The Influence of Alumina Bubbles on the Properties of Lightweight Corundum–Spinel Refractory
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
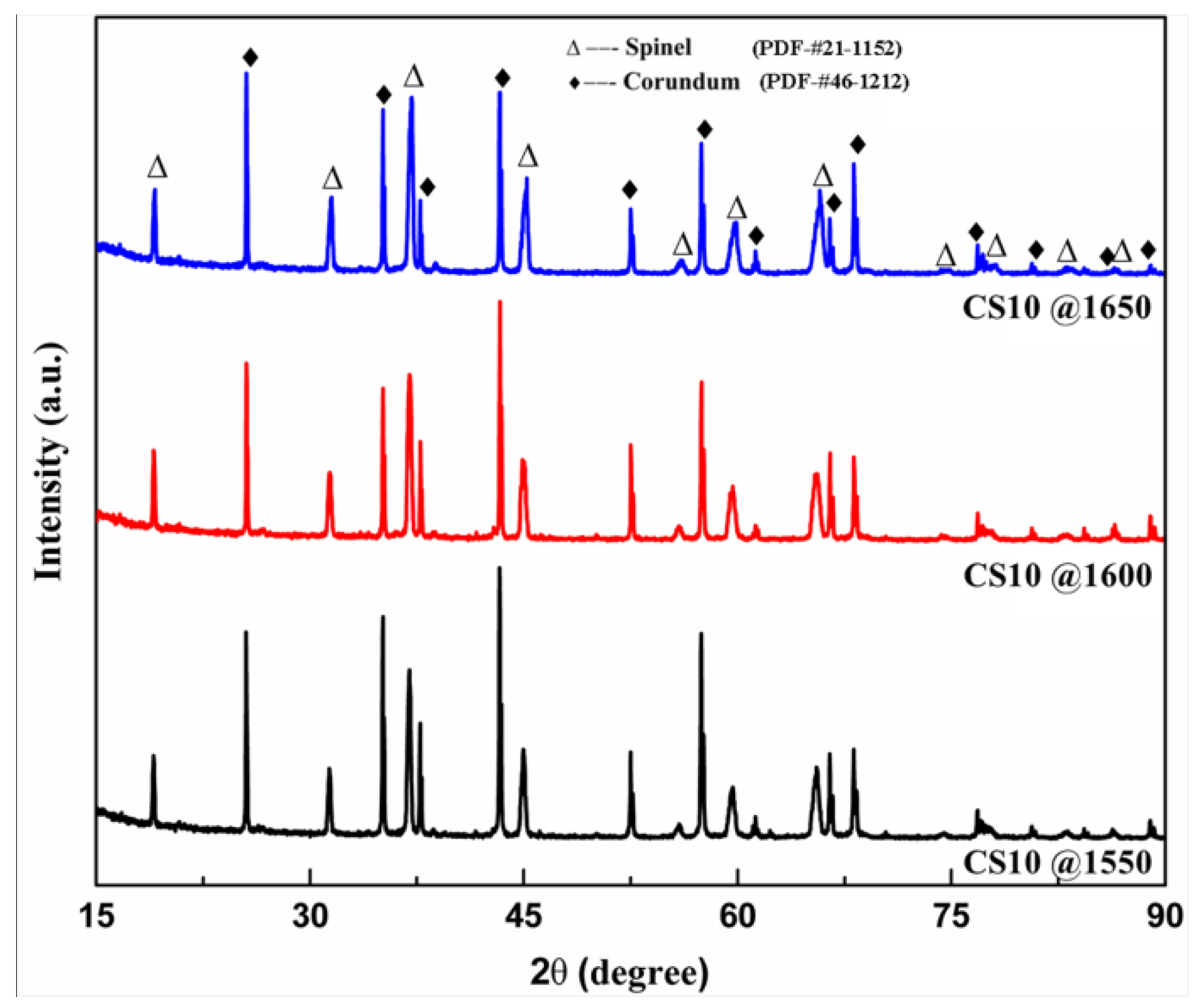
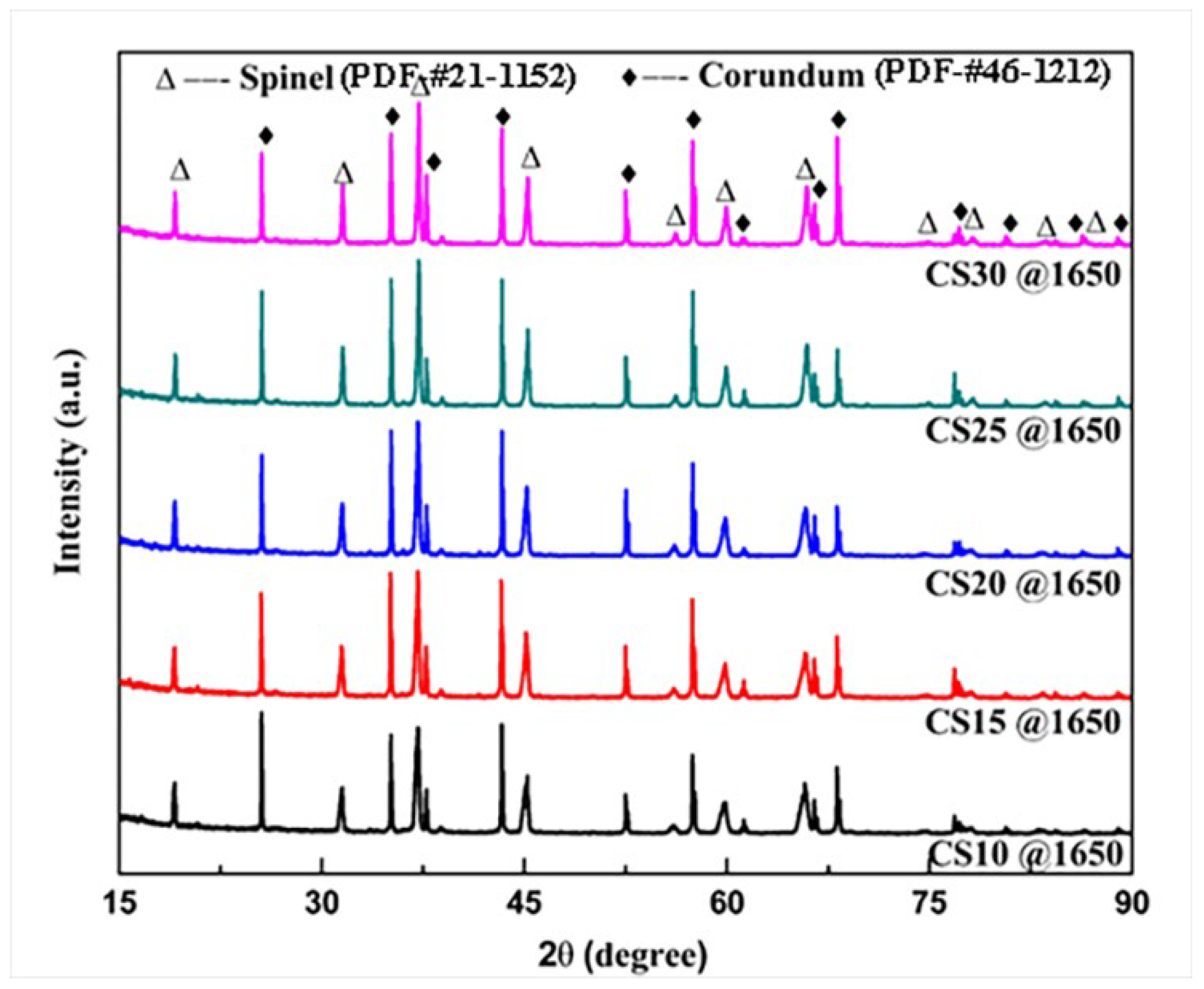
3.1. Phase Compositions
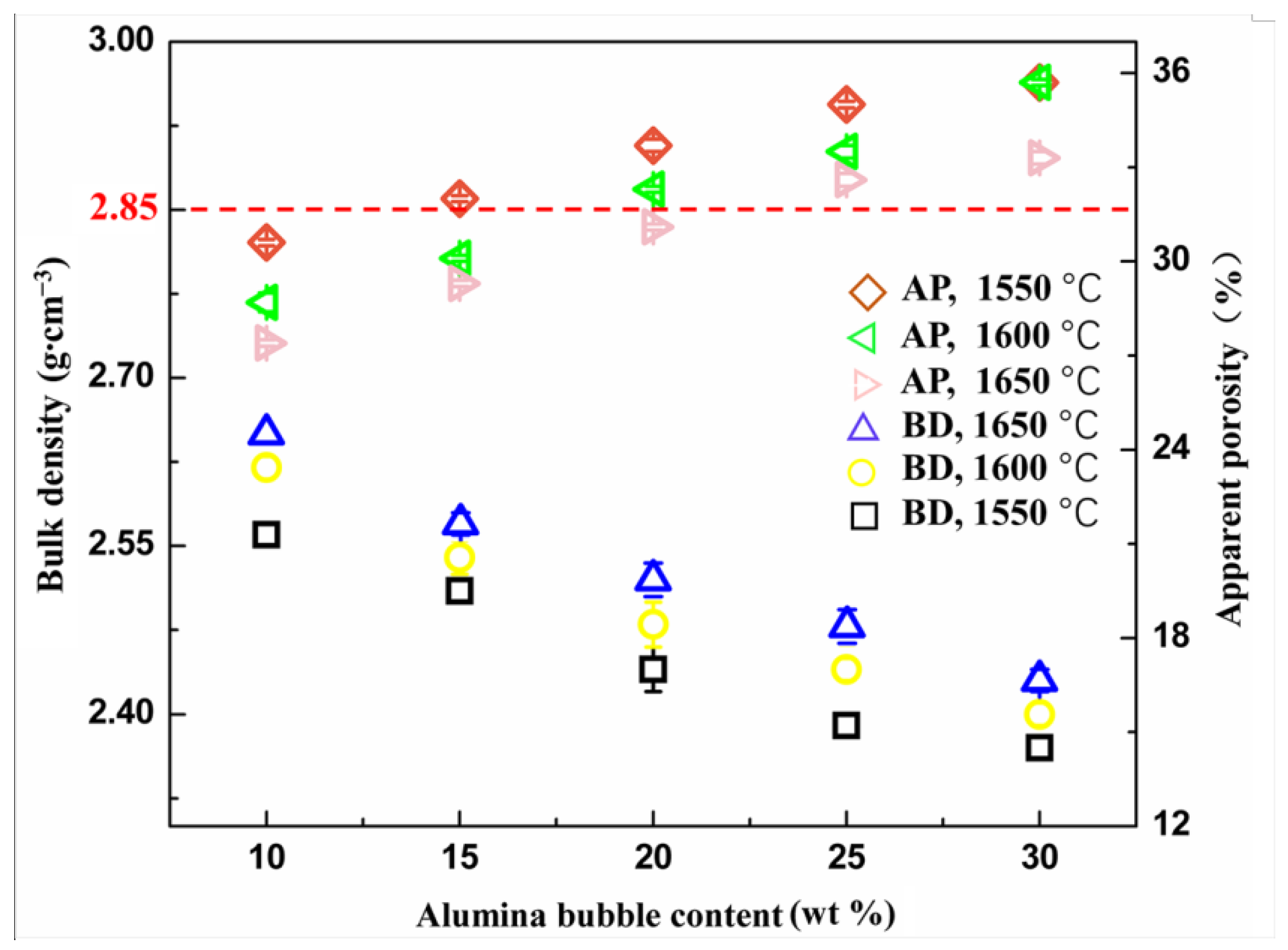
3.2. Physical Properties
3.3. Compressive Strength
3.4. Performance in Use
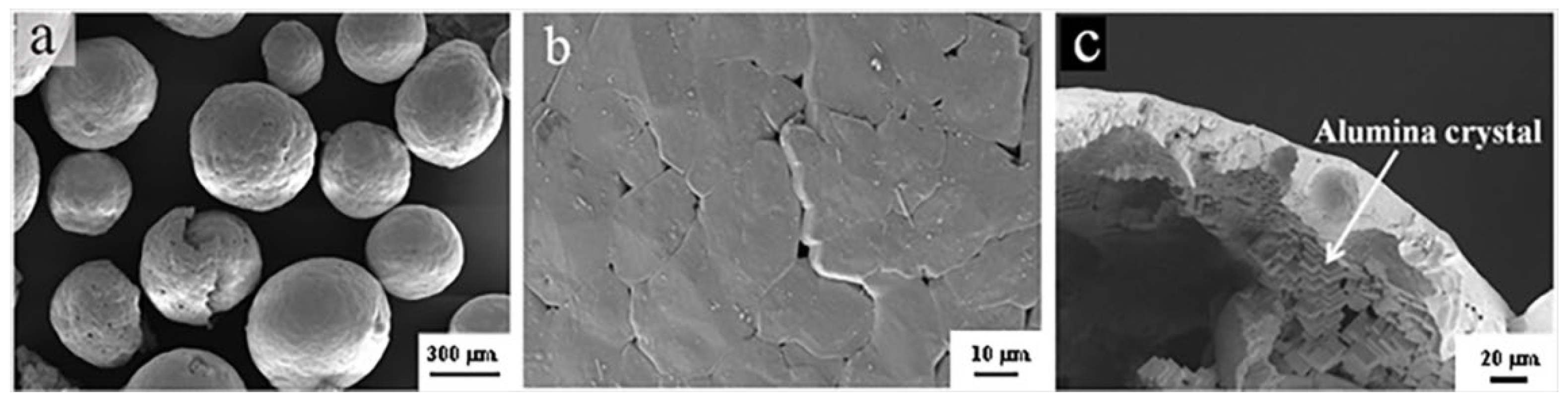
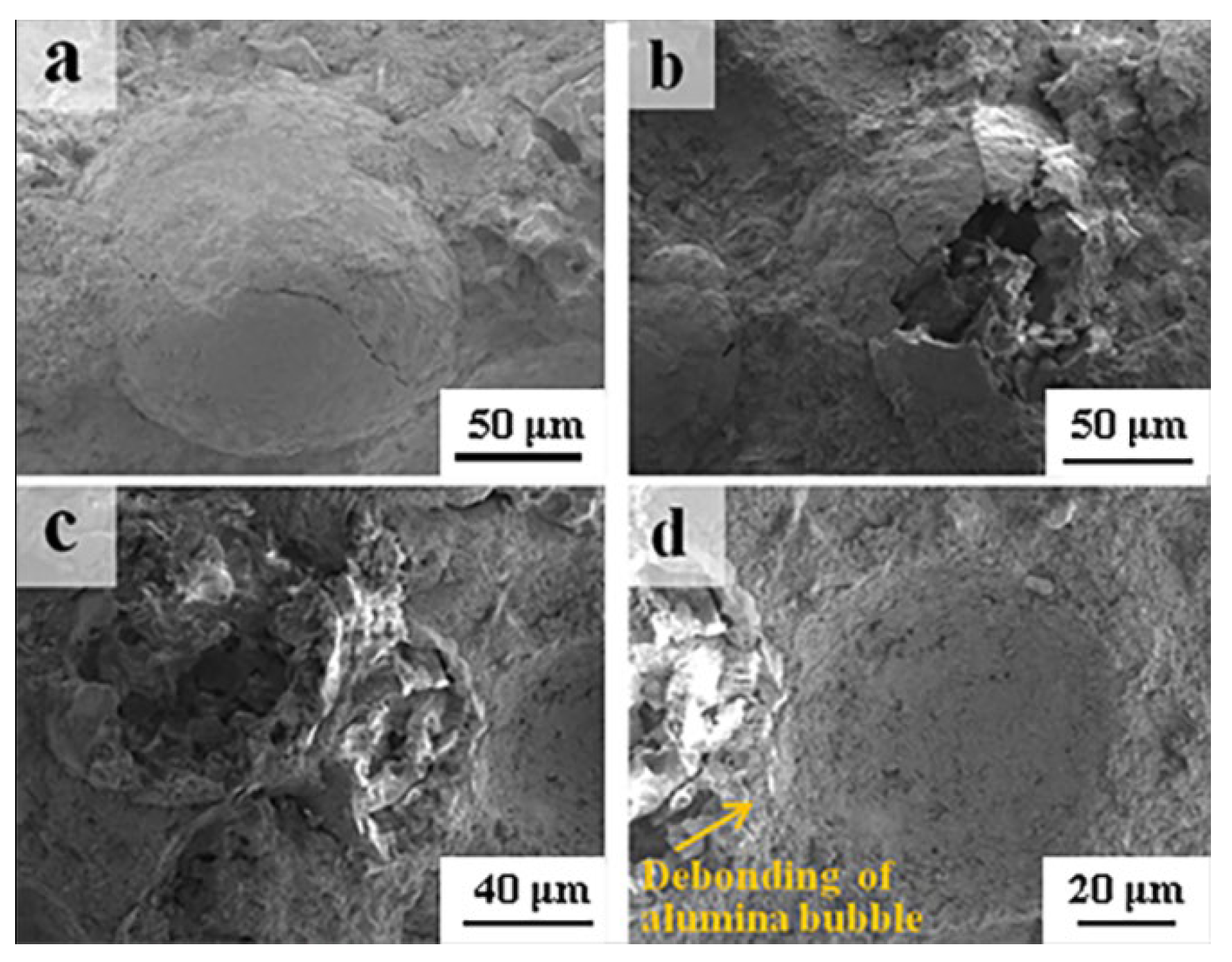
3.5. Fracture Morphologies
4. Conclusions
- (1)
- After the introduction of alumina bubble particles as a substitute of medium-sized tabular corundum aggregate, the bulk density of the refractory decreased dramatically and realized the light weight of the corundum–spinel refractory. The obtained refractory also possessed high compress strength and refractoriness under load.
- (2)
- The overall performance of the lightweight refractory was mainly dominated by the content of alumina bubbles. The sintering temperature also had a significant influence on the properties of the refractory; increasing the temperature was beneficial for improving the mechanical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, W.; Chen, Z.; Yan, W.; Schafföner, S.; Li, G.; Li, Y.; Jia, C. Advanced lightweight periclase-magnesium aluminate spinel refractories with high mechanical properties and high corrosion resistance. Constr. Build. Mater. 2021, 291, 123388. [Google Scholar] [CrossRef]
- Bartha, P. The cement rotary kiln and its refractory lining. Interceram 2004, 53, 14–17. [Google Scholar]
- Engin, T.; Ari, V. Energy auditing and recovery for dry type cement rotary kiln systems-A case study. Energy Convers. Manag. 2005, 46, 551–562. [Google Scholar] [CrossRef]
- Kantorek, M.; Jesionek, K.; Polesek, S.; Paweł, Z.; Michał, S.; Janusz, B. Thermal utilization of meat-and-bone meal using the rotary kiln pyrolyzer and the fluidized bed boiler-The performance of pilot-scale installation. Renew. Energy 2021, 164, 1447–1456. [Google Scholar] [CrossRef]
- Paramonov, A. Heating furnaces efficiency improvement. Procedia Eng. 2015, 113, 181–185. [Google Scholar] [CrossRef][Green Version]
- Hou, Q.; Luo, X.; Xie, Z.; Li, Y.; An, D.; Li, J. Preparation and characterization of microporous magnesia-based refractory. Int. J. Appl. Ceram. Technol. 2020, 17, 1830. [Google Scholar] [CrossRef]
- Dong, T.; Wang, X.; Peng, C.; Wen, Z.; Zhou, X. Preparation of lightweight mullite refractory by foaming method. J. Wuhan Univ. Technol. 2009, 32, 184–187. [Google Scholar]
- Chen, R.; He, P.; Wang, N.; Mou, J.; Gan, F. Development of a low density castable for steel ladle. In Proceedings of the Unified International Technical Conference on Refractories, Osaka, Japan, 19–22 October 2003. [Google Scholar]
- Yan, W.; Li, N.; Han, B.Q. Influence of microsilica content on the slag resistance of castables containing porous corundum-spinel aggregates. Int. J. Appl. Ceram. Technol. 2008, 5, 633–640. [Google Scholar] [CrossRef]
- Fu, L.; Gu, H.; Huang, A.; Or, S.; Zou, Y.; Zhang, M. Design, fabrication and properties of lightweight wear lining refractories: A review. J. Eur. Ceram. Soc. 2022, 3, 42. [Google Scholar] [CrossRef]
- Chen, Q.; Li, T.; Gao, J.; Zuo, Q.; Zhang, Q. Improved comprehensive properties of Al2O3-MgO-C refractories containing lightweight tabular alumina aggregates. Ceram. Int. 2023, 49, 17818–17826. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, A.; Zhu, X.; Gu, H.; Fu, L. Dynamic slag/refractory interaction of lightweight Al2O3-MgO castable for refining ladle. Ceram. Int. 2015, 41, 8149–8154. [Google Scholar] [CrossRef]
- Zou, Y.; Gu, H.; Huang, A.; Huo, Y.; Fu, L.; Li, Y. Characterisation and properties of low-conductivity microporous magnesia based aggregates with in-situ intergranular spinel phases. Ceram. Int. 2021, 47, 11063–11071. [Google Scholar] [CrossRef]
- Yin, H.; Liu, Y.; Tang, Y.; Yuan, H.; Xin, Y.; Gao, K.; Zuo, B. Effect of Al2O3@CaCO3 spherical particles on microstructures, phase compositions and performances of lightweight MA spinel-corundum refractories. Ceram. Int. 2021, 47, 31548–31554. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, W.; Schafföner, S.; Wu, H.; Han, B.; Zhang, J.; Li, Y. Microstructures and strength of microporous MgO-Mg(Al,Fe)2O4 refractory aggregates. J. Eur. Ceram. Soc. 2023, 43, 2654–2662. [Google Scholar] [CrossRef]
- Fu, L.; Huang, A.; Gu, H.; Zhang, M.; Lian, P. Fabrication and characterization of lightweight microporous alumina with guaranteed slag resistance. Ceram. Int. 2016, 42, 8724–8728. [Google Scholar] [CrossRef]
- Yan, W.; Chen, Q.; Lin, X.; Chen, J.; Li, N. Lightweight corundum-mullite refractories: II, Effects of porous aggregates on the slag resistances of corundum-mullite refractories. J. Ceram. Process. Res. 2016, 17, 313–317. [Google Scholar]
- Lin, X.; Yan, W.; Ma, S.; Chen, Q.; Li, N.; Han, B.; Wei, Y. Corrosion and adherence properties of cement clinker on porous periclase-spinel refractory aggregates with varying spinel content. Ceram. Int. 2017, 43, 4984–4991. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, H.; Tang, Y.; Xin, Y.; Yuan, H.; Ren, X.; Wan, Q. Synthesis mechanism and properties of lightweight mullite-corundum refractories obtained through high temperature liquid-assisted micrometer-scale Kirkendall effect. Ceram. Int. 2021, 47, 9234–9244. [Google Scholar] [CrossRef]
- Sarkar, R.; Sengupta, S. Gradient Refractories: A New Concept for Refractory Linings. Interceram Int. Ceram. Rev. 2019, 68, 28–33. [Google Scholar] [CrossRef]
- Yin, H.; Xin, Y.; Dang, J.; Gao, K.; Tang, Y.; Yuan, H. Preparation and properties of lightweight corundum-spinel refractory with density gradient. Ceram. Int. 2018, 44, 20478–20483. [Google Scholar] [CrossRef]
- Xin, Y.; Yin, H.; Tang, Y.; Yuan, H.; Ren, X.; Gao, K.; Wan, Q.; Liu, Y. Formation mechanism of MgSrAl10O17 and its effect on the mechanical performance of lightweight Al2O3-MgAl2O4 refractories. Ceram. Int. 2020, 46, 11075–11079. [Google Scholar] [CrossRef]
- Sheng, G. Preparation and exploitation of high strength alumina bubbles product. Ceram. Eng. 1999, 33, 23–24. [Google Scholar]
- Permikina, N.; Evdokimova, Z.; Kukuruzov, A.; Kukuruzov, P.; Panov, G. Thermal insulation materials based on spherical corundum. Refract. Ind. Ceram. 1987, 28, 421–424. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Lu, J.; Chen, H. Effect of binder on the structure and mechanical properties of lightweight bubble alumina ceramic. Ceram. Int. 2012, 38, 657–662. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Ouyang, D.; Wang, X.; Li, S.; Chen, R. Effects of alumina bubble addition on the properties of mullite castables. J. Alloys Compd. 2018, 735, 327–337. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Ouyang, D.; Chen, R.; Li, S. The impact of alumina bubble particle size on the microstructure and physical properties of mullite castables. Ceram. Int. 2019, 45, 1928–1939. [Google Scholar]
- GB/T5989-2008; Refractory Products. Determination of Refractoriness-Under-Load. Differential Method with Rising Temperature. Standardization Administration of China: Beijing, China, 2008.
- ISO 8894-2:1990; Refractory Materials—Determination of Thermal Conductivity—Part 2: Hot-Wire Method (Parallel). ISO: Geneva, Switzerland, 1990.



| Bulk Density g/cm3 | Apparent Porosity % | Medium Diameter μm | Breakage Rate % | |
|---|---|---|---|---|
| Activated alumina | ≥3.95 | — | 2.6 | — |
| Fused magnesia | ≥3.45 | — | 13 | — |
| Tabular corundum | ≥3.50 | ≥5 | — | — |
| Alumina bubble | ~1.25 | — | — | ≤15 |
| Al2O3 | MgO | Na2O | CaO | Fe2O3 | SiO2 | TiO2 | |
|---|---|---|---|---|---|---|---|
| Activated alumina | ≥99.5 | ≤0.07 | ≤0.1 | ≤0.02 | ≤0.03 | ≤0.03 | — |
| Fused magnesia | ≤0.21 | ≥96.6 | — | ≤1.55 | ≤0.73 | ≤0.9 | ≤0.01 |
| Tabular corundum | ≥99.2 | — | ≤0.40 | — | ≤0.02 | ≤0.09 | — |
| Alumina bubble | ≥98.5 | — | — | — | — | — | — |
| Sample | Sintering Temperature (°C) | Matrix (wt%) | Aggregate (wt%) | |
|---|---|---|---|---|
| Alumina Bubble (0–1 mm) | Tabular Corundum (1–3 mm) | |||
| CS10 | 1550, 1600, 1650 | 40% | 10% | 50% |
| CS15 | 1550, 1600, 1650 | 40% | 15% | 45% |
| CS20 | 1550, 1600, 1650 | 40% | 20% | 40% |
| CS25 | 1550, 1600, 1650 | 40% | 25% | 35% |
| CS30 | 1550, 1600, 1650 | 40% | 30% | 30% |
| Sample | Thermal Conductivity (W/(m·K)) |
|---|---|
| CS30@1650 | 1.52 |
| CS25@1650 | 1.60 |
| CS20@1650 | 2.01 |
| CS15@1650 | 2.10 |
| CS10@1650 | 2.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Y.; Jian, Y.; Yin, H.; Tang, Y.; Yuan, H.; Liu, Y. The Influence of Alumina Bubbles on the Properties of Lightweight Corundum–Spinel Refractory. Materials 2023, 16, 5908. https://doi.org/10.3390/ma16175908
Xin Y, Jian Y, Yin H, Tang Y, Yuan H, Liu Y. The Influence of Alumina Bubbles on the Properties of Lightweight Corundum–Spinel Refractory. Materials. 2023; 16(17):5908. https://doi.org/10.3390/ma16175908
Chicago/Turabian StyleXin, Yalou, Yunling Jian, Hongfeng Yin, Yun Tang, Hudie Yuan, and Yuchi Liu. 2023. "The Influence of Alumina Bubbles on the Properties of Lightweight Corundum–Spinel Refractory" Materials 16, no. 17: 5908. https://doi.org/10.3390/ma16175908
APA StyleXin, Y., Jian, Y., Yin, H., Tang, Y., Yuan, H., & Liu, Y. (2023). The Influence of Alumina Bubbles on the Properties of Lightweight Corundum–Spinel Refractory. Materials, 16(17), 5908. https://doi.org/10.3390/ma16175908





