Abstract
This study focuses on fabricating cobalt particles deposited on graphitic carbon nitride (Co/gCN) using annealing, microwave-assisted and hydrothermal syntheses, and their employment in hydrogen and oxygen evolution (HER and OER) reactions. Composition, surface morphology, and structure were examined using inductively coupled plasma optical emission spectroscopy, X-ray photoelectron spectroscopy, and X-ray diffraction. The performance of Co-modified gCN composites for the HER and OER were investigated in an alkaline media (1 M KOH). Compared to the metal-free gCN, the modification of gCN with Co enhances the electrocatalytic activity towards the HER and OER. Additionally, thermal annealing of both Co(NO3)2 and melamine at 520 °C for 4 h results in the preparation of an effective bifunctional Co3O4/gCN catalyst for the HER with the lower Eonset of −0.24 V, a small overpotential of −294.1 mV at 10 mA cm−2, and a low Tafel slope of −29.6 mV dec−1 in a 1.0 M KOH solution and for the OER with the onset overpotential of 286.2 mV and overpotential of 422.3 mV to achieve a current density of 10 mA cm−2 with the Tafel slope of 72.8 mV dec−1.
1. Introduction
Hydrogen demand in 2021 was 94 million tons (Mt), above pre-pandemic levels (91 Mt in 2019), and accounted for about 2.5% of global final energy consumption [1]. Most of the increase was driven by traditional uses in the refining and industrial sectors. However, demand for new applications increased to about 40 Mt (60% more than in 2020). Hydrogen demand is estimated to reach 115 Mt by 2030. This compares with the 130 Mt needed to meet existing climate change commitments made to date by governments around the world and with nearly 200 Mt required by 2030 to achieve net zero emissions by 2050 [1]. In the face of the global energy crisis, the efficient production of hydrogen is becoming one of the mainstays of the hydrogen economy and sustainable development. However, the vast majority of current hydrogen (about 95%) is produced by steam methane reforming and coal gasification processes, which produce CO2 and require carbon capture and storage to reduce the environmental impact [2,3]. At the same time, fossil fuels are inevitably consumed. A cleaner and commercially viable alternative is electrochemical hydrogen production through water splitting (WS), although clean hydrogen energy can also be produced from renewable sources (e.g., solar, wind, geothermal, and tidal).
Clean hydrogen production by electrocatalytic WS has recently become a major current priority [4,5,6,7,8]. The electrochemical WS process is based on electrocatalytic reactions, such as the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), which are critical for the large-scale development of clean energy alternatives. Both reactions require efficient HER/OER electrocatalysts to reduce the activation energy and overpotentials [4,9,10]. Noble metals, such as Pt or Pt-group materials (PGMs, including Pt, Pd, Ru, Ir, and Rh), have been the most widely studied and are currently considered as state-of-the-art for water splitting [11,12,13,14,15,16]. Given the impressively high cost of precious metals and their scarcity in nature, significant progress has been made in the search for alternative, cost-effective, and innovative substitutes. As evidence of this, a rapidly increasing number of publications has been reported on earth-abundant transition/noble metal-free (TMs, M=Co, Ni, Fe, Mn, and Mo) and TMs-based alloys or non-metallic (TMXs, X=N, O, S, C, P, etc.) compounds, aimed to function as active HER/OER electrocatalysts [17,18,19,20,21]. TMXs have received much attention due to their compositions, ease of application for large-scale production, distinctive structural features, abundant active sites, and tunable electronic properties. Co, Ni, and Fe are typically characterized as the most powerful materials for WS [22,23,24,25]. Among them, Co-based electrocatalysts, including cobalt oxides [26], hydroxides [27], phosphides [28], sulfides [29], nitrides [30,31], and selenides [32], play a rather significant role in WS and are widely used in HER or OER [33,34]. However, their catalytic performance and stability do not yet meet the requirements for use in practical applications. Many electrocatalysts based on cobalt suffer from poor electrical conductivity and hence a low charge transfer efficiency [33,34]. Efficient and stable Co-based electrocatalytic materials with sufficient intrinsic electronic structure and unlimited active sites on the surface for optimized WS remain challenging.
Strategies to improve the performance of electrocatalysts are mainly based on increasing the intrinsic activity, the number of exposed active sites, and the charge transfer from the conductive support to the catalyst surface [35,36,37,38]. They are closely supported by different engineering approaches [20,35,36,39,40,41,42,43]. Recently, incorporating Co-based catalytic materials onto suitable substrates, or even growing on 3D substrates, has become a convenient way to improve the active site dispersion and catalytic activity of the catalyst [44,45]. In this regard, carbon-based materials have been the most hotly pursued due to their suitability in various catalytic reactions, high stability, large surface area, ease of processing, and low production costs [46,47]. Coupling transition metal-based materials of different structures and morphologies, such as porous carbon [48], carbon cloth [41], carbon nanofibers [42], carbon paper [43], carbon nanotubes [20], graphitic carbon [44], reduced graphene oxide [45], nitrogen-doped graphene [46], and graphene-based nanostructures [49], has been extensively studied for HER/OER and has proven to be an additional effective strategy to increase the electrocatalytic efficiency by controlling the structural morphology [41,42,43,44,45,46,47,48,49,50].
Among the numerous carbon-based materials, graphitic carbon nitride (g−C3N4) has been pointed out to have great potential in a variety of photocatalytic and electrocatalytic applications due to its thermal, mechanical, electrical, and tunable physicochemical properties; metal-free nature; non-toxicity; low cost; optical response in the visible light range; ease of preparation; environmental friendliness; biocompatibility; and chemical inertness [48,49,50,51,52,53,54]. Unlike various carbon-based materials, g−C3N4 is a type of polymeric semiconductor with a graphite-like structure containing a large number of N atoms in the form of specific tri-s-triazine rings, beneficial for catalytic activity. It (g−C3N4) has high stability due to strong covalent bonds between the carbon and nitrogen atoms and can effectively serve as an electrocatalyst for water splitting, being active for both OER and HER reactions [49,52]. However, low conductivity and limited availability of redox sites limit the applicability of pristine g−C3N4 [48]. Considerable efforts have been applied to address these shortcomings of g−C3N4.
Numerous strategies have been employed to enhance the functionality of g−C3N4, including diverse synthesis methods and conditions that allow obtaining g−C3N4-based materials in different morphologies and sizes of nanoparticles with the required unique properties for improved catalytic performance. Commonly used synthesis techniques are electrodeposition, physical and chemical vapor deposition, microwave-assisted processes, thermal condensation, hydrothermal and solvothermal synthesis routes, sol-gel processes, thermochemical reactions such as pyrolysis and combustion, etc. [49,51,52,55,56,57]. Thermal condensation approaches enable the degree of condensation to be controlled by determining the formation of either melon or g−C3N4. Heating the melamine at different temperatures makes it possible to obtain different morphologies of g−C3N4, ranging from nanosheets to rolled nanosheets, nanotubes, and nanoflakes with nanoparticles, depending on the thermal polymerization temperatures of 500, 520, and 540 °C, respectively [58]. Solvothermal methods for synthesizing g−C3N4 offer significant advantages, such as the formation of uniform and fine particles, exceptionally high surface area and overall porosity, and low energy consumption [51]. However, they are still time-consuming, requiring several hours to complete particle formation and crystallization. Microwave heating methods provide a wide reaction range and short reaction times suitable for industrial-scale production [59]. Synthesis of g−C3N4 using this technique for only 30 min led to the formation of submicron spheres and a large surface area of 90 m2 g−1, with improved photocatalytic efficiency [59]. However, the specimen’s morphology is not easily subject to specific regulation within this method. Hydrothermal syntheses are simple and promising methods that can be used to increase a sample’s crystallinity, crystal size, and shape and obtain a product of high purity and stability [60]. However, they are inconvenient for monitoring the growth process of the sample as the preparation process is heavily dependent on the reaction duration, pH, pressure, temperature, and the type of solvent used.
Beyond this, the functionality of g−C3N4-based materials can be significantly improved by compounding with conductive counterparts, surface modification, metal/non-metal doping, hybridization, heterostructure formation, etc. [51]. Studies on the bonding of g-C3N4 to Co-based materials have progressed. Different synthesis approaches have been used. A novel hybrid of Co3O4 embedded in tubular nanostructures of g−C3N4 was recently synthesized using a simple chemical method at low temperatures, which showed excellent performance for both OER and HER [61]. The synergistic effect, high surface area, and unique structure made maximum redox sites more easily available for catalysis and provided faster ionic and electronic conduction. The tubular nanostructured hybrid Co3O4@GCN (graphitic carbon nitride) signified the lowest overpotential of 120 mV (at 10 mA cm−2) and a current density of 147 mA cm−2 for OER, outperforming the benchmark catalysts of IrO2 and RuO2. At the same time, it showed a small onset potential of −0.03 V towards HER, very close to the onset potential of commercial Pt/C (−0.01 V). The presence of cobalt, carboxyl/hydroxyl groups, and partially negative nitrogen centers were considered to favor the absorption of the water molecules on the surface, contributing greatly to the initial step of OER and HER. S. Vignesh et al. [62] developed an effective, highly dispersed Co3O4 growth on a g−C3N4 surface coupled with quantum dots (QDs) on a ZnS composite via a facile hydrothermal synthesis. It exhibited enhanced HER performance with a small Tafel slope of 71 mV dec−1 and a low overpotential of 304 mV at 10 mA cm−2. The Co3O4 and ZnS QDs served as a bridging junction, which functioned as a good electron transfer channel. It provided abundant active sites and effectively prevented the agglomeration of the g−C3N4 nanosheets during the HER process. Zahra et al. [63] developed a cobalt–nickel sulfide on a g−C3N4 via a simple hydrothermal synthesis. A three-dimensional nickel foam electrode with a well-defined hierarchical flower-like pattern showed excellent catalytic activity for WS reactions with a low overpotential of 160 mV at 10 mA cm−2 for HER and 310 mV at 30 mA cm−2 for OER in alkaline media. The authors identified a controllable morphological effect to enhance the electrochemical performance and showed that optimized g−C3N4 concentration plays a vital role in inducing the formation of hierarchical flower-like patterns, which not only prevents agglomeration but also provides a porous structure for enhanced gas diffusivity and charge transfer rates due to the synergistic carbon and nitrogen bonding with Co and NiS. Y. Shen et al. [64] prepared g−C3N4@Co(OH)2 nanowires using a plasma modification strategy. The developed catalyst was subjected to a 60 s plasma treatment, which generated more exposed active edge sites and increased the number of Co3+ active sites for enhanced OER activity, displaying an overpotential of 329 mV. B. Shalini Reghunath et al. [65] prepared an effective cobalt ferrite/graphitic carbon nitride/N doped graphene QDs electrocatalyst using hydrothermal synthesis. Notably, this electrocatalyst demonstrated a very low overpotential towards HER of 287 mV at 10 mA cm−2 with a Tafel slope of 94 mV dec−1 and an overpotential towards OER of 445 mV at 10 mA cm−2 with a Tafel slope of 69 mV dec−1. S.Y. Ejeta et al. [66] presented Co-incorporated graphitic carbon nitride as a bifunctional catalyst for electrochemical WS in acidic media. The g−C3N4 powder was synthesized using pyrolysis of melamine and exfoliation via strong sonication, while the reduction of the Co precursor with sodium borohydride synthesized the composite of Co on g−C3N4. It showed excellent catalytic activity for WS reactions with a minimum overpotential of ~85 mV and ~530 mV for HER and OER, respectively, at 10 mA cm−2. It also had a current density of 10 mA cm−2 at a cell voltage of 1.84 V, slightly lower than the benchmark catalyst (Pt/Cp).
Various strategies for synthesizing high-performance transition metal-modified g-C3N4-based catalysts, including annealing, microwave-assisted, and hydrothermal approaches, have been the subject of considerable research interest [62,63,64,65,66]. Each strategy has its own advantages and disadvantages. For example, thermal annealing is inexpensive, simple, and easy to operate [67,68]. However, it is often associated with aggregation phenomena and produces a low surface area catalyst, which limits its use for WS. Hydrothermal synthesis can produce crystallized nanoparticles of the desired shape and size with enhanced chemical activity [62,63]. But the synthesis is carried out under high pressure and requires expensive autoclaves. It is difficult to control the synthesis process and raises safety concerns. Microwave-assisted synthesis is an advantageous, highly effective, fast, and environmentally friendly process. It is often combined with other methods and allows the production of nanostructures of various shapes and sizes [66,69,70]. In addition, uniform microwave heating results in a homogeneous, uniform particle size distribution. However, it depends on microwave heating frequencies and therefore carries the risk of producing an impure synthesised compound.
Although many review articles have been published on the synthesis and application of modified g-C3N4-based materials for HER and OER, the state of the art in research is not fully understood and explored. The modification of g-C3N4 with a co-catalyst is achieved by various means and synthesis methods that determine the structure of the catalyst, the particle size and distribution, and thus the catalytic performance. Controlling and combining the relevant preparation methods and approaches can yield g−C3N4 with different morphologies and unique physicochemical properties required for various applications. However, correlations between the various synthesis methods (for a given catalyst and a given catalytic process) have rarely been reported [70,71].
In this study, different preparation methods—thermal annealing, microwave-assisted, and hydrothermal methods—were used to synthesize Co-supported g-C3N4. The obtained catalysts were characterized in terms of composition, structure, and catalytic activities. It was shown that thermal annealing is a cost-effective and simple approach for preparing the bifunctional Co3O4/gC3N4 catalyst for its applications in electrochemical WS.
2. Materials and Methods
2.1. Materials and Synthesis
Materials involved included melamine (99%), Co(II) nitrate (Co(NO3)2, 99%), Co(II) acetylacetonate (C10H14CoO4, 99%), Co(II) chloride (CoCl2·6H2O, 98%), sodium chloride (NaCl, 98%), tetraethylene glycol (TEG, HO(CH2CH2O)3CH2CH2OH, 99%), hexamethylenetetramine (HMT, 99–100.5%), sodium hydroxide (NaOH, 98.8%), ethanol (C2H5OH, 96%), and 5 wt.% Nafion were purchased from Merck KGaA supplier (Darmstadt, Germany).
The gCN was prepared using thermal annealing of melamine at 520 °C for 4 h in a closed high-alumina crucible. A 5 °C/min rate was used to obtain the 520 °C temperature. After synthesis, it was ground into a fine powder.
Co-supported gCN catalysts were prepared using three different methods. One was a thermal treatment of both Co(NO3)2 and melamine at 520 °C for 4 h, with the resulting catalyst denoted as Co3O4/gCN. The other two were microwave (MWS) and hydrothermal (HTS) syntheses, which resulted in the preparation of catalysts denoted as Co/gCN-MWS and Co/gCN-HTS, respectively. Briefly, for MWS, a reaction mixture containing 30 mg of synthesized gCN, 14.55 mg of Co(II) acetylacetonate, and 8 mL of TEG was placed in the microwave reactor Monowave 300 (Anton Paar, Graz, Austria). The synthesis was carried out at 270 °C for 1 h. After synthesis, the obtained catalyst was filtered, washed with ethanol and ultra-pure water with a resistivity of 18.2 MΩ cm−1, and then dried in a vacuum oven at 80 °C for 2 h.
For the hydrothermal synthesis, 150 mg of CoCl2·6H2O, 600 mg of HMT, and 375 mg of NaCl were dissolved in 30 mL of water and ethanol solution (the volume ratio being 1:5). The reaction mixture was stirred for 15 min. Then, 40 mg of gCN was added to the mixture and stirred for 4 h. The synthesis was carried out at 120 °C for 8 h in an autoclave.
2.2. Materials’ Characterization
The crystallinity of studied catalysts was measured using an X-ray diffractometer D2 PHASER (Bruker, Karlsruhe, Germany). The XRD patterns were recorded in the 2θ range 10–90°.
X-ray photoelectron spectroscopy (XPS) was used for the samples’ chemical characterization using Kratos AXIS Supra+ spectrometer (Kratos Analytical, UK, Manchester, 2019) with the monochromatic Al Kα (1486.6 eV) X-ray radiation powered at 225 W. A low electron flood gun was used as a charge neutralizer. The base pressure in the analysis chamber was less than 1 × 10−8 mbar. The survey spectra for each sample were recorded at a pass energy of 80 eV with a 1 eV energy step and high-resolution spectra (pass energy—10 eV, in 0.1 eV steps) over individual element peaks. The binding energy scale was calibrated by setting the adventitious carbon peak at 284.8 eV. Avantage software (Version V5, Thermo Scientific, East Grinstead, UK) was used for the conversion of XPS data to VAMAS format and their processing.
Raman spectra were recorded using in Via Raman (Renishaw, Wotton-under Edge, UK) spectrometer equipped with a thermoelectrically cooled (−70 °C) CCD camera and microscope. Raman spectra were excited with 830 nm radiation from a diode laser (Renishaw, Wotton-under Edge, UK). The 20×/0.40 NA objective lens and 830 lines/mm grating were used to record the Raman spectra. The accumulation time was 200 s. To avoid damage of the sample, the laser power at the sample was restricted to 1.7 mW. The Raman frequencies were calibrated using the polystyrene standard. The spectra were background-corrected using a 6-order function fit. Parameters of the bands were determined by fitting the experimental spectra with Gaussian and Lorentzian shape components using GRAMS/A1 8.0 (Thermo Scientific, Waltham, MA, USA) software
The composition of the catalyst was analyzed using the spectrometer Optima 7000DV (Perkin Elmer, Waltham, MA, USA).
2.3. Electrochemical Measurements
The experiments were performed using a potentiostat/galvanostat PGSTAT100 (Metrohm Autolab B. V., Utrecht, The Netherlands). The three-electrode standard cell was used, where a glassy carbon (GC) electrode modified with the synthesized catalysts ink was used as the working electrode. The geometric surface area of the GC electrode was 0.196 cm2 as the counter and reference electrodes employed an Ag/AgCl (3 M KCl) and GC road, respectively. Linear sweep voltammograms (LSVs) were recorded in an N2-saturated 1 M KOH solution at a scan rate of 2 mV s−1. All reported potential values were referred to as “ERHE”—reversible hydrogen electrode according to Equation (1):
where EAg/AgCl (3 M KCl) = 0.210 V.
ERHE = Emeasured + 0.059·pH + EAg/AgCl (3 M KCl)
Current densities for HER and OER presented in this paper were normalized to the geometric area of catalysts.
The catalyst’s ink was obtained by mixing 10 mg of the synthesized catalysts with 1980 μL deionized water and ethanol solution ultrasonically for 2 h, with the volume ratio being 1:1 and 20 μL 5wt.% Nafion solution. Then, 10 μL of the obtained ink was dripped on a pre-prepared GC electrode and dried at 80 °C for 4 h.
Electrochemical impedance spectroscopy (EIS) experiments were carried out on a Zahner Zennium electrochemical workstation (Zahner-elektrik, Kronach-Gundelsdorf, Germany). The spectra were obtained potentiostatically in a deaerated 1 M KOH solution using a 10 mV potential perturbation amplitude and in the frequency region from 10 kHz to 0.1 Hz.
For the determination of the electrochemically active surface area (ECSA) of catalysts, the double layer capacitance (Cdl) was determined by recording CV curves at various scan rates under the non-faradaic region followed by the calculation of the slope of the curve obtained by plotting the difference in anodic and cathodic current against the scan rate [72,73,74]. From the CVs, the charging current, Ic, of the electrodes at each scan rate was determined via Equation (2):
Ic [A] = (Ianodic−Icathodic)OCP
Cdl values were evaluated by plotting a graph of charging current vs. scan rate and calculating the slope, as shown by Equation (3):
Slope = Cdl [F] = ΔIC [A]/Δν [V s−1]
Then, the ECSA values were calculated using the specific capacitance (Cs) of 40 μF cm−2 [72,73,74] and Equation (4):
ECSA [cm2] = Cdl [μF]/Cs [μF cm−2]
The stability test of the Co3O4/gCN catalyst was carried out for 1000 cycles at a constant scan rate of 100 mV s−1 at the rotation speed of 1600 rpm, after which the stable polarization curve was recorded at 2 mV s−1 for comparison with the initial curve.
3. Results and Discussion
3.1. Characterization of Catalysts
The XRD pattern of as-prepared gCN exhibited a typical pattern, with two broad peaks centered approximately at 12.7° and 27.3° (Figure 1a), and can be assigned to the (100) and (002), respectively, planes of the trigonal N bond of tri-s-triazine and the layered packing of conjugated aromatic units in g−C3N4, respectively [58,59,60,75]. Figure 1b presents the XRD pattern for Co3O4/gCN obtained by annealing Co(NO3)2 and melamine at 520 °C for 4 h. The profile shows the diffraction peaks obtained at 2θ = 18.991, 31.308, 36.848, 38.587, 44.816, 59.379, and 65.238° attributed to crystallographic planes (111), (220), (311), (222), (400), (511), and (440), respectively, of the Co3O4 phase (ICDD # 01-078-1969). This means that Co3O4 can be readily obtained using a simple one-step annealing procedure with high crystallinity and without any impurities, confirming the high purity of the prepared Co3O4.
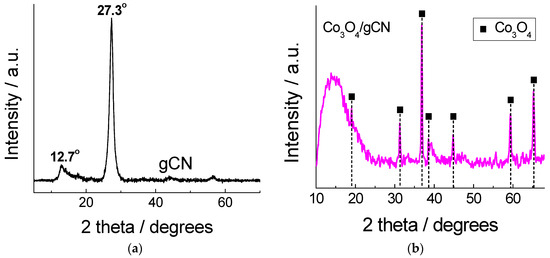
Figure 1.
XRD patterns for (a) gCN and (b) Co3O4/gCN.
XPS was used to investigate the electronic structure and surface composition of the as-prepared gCN, Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS catalysts. XPS analysis data on the elemental composition of the investigated catalysts are given in Table 1, whereas XPS spectra for C 1s, N 1s, O 1s, and Co 2p are presented in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6.

Table 1.
The elemental composition of the tested catalysts.
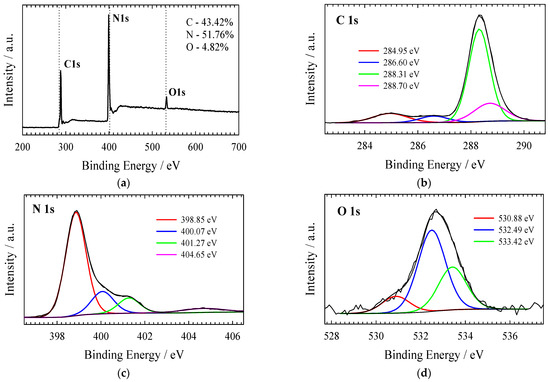
Figure 2.
(a) The survey and high-resolution XPS spectra of (b) C 1 s, (c) N 1 s, and (d) O 1 s for gCN obtained by annealing the melamine at 520 °C for 4 h.
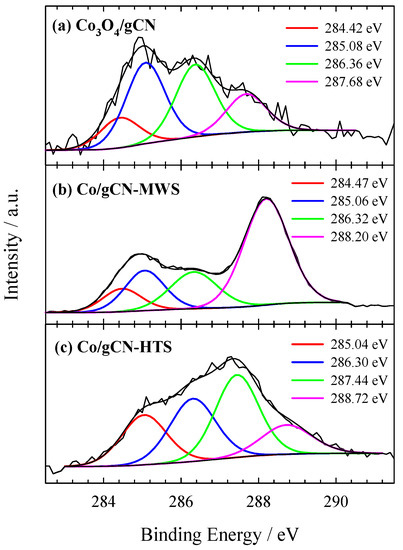
Figure 3.
High-resolution XPS spectra of C 1s for (a) Co3O4/gCN, (b) Co/gCN-MWS, and (c) Co/gCN-HTS.
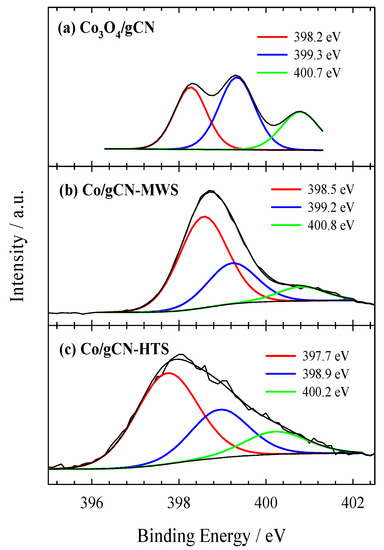
Figure 4.
High-resolution XPS spectra of N 1s for (a) Co3O4/gCN, (b) Co/gCN-MWS, and (c) Co/gCN-HTS.
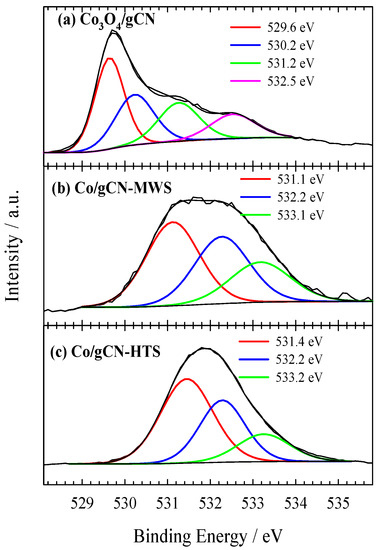
Figure 5.
High-resolution XPS spectra of O 1s for (a) Co3O4/gCN, (b) Co/gCN-MWS, and (c) Co/gCN-HTS.
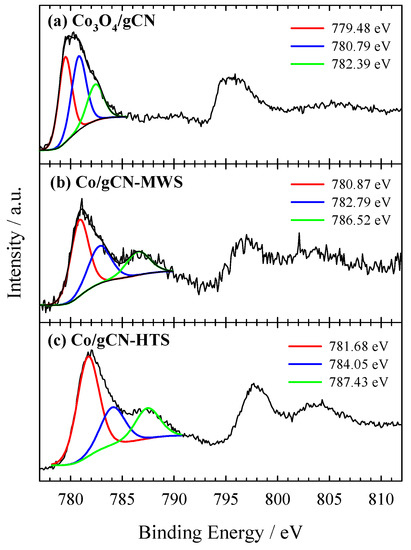
Figure 6.
High-resolution XPS spectra of Co 2p for (a) Co3O4/gCN, (b) Co/gCN-MWS, and (c) Co/gCN-HTS.
The survey scan of the XPS for the pristine g-C3N4 shows that the synthesized material is mainly composed of carbon and nitrogen, with the weakly pronounced O1s peak (Figure 2a) possibly originating from adsorbed oxygen. The high-resolution XPS of the C 1s spectrum (Figure 2b) for the g-C3N4 shows four peaks centered at around 284.95 eV, 286.60 eV, 288.31 eV, and 288.70 eV. The strongest peak at 288.31 eV corresponds to sp2 bonded carbon (N−C=N), and the weaker one at 284.95 eV is attributed to graphitic carbon, which is typically found in the XPS spectrum characterizing g-C3N4 [76]. The high-resolution XPS of the N 1s spectrum (Figure 2c) can be deconvoluted into four peaks located at 398.85 eV, 400.07 eV, 401.27 eV, and 404.65 eV that can be attributed to sp2-hybridized nitrogen (C–N=C), tertiary nitrogen N–(C)3 groups, amino functions caring hydrogen (C–N–H) and π-excitation, respectively [58,76,77]. O 1s signals at 530.89 eV, 532.49 eV, and 533.42 eV in Figure 2d are attributed to the oxygen of O=C, O-C, and OH, respectively [76]. Similarly, from the high-resolution C 1s spectrum of oxidized g–C3N4, two new C1s peaks at 286.60 and 288.70 eV can be deconvoluted, which are attributed to the carbon of O-C and O=C-O, respectively [76].
The XPS spectra show differences in both shape and peak position depending on the catalyst preparation conditions. The high-resolution XPS of the C 1s spectrum (Figure 3a) for the Co3O4/gCN catalyst can be resolved into several peaks centered at around 284.42, 285.08, 286.36, and 287.68 eV. This confirmed the existence of the g–C3N4 phase corresponded to adventitious sp2 C–C carbon species (284.42 eV), sp2 hybridized carbon atoms in N–C–N (286.38 eV), and sp2 C=N bond (287.36 eV) in the s-triazine ring, respectively [58,59,78]. In addition, carbon at 285.08 eV and 287.68 eV can be resolved into C–O and CO=, respectively, indicating a strong interaction between g–C3N4 and Co3O4 [78].
The N 1s signal shown in Figure 4a is located in the binding energy region of 398–401 eV and can be fitted into three peaks located at 398.27 eV, 399.33 eV, and 400.78 eV for sp2-hybridized nitrogen in triazine rings (C–N=C), tertiary nitrogen N–(C)3 groups, amino functions caring hydrogen (C–N–H), respectively [58,78]. Furthermore, the peak at 399.33 eV is prevailing, accounting for 42.74 at.%. This binding energy area covers the binding energy region required for the N−Co bond, which is difficult to distinguish [64,77,79]. The binding energy of pyridine N in the Co3O4/g−CN sample (398.27 eV) decreased by 0.58 eV compared with that of pristine in g−C3N4 (398.85 eV), reflecting the interfacial interaction between g–C3N4 and Co3O4.
O 1s signals in Figure 5a at 529.64 eV (36.81 at.%) and 530.23 eV (26.76 at.%) are the dominant peaks corresponding to the Co−OC and the CoOCo bonds, respectively, suggesting that the C groups of g–C3N4 serve as the nucleation and anchoring sites for Co3O4 [78]. The binding energies of O 1s peaks at 531.26 eV and 532.53 are attributed to adsorbed oxygen species (water, oxygen, CO2) [58,59,66,80]. In Figure 6a, the high-resolution XPS spectrum of Co 2p presents dominant peaks at 779.9 eV (2p3/2) and 795.5 eV (2p1/2), which are accompanied by shake-up satellite peaks at approximately 790.4 eV and 805.7 eV, respectively. The results show the occurrence of high-spin Co oxide species, indicating an admixture of Co2+ and Co3+, the two cations present in the spinel structure of Co3O4 [64,78,80,81]. The Co 2p3/2 and Co 2p1/2 peaks of the Co species at 779.9 and 795.5 eV, giving a spin-energy separation of 15.6 eV, is the characteristic of a Co3O4 phase and matches well with the reported data [82,83,84]. Meanwhile, the deconvolution of the XPS spectrum of Co 2p3/2 confirms the presence of different oxidation states of Co atoms as Co3O4 (779.48 eV), and Co(II) oxides/hydroxides, including possible Co–Nx structures, CoO, CoOOH, Co(OH)2 (780.79 eV, 782.39 eV), with overlapping binding energies of different oxide and hydroxide forms [64,77]. In addition, the peak with a binding energy of 779.48 eV, attributed to the Co3O4 species, clearly dominates over the other identified peaks, accounting for 40.22 at.%, thus confirming the successful synthesis of Co3O4 giving rise to the formation of a hybrid nanostructure with g–C3N4 within the prepared Co3O4/gCN catalyst.
In the case of Co/gCN-MWS and Co/gCN-HTS catalysts, the XPS data for Co 2p show the peak shift to the region of higher binding energy, indicating the disappearance of Co3O4 species and the predominance of different forms of Co(II)-based substances (Figure 6b,c). At the same time, the XPS data for O 1s are also detected at higher binding energy values of 531.12, 532.27, and 533.17 eV for the Co/gCN-MWS catalyst and 531.45, 532.28, and 533.25 eV for the Co/gCN-HTS catalyst, corresponding to adsorbed oxygen species (Figure 5b,c). In contrast, the N 1s signal for these catalysts is located almost in the same binding energy region of approximately 398–401 eV for the Co3O4/gCN catalyst, but the intensities of the deconvoluted peaks differ considerably (Figure 4b,c). The above results have shown that both Co3O4 and g–C3N4 were successfully synthesized to form a hybrid nanostructure in the prepared Co3O4/gCN catalyst, whereas, in the case of Co/gCN-MWS and Co/gCN-HTS catalysts, the Co(II)-based substances were dominant.
Figure 7 compares 830 nm excited Raman spectra of differently prepared Co/gCN samples and graphitic carbon nitride. The Raman spectrum from a sample prepared by thermal treatment of both Co(NO3)2 and melamine (Co3O4/gCN) shows intense and well-defined bands at 197, 483, 522, 621, and 692 cm−1 assigned to the F(3)2g, Eg, F(2)2g, F(1)2g, and A1g Raman active modes of the spinel Co3O4 structure, respectively [85,86,87]. The peak position of the A1g mode coincides well with the high-quality crystal structure [87]. It was demonstrated that defective structure results in a shift of this mode to lower wavenumbers [87,88]. The presence of the gCN matrix can be visible from very-low-intensity bands in the frequency region from 700 to 1400 cm−1 [89,90,91]. The Raman spectra from other samples differ considerably compared with Co3O4/gCN (Figure 7).
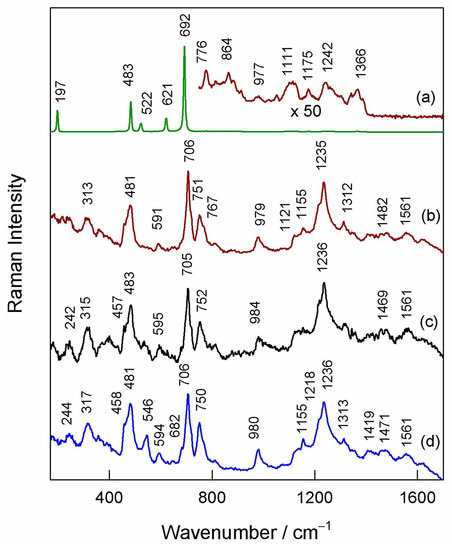
Figure 7.
Raman spectra of samples: (a) Co3O4/gCN, (b) Co/gCN-HTS, (c) Co/gCN-MWS), and (d) gCN. Spectra are normalized to the intensity of the most intense band. The excitation wavelength is 830 nm (1.7 mW).
No bands characteristic of crystalline Co3O4 are visible in these spectra; all observed features can be assigned to vibrations of the gC3N4 structure [89,90,91]. Thus, two strong bands located at 706 and 1235 cm−1 were assigned to the breathing vibrations of the s-triazine ring [90]. The low-intensity feature at 1561 cm−1 might be related to the graphitic G band, while the low-intensity band near 1469–1482 cm−1 might be related to the presence of amorphous carbon. It should be noted that the Raman spectrum of the gC3N4 matrix in the case of sample Co3O4/gCN is different compared with the other three structures. This might be related to the modification of graphitic carbon nitride structure because of the presence of the Co3O4 phase. The absence of Co3O4 bands in the spectra of samples prepared by microwave-assisted and hydrothermal-assisted methods suggests that the structure of cobalt oxides is amorphous.
3.2. Investigation of Electrocatalyst Activity for HER
The electrocatalytic activity of synthesized catalysts was investigated for HER and OER in an alkaline medium. The HER polarization curves recorded on the investigated Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS catalysts in alkaline media are shown in Figure 8a, whereas data of the electrochemical performance of the tested catalysts are given in Table 2.
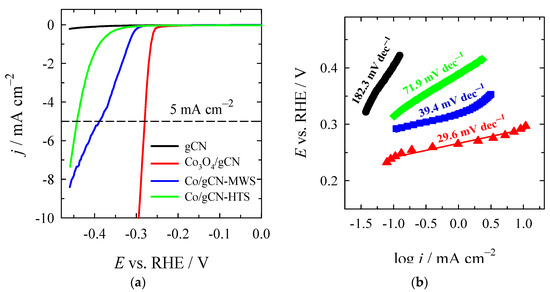
Figure 8.
(a) HER polarization curves of gCN, Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS catalysts in 1 M KOH solution at a potential scan rate of 2 mV s−1; (b) The corresponding Tafel slopes for each catalyst.

Table 2.
Electrochemical performance of the tested catalysts toward HER in alkaline media.
As seen, the lowest onset potential (Eonset) of −0.24 V for the HER exhibits the Co3O4/gCN catalyst compared with Co/gCN-MWS, Co/gCN-HTS, and gCN catalysts (Table 2). Additionally, the latter catalyst shows a significantly higher current density and the lowest overpotential of −280.5 mV for the HER to reach a current density of 5 mA cm−2 (η5) (Figure 8a), followed by Co/gCN-MWS (−389.4 mV) and Co/gCN-HTS (−444.1 mV). The overpotential value at a current density of 10 mA·cm−2 (η10) for Co3O4/gCN was found to be −294.1 mV (Figure 8a).
The reaction kinetics and mechanism of the as-prepared catalysts can be evaluated based on Tafel slopes determined from the following equation (Equation (5)) [76]:
η is the overpotential, b is the Tafel slope, j is the experimental current density, and j0 is the exchange current density. The plot of η versus log j represents the Tafel slope. It is widely accepted that HER proceeds by either the Volmer–Heyrovsky or Volmer–Tafel mechanisms, and in alkaline media, it involves three main steps, as shown in Equations (6) to (8) [63]:
Hads denotes the H2 adsorbed to the metal sites, where * represents the metal sites. The theoretical Tafel slopes in the aforementioned reaction steps are 120 mV dec−1, 40 mV dec−1, and 30 mV dec−1, respectively. Figure 8b shows the Tafel slopes of gCN, Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS catalysts, pointing to the rate-determining step and the likely mechanism associated with electrocatalytic hydrogen generation. The Co3O4/gCN catalyst was found to have the lowest Tafel slope of 29.6 mV dec−1 compared to Co/gCN-MWS (39.4 mV dec−1), Co/gCN-HTS (71.9 mV dec−1), and gCN (182.3 mV dec−1). This predicts the favorable HER kinetics following the Volmer–Tafel mechanism on the Co3O4/gCN catalyst, suggesting that the Tafel recombination step is the dominating process, indicating that the rapid charge transfer process is present. In the case of Co/gCN-MWS and Co/gCN-HTS materials, the Heyrovsky reaction is the rate-determining step, and the Volmer–Heyrovsky reaction is expected to take place. Likely, the lower performance of Co/gCN-MWS and Co/gCN-HTS towards HER, as compared to Co3O4/gCN, is related to the poor adsorption of H+ ions onto the catalyst surface.
η = b · log j/j0
* + H2O + e− ↔ *Hads + OH− (Volmer step)
*Hads + e− + H2O ↔ H2 + OH− + * (Heyrovsky step)
2*Hads ↔ H2 + * (Tafel step)
Among the investigated catalysts, the lower Eonset of −0.24 V, a small overpotential of −280.5 mV at 5 mA cm−2, and a low Tafel slope of 29.6 mV dec−1 of Co3O4/gCN indicate that this catalyst prepared by annealing Co(NO3)2 together with melamine affords the fabrication of efficient catalyst with the highest HER activity in an alkaline medium.
3.3. Investigation of Electrocatalyst Activity for OER
The activity of catalysts for OER was further evaluated. The obtained data are presented in Figure 9 and Table 3. Figure 9a,b illustrates the OER polarization curves and the corresponding Tafel slopes recorded on the gCN, Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS at a slow scan rate of 2 mV s−1 in N2-saturated 1 M KOH solution. The summarized data are also given in Table 3.
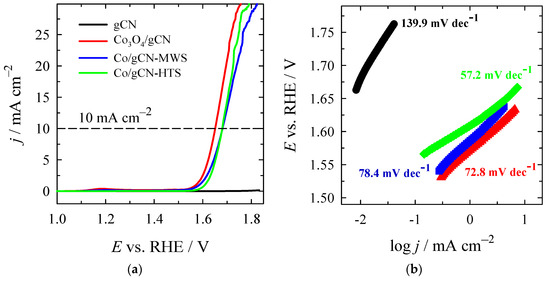
Figure 9.
(a) OER polarization curves of gCN, Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS catalysts in N2-saturated 1 M KOH solution at a potential scan rate of 2 mV s−1; (b) The corresponding Tafel slopes for each catalyst.

Table 3.
Electrochemical performance of the tested catalysts toward OER in alkaline media.
Bare gCN shows poor activity for OER with a low current density, even at high overpotential. On the contrary, all of the Co-modified gCNs gave much higher current densities and lower overpotentials than gCN, meaning a significant improvement in catalytic activity toward OER. Eonset values were found in a gradually increasing order, as follows: Co3O4/gCN (1.5162 V) < Co/gCN-MWS (1.5230 V) < Co/gCN-HTS (1.5394 V) < gCN (1.6404 V), with overpotential values of 286.2, 293.0, 309.4, and 410.4 mV, respectively (Table 3). Overpotentials to achieve the current density of 10 mA·cm−2 were found as 422.3, 451.3, and 450.9 mV for Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS, respectively (Table 3). The Tafel slope of Co/gCN-HTS (57.2 mV dec−1) is lower than those of Co3O4/gCN and Co/gCN-MWS (Figure 9b, Table 3), indicating better catalytic activity for the OER. A 4e− mechanism is widely accepted for the OER process. The steps of the reaction in alkaline media can be represented by Equations (9)–(12) [29,65,92]:
where * denotes the electrocatalyst’s adsorption site; similarly, during OER, the adsorbed intermediates are OH*, O*, and OOH*. The first step of the OER process, denoted by Equation (9), is the electrosorption of OH− onto the active sites of the catalyst’s surface. Higher oxidation state metal species are more susceptible to adsorb OH−, accelerate the multielectron transportation process, and, hence can therefore enhance the OER process [29,92]. According to the XPS analysis, the synthesized catalyst Co3O4/gCN has the highest valence state of the rest prepared and is considered to be the most favorable for the adsorption of OH−. In addition, it is generally accepted that during the OER process, the phase transformation of Co-based materials, such as oxides (Co3O4), to hydroxides or oxyhydroxides plays a rather significant role [92]. In this view, the Co3O4/gCN catalyst with the lowest onset overpotential of 286.2 mV and the lowest overpotential of 422.3 mV reaches 10 mA cm−2 with a Tafel slope of 72.8 mV dec−1. This is predicted to have the most perfect OER reaction kinetics compared to the other catalysts studied in the current work. The electrocatalytic behavior of the Co3O4/gCN catalyst and those obtained by microwave-assisted and hydrothermal syntheses are also compared with previously reported works and are presented in Table 4. These overpotentials are comparable to those previously reported for state-of-the-art non-precious metal catalysts for WS in alkaline media.
* + OH− ↔ e− + OH*
OH− + OH* ↔ e− + O* + H2O
OH− + O* ↔ e− + OOH*
OH− + OOH* ↔ H2O +*+ e− + O2

Table 4.
Electrochemical parameters of different Co-based gCN catalysts.
The above results demonstrate that Co3O4/gCN could act as a bifunctional catalyst due to the notable activity towards HER and OER, and for total WS in practical applications, it is a promising alternative to noble metal-based electrocatalysts. The potential to produce the Co3O4/gCN catalyst with a high crystallinity of high-purity Co3O4 using a simple annealing procedure contributes to significantly improved HER and OER performances. Appropriate incorporation of Co species into the tri-s-triazine moieties creates unique active redox sites of Co–(N)–C for pronounced HER and OER activities.
3.4. Determination of ECSAs of Electrocatalysts
For the determination of ECSA, the Cdl values were determined from CV curves recorded at various scan rates under the non-faradic region (Figure 10a–c), followed by the calculation of the slope of the curve obtained by plotting the difference in anodic and cathodic current against the scan rate (Figure 10d). ECSA values were calculated by normalizing the Cdl to the specific capacitance (40 μF cm−2). Cdl was found to be 66, 60, and 36 µF for Co3O4/gCN, Co/gCN-MWS, and Co/gCN-HTS (Figure 10d), whereas the calculated ECSA values were 1.65, 1.5, and 0.9 cm2 for Co3O4/gCN, Co/gCN-MWs, and Co/gCN-HTS, respectively. The higher ECSA of Co3O4/gCN than Co/gCN-MWS and Co/gCN-HTS represents the higher number of active sites and, consequently, larger electrocatalytic activity of this catalyst for HER and OER.
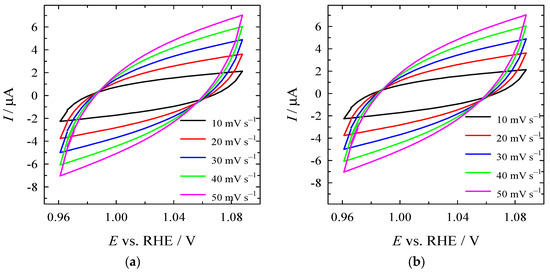
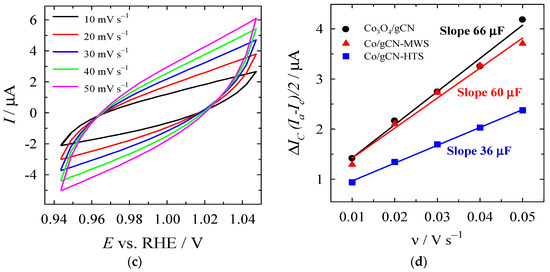
Figure 10.
CVs of (a) Co3O4/gCN, (b) Co/gCN-MWS, and (c) Co/gCN-HTS in N2-saturated 1 M KOH in the non-faradaic potential region at different scan rates. (d) Capacitive current as a function of scan rate.
Additionally, the stability of the Co3O4/gCN catalyst was tested for 1000 cycles at a constant scan rate of 100 mV s−1 at a rotation rate of 1600 rpm in N2-saturated 1 M KOH. Figure 11 presents the LSVs before and after 1000 cycles recorded at 2 mV s−1 at 1600 rpm. The LSV curve exhibited almost no differences from the initial test before the CV cycles (Figure 11) at a constant current density of 10 mA cm−2, indicating high stability for HER and maintaining the same morphology and structure after the HER test.
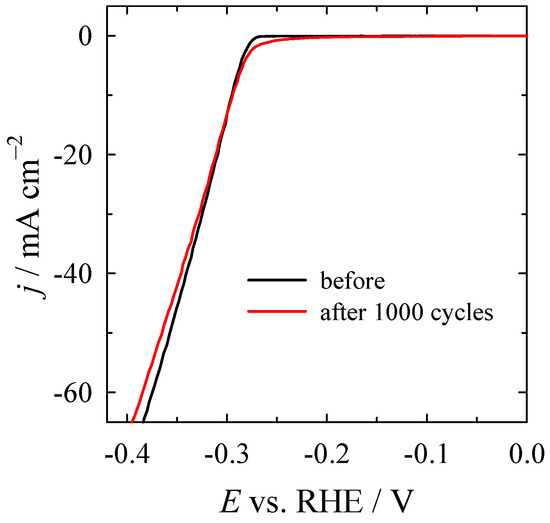
Figure 11.
LSV curves before and after 1000 cycles for HER for Co3O4/gCN recorded at 2 mV s−1 at 1600 rpm.
3.5. EIS Measurements
EIS was used to obtain more detailed information on the interfacial kinetics of these catalysts. A relatively large overpotential (400 mV) was chosen so that the spectral response would be indicative of the catalytic Faradaic reactions rather than any capacitance-dominated processes. From the profile of the Nyquist plots (Figure 12a), it is apparent that the impedance magnitude is smallest for the Co3O4/gCN film, corresponding well to LSV data (Figure 8a). The Co/gCN-MWS and HTS catalysts perform slightly worse, which is indicated by their larger impedances at the same overpotential. Bare gCN is included for comparison in the inset of Figure 12a and, at the same conditions, shows virtually no charge transfer activity.
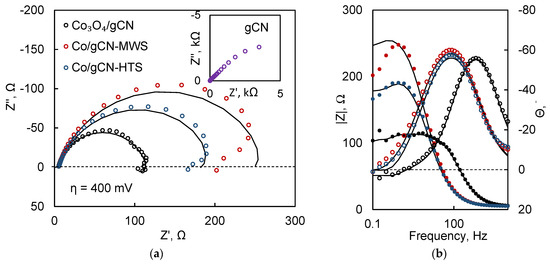
Figure 12.
EIS spectra of catalytic and gCN films, obtained at 400 mV overpotential in Nyquist (a) and Bode (b) coordinates. Solid lines show fits to the equivalent circuit in Figure 13.
Another feature of the spectra is the emerging low-frequency inductive loops, particularly apparent for the Co3O4/gCN sample. This phenomenon is said to occur due to the slow relaxation of adsorbed reaction intermediates and characterizes a mechanism with two electrochemical steps [97,98]. This process can be either HER or ORR, but EIS does not allow us to distinguish them without additional experimentation. The Bode plots (Figure 12b) also reveal an interesting feature: for the Co3O4/gCN catalyst, the phase maximum emerges at significantly higher frequencies (~1 kHz). This means that the current responds more swiftly to potential perturbation, and the catalytic reaction occurs at a faster rate.
Equivalent circuit fitting was carried out to gain quantitative data on the performance of these catalysts. To fit a spectrum with an inductive loop with no mass transport limitations, typically, an equivalent circuit, as shown in Figure 13, is used [99].
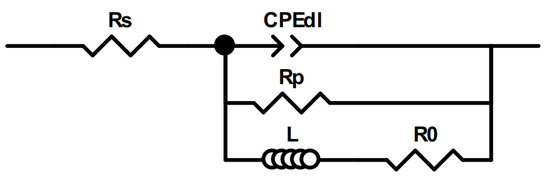
Figure 13.
The equivalent electric circuit used to fit EIS data.
In this circuit, Rs is the solution resistance, CPEdl is a constant phase element that accounts for the double layer capacitance of an inhomogeneous surface; Rp is the resistance of the Faradaic process, here approximated as the charge transfer resistance; and L and R0 account for the low-frequency inductance.
CPE values were recalculated into capacitance by Equation (13) [99], and the results are shown in Table 5.

Table 5.
Equivalent circuit fitting data of catalytic films.
The values of the low-frequency resistance Rp correspond most directly to the rate-determining step of the catalytic reaction, and the lowest value (109.5 Ω) is calculated for Co3O4/gCN. The inductive elements (L, R0) occur due to the slow adsorption of reaction intermediates on the surface of the catalyst and the delay between coverage and removal during potential modulation [99]. Therefore, lower equivalent circuit element values mean faster surface relaxation [100], and the Co3O4/gCN film potentially has the fastest adsorbate relaxation kinetics.
4. Conclusions
Cobalt-particle-supported graphitic carbon nitride catalysts were obtained using three different methods: materials annealing, microwave-assisted, and hydrothermal syntheses. It was found that the catalyst prepared via the annealing of Co(NO3)2 with melamine at 520 °C exhibited the highest activity for hydrogen evolution compared to the other Co-supported gCN catalysts prepared via microwave-assisted and hydrothermal syntheses. The Co3O4/gCN catalyst shows the highest current density and gives the lowest overpotential of −280.5 mV for HER to reach a current density of 5 mA cm−2 in 1 M KOH. Overpotentials to reach the current density of 5 mA·cm−2 for HER were found in a gradually increasing order, as follows: Co3O4/gCN (−280.5 mV) < Co/gCN-MWS (−389.4 mV) < Co/gCN-HTS (−444.1 mV). Overpotentials to reach the current density of 10 mA·cm−2 for OER are in a gradually increasing order, as follows: Co3O4/gCN (422.3 mV) < Co/gCN-HTS (450.9 mV) < Co/gCN-MWS (451.3 mV).
Thermal annealing of both Co(NO3)2 and melamine at 520 °C for 4 h results in the preparation of an efficient Co3O4/gCN catalyst for the HER with the lower Eonset of −0.24 V, a small overpotential of −294.1 mV at −10 mA cm−2, and a low Tafel slope of −29.6 mV dec−1. Additionally, it has the lowest onset overpotential of 286.2 mV and the lowest overpotential of 422.3 mV to reach a current density of 10 mA cm−2 with the Tafel slope of 72.8 mV dec−1 for OER compared to Co/gCN-MWS and Co/gCN-HTS. Importantly, the annealing treatment of Co(NO3)2 with melamine to obtain the Co3O4/gCN catalyst is a potential method for its practical applications in electrochemical water splitting.
EIS analysis of the films revealed that the Co3O4/gCN film has the fastest adsorbate relaxation kinetics, which is likely one reason for its improved electrocatalytic activity.
Author Contributions
Conceptualization, A.Z., E.N. and L.T.-T.; methodology, V.J., J.V., R.L., G.N. and D.U.; validation, A.B., data curation, V.J., J.V. and D.U.; writing—original draft preparation, A.Z., D.S., R.L., G.N. and L.T.-T.; writing—review and editing, E.N., D.S. and A.B.; supervision, A.Z. and E.N.; project administration, E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Social Fund (project No. 09.3.3-LMT-K-712-23-0188) under a grant agreement with the Research Council of Lithuania (LMTLT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IEA. Global Hydrogen Review 2022, IEA, Paris. License: CC BY 4.0. 2022. Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 1 July 2023).
- Hydrogen to the rescue. Nat. Mater. 2018, 17, 565. [CrossRef] [PubMed]
- Hydrogen Council. Hydrogen Insights: A Perspective on Hydrogen Investment, Market Development and Cost Competitiveness; Hydrogen Council; McKinsey & Company: Hong Kong, China, 2021. [Google Scholar]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, Y.; Feng, X.; Yu, X.; Gao, M.; Yu, S. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, e2007100. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, L.; Shi, J. Anion-containing noble-metal-free bifunctional electrocatalysts for overall water splitting. ACS Catal. 2018, 8, 3688–3707. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Kaneva, M.V.; Gulina, L.B.; Tolstoy, V.P. Pt nanoparticles synthesized by successive ionic layers deposition method and their electrocatalytic properties in hydrogen evolution reaction during water splitting in the acidic medium. J. Alloy. Compn. 2022, 901, 163640. [Google Scholar] [CrossRef]
- Nichols, F.; Lu, J.E.; Mercado, R.; Dudschus, R.; Bridges, F.; Chen, S.W. Platinum oxide nanoparticles for electrochemical hydrogen evolution: Influence of platinum valence state. Chem.-Eur. J. 2020, 26, 4136–4142. [Google Scholar] [CrossRef]
- Pan, D.; Liu, Q.; Nichols, F.; Mercado, R.; Kuo, H.-L.; Lu, J.Q.; Bridges, F.; Chen, S. Impacts of ruthenium valence state on the electrocatalytic activity of ruthenium ion-complexed graphitic carbon nitride/reduced graphene oxide nanosheets towards hydrogen evolution reaction. J. Colloid Interface Sci. 2023, 629, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Y.; Torres-Pinto, A.; LaGrow, A.P.; Diaconescu, V.M.; Simonelli, L.; Sampaio, M.J.; Bondarchuk, O.; Amorim, I.; Araujo, A.; et al. Single-atom Ir and Ru anchored on graphitic carbon nitride for efficient and stable electrocatalytic/photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2022, 310, 121318. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Guo, S. Noble metal-free electrocatalytic materials for water splitting in alkaline electrolyte. EnergyChem 2021, 3, 100053. [Google Scholar] [CrossRef]
- Li, S.; Li, E.; An, X.; Hao, X.; Guan, G. Transition metal-based catalysts for electrochemical water splitting at high current density: Current status and perspectives. Nanoscale 2021, 13, 12788–127817. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Feng, C.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Non-noble metal electrocatalysts for the hydrogen evolution reaction in water electrolysis. Electrochem. Energy Rev. 2021, 4, 473–507. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.-S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Ni, B.-J. Cost-effective catalysts for renewable hydrogel production via electrochemical water splitting: Recent advances. Curr. Opin. Green Sustain. Chem. 2021, 27, 100398. [Google Scholar] [CrossRef]
- Du, P.; Eisenberg, R. Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges. Energy Environ. Sci. 2012, 5, 6012–6021. [Google Scholar] [CrossRef]
- Cao, X.; Wang, T.; Jiao, L. Transition-metal (Fe, Co, and Ni)-based nanofiber electrocatalysts for water splitting. Adv. Fiber Mater. 2021, 3, 210–228. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.; Wang, E. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ai, H.; Chen, M.; Zhou, P.; Li, B.; Liu, D.; Du, X.; Lo, K.H.; Ng, K.; Wang, S.; et al. Multi-phase heterostructure of CoNiP/CoxP for enhanced hydrogel evolution under alkaline and seawater conditions by promoting H2O dissociation. Small 2021, 17, 2007557. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Ma, A.; Abbas, S.A.; Kim, H.Y.; Choe, H.R.; Jo, S.Y.; Nam, K.M. A new synthetic approach to cobalt oxides: Designed phase transformation for electrochemical water splitting. Chem. Eng. J. 2021, 415, 127958. [Google Scholar] [CrossRef]
- Duraivel, M.; Nagappan, S.; Park, K.H.; Prabakar, K. Hierarchical 3D flower like cobalt hydroxide as an efficient bifunctional electrocatalyst for water splitting. Electrochim. Acta 2022, 411, 140071. [Google Scholar] [CrossRef]
- Wang, Q.; He, R.; Yang, F.; Tian, X.; Sui, H.; Feng, L. An overview of heteroatom doped cobalt phosphide for efficient electrochemical water splitting. Chem. Eng. J. 2023, 456, 141056. [Google Scholar] [CrossRef]
- Bian, H.; Chen, T.; Chen, Z.; Liu, J.; Li, Z.; Du, P.; Zhou, B.; Zeng, X.; Tang, J.; Liu, C. One-step synthesis of mesoporous cobalt sulfides (CoSx) on the metal substrate as an efficient bifunctional electrode for overall water splitting. Electrochim. Acta 2021, 389, 138786. [Google Scholar] [CrossRef]
- Xue, Z.; Kang, J.; Guo, D.; Zhu, C.; Li, C.; Zhang, X.; Chen, Y. Self-supported cobalt nitride porous nanowire arrays as bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2018, 273, 229–238. [Google Scholar] [CrossRef]
- Zou, H.; Li, G.; Duan, L.; Kou, Z.; Wang, J. In situ coupled amorphous cobalt nitride with nitrogen-doped graphene aerogel as a trifunctional electrocatalyst towards Zn-air battery deriven full water splitting. Appl. Catal. B Environ. 2019, 259, 118100. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Ke, N.; Dong, B.; Huang, A.; Tan, C.; Yin, L.; Xu, X.; Hao, L.; Xian, Y.; et al. Self-supported cobalt/cobalt selenide heterojunction for highly efficient overall water splitting. J. Alloys Compd. 2022, 925, 166683. [Google Scholar] [CrossRef]
- Huang, C.; Qin, P.; Luo, Y.; Ruan, Q.; Liu, L.; Wu, Y.; Li, Q.; Xu, Y.; Liu, R.; Chu, P.K. Recent progress and perspective of cobalt-based catalysts for water splitting: Design and nanoarchitectonics. Mater. Today Energy 2022, 23, 10091. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Peng, X.; Jin, X.; Gao, B.; Liu, Z.; Chu, P.K. Strategies to improve cobalt-based electrocatalysts for electrochemical water splitting. J. Catal. 2021, 398, 54–66. [Google Scholar] [CrossRef]
- Deng, R.; Guo, M.; Wang, C.; Zhang, Q. Recent advances in cobalt phosphide-based materials for electrocatalytic water splitting: From catalytic mechanism and synthesis method to optimization design. Nano Mater. Sci. 2022. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Feng, X. Support and interface effects in water-splitting electrocatalysts. Adv. Mater. 2019, 31, 1808167. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nųrskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Xu, G.; Wang, L. Recent advances in interface engineering strategy for highly-efficient electrocatalytic water splitting. InfoMat 2023, 5, 12377. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Dinh, C.-T.; Jain, A.; de Arquer, F.P.G.; De Luna, P.; Li, J.; Wang, N.; Zheng, X.; Cai, J.; Gregory, B.Z.; Voznyy, O.; et al. Multi-site electrocatalysts for hydrogen evolution in neutral media by destabilization of water molecules. Nat. Energy 2018, 4, 107. [Google Scholar] [CrossRef]
- Lee, M.G.; Yang, J.W.; Kwon, H.R.; Jang, H.W. Crystal facet and phase engineering for advanced water splitting. CrystEngComm 2022, 24, 5838–5864. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Talukdar, M.; Deb, P. Recent progress in research on multifunctional graphitic carbon nitride: An emerging wonder material beyond catalyst. Carbon 2022, 192, 308–331. [Google Scholar] [CrossRef]
- Govindaraju, V.R.; Sureshkumar, K.; Ramakrishnappa, T.; Muralikrishna, S.; Samrat, D.; Pai, R.K.; Kumar, V.; Vikrant, K.; Kim, K.-H. Graphitic carbon nitride composites as electro catalysts: Applications in energy conversion/storage and sensing system. J. Clean. Prod. 2021, 320, 128693. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Graphitic carbon nitride-based catalysts and their applications: A Review. Nano-Struct. Nano-Objects 2020, 24, 100577. [Google Scholar] [CrossRef]
- Konga, L.; Wangb, J.; Ma, F.; Sunb, M.; Quana, J. Graphitic carbon nitride nanostructures: Catalysis. Appl. Mater. Today 2019, 16, 388–424. [Google Scholar] [CrossRef]
- Besharat, F.; Ahmadpoor, F.; Nezafat, Z.; Nasrollahzadeh, M.; Manwar, N.R.; Fornasiero, P.; Gawande, M.B. Advances in carbon nitride-based materials and their electrocatalytic applications. ACS Catal. 2022, 12, 5605–5660. [Google Scholar] [CrossRef]
- Baudys, M.; Paušová, Š.; Praus, P.; Brezová, V.; Dvoranová, D.; Barbieriková, Z.; Krýsa, J. Graphitic carbon nitride for photocatalytic air treatment. Materials 2020, 13, 3038. [Google Scholar] [CrossRef] [PubMed]
- Sudhaik, A.; Raizada, P.; Khan, A.A.P.; Singh, A.; Singh, P. Graphitic carbon nitride-based upconversion photocatalyst for hydrogen production and water purification. Nanofabrication 2022, 7, 280–313. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini review on the structure and properties (photocatalysis), and preparation techniques of graphitic carbon nitride nano-based particle, and its applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Pourmadadi, M.; Rahmani, E.; Eshaghi, M.M.; Shamsabadipour, A.; Ghotekar, S.; Rahdar, A.; Ferreira, L.F.R. Graphitic carbon nitride (g-C3N4) synthesis methods, surface functionalization, and drug delivery applications: A review. J. Drug Deliv. Sci. Technol. 2023, 79, 104001. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g–C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, Z.; Zhao, H.; Lou, Z.; Liao, Y.; Xue, C. Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light. RSC Adv. 2015, 5, 49317–49325. [Google Scholar] [CrossRef]
- Hu, C.; Chu, Y.-C.; Wang, M.-S.; Xiao-Han, W. Rapid synthesis of g-C3N4 spheres using microwave-assisted solvothermal method for enhanced photocatalytic activity. J. Photochem. Photobiol. A Chem. 2017, 348, 8–17. [Google Scholar] [CrossRef]
- Chen, H.; Fan, Y.; Xu, H.; Cui, D.; Xuea, C.; Zhanga, W. A facile and green microwave hydrothermal method for fabricating g-C3N4 nanosheets with improved hydrogen evolution performance. J. Alloys Compd. 2021, 863, 158448. [Google Scholar] [CrossRef]
- Tahir, M.; Mahmood, N.; Zhang, X.; Mahmood, T.; Butt, F.K.; Aslam, I.; Tanveer, M.; Idrees, F.; Khalid, S.; Shakir, I.; et al. Bifunctional catalysts of Co3O4@GCN tubular nanostructured (TNS) hybrids for oxygen and hydrogen evolution reactions. Nano Res. 2015, 8, 3725–3736. [Google Scholar] [CrossRef]
- Vignesh, S.; Kim, H. Rational construction of efficient ZnS quantum dots-supported g-C3N4 with Co3O4 heterostructure composite for bifunctional electrocatalytic hydrogen evolution reaction and environmental pollutant degradation. J. Alloys Compd. 2023, 942, 169077. [Google Scholar] [CrossRef]
- Zahra, R.; Pervaiz, E.; Baig, M.M.; Rabi, O. Three-dimensional hierarchical flowers-like cobalt-nickel sulfide constructed on graphitic carbon nitride: Bifunctional non-noble electrocatalyst for overall water splitting. Electrochim. Acta 2022, 418, 140346. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Y.; Fang, S.; Park, J.K.; Xu, H. Plasma-modified graphitic C3N4@Cobalt hydroxide nanowires as a highly efficient electrocatalyst for oxygen evolution reaction. Heliyon 2022, 8, e11573. [Google Scholar] [CrossRef]
- Reghunath, B.S.; Rajasekaran, S.; KR, S.D.; Pinheiro, D.; UC, J.R.J. N-doped graphene quantum dots incorporated cobalt ferrite/graphitic carbon nitride ternary composite for electrochemical overall water splitting. Int. J. Hydrogen Energy 2023, 48, 2906–2919. [Google Scholar] [CrossRef]
- Ejeta, S.Y.; Imae, T. Cobalt incorporated graphitic carbon nitride as a bifunctional catalyst for electrochemical water-splitting reactions in acidic media. Molecules 2022, 27, 6445. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; An, P.; Chen, L.; Sun, S.; Xie, Z.; Chen, M.; Jiang, D. Integrating Ru-modulated CoP nanosheets binary co-catalyst with 2D g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution activity. J. Colloid Interface Sci. 2021, 585, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yang, X.; Chen, Y.; Huang, H.; Hu, J.; Wen, B. Fabrication of alveolate g-C3N4 with nitrogen vacancies via cobalt introduction for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 24792–24806. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Liao, J.; Wei, X. Microwave-assisted synthesis of graphitic carbon nitride/CuO nanocomposites and the enhancement of catalytic activities in the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2019, 499, 143875. [Google Scholar] [CrossRef]
- Daş, E.; Gürsel, S.A.; Şanli, L.I.; Yurtcan, A.B. Comparison of two different catalyst preparation methods for graphene nanoplatelets supported platinum catalysts. Int. J. Hydrogen Energy 2016, 41, 9755–9761. [Google Scholar] [CrossRef]
- Rana, A.G.; Schwarze, M.; Tasbihi, M.; Sala, X.; García-Antón, J.; Minceva, M. Influence of cocatalysts (Ni, Co, and Cu) and synthesis method on the photocatalytic activity of exfoliated graphitic carbon nitride for hydrogen production. Nanomaterials 2022, 12, 4006. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Subrata, K. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Cossar, E.; Houache, M.S.E.; Zhang, Z.; Baranova, E.A. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Niyitanga, T.; Kim, H. Time-dependent oxidation of graphite and cobalt oxide nanoparticles as electrocatalysts for the oxygen evolution reaction. J. Electroanal. Chem. 2022, 914, 116297. [Google Scholar] [CrossRef]
- Negro, P.; Cesano, F.; Casassa, S.; Scarano, D. Combined DFT-D3 computational and experimental studies on g-C3N4: New insight into structure, optical, and vibrational properties. Materials 2023, 16, 3644. [Google Scholar] [CrossRef]
- Zhu, G.X.; Lu, T.L.; Han, L.; Zhan, Y.Z. Graphitic carbon nitride (g–C3N4) as an efficient metal-free Fenton-like catalyst for degrading organic pollutants: The overlooked non-photocatalytic activity. Water Sci. Technol. 2020, 81, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-W.; Li, K.; Yu, Y.-X.; Zhang, W.-D. Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution. Appl. Surf. Sci. 2017, 392, 608–615. [Google Scholar] [CrossRef]
- Yang, Q.; An, J.; Xu, Z.; Liang, S.; Wang, H. Performance and mechanism of atrazine degradation using Co3O4/g−C3N4 hybrid photocatalyst with peroxymonosulfate under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126161. [Google Scholar] [CrossRef]
- Morozan, A.; Jégou, P.; Jousselme, B.; Palacin, S. Electrochemical performance of annealed cobalt–benzotriazole/CNTs catalysts towards the oxygen reduction reactionw. Phys. Chem. Chem. Phys. 2011, 13, 21600–21607. [Google Scholar] [CrossRef]
- Yusuf, B.A.; Xie, M.; Yaseen, W.; Xie, J.; Xu, Y. Hierarchical ultrathin defect-rich CoFe2O4@BC nanoflowers synthesized via a temperature regulated strategy with outstanding hydrogel evolution reaction activity. Inorg. Chem. Front. 2021, 8, 1455–14467. [Google Scholar] [CrossRef]
- Rabani, I.; Zafar, R.; Subalakshmi, K.; Kim, H.-S.; Bathula, C.; Seo, Y.-S. A facile mechanochemical preparation of Co3O4@g−C3N4 for application in supercapacitors and degradation of pollutants in water. J. Hazard. Mater. 2021, 407, 124360. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Qiao, W.; Shao, B.; Zhao, Q.; Zhang, L.; Fan, Z. Rapid microwave-assisted synthesis of graphene nanosheet/Co3O4 composite for supercapacitors. Electrochim. Acta 2010, 55, 6973–6978. [Google Scholar] [CrossRef]
- Kumar, R.; Soamb, A.; Sahajwalla, V. Carbon coated cobalt oxide (CC−Co3O4) as electrode material for supercapacitor applications. Mater. Adv. 2021, 2, 2918–2923. [Google Scholar] [CrossRef]
- Liu, L.; Fang, Z.J.L.; Xu, H.; Zhang, H.; Gu, X.; Wang, Y. Probing the crystal plane effect of Co3O4 for enhanced electrocatalytic performance toward efficient overall water splitting. ACS Appl. Mater. Interfaces 2017, 9, 27736–27744. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, L. Synthesis of sphere-like Co3O4 nanocrystals via a simple polyol route. Mater. Lett. 2007, 61, 4894–4896. [Google Scholar] [CrossRef]
- Heydariyan, Z.; Monsef, R.; Salavati-Niasari, M. Insights into impact of Co3O4-CeO2 nanocomposites on the electrochemical hydrogen storage performance of g-C3N4: Pechini preparation, structural design and comparative study. J. Alloys Compd. 2022, 924, 166564. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, S.; Zhu, C.; Shi, J.; Su, C.; Yang, J.; Wang, M.; Li, J.; Li, J.; Liu, P.; et al. Rational construction of high-active Co3O4 electrocatalysts for oxygen evolution reaction. Nano Res. 2023, 16, 624–633. [Google Scholar] [CrossRef]
- Ma, X.; Yu, X.; Yang, X.; Lin, M.; Ge, M. Hydrothermal synthesis of a novel double-sided nanobrush Co2O4 catalyst and its catalytic performance for benzene oxidation. ChemCatChem 2019, 11, 1214–1221. [Google Scholar]
- Zinin, P.V.; Ming, L.-C.; Sharma, S.K.; Khabashesku, V.N.; Liu, X.; Hong, S.; Endo, S.; Acosta, T. Ultraviolet and near-infrared Raman spectroscopy of graphitic C3N4 phase. Chem. Phys. Lett. 2009, 472, 69–73. [Google Scholar] [CrossRef]
- Liu, S.; Ke, J.; Sun, H.; Liu, J.; Tade, M.O.; Wang, S. Size dependence of uniformed carbonspheres in promoting graphitic nitride toward enhanced photocatalysis. Appl. Catal. B Environ. 2017, 204, 358–364. [Google Scholar] [CrossRef]
- Maslana, K.; Kalenczuk, R.J.; Zielinska, B.; Mijowska, E. Synthesis and characterization of nitrogen-doped carbon naotubes derived from g-C3N4. Materials 2020, 13, 1349. [Google Scholar] [CrossRef]
- Plevová, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the oxygen evolution reaction in alkaline and neutral media. A comparative review. J. Power Sources 2021, 507, 230072. [Google Scholar] [CrossRef]
- Trotochaud, L.; Ranney, J.K.; Williams, K.N.; Boettcher, S.W. Solution-cast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 2012, 134, 17253–17261. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.B.; Tamilarasan, S.; Thangavelu, S. Layered porous graphitic carbon nitride stabilized effective Co2SnO4 inverse spinel as a bifunctional electrocatalyst for overall water splitting. Langmuir 2022, 38, 7833–7845. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-K.; Moru, S.; Tonda, S. Cobalt-coordinated sulfur-doped graphitic carbon nitride on reduced graphene oxide: An efficient metal−(N,S)−C-class bifunctional electrocatalyst for overall water splitting in alkaline media. ACS Sustain. Chem. Eng. 2019, 7, 15373–15384. [Google Scholar] [CrossRef]
- Ahmed, I.; Biswas, R.; Patil, R.A.; Halder, K.K.; Singh, H.; Banerjee, B.; Kumar, B.; Ma, Y.-R.; Haldar, K.K. Graphitic Carbon Nitride Composites with MoO3-Decorated Co3O4 Nanorods as Catalysts for Oxygen and Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 12672–12681. [Google Scholar] [CrossRef]
- Singh, R.K.; Devivaraprasad, R.; Kar, T.; Chakraborty, A.; Neergat, M. Electrochemical impedance spectroscopy of oxygen reduction reaction (ORR) in a rotating disk electrode configuration: Effect of ionomer content and carbon-support. J. Electrochem. Soc. 2015, 162, F489–F498. [Google Scholar] [CrossRef]
- Chatenet, M.; Genies-Bultel, L.; Aurousseau, M.; Durand, R.; Andolfatto, F. Oxygen reduction on silver catalysts in solutions containing various concentrations of sodium hydroxide—Comparison with platinum. J. Appl. Electrochem. 2002, 32, 1131–1140. [Google Scholar] [CrossRef]
- Choudhury, D.; Das, R.; Maurya, R.; Gupta, G.; Neergat, M. Low-frequency inductive features in the electrochemical impedance spectra of mass-transport limited redox reactions. Phys. Chem. Chem. Phys. 2023, 25, 10966–10976. [Google Scholar] [CrossRef]
- Priyadarshani, D.; Choudhury, D.; Joy, M.E.; Kottantharayil, A.; Neergat, M. Electrochemical investigation of Si of various dopant concentrations at negative overpotentials in aqueous electrolyte. J. Phys. Chem. C 2021, 125, 27736–27746. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).