K.; Goeva, L.V.; Grachev, A.V.; Avdeeva, V.V.; Malinina, E.A.; Shaulov, A.Y.; Berlin, A.A.; Kuznetsov, N.T. Thermal oxidation of the decahydro-closo-decaborate anion B10H102− in a silicate matrix. Inorg. Mater. 2015, 51, 498–502. [Google Scholar] [CrossRef]
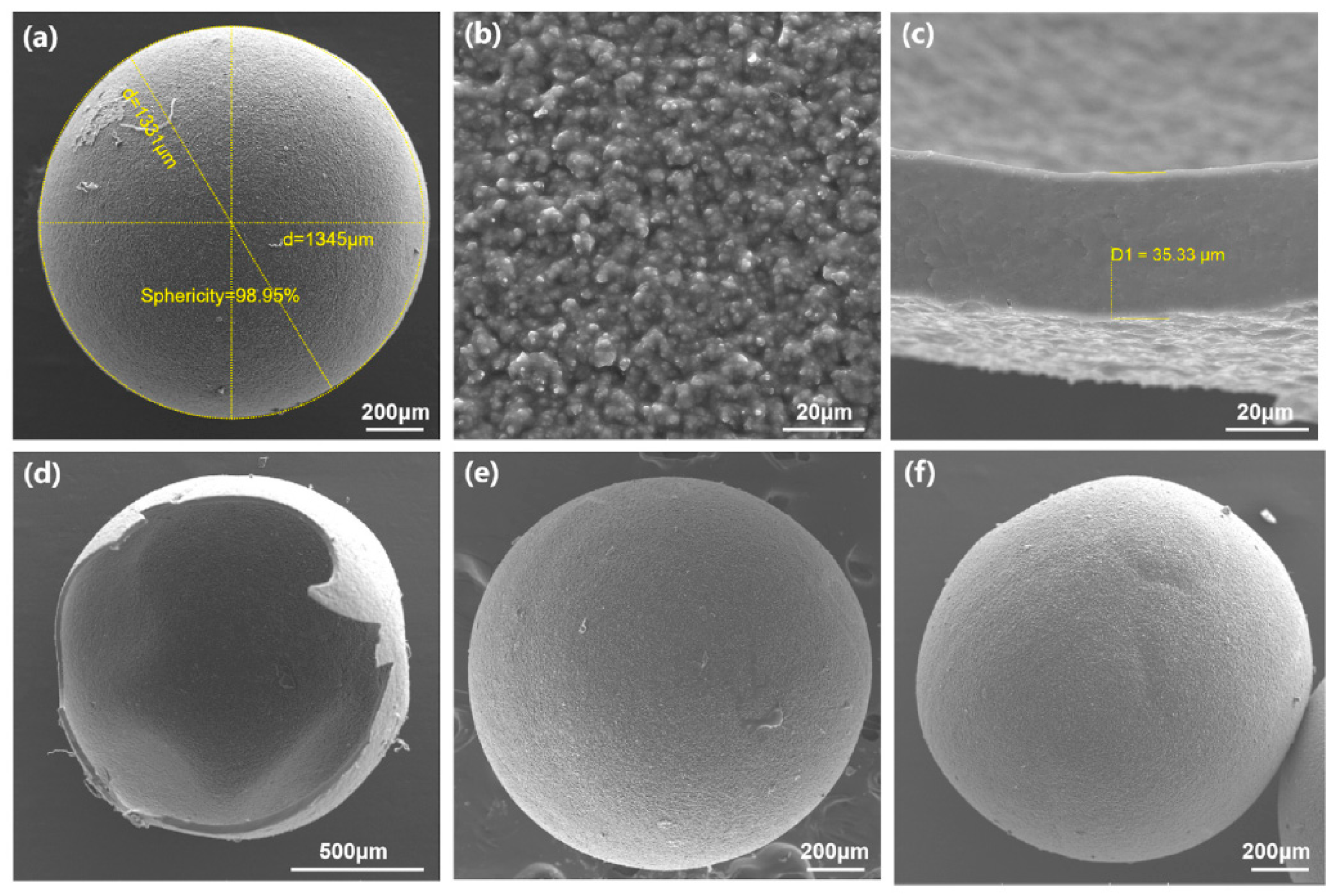
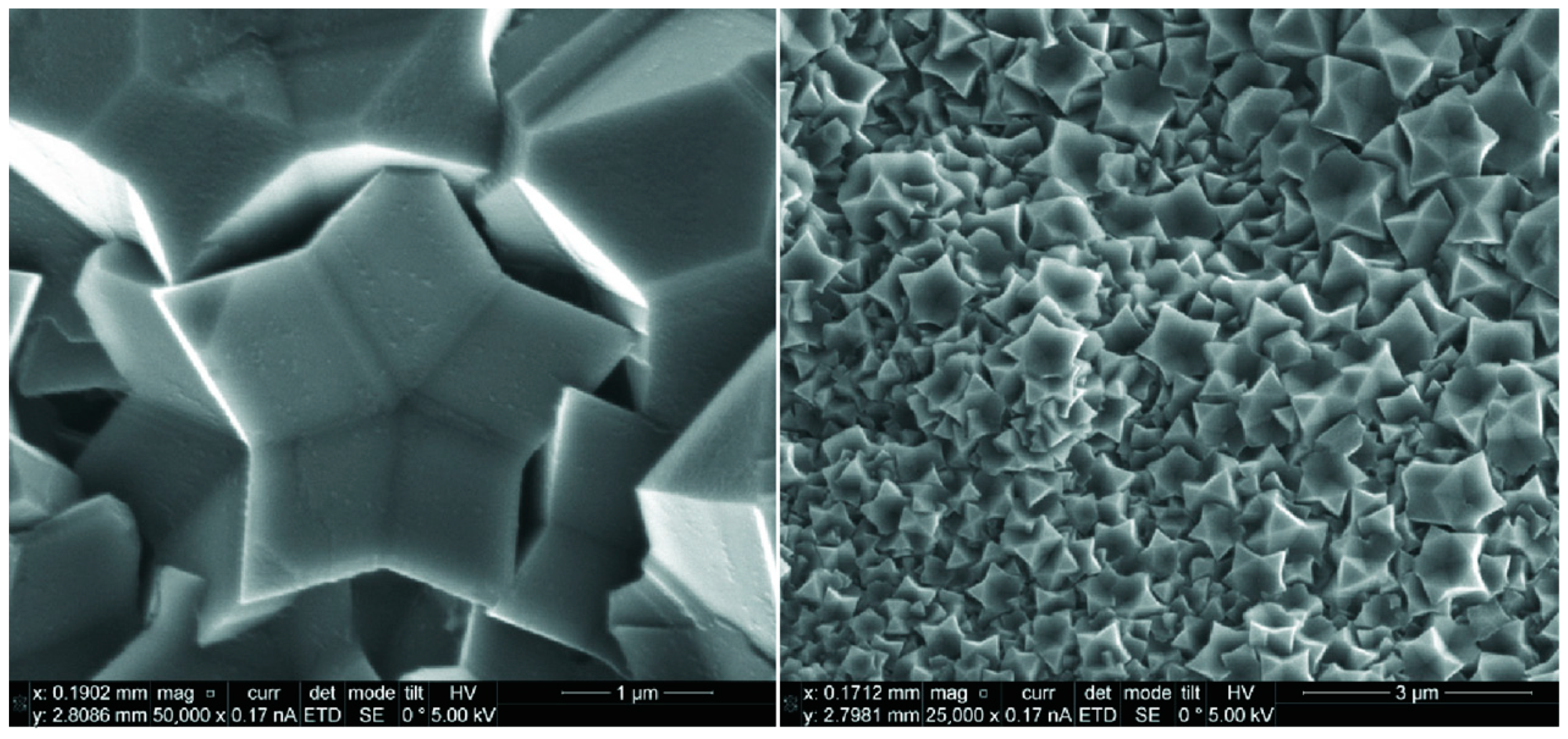
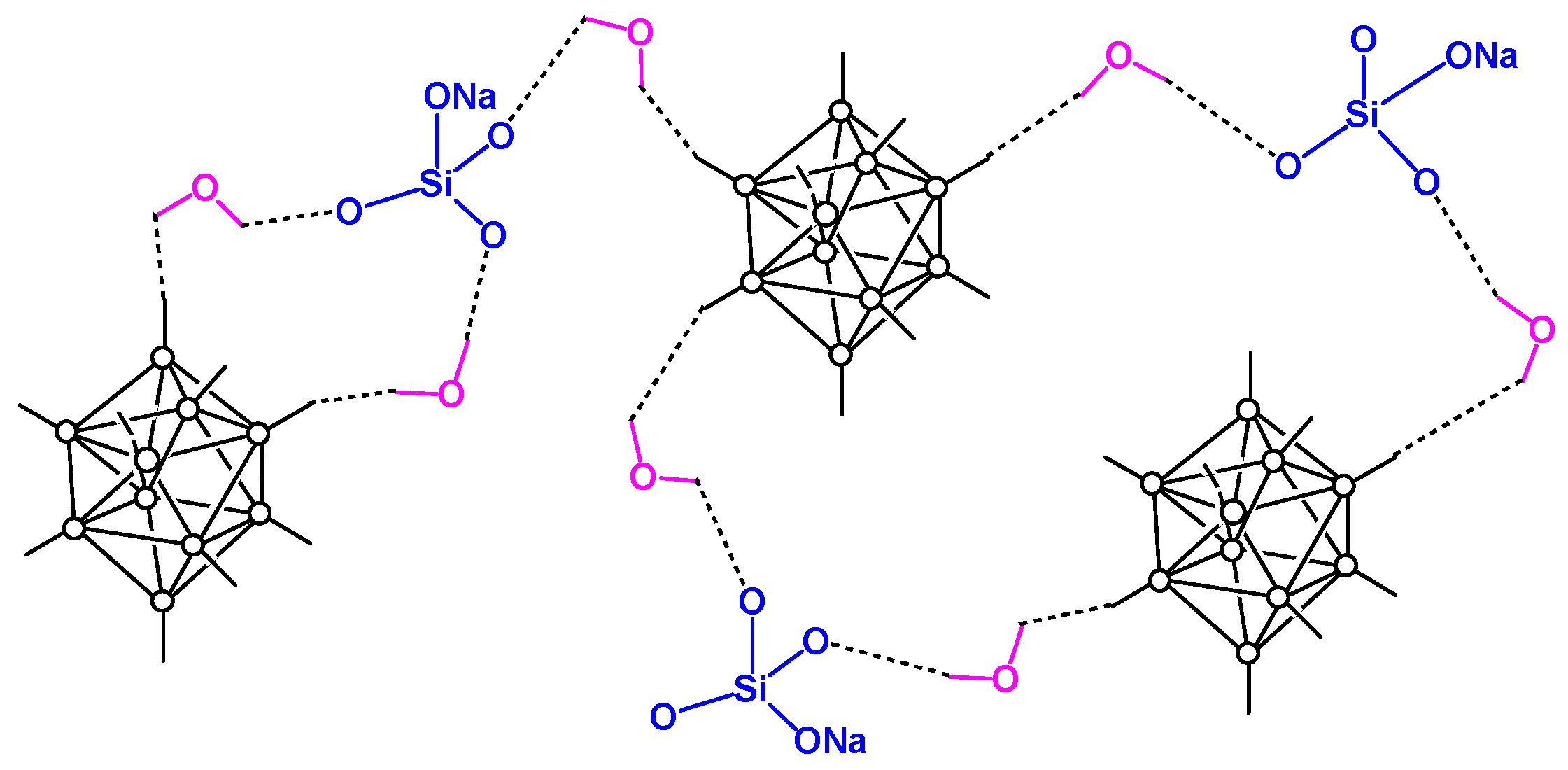
- Skachkova, V.K.; Malinina, E.A.; Goeva, L.V.; Grachev, A.V.; Avdeeva, V.V.; Kozerozhets, I.V.; Shaulov, A.Y.; Berlin, A.A.; Kuznetsov, N.T. Morphology of supramolecular structures based on sodium silicates and [B10R10]2– (R = H, Cl) boron cluster anions. Inorg. Mater. 2021, 57, 1173–1177. [Google Scholar] [CrossRef]
- Malinina, E.; Skachkova, V.; Goeva, L.; Grachev, A.; Lyubimova, G.; Avdeeva, V.; Kozerozhets, I.; Shaulov, A.; Berlin, A.; Kuznetsov, N. Thermomechanical properties of compositions based on polysilicates modified with boron cluster anions or SiO2 nanoparticles. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 201–208. [Google Scholar] [CrossRef]
- Skachkova, V.K.; Malinina, E.A.; Goeva, L.V.; Grachev, A.V.; Avdeeva, V.V.; Shaulov, A.Y.; Berlin, A.A.; Kuznetsov, N.T. Polycondensation of water glass sodium silicates in the presence of [BnXn]2– (n = 10, 12; X = H, Cl) boron cluster anions. Inorg. Mater. 2020, 56, 657–661. [Google Scholar] [CrossRef]
- Skachkova, V.K.; Goeva, L.V.; Grachev, A.V.; Kochneva, I.K.; Malinina, E.A.; Shaulov, A.Y.; Berlin, A.A.; Kuznetsov, N.T. Composites based on triethylammonium dodecahydro-closo-dodecaborate ((Et 3NH)2[B12H12]) and sodium silicate water glass. Inorg. Mater. 2017, 53, 207–211. [Google Scholar] [CrossRef]
- Malinina, E.A.; Skachkova, V.K.; Kozerozhets, I.V.; Avdeeva, V.V.; Goeva, L.V.; Buzanov, G.A.; Shaulov, A.Y.; Berlin, A.A.; Kuznetsov, N.T. Formation of nanoscale sodium dodecahydro-closo-dodecaborate Na2[B12H12] on the surface of a silicate matrix. Dokl. Chem. 2019, 484, 1–4. [Google Scholar] [CrossRef]
- Goeva, L.V.; Malinina, E.A.; Avdeeva, V.V.; Kuznetsov, N.T.; Skachkova, V.K.; Shaulov, A.Y.; Grachev, A.V.; Berlin, A.A. Boron-Containing Neutron Shielding Material. RF Patent RU2550156C1, 10 May 2016. [Google Scholar]
- Popov, V.A.; Zhizhin, K.Y.; Malinina, E.A.; Ketsko, V.A.; Kuznetsov, N.T. A possibility of using mechanical alloying for developing metal matrix composites with light-weight reinforcements. J. Alloys Compd. 2007, 434–435, 451–454. [Google Scholar] [CrossRef]
- Popov, V.A.; Zhizhin, K.Y.; Kuznetsov, N.T.; Staudhammer, K.P.; Retivov, V.M. Investigation of the possibility of application of boron clusters in composite materials with metal matrix. Adv. Mater. Res. 2009, 59, 96–100. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeeva, V.V.; Nikiforova, S.E.; Malinina, E.A.; Sivaev, I.B.; Kuznetsov, N.T. Composites and Materials Prepared from Boron Cluster Anions and Carboranes. Materials 2023, 16, 6099. https://doi.org/10.3390/ma16186099
Avdeeva VV, Nikiforova SE, Malinina EA, Sivaev IB, Kuznetsov NT. Composites and Materials Prepared from Boron Cluster Anions and Carboranes. Materials. 2023; 16(18):6099. https://doi.org/10.3390/ma16186099
Chicago/Turabian StyleAvdeeva, Varvara V., Svetlana E. Nikiforova, Elena A. Malinina, Igor B. Sivaev, and Nikolay T. Kuznetsov. 2023. "Composites and Materials Prepared from Boron Cluster Anions and Carboranes" Materials 16, no. 18: 6099. https://doi.org/10.3390/ma16186099
APA StyleAvdeeva, V. V., Nikiforova, S. E., Malinina, E. A., Sivaev, I. B., & Kuznetsov, N. T. (2023). Composites and Materials Prepared from Boron Cluster Anions and Carboranes. Materials, 16(18), 6099. https://doi.org/10.3390/ma16186099