Study on the Deterioration Mechanism of Magnesium Oxychloride Cement under an Alkaline Environment
Abstract
1. Introduction
2. Materials and Experiment
2.1. Principal Raw Materials
2.2. Preparation of MOC Samples
2.3. Immersion Experiment
2.4. Characterization
3. Results
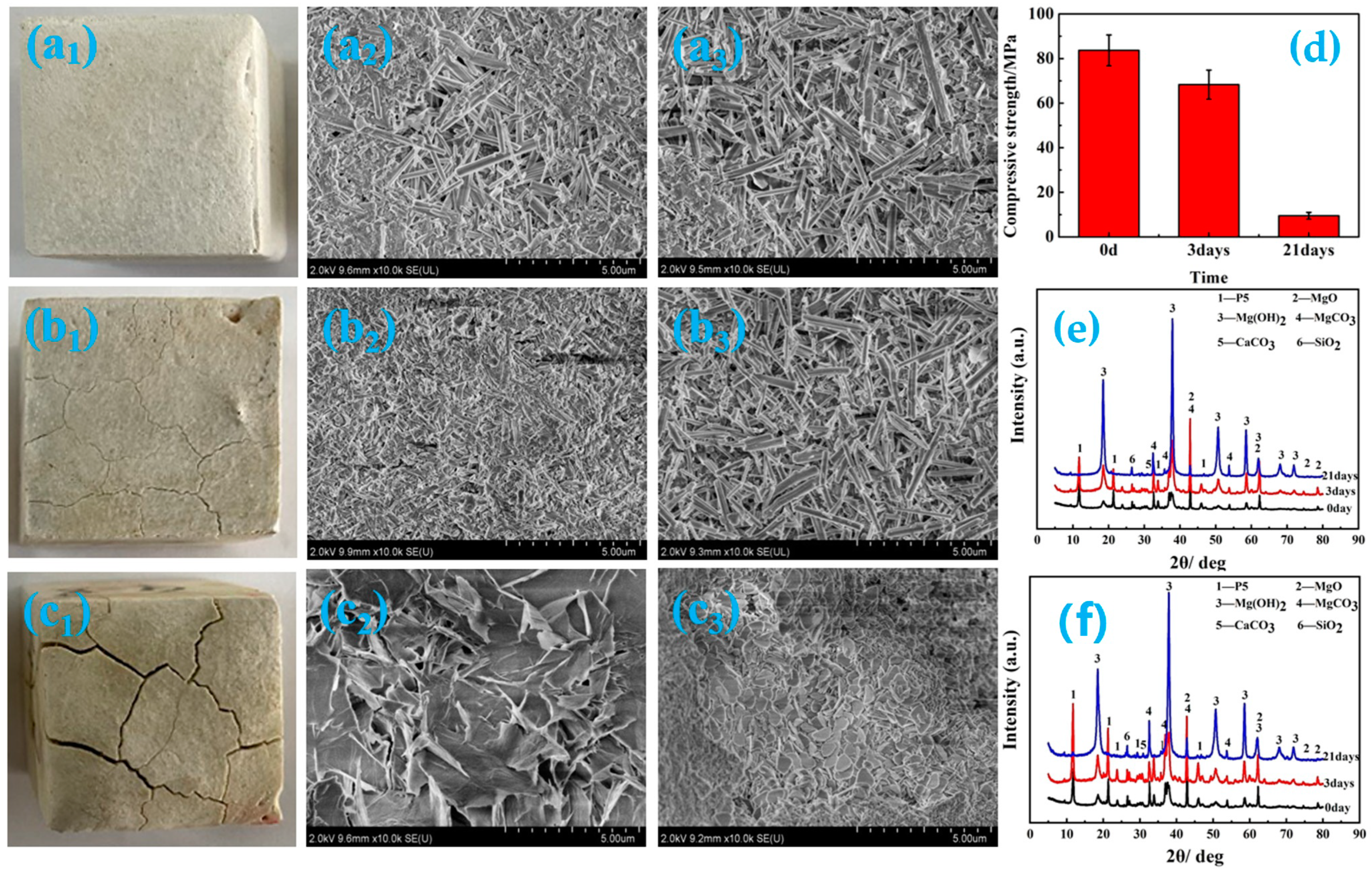
3.1. Macroscopic Morphology Analyses
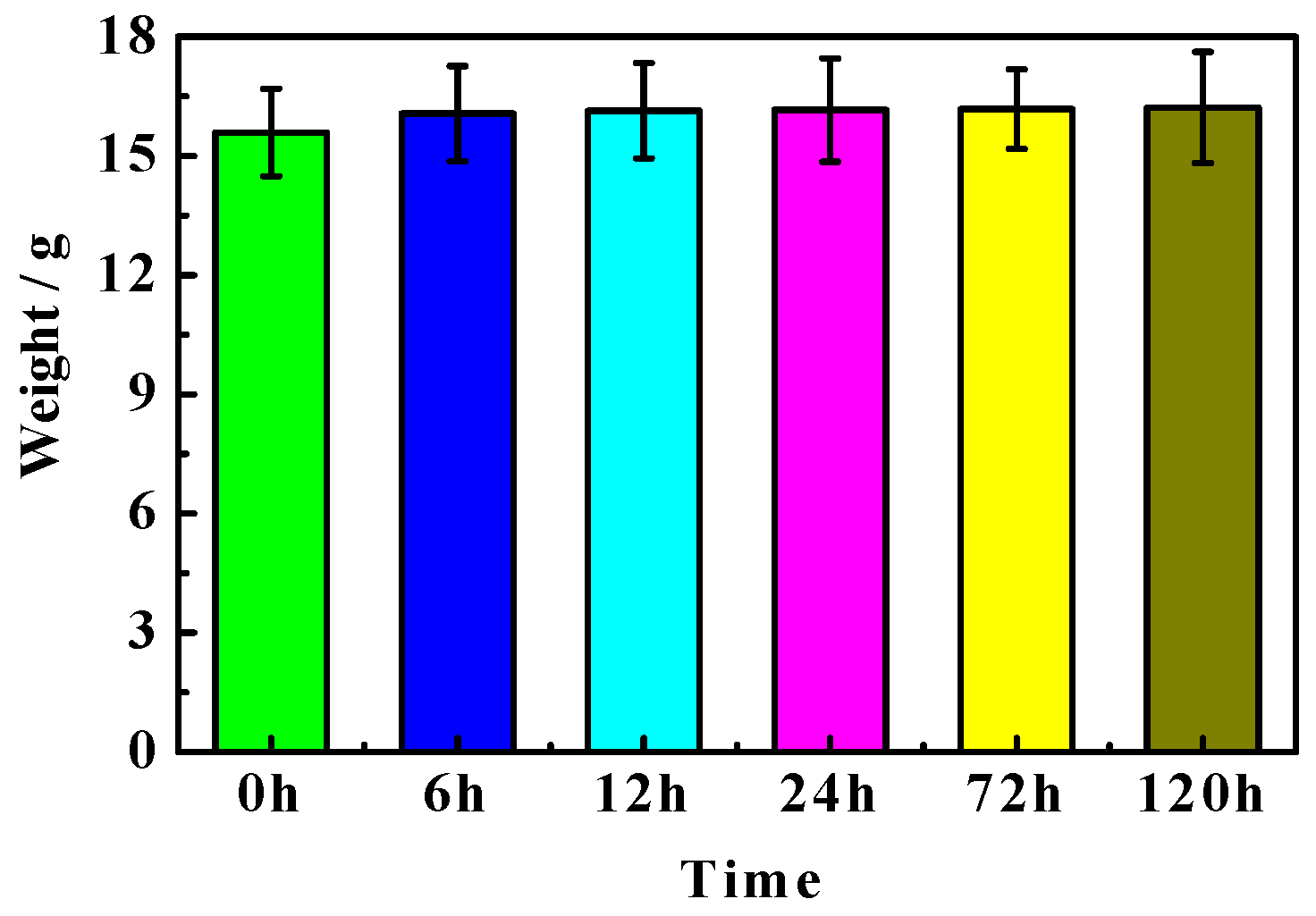
3.2. Weight Analyses
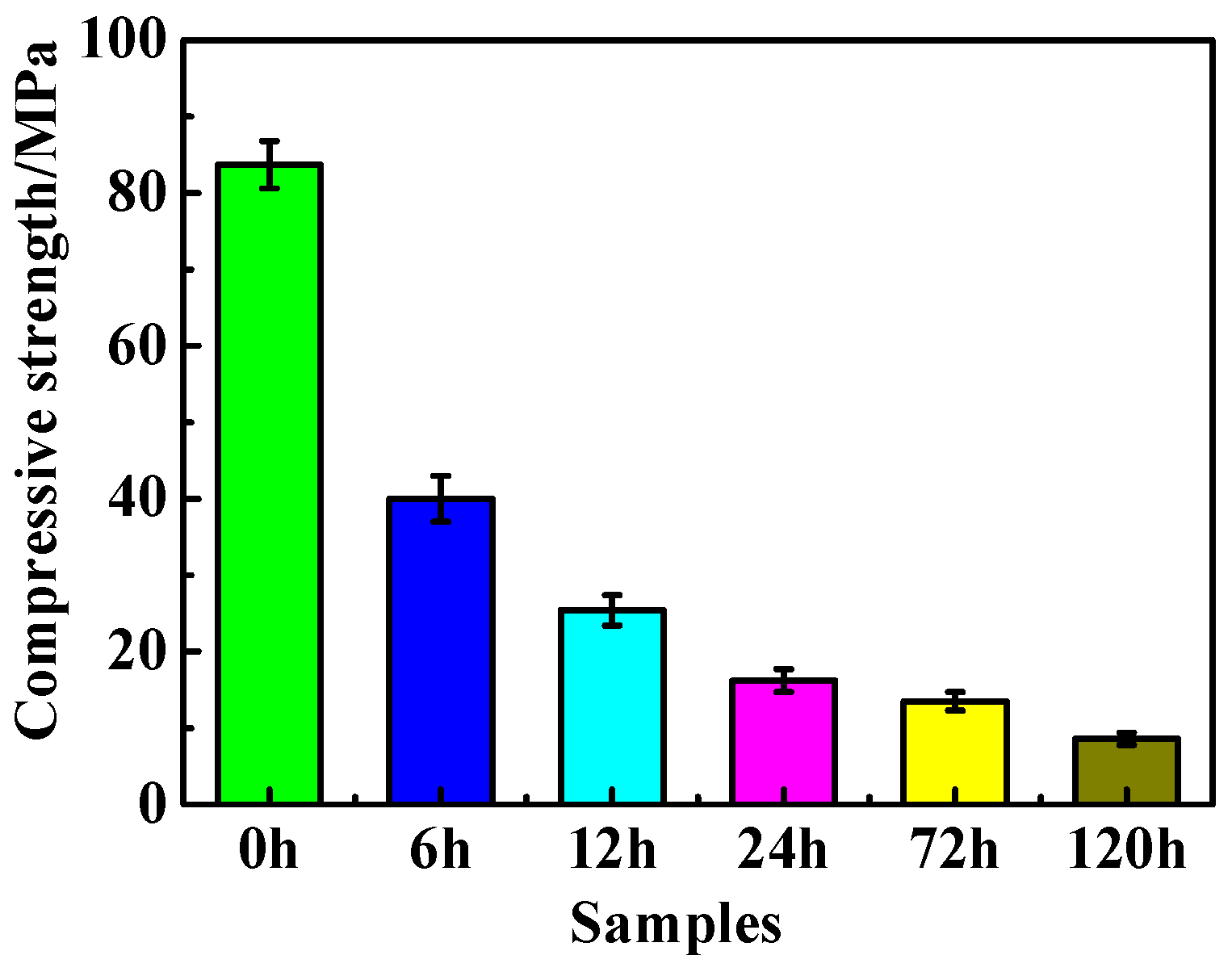
3.3. Compressive Strength Analyses
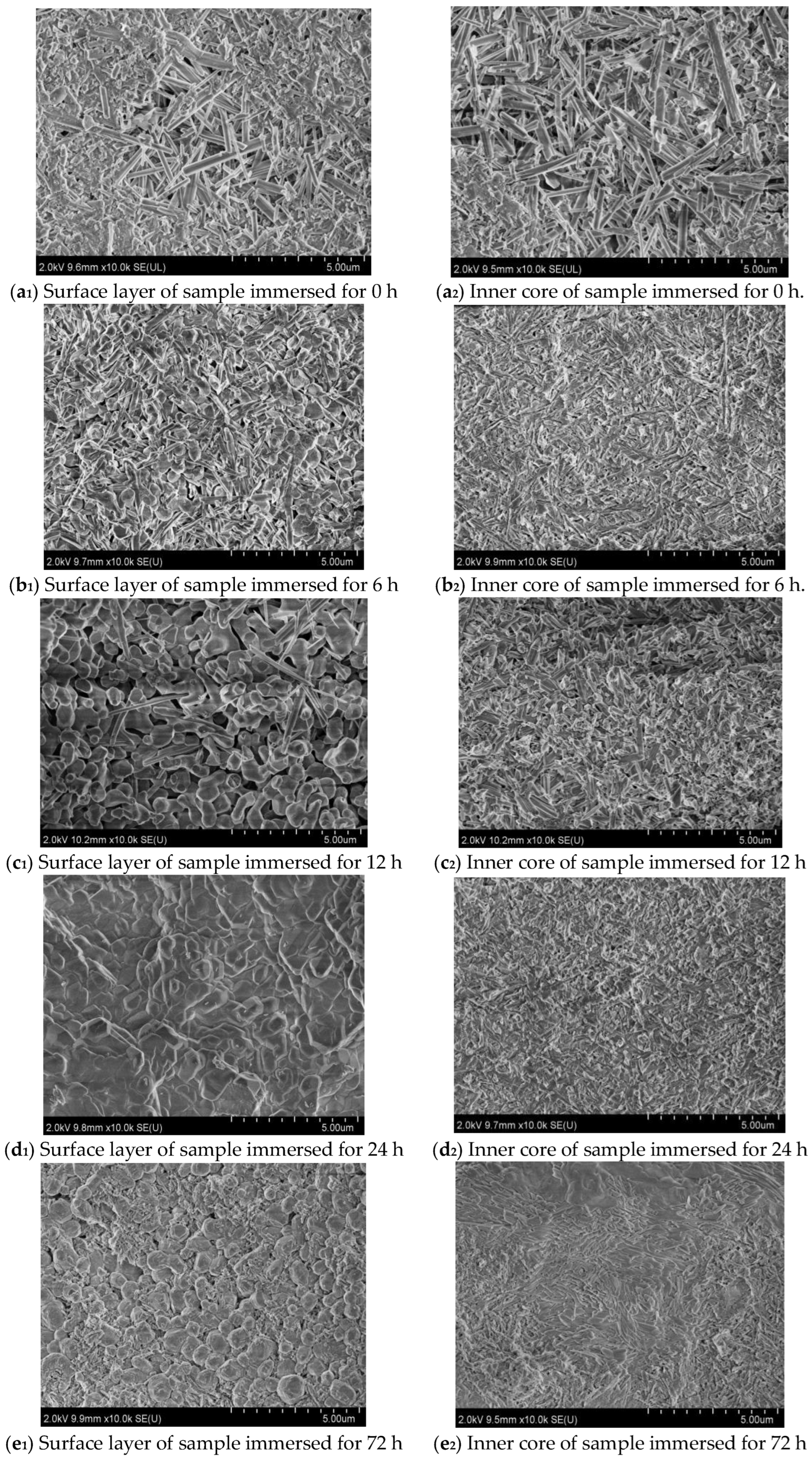
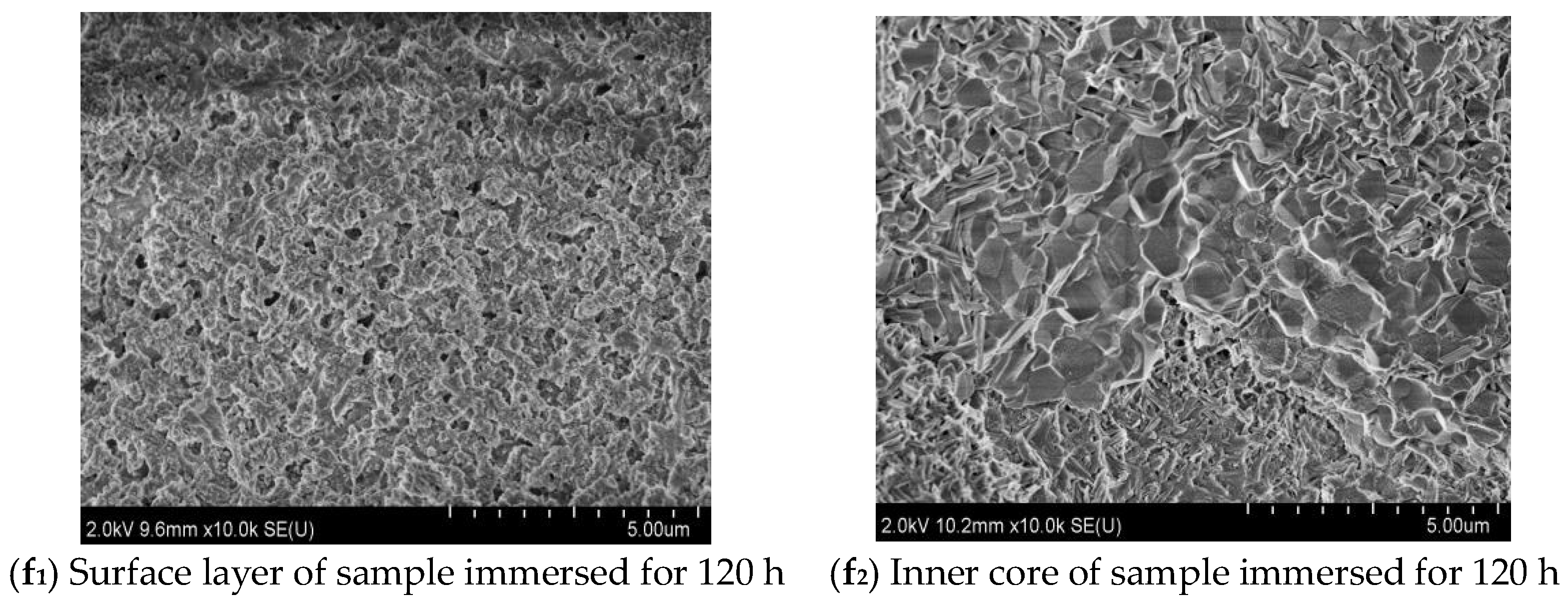
3.4. Microscopic Morphology Analyses
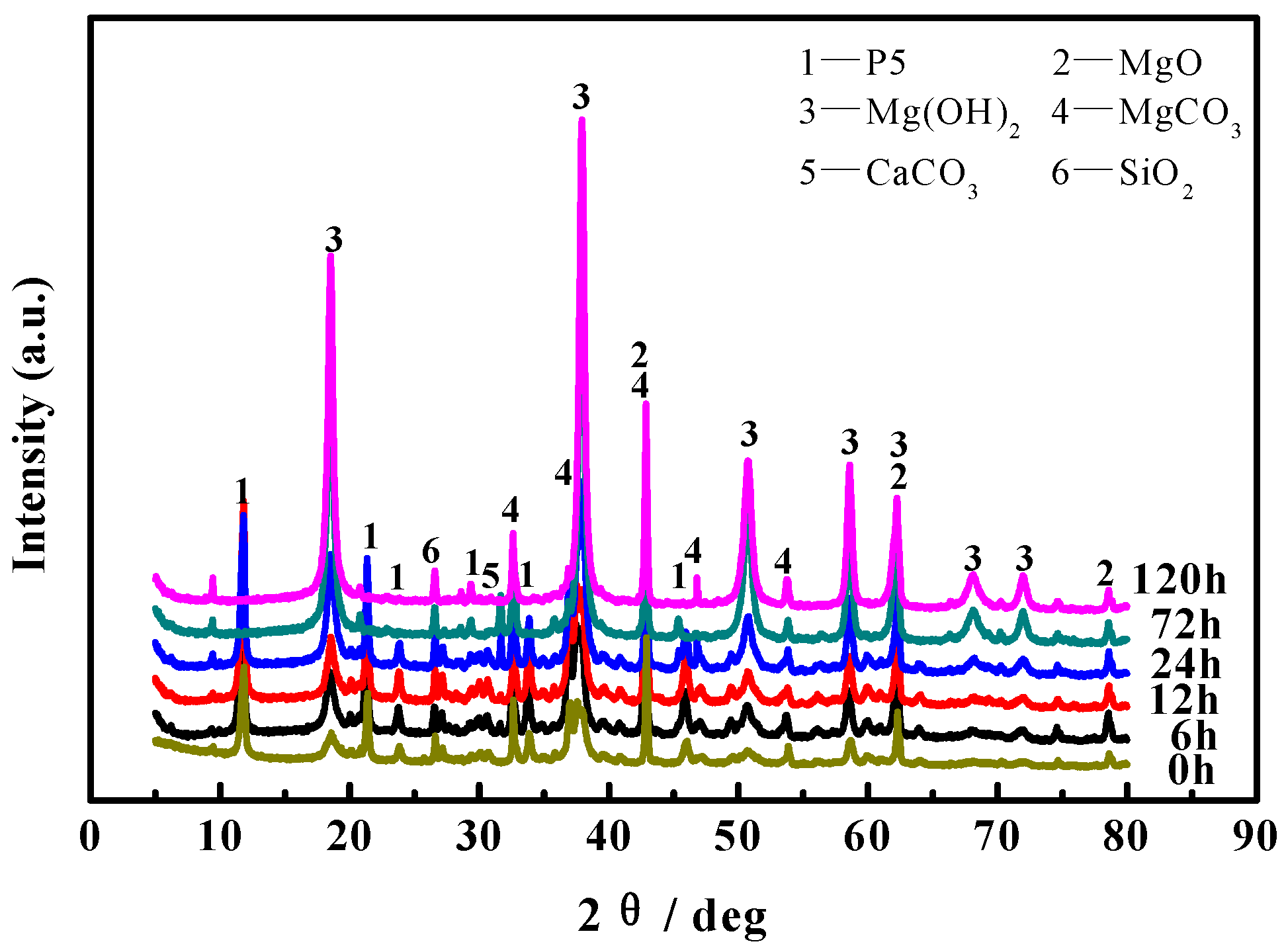
3.5. Phase Composition Analyses
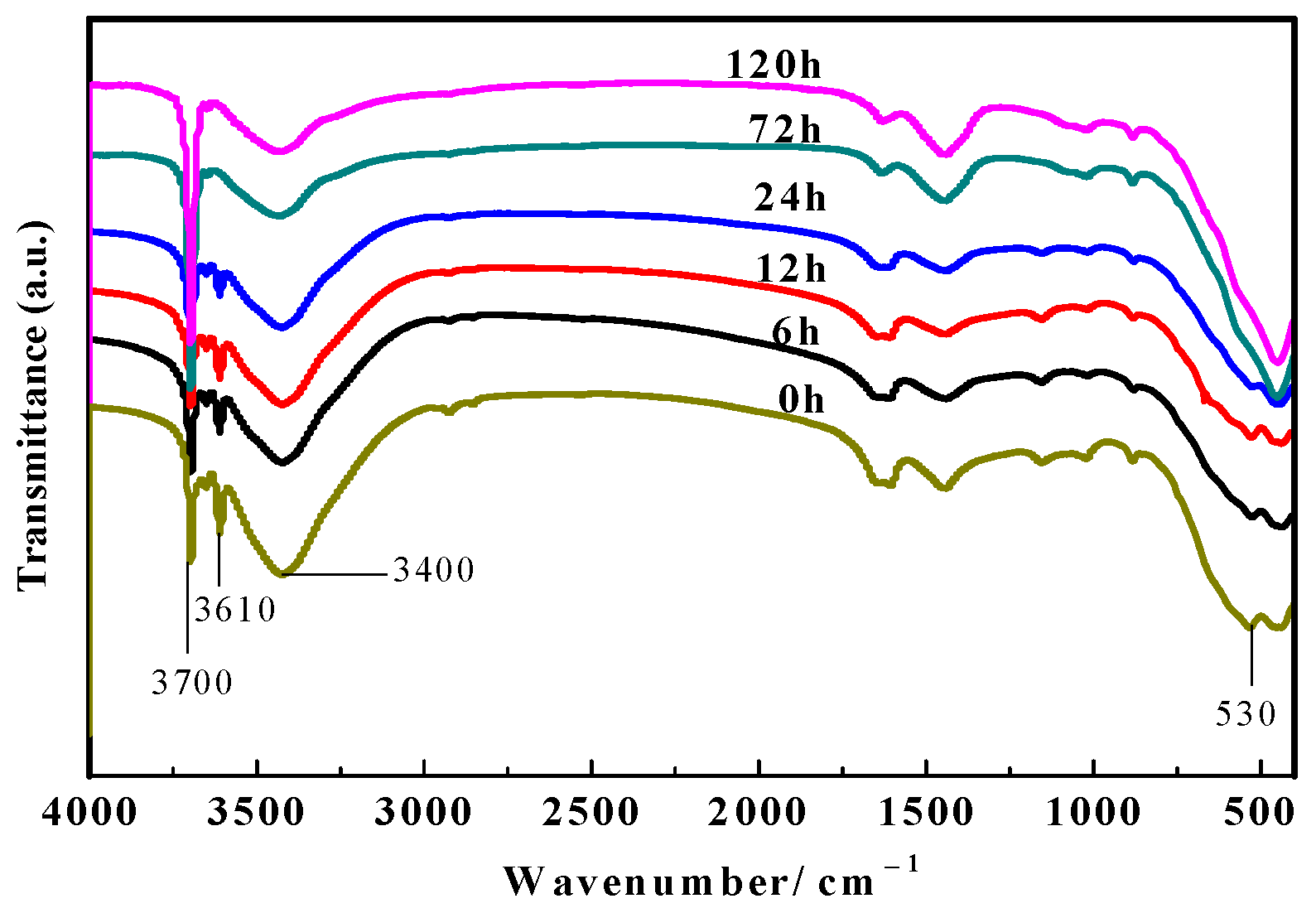
3.6. FT-IR Spectra Analyses
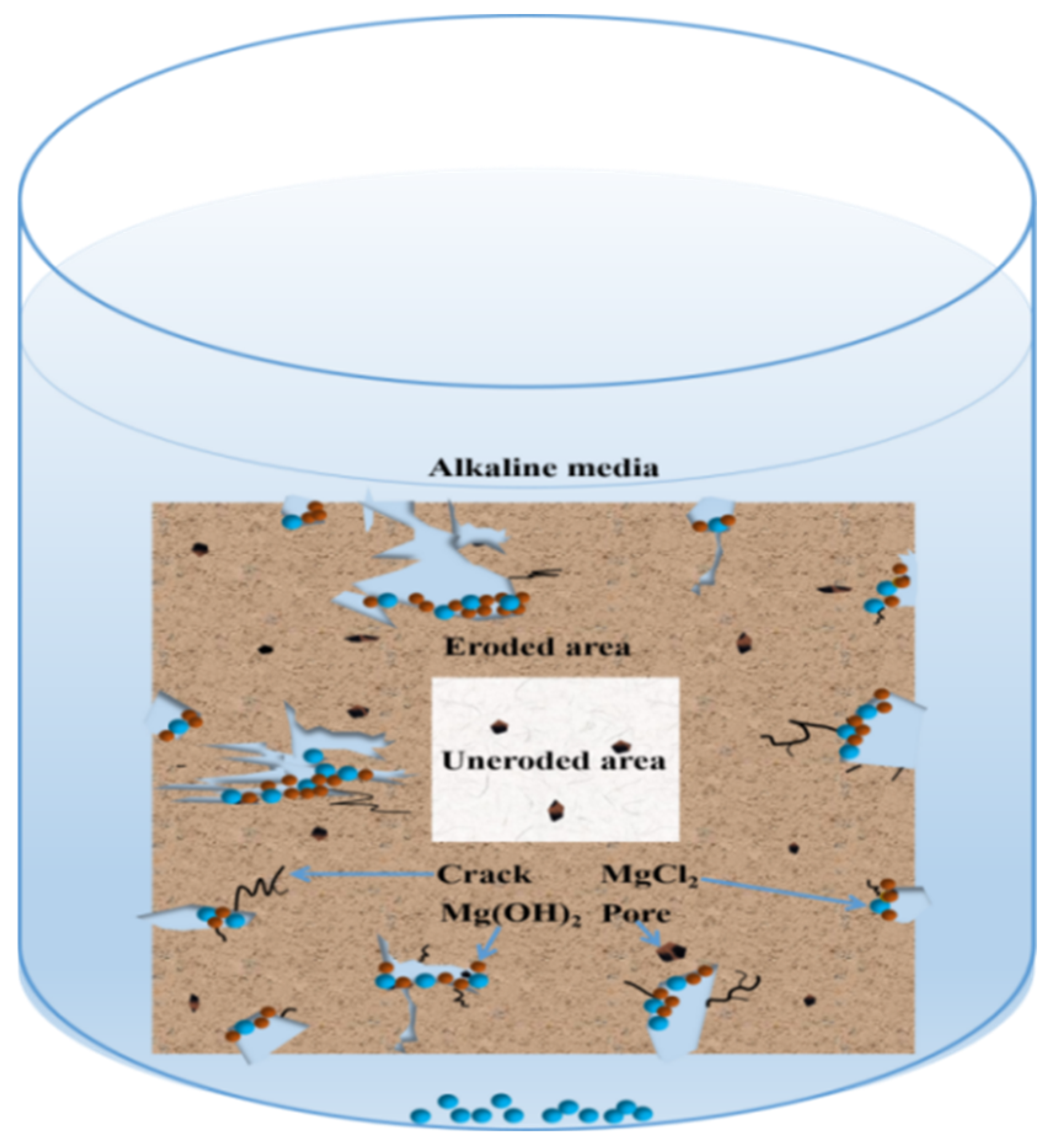
4. Discussion
5. Conclusions
- The deterioration of the MOC sample in an alkaline environment is mainly due to the fact that the alkaline environment provides more OH− ions, which can react with P 5 in the sample, giving rise to a faster decomposition of the main strength phase P 5 in the sample. This further results in a substantial reduction in the strength of the sample and its gradual deterioration.
- Immersion time also exhibits a significant effect on the MOC sample. With the prolongation of the immersion time of the sample in an alkaline medium, a greater number of OH− ions can enter the sample, which causes increasing P 5 to undergo a hydrolysis reaction, and results in more serious deterioration for the sample. The MOC slurry sample is mainly composed of P 5, Mg(OH)2 and MgO. Meanwhile, it possesses a gel-like and a needle-like micro-morphology, which are interleaved with each other and relatively compact. As a result, the MOC slurry sample has a high compressive strength of 83.7 MPa. After soaking for 6 h in an alkaline medium, the main phase composition of the surface layer of the MOC sample changes to MgO and Mg(OH)2, and the microscopic morphology is also dominated by a round-sheet, resulting in a sharp decrease in compressive strength (52.2%). When the immersion time is extended to 72 h, OH− ions have already been immersed into the inner core of the sample, giving rise to the disappearance of P 5 from the entire sample. Meanwhile, both the surface layer and inner core of the sample exhibit a round-sheet morphology, and a powdering phenomenon also appears on the surface layer. Therefore, the compressive strength of the sample is reduced to 13.5 MPa, which is only 20% of its strength when dipped into water. After immersion for 120 h (3 days), the compressive strength of the sample is as low as 8.6 MPa, which is weaker than that when it has been steeped in water for 21 days (9.5 MPa), presenting a faster deterioration rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Qinghai Institute of Salt Lake, Chinese Academy of Sciences. 60 Years of Salt Lake Research of Chinese Academy of Science; Science Press: Beijing, China, 2015. [Google Scholar]
- Chang, C.G.; Dong, J.M.; Xiao, X.Y.; Zheng, W.X.; Wen, J.; Li, Y.; Huang, Q.; Man, Y. Long-term mechanical properties and micro mechanism of magnesium oxychloride cement concrete. Adv. Cem. Res. 2020, 32, 371–378. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.S.; Yao, N.; Zhang, A. Recent progress of magnesium oxychloride cement: Manufacture, curing, structure and performance. Constr. Build. Mater. 2020, 255, 119381. [Google Scholar] [CrossRef]
- Sorel, S.T. Sur un nouveau ciment magnesien. C. R. Hebd. Seances Acad. Sci. 1867, 65, 102–104. [Google Scholar]
- Paterson, J.H. Magnesium oxychloride cement. J. Soc. Chem. Ind. 1924, 43, 215–218. [Google Scholar] [CrossRef]
- Sorre, C.A.; Armstrong, C.R. Reactions and equilibria in magnesium oxychloride cements. J. Am. Ceram. Soc. 1976, 59, 51–54. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Zhang, Y.X.; Soe, K.; Pulham, M. Recent development in magnesium oxychloride cement. Struct. Concr. 2018, 19, 1290–1300. [Google Scholar] [CrossRef]
- Xu, B.W.; Ma, H.Y.; Hu, C.L.; Yang, S.Q.; Li, Z.J. Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Constr. Build. Mater. 2016, 102, 613–619. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Lauermannová, A.; Antončík, F.; Pavlíková, M.; Záleská, M.; Sedmidubský, D. Car-bon Dioxide Uptake by MOC-Based Materials. Appl. Sci. 2020, 10, 2254. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Pivák, A.; Pavlík, Z. Experimental Analysis of MOC Composite with a Waste-Expanded Polypropylene-Based Aggregate. Materials 2018, 11, 931. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wei, L.Z.; Yu, J.T.; Yu, K.Q. Mechanical properties of high ductile magnesium oxychloride ce-ment-based composites after water soaking. Cem. Concr. Compos. 2019, 97, 248–258. [Google Scholar] [CrossRef]
- Zuo, Y.F.; Xiao, J.H.; Wang, J.; Liu, W.J.; Li, X.G.; Wu, Y.Q. Preparation and characterization of fire retardant straw/magnesium cement composites with an organic-inorganic network structure. Constr. Build. Mater. 2018, 171, 404–413. [Google Scholar] [CrossRef]
- Chang, C.G.; Dong, J.M.; Zheng, W.X.; Wen, J.; Yan, F.Y.; Xiao, X.Y.; An, L.Y. Long-term Properties of Aeolian Sand-magnesium Oxychloride Cement Composites and Its Engineering Application. J. Wuhan Univ. Technol. Sci. Ed. 2023, 38, 842–848. [Google Scholar] [CrossRef]
- Edan, H.H.; Hilal, N.; Sor, N.H.; Tawfik, T.A. Durability and Hardened Characteristics with SEM Analysis of Eco-Efficient Self-Compacting Concrete Partially Contained Waste Walnut Shell Particles as Fine Aggregate. Iran. J. Sci. Technol. Trans. Civ. Eng. 2023, 52, 1–17. [Google Scholar] [CrossRef]
- Alani, N.Y.; Al-Jumaily, I.A.; Hilal, N. Performance of self-compacting concrete containing nano clay at ele-vated temperatures and MgSO4 attack. Eur. J. Environ. Civ. Eng. 2023, 27, 3001–3019. [Google Scholar] [CrossRef]
- Chang, C.G.; An, L.Y.; Zheng, W.X.; Wen, J.; Dong, J.M.; Yan, F.Y.; Xiao, X.Y. Research and Engineering Applica-tion of Salt Erosion Resistance of Magnesium Oxychloride Cement Concrete. Materials 2021, 14, 7880. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.G.; An, L.Y.; Lin, R.; Wen, J.; Dong, J.M.; Zheng, W.X.; Yan, F.Y.; Xiao, X.Y. Effect of Calcination Tem-perature on Mechanical Properties of Magnesium Oxychloride Cement. Materials 2022, 15, 607. [Google Scholar] [CrossRef]
- Chang, C.G.; Wen, J.; Dong, J.M.; Zheng, W.X.; Yan, F.Y.; Xiao, X.Y. Effect of carbonization on evolution process of microstructure of magnesium oxychloride cement. Inorg. Chem. Ind. 2022, 54, 61–65. (In Chinese) [Google Scholar]
- Chang, C.G.; An, L.Y.; Dong, J.M.; Zheng, W.X.; Wen, J.; Yan, F.Y.; Xiao, X.Y. Study on Deterioration Process of Magnesium Oxychloride Cement under the Environment of Dry–Wet Cycles. Materials 2023, 16, 1817. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.F. Rapid Assessment Method for Water Resistance of Magnesium Oxychloride Cement Basedon Measurement of High-temperature Erosion Thicknes. J. Chin. Ceram. Soc. 2014, 42, 1047–1054. [Google Scholar]
- GB/T 17671-1999; Method of Testing Cements-Determination of Strength. The State Bureau of Quality and Technical Supervision: Beijing, China, 1999.
- Li, Y.; Li, Z.J.; Pei, H.F.; Yu, H.F. The inflfluence of FeSO4 and KH2PO4 on the performance of magnesium ox-ychloride cement. Constr. Build. Mater. 2016, 102, 233–238. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Zhang, X.X.; Zheng, W.X.; Chang, C.G.; Ren, X.F.; Zeng, J.B.; Hai, C.X.; Shen, Y. Experimental in-vestigation of thermal and mechanical properties of magnesium oxychloride cement with form-stable phase change material. Constr. Build. Mater. 2018, 186, 670–677. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Pivák, A.; Maruiak, S.; Jankovsk, O.; Lauermannová, A.M.; Lojka, M.; Antončík, F.; Pavlík, Z. MOC Doped with Graphene Nanoplatelets: The Influence of the Mixture Preparation Technology on Its Properties. Materials 2021, 14, 1450. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Zhang, Y.X.; Soe, K.; Hutchison, W.D.; Timmer, H.; Poblete, M.R. Effect of fly ash on mechanical prop-erties of magnesium oxychloride cement under water attack. Struct. Concr. 2020, 21, 1181–1199. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Chang, C.G.; Zheng, W.X.; Fang, J.H.; Huang, S.J.; Wen, J.; Dong, J.M.; An, S.X. Study on Mechanical Property of Pavement Built with Magnesium Oxychloride Cement Concrete. J. Salt Lake Res. 2016, 24, 50–54. (In Chinese) [Google Scholar]
- Zhang, Z.Y.; Dai, C.L.; Zhang, Q.C.; Guo, B.Z.; Liu, W.L. Study on formation mechanism of phase 5 and phase 3. Sci. China Ser. B 1991, 1, 82–89. (In Chinese) [Google Scholar]



| Composition | MgCl2 | NaCl | MgSO4 | KCl | CaCl2 | Water-Insoluble Matter | H2O |
|---|---|---|---|---|---|---|---|
| Content (wt.%) | 44.90 | 0.13 | 0.06 | 0.01 | 0.03 | 0.27 | 54.6 |
| Composition | MgO | MgCO3 | CaCO3 | Free CaO | Acid-Insoluble Matter |
|---|---|---|---|---|---|
| Content (wt.%) | 69.52 | 19.80 | 1.34 | 0.38 | 8.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, L.; Chang, C.; Yan, F.; Peng, J. Study on the Deterioration Mechanism of Magnesium Oxychloride Cement under an Alkaline Environment. Materials 2023, 16, 5924. https://doi.org/10.3390/ma16175924
An L, Chang C, Yan F, Peng J. Study on the Deterioration Mechanism of Magnesium Oxychloride Cement under an Alkaline Environment. Materials. 2023; 16(17):5924. https://doi.org/10.3390/ma16175924
Chicago/Turabian StyleAn, Lingyun, Chenggong Chang, Fengyun Yan, and Jianhong Peng. 2023. "Study on the Deterioration Mechanism of Magnesium Oxychloride Cement under an Alkaline Environment" Materials 16, no. 17: 5924. https://doi.org/10.3390/ma16175924
APA StyleAn, L., Chang, C., Yan, F., & Peng, J. (2023). Study on the Deterioration Mechanism of Magnesium Oxychloride Cement under an Alkaline Environment. Materials, 16(17), 5924. https://doi.org/10.3390/ma16175924






