Transition Metal Borides for All-in-One Radiation Shielding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compound Selection
2.2. Theoretical Calculations
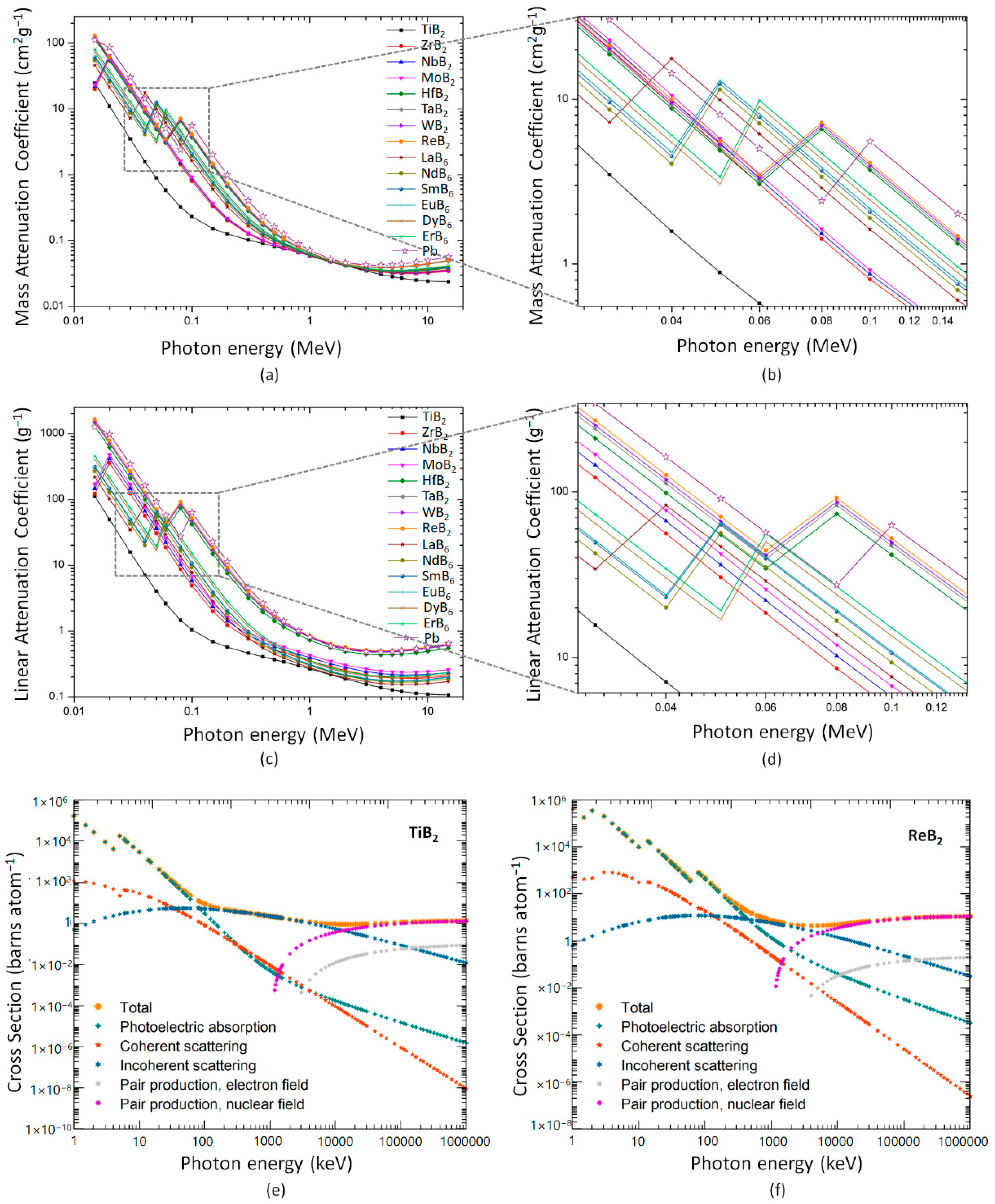
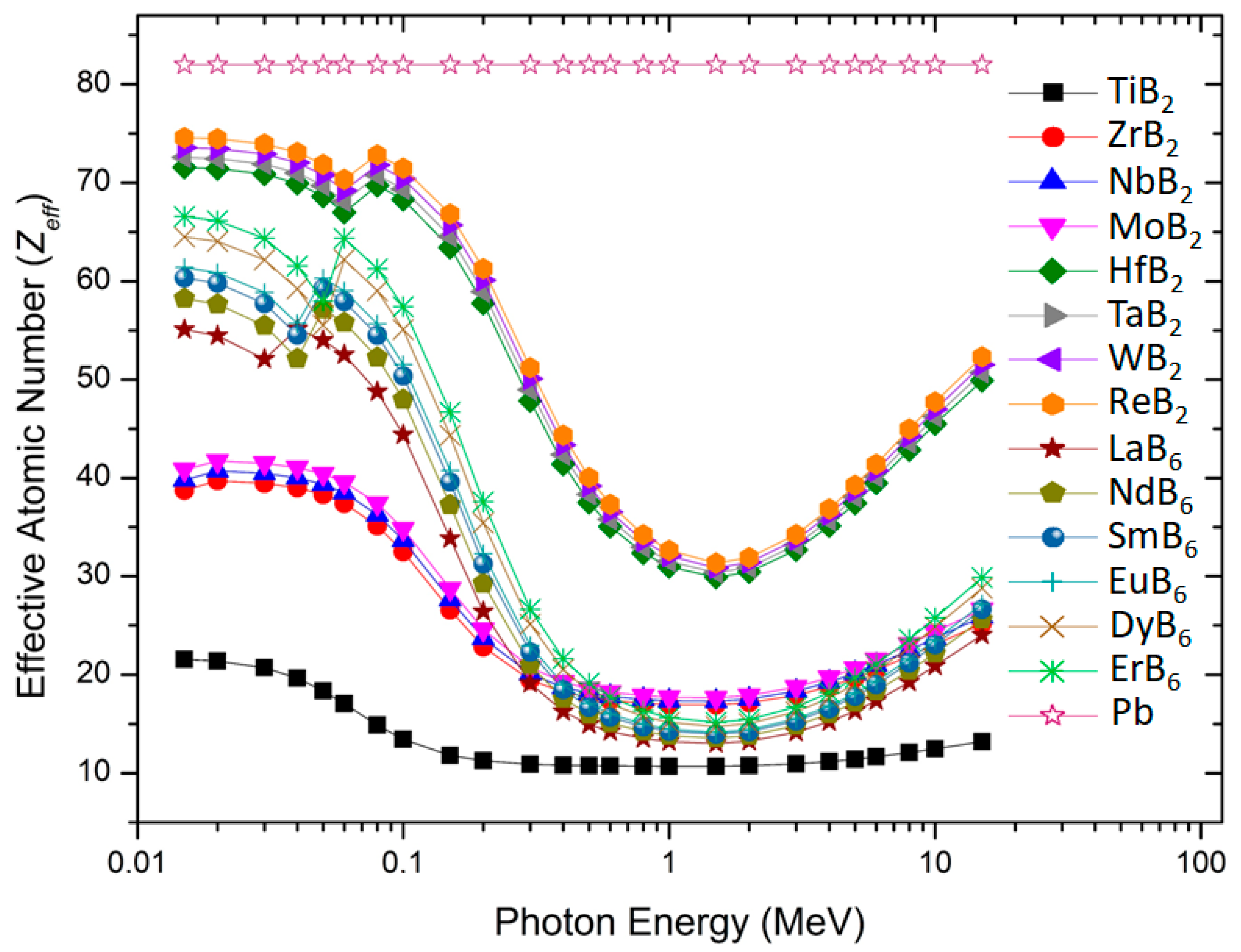
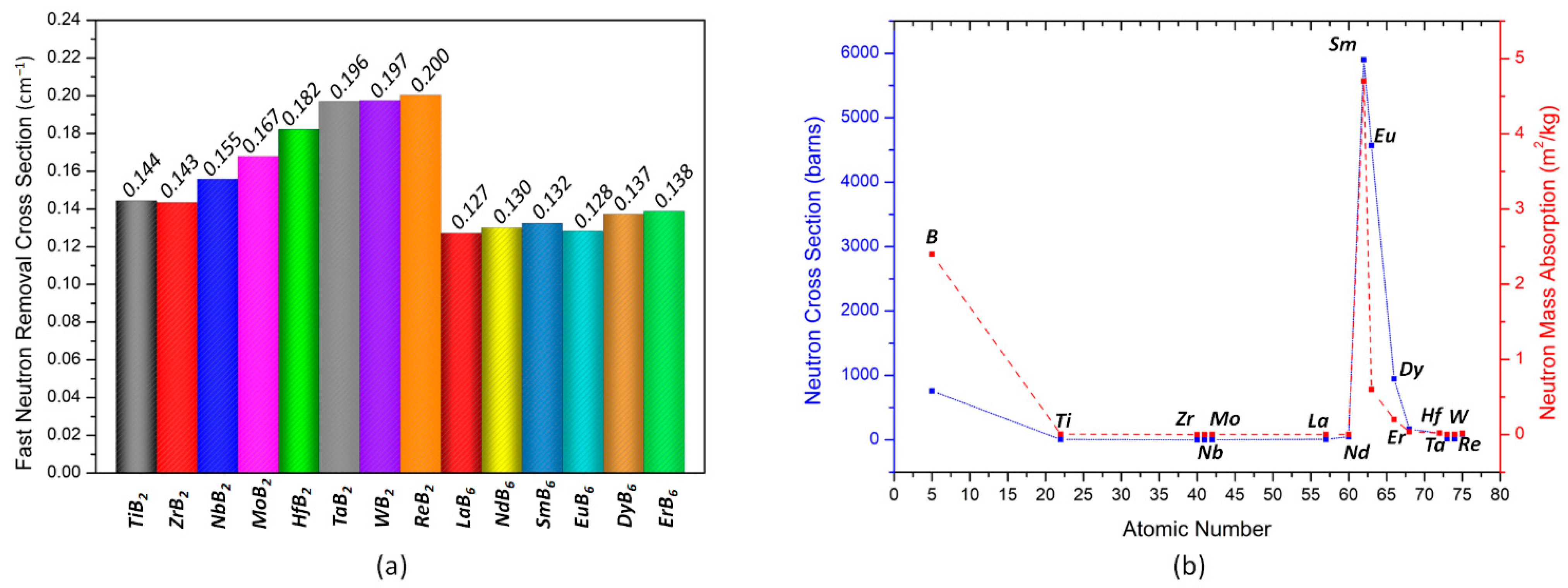
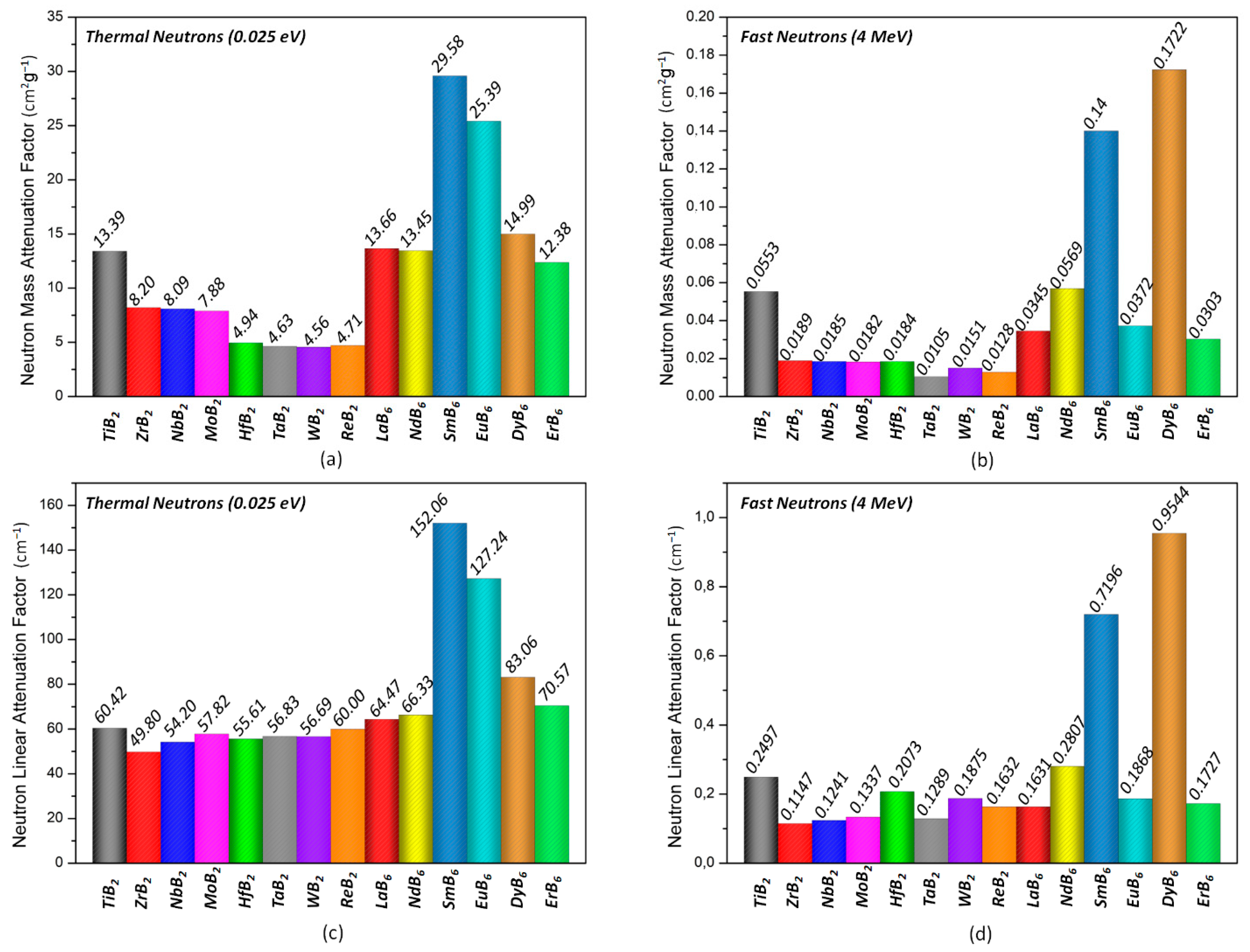
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.K.; Keole, S.R. Proton Therapy in the Adolescent and Young Adult Population. Cancers 2023, 15, 4269. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arias, P.; Vergara, D.; Antón-Sancho, Á. Global Review of International Nuclear Waste Management. Energies 2023, 16, 6215. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. Review: The Energy Implications of Averting Climate Change Catastrophe. Energies 2023, 16, 6178. [Google Scholar] [CrossRef]
- Sidhu, A.; Khan, N.; Phillips, C.; Briones, J.; Kapoor, A.; Zalewski, P.; Fleshner, N.E.; Chow, E.; Emmenegger, U. Prevalence and Prognostic Implications of PSA Flares during Radium-223 Treatment among Men with Metastatic Castration Resistant Prostate Cancer. J. Clin. Med. 2023, 12, 5604. [Google Scholar] [CrossRef] [PubMed]
- Tuieng, R.J.; Cartmell, S.H.; Kirwan, C.C.; Sherratt, M.J. The Effects of Ionising and Non-Ionising Electromagnetic Radiation on Extracellular Matrix Proteins. Cells 2021, 10, 3041. [Google Scholar] [CrossRef]
- Avcıoğlu, S.; Buldu, M.; Kaya, F.; Kaya, C. Chapter 20—Synthesis of nuclear-grade nano-sized boron carbide powders and its application in LDPE matrix composites for neutron shielding. In Composite Materials: Manufacturing, Properties, and Applications; Low, I.-M., Dong, Y., Eds.; Elsevier: Cambridge, MA, USA; Amsterdam, The Netherlands, 2021; pp. 543–579. ISBN 978-0-12-820512-9. [Google Scholar]
- AVCIOĞLU, S. LDPE matrix composites reinforced with dysprosium-boron containing compounds for radiation shielding applications. J. Alloys Compd. 2022, 927, 166900. [Google Scholar] [CrossRef]
- Sunitha, Y.; Kumar, S. 10B/11B isotopic ratio and atomic composition of boron carbide: Determination by proton induced γ-ray emission and proton elastic backscattering spectrometry. Appl. Radiat. Isot. 2017, 128, 28–35. [Google Scholar] [CrossRef]
- UZUN, S. Investigation of boron element and its use in nuclear reactors. J. Boron 2023, 8, 25–30. [Google Scholar] [CrossRef]
- Pitto-Barry, A. Polymers and boron neutron capture therapy (BNCT): A potent combination. Polym. Chem. 2021, 12, 2035–2044. [Google Scholar] [CrossRef]
- Monti Hughes, A.; Hu, N. Optimizing Boron Neutron Capture Therapy (BNCT) to Treat Cancer: An Updated Review on the Latest Developments on Boron Compounds and Strategies. Cancers 2023, 15, 4091. [Google Scholar] [CrossRef]
- Avcıoğlu, S.; Buldu, M.; Kaya, F.; Üstündağ, C.B.; Kam, E.; Menceloğlu, Y.Z.; Kaptan, H.Y.; Kaya, C. Processing and properties of boron carbide (B4C) reinforced LDPE composites for radiation shielding. Ceram. Int. 2020, 46, 343–352. [Google Scholar] [CrossRef]
- Özcan, M.; Avcıoğlu, S.; Kaya, C.; Kaya, F. Boron carbide reinforced electrospun nanocomposite fiber mats for radiation shielding. Polym. Compos. 2023, 44, 4155–4167. [Google Scholar] [CrossRef]
- Özcan, M.; Kam, E.; Kaya, C.; Kaya, F. Boron-containing nonwoven polymeric nanofiber mats as neutron shields in compact nuclear fusion reactors. Int. J. Energy Res. 2022, 46, 7441–7450. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T.W. Polymer-composite materials for radiation protection. ACS Appl. Mater. Interfaces 2012, 4, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Almurayshid, M.; Alsagabi, S.; Alssalim, Y.; Alotaibi, Z.; Almsalam, R. Feasibility of polymer-based composite materials as radiation shield. Radiat. Phys. Chem. 2021, 183, 109425. [Google Scholar] [CrossRef]
- Okonkwo, U.C.; Idumah, C.I.; Okafor, C.E.; Ezeani, E.O. Construction of radiation attenuating polymeric nanocomposites and multifaceted applications: A review. Polym. Plast. Technol. Mater. 2023, 62, 1639–1661. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, Z.; Li, J.; Guo, Y.; Yang, G.; Yu, Y.; Zhang, J. The Advancement of Neutron-Shielding Materials for the Transportation and Storage of Spent Nuclear Fuel. Materials 2022, 15, 3255. [Google Scholar] [CrossRef]
- Yasin, T.; Khan, M.N. High density polyethylene/boron carbide composites for neutron shielding. e-Polymers 2008, 8, 670–676. [Google Scholar] [CrossRef]
- Salimi, M.; Ghal-Eh, N.; Amirabadi, E.A. Characterization of a new shielding rubber for use in neutron–gamma mixed fields. Nucl. Sci. Tech. 2018, 29, 36. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Gwaily, S.E.; Makarious, A.S.; El-Sayed Abdo, A. Ethylene-propylene diene rubber/low density polyethylene/boron carbide composites as neutron shields. Polym. Degrad. Stab. 1995, 50, 235–240. [Google Scholar] [CrossRef]
- Avcıoğlu, C.; Bekheet, M.F.; Artır, R. Radiation shielding and mechanical properties of mullite-zirconia composites fabricated from investment-casting shell waste. J. Mater. Res. Technol. 2023, 24, 5883–5895. [Google Scholar] [CrossRef]
- Kassem, S.M.; Abdel Maksoud, M.I.A.; Ghobashy, M.M.; El Sayed, A.M.; Ebraheem, S.; Helal, A.I.; Ebaid, Y.Y. Novel flexible and lead-free gamma radiation shielding nanocomposites based on LDPE/SBR blend and BaWO4/B2O3 heterostructures. Radiat. Phys. Chem. 2023, 209, 110953. [Google Scholar] [CrossRef]
- Kaur, T.; Sharma, J.; Singh, T. Review on scope of metallic alloys in gamma rays shield designing. Prog. Nucl. Energy 2019, 113, 95–113. [Google Scholar] [CrossRef]
- Kharita, M.H.; Yousef, S.; AlNassar, M. Review on the addition of boron compounds to radiation shielding concrete. Prog. Nucl. Energy 2011, 53, 207–211. [Google Scholar] [CrossRef]
- Sallam, O.I.; Madbouly, A.M.; Elalaily, N.A.; Ezz-Eldin, F.M. Physical properties and radiation shielding parameters of bismuth borate glasses doped transition metals. J. Alloys Compd. 2020, 843, 156056. [Google Scholar] [CrossRef]
- Sallam, O.I.; Madbouly, A.M.; Ezz-Eldin, F.M. Impact of Nd3+ additive on the radiation shielding competence of borosilicate glasses fabricated from agro-waste materials. J. Non-Cryst. Solids 2022, 590, 121691. [Google Scholar] [CrossRef]
- Kavaz, E.; Ersundu, M.Ç.; Ersundu, A.E.; Tekin, H.O. Synthesis and characterization of newly developed phosphate-based glasses through experimental gamma-ray and neutron spectroscopy methods: Transmission and dose rates. Ceram. Int. 2022, 48, 13842–13849. [Google Scholar] [CrossRef]
- Ilik, E.; Kavaz, E.; Kilic, G.; Issa, S.A.M.; Zakaly, H.M.H.; Tekin, H.O. A closer-look on Copper(II) oxide reinforced Calcium-Borate glasses: Fabrication and multiple experimental assessment on optical, structural, physical, and experimental neutron/gamma shielding properties. Ceram. Int. 2022, 48, 6780–6791. [Google Scholar] [CrossRef]
- Al-Ghamdi, H.; Almuqrin, A.H.; Koubisy, M.S.I.; Mahmoud, K.A.; Sayyed, M.I.; Darwish, M.A.; Henaish, A.M.A. Zinc-lead-borate glasses doped with dysprosium oxide: Structure, optical, and radiation shielding features. Optik 2021, 246, 167765. [Google Scholar] [CrossRef]
- Kavaz, E.; Ilik, E.; Kilic, G.; ALMisned, G.; Tekin, H.O. Synthesis and experimental characterization on fast neutron and gamma-ray attenuation properties of high-dense and transparent Cadmium oxide (CdO) glasses for shielding purposes. Ceram. Int. 2022, 48, 23444–23451. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Elmahroug, Y.; Kumar, A.; Tekin, H.O.; Rekik, N.; Dong, M.; Lee, D.-E.; Yoon, J.; Park, T. Detailed Inspection of γ-ray, Fast and Thermal Neutrons Shielding Competence of Calcium Oxide or Strontium Oxide Comprising Bismuth Borate Glasses. Materials 2021, 14, 2265. [Google Scholar] [CrossRef] [PubMed]
- ALMisned, G.; Tekin, H.O.; Kavaz, E.; Bilal, G.; Issa, S.A.M.; Zakaly, H.M.H.; Ene, A. Gamma, Fast Neutron, Proton, and Alpha Shielding Properties of Borate Glasses: A Closer Look on Lead (II) Oxide and Bismuth (III) Oxide Reinforcement. Appl. Sci. 2021, 11, 6837. [Google Scholar] [CrossRef]
- Sallam, O.I.; Madbouly, A.M.; Moussa, N.L.; Abdel-Galil, A. Impact of radiation on CoO-doped borate glass: Lead-free radiation shielding. Appl. Phys. A 2022, 128, 70. [Google Scholar] [CrossRef]
- Sallam, O.I.; Issa, S.A.M.; Rashad, M.; Madbouly, A.M.; Tekin, H.O.; Badawi, A.; Hamdy, A.; Zakaly, H.M.H. Impact of molybdenum on optical, structure properties and gamma radiation shielding parameters of bor-ophosphate glass: Intensive experiment investigations. Radiat. Phys. Chem. 2022, 198, 110140. [Google Scholar] [CrossRef]
- Madbouly, A.M.; Sallam, O.I.; Issa, S.A.M.; Rashad, M.; Hamdy, A.; Tekin, H.O.; Zakaly, H.M.H. Experimental and FLUKA evaluation on structure and optical properties and γ-radiation shielding capacity of bismuth borophosphate glasses. Prog. Nucl. Energy 2022, 148, 104219. [Google Scholar] [CrossRef]
- Xiao, M.; Qin, Q.; He, X.; Li, F.; Wang, X. Shielding Capability Research on Composite Base Materials in Hybrid Neutron-Gamma Mixed Radiation Fields. Materials 2023, 16, 2084. [Google Scholar] [CrossRef]
- Arif Sazali, M.; Alang Md Rashid, N.K.; Hamzah, K. A review on multilayer radiation shielding. IOP Conf. Ser. Mater. Sci. Eng. 2019, 555, 12008. [Google Scholar] [CrossRef]
- Daneshvar, H.; Milan, K.G.; Sadr, A.; Sedighy, S.H.; Malekie, S.; Mosayebi, A. Multilayer radiation shield for satellite electronic components protection. Sci. Rep. 2021, 11, 20657. [Google Scholar] [CrossRef]
- Pangilinan, L.E.; Hu, S.; Hamilton, S.G.; Tolbert, S.H.; Kaner, R.B. Hardening Effects in Superhard Transition-Metal Borides. Acc. Mater. Res. 2022, 3, 100–109. [Google Scholar] [CrossRef]
- Zoli, L.; Galizia, P.; Silvestroni, L.; Sciti, D. Synthesis of group IV and V metal diboride nanocrystals via borothermal reduction with sodium borohydride. J. Am. Ceram. Soc. 2018, 101, 2627–2637. [Google Scholar] [CrossRef]
- MERTDİNÇ, S.; AĞAOĞULLARI, D.; ÖVEÇOĞLU, M.L. TaB/TaB2 Tozlarının Mekanokimyasal Sentezleme ile Üretimi ve Karakterizasyonu. AKÜ FEMÜBİD 2019, 19, 465–473. [Google Scholar]
- Yan, G.; Feng, Y.; Fu, B.; Liu, C.; Ji, P.; Zhang, P.; Zhou, L. Supercondcuting properties in MgB2/Fe wires prepared by PIT method. Chin. Sci. Bull. 2003, 48, 1331–1333. [Google Scholar] [CrossRef]
- Materials Explorer. Materials Data on LaB6 by Materials Project; Materials Explorer: Riverside, CA, USA, 2022. [Google Scholar]
- Materials Project App. Materials Data on LuB6 (SG:221) by Materials Project; Materials Explorer: Riverside, CA, USA, 2014. [Google Scholar]
- Cahill, J.T.; Graeve, O.A. Hexaborides: A review of structure, synthesis and processing. J. Mater. Res. Technol. 2019, 8, 6321–6335. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Sakar, E.; Guler, O.; Alım, B.; Say, Y.; Dikici, B. A comprehensive study on structural properties, photon and particle attenuation competence of CoNiFeCr-Ti/Al high entropy alloys (HEAs). J. Alloys Compd. 2023, 931, 167561. [Google Scholar] [CrossRef]
- Şakar, E.; Alım, B.; Ertap, H.; Karabulut, M. An extensive survey on radiation protection features of novel hafnium iron-borophosphate glasses: Experimental and theoretical study. Prog. Nucl. Energy 2023, 160, 104690. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Güngör, O.; Yılmaz, H. An online software to simulate the shielding properties of materials for neutrons and photons: NGCal. Radiat. Phys. Chem. 2021, 185, 109519. [Google Scholar] [CrossRef]
- Hila, F.C.; Asuncion-Astronomo, A.; Dingle, C.A.M.; Jecong, J.F.M.; Javier-Hila, A.M.V.; Gili, M.B.Z.; Balderas, C.V.; Lopez, G.E.P.; Guillermo, N.R.D.; Amorsolo, A.V. EpiXS: A Windows-based program for photon attenuation, dosimetry and shielding based on EPICS2017 (ENDF/B-VIII) and EPDL97 (ENDF/B-VI.8). Radiat. Phys. Chem. 2021, 182, 109331. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Yuan, Y.; Cui, K.; Wei, W.; Wu, J.; Qin, W.; Wu, X. Spatially confined Bi2O3–Ti3C2T hybrids reinforced epoxy composites for gamma radiation shielding. Compos. Commun. 2022, 34, 101252. [Google Scholar] [CrossRef]
- Hubbell, J.H. Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 keV to 100 GeV; U.S. Department of Commerce, The National Standard Reference Data System: Washington, DC, USA, 1969.
- Eisa, M.H.; Shen, H.; Yao, H.Y.; Mi, Y.; Zhou, Z.Y.; Hu, T.D.; Xie, Y.N. Studies on absorption coefficient near edge of multi elements. J. Quant. Spectrosc. Radiat. Transf. 2005, 96, 503–511. [Google Scholar] [CrossRef]
- Cremer, J.T. Chapter 4—X-ray Optics. In Advances in Imaging and Electron Physics; Cremer, J.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 497–559. ISBN 1076-5670. [Google Scholar]
- Zegaoui, A.; Derradji, M.; Ghouti, H.A.; Medjahed, A.; Zu, L.-W.; Liu, W.-B.; Wang, J.; Dayo, A.Q.; Liu, Y.-G. Synergetic effects of short carbon/basalt hybrid fibers on the mechanical, thermal and nuclear shielding properties of DCBA/BA-a resin composites. Compos. Commun. 2019, 15, 179–185. [Google Scholar] [CrossRef]
- Crompton, T.R. Chapter 4—Metals in Surface, Ground, and Mineral Waters. In Determination of Metals in Natural Waters, Sediments and Soils; Crompton, T.R., Ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2015; pp. 161–178. ISBN 978-0-12-802654-0. [Google Scholar]
- Singh, V.P.; Badiger, N.M. Investigation of Gamma and Neutron Shielding Parameters for Borate Glasses Containing NiO and PbO. Phys. Res. Int. 2014, 2014, 954958. [Google Scholar] [CrossRef]
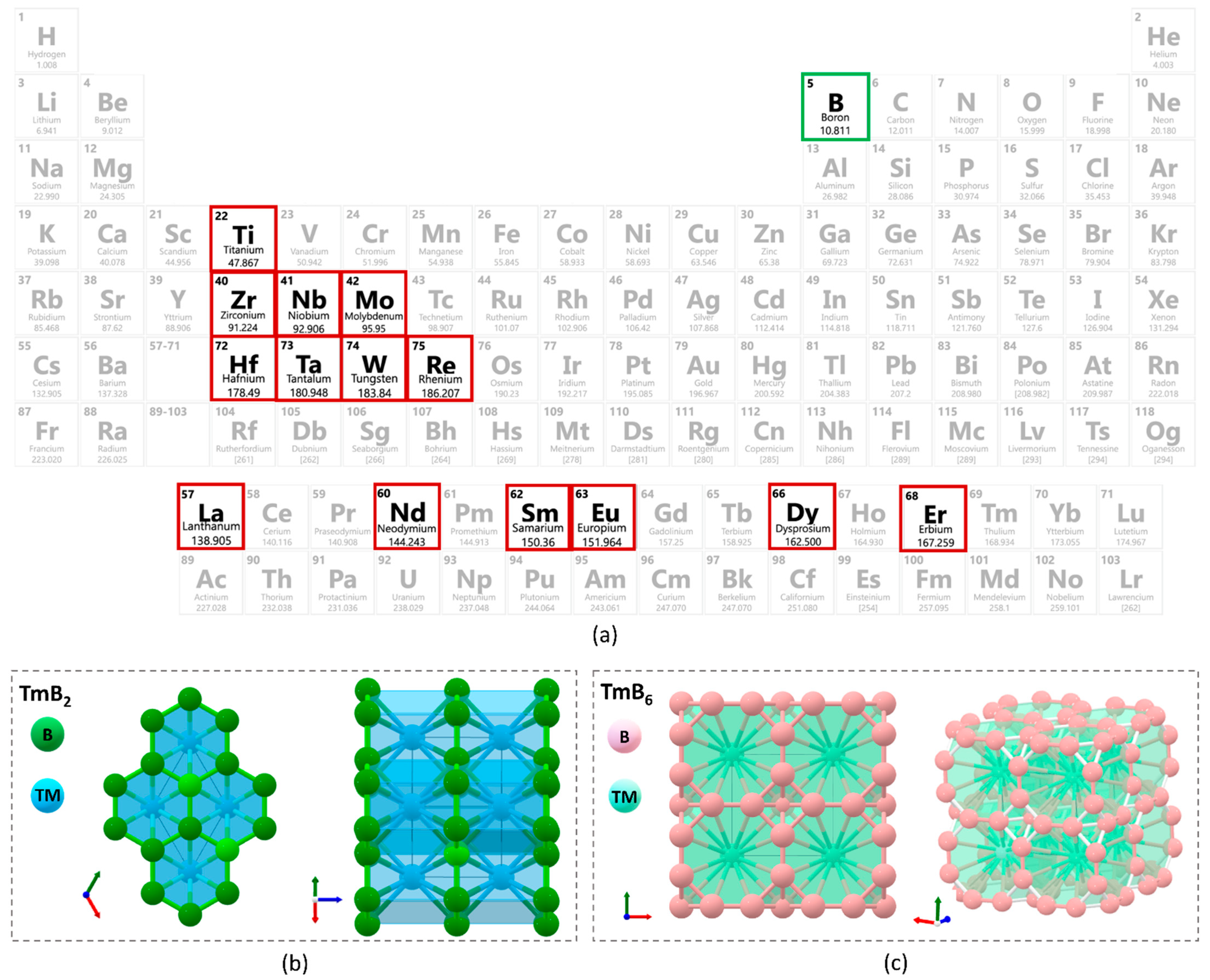

| Di-Borides | ||||||||
|---|---|---|---|---|---|---|---|---|
| TiB2 | ZrB2 | NbB2 | MoB2 | HfB2 | TaB2 | WB2 | ReB2 | |
| Density (g/cm3) | 4.52 | 6.07 | 6.78 | 7.33 | 11.24 | 12.27 | 12.42 | 12.62 |
| Hexa-Borides | ||||||||
| LaB6 | NdB6 | SmB6 | EuB6 | DyB6 | ErB6 | |||
| Density (g/cm3) | 4.72 | 4.93 | 5.14 | 5.01 | 5.54 | 5.70 | ||
| The Mass Attenuation Factors | The Linear Attenuation Factors | |||
|---|---|---|---|---|
| Thermal Neutrons (0.025 eV) | Fast Neutrons (4 MeV) | Thermal Neutrons (0.025 eV) | Fast Neutrons (4 MeV) | |
| B4C | 33.51172 | 0.07678 | 84.44954 | 0.19349 |
| SmB6 | 29.58439 | 0.14001 | 152.06374 | 0.71963 |
| DyB6 | 14.99297 | 0.17228 | 70.57155 | 0.17271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avcıoğlu, C.; Avcıoğlu, S. Transition Metal Borides for All-in-One Radiation Shielding. Materials 2023, 16, 6496. https://doi.org/10.3390/ma16196496
Avcıoğlu C, Avcıoğlu S. Transition Metal Borides for All-in-One Radiation Shielding. Materials. 2023; 16(19):6496. https://doi.org/10.3390/ma16196496
Chicago/Turabian StyleAvcıoğlu, Celal, and Suna Avcıoğlu. 2023. "Transition Metal Borides for All-in-One Radiation Shielding" Materials 16, no. 19: 6496. https://doi.org/10.3390/ma16196496
APA StyleAvcıoğlu, C., & Avcıoğlu, S. (2023). Transition Metal Borides for All-in-One Radiation Shielding. Materials, 16(19), 6496. https://doi.org/10.3390/ma16196496





