Photocatalytic Activity of N-Doped ZrO2 Thin Films Determined by Direct and Indirect Irradiation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
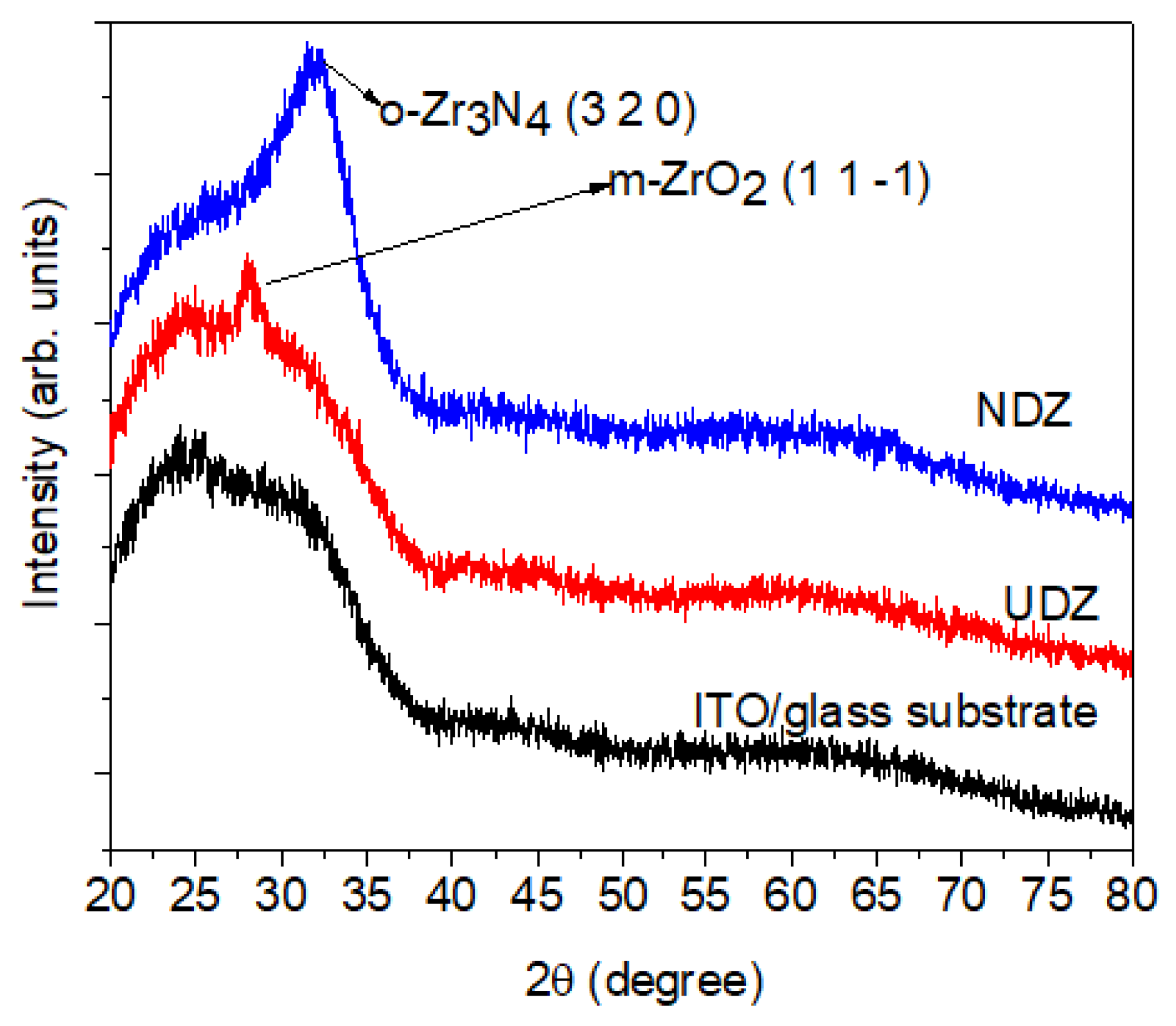
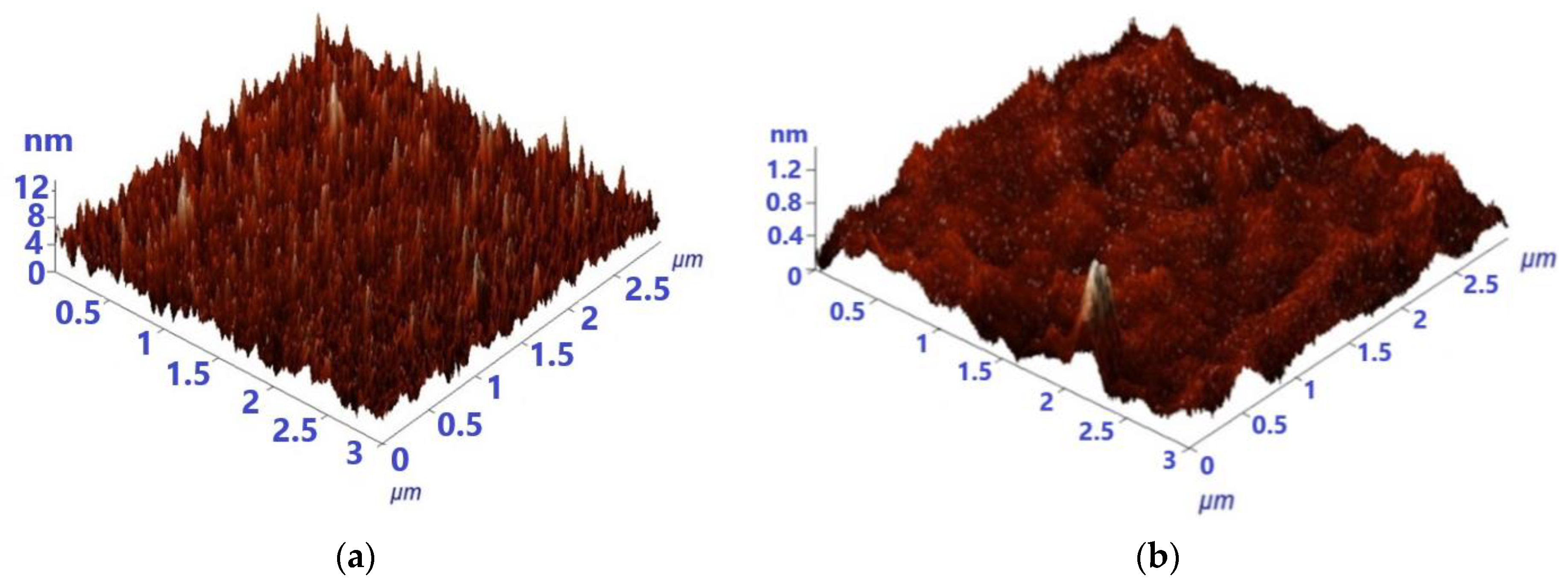
3.1. Structural and Surface Topography Analysis
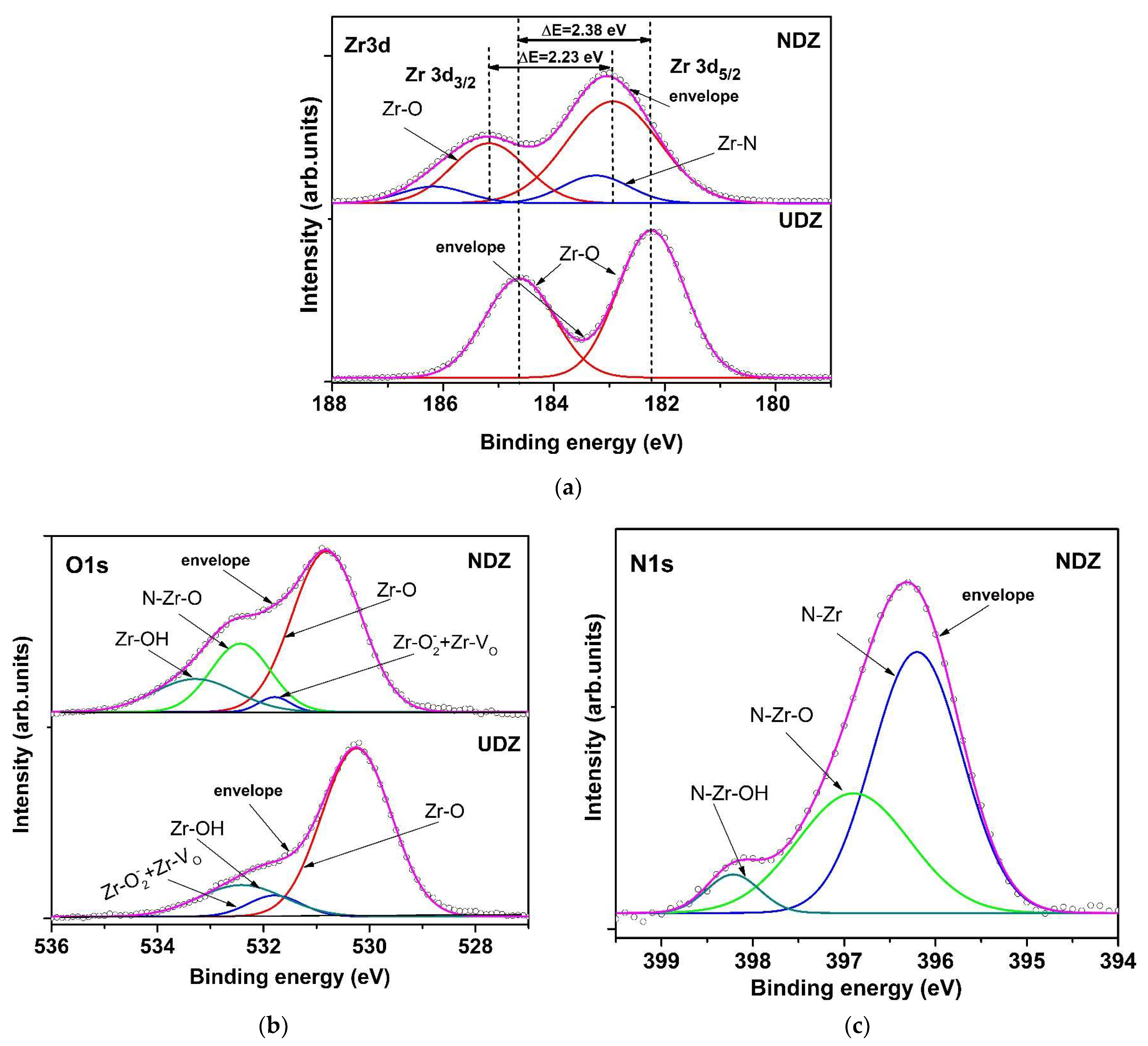
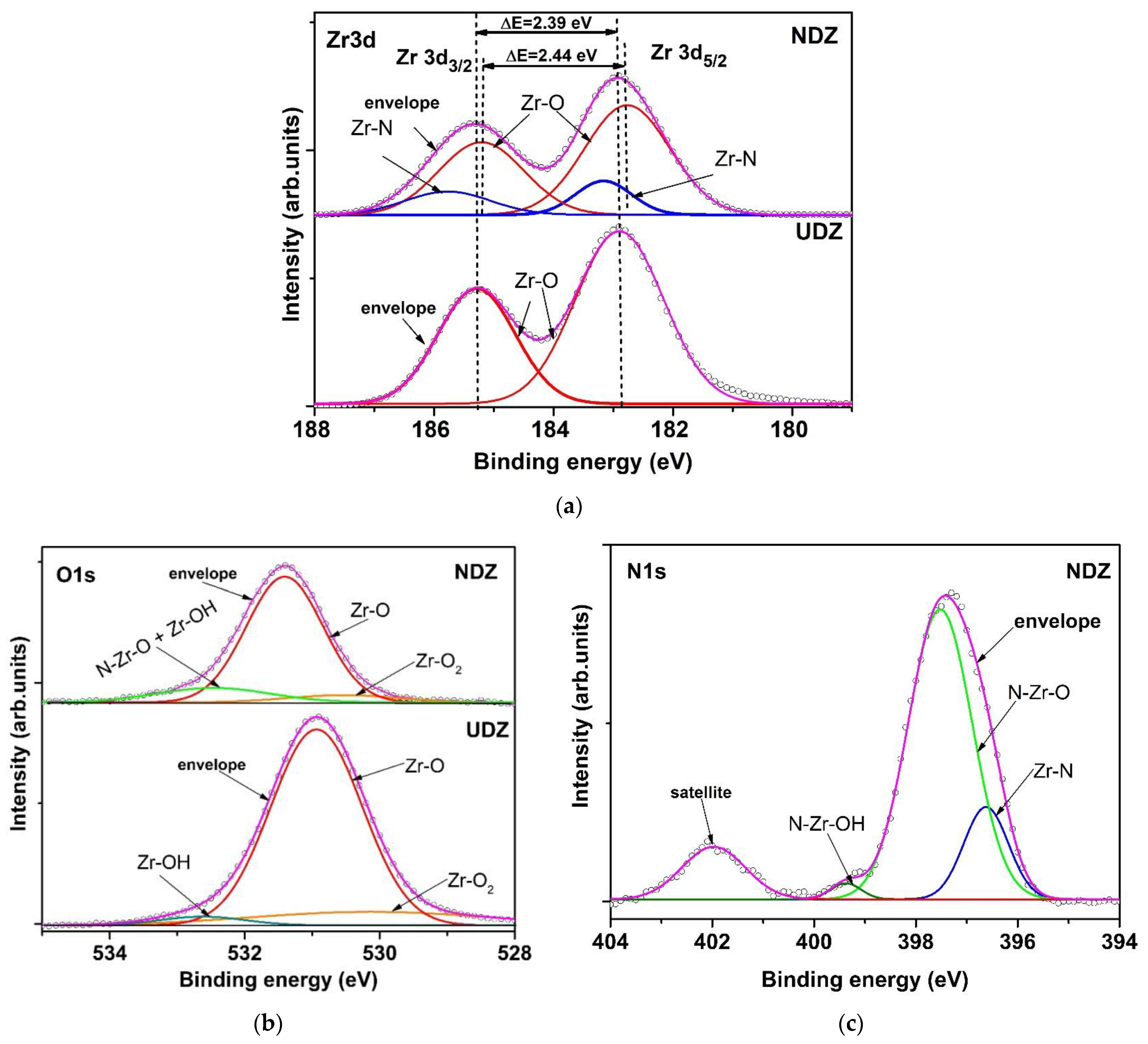
3.2. X-ray Photoelectron Spectroscopy Analysis
3.3. Optical Bandgap
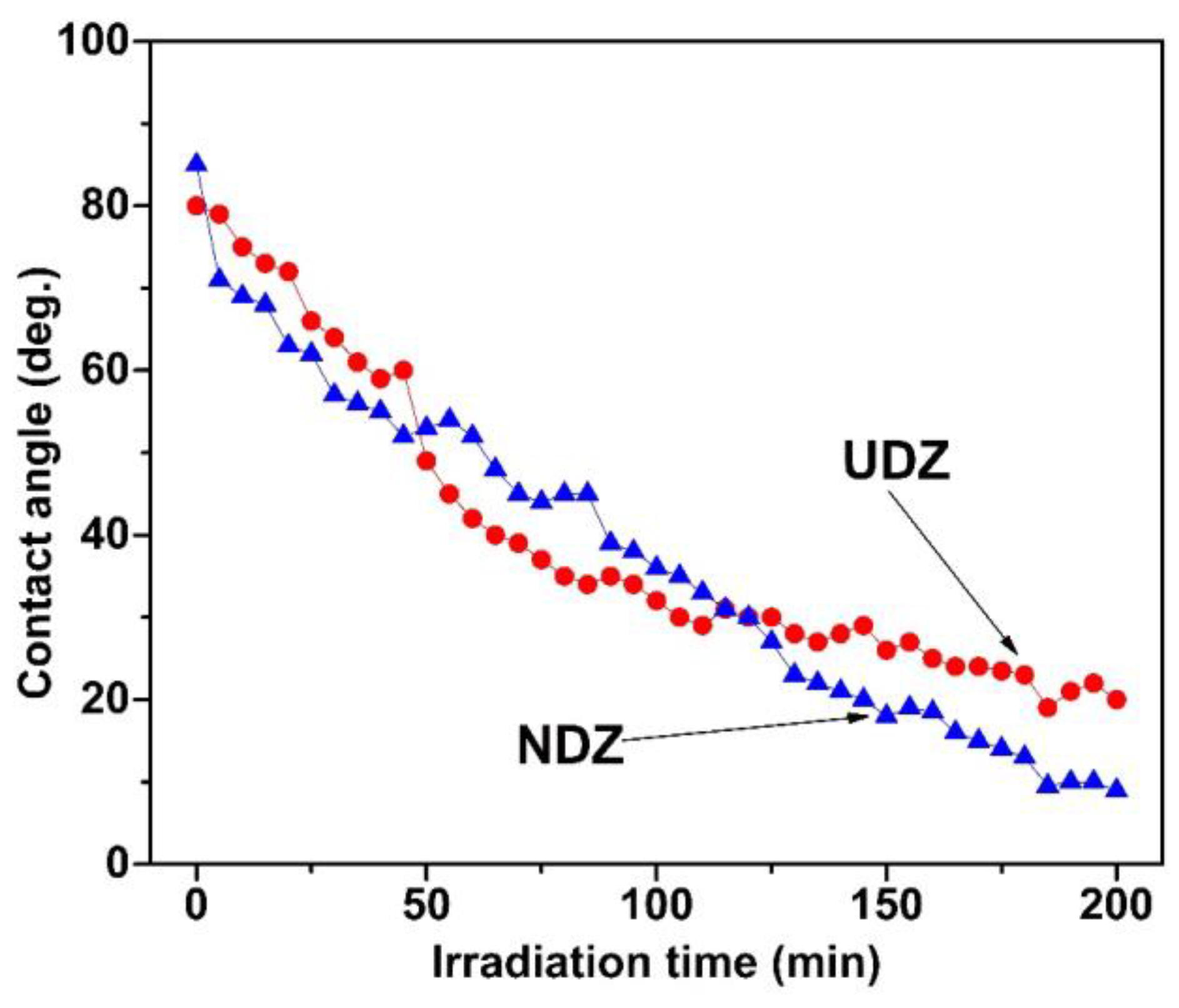
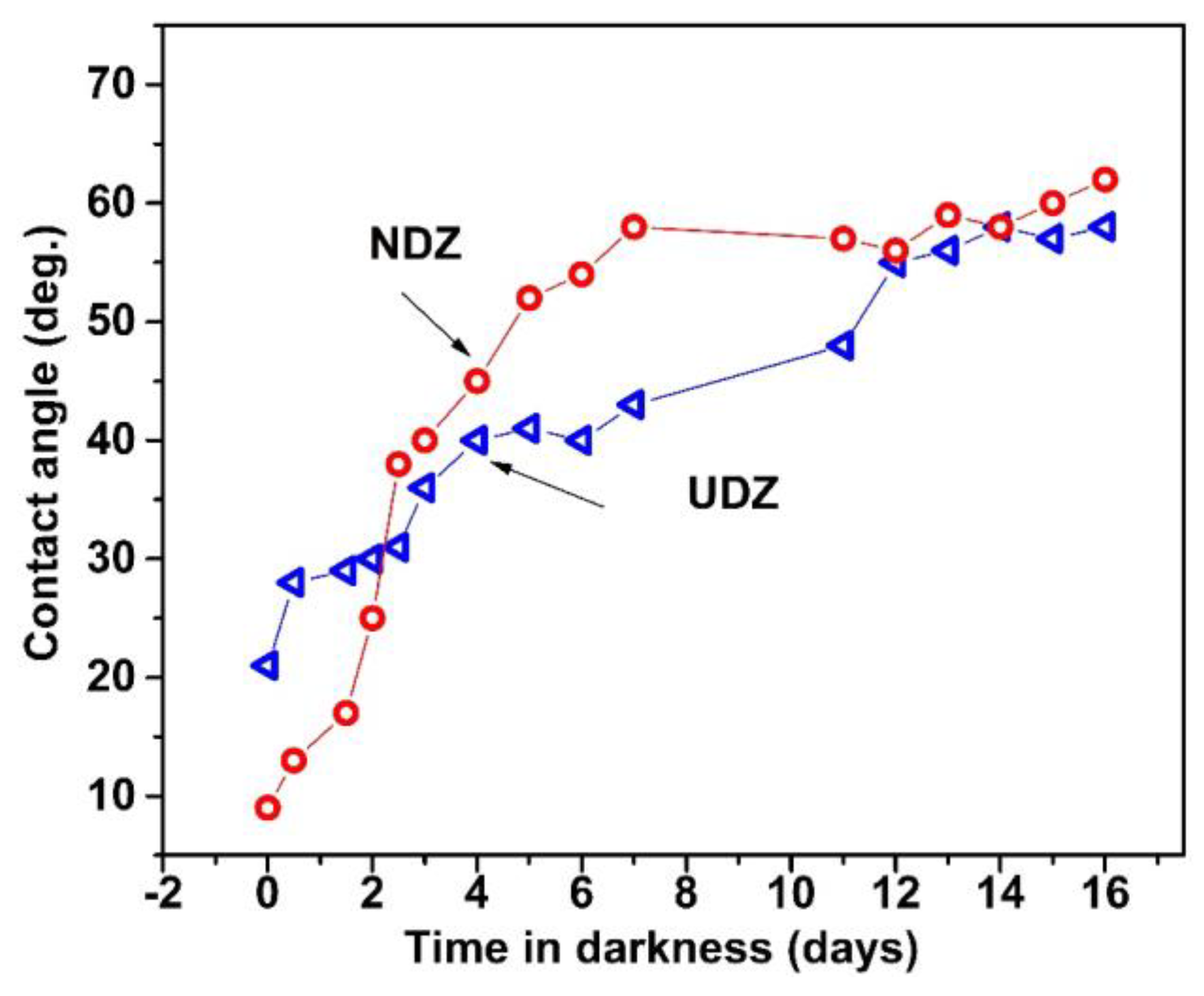
3.4. Hydrophilic Properties
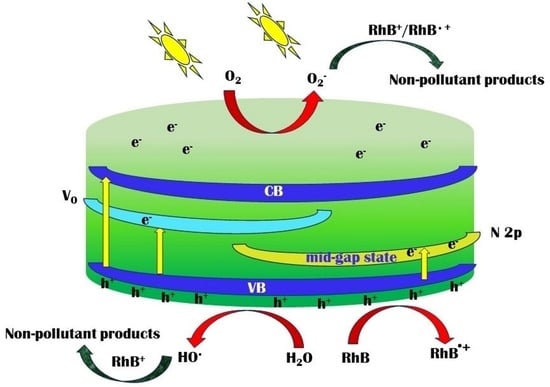
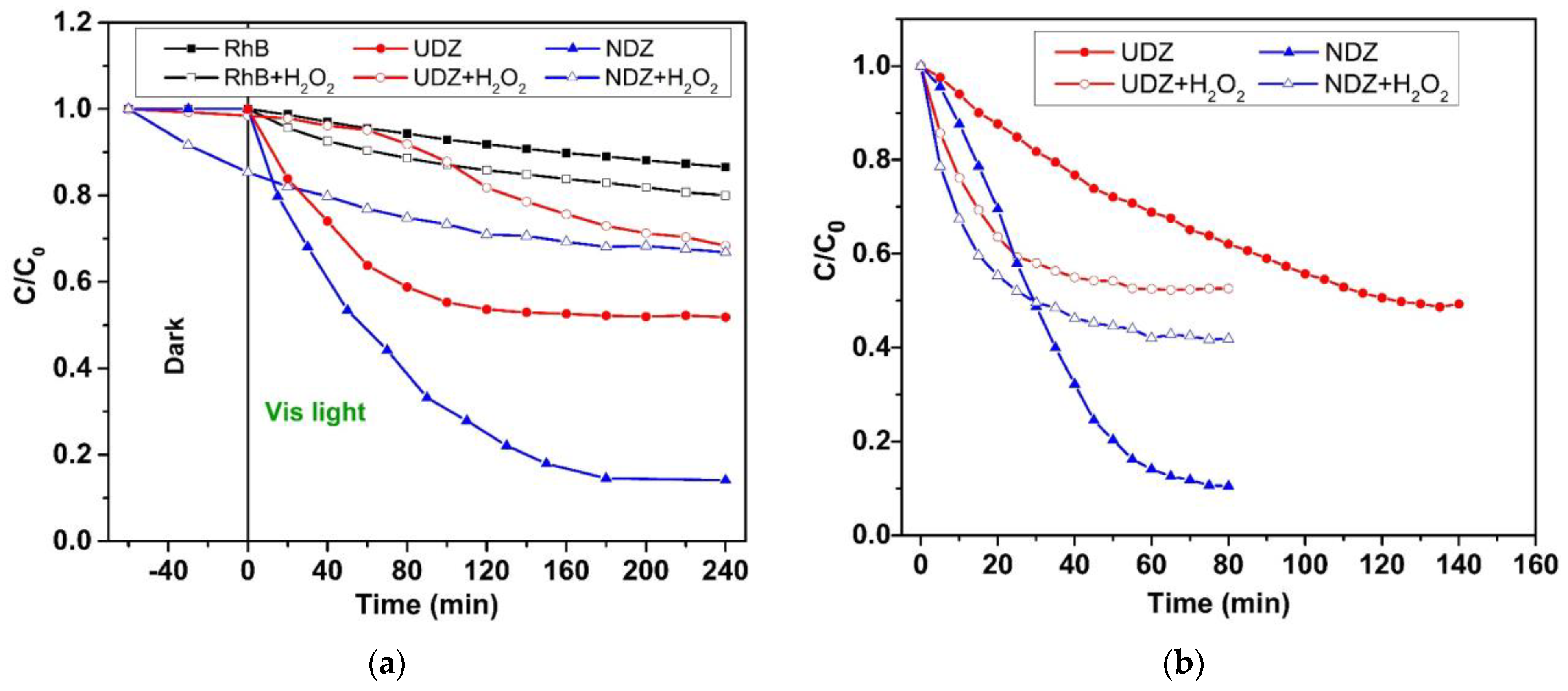
3.5. Photocatalytic Activity
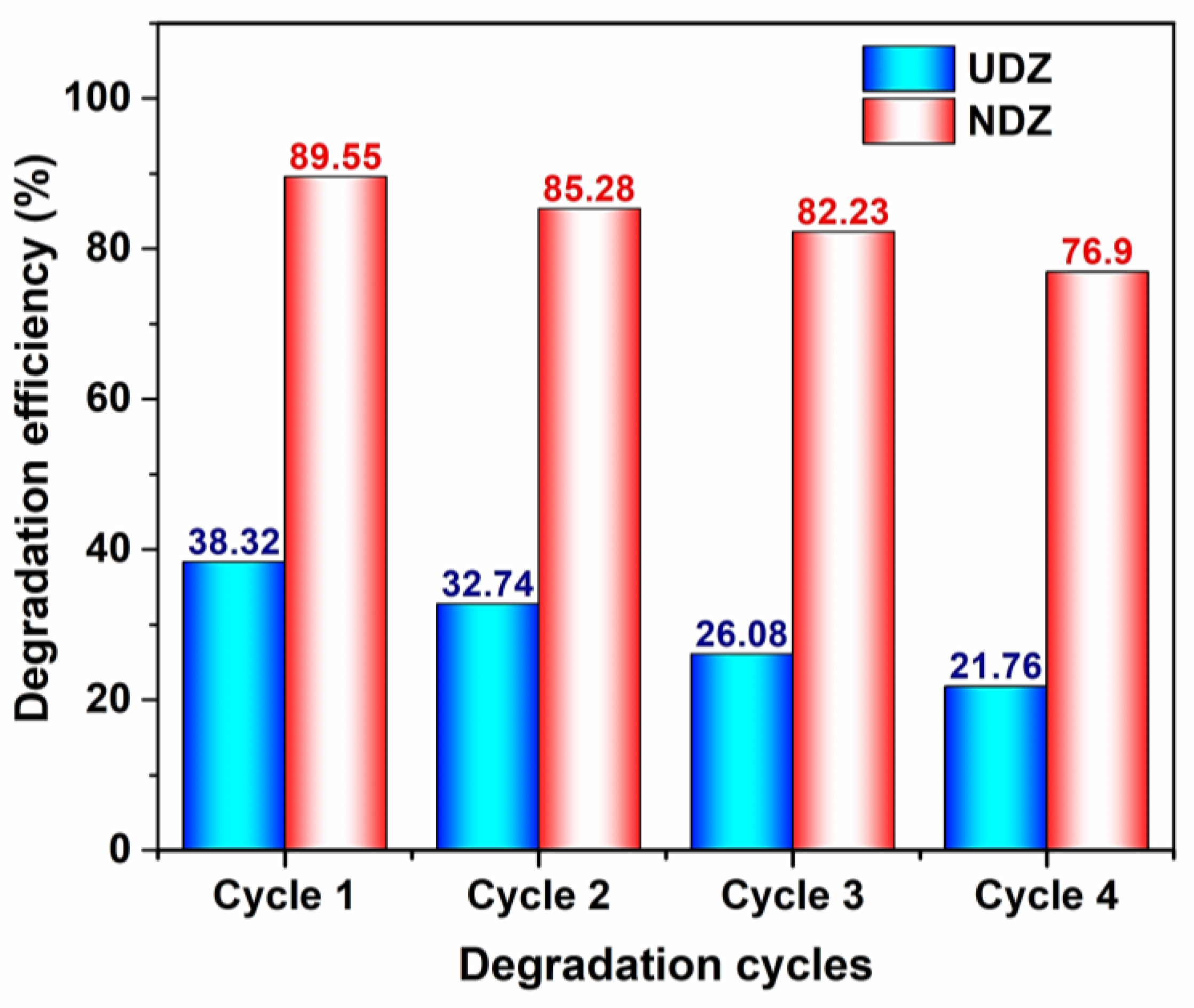
3.6. Stability, Reusability, and Thin Film Performance
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedanekar, R.S.; Shaikh, S.K.; Rajpure, K.Y. Thin film photocatalysis for environmental remediation: A status review. Curr. Appl. Phys. 2020, 20, 931–952. [Google Scholar] [CrossRef]
- Bandara, W.R.L.N.; de Silva, R.M.; de Silva, K.M.N.; Dahanayake, D.; Gunasekara, S.; Thanabalasingam, K. Is nano ZrO2 a better photocatalyst than nano TiO2 for degradation of plastics? RSC Adv. 2017, 7, 46155–46163. [Google Scholar] [CrossRef]
- Boelee, E.; Geerling, G.; van der Zaan, B.; Blauw, A.; Vethaak, A.D. Water and health: From environmental pressures to integrated responses. Acta Trop. 2019, 193, 217–226. [Google Scholar] [CrossRef]
- Hojabri, A. Structural and optical characterization of ZrO2 thin films grown on silicon and quartz substrates. J. Theor. Appl. Phys. 2016, 10, 219–224. [Google Scholar] [CrossRef]
- Frenți, M.; Mița, C.; Cornei, N.; Tiron, V.; Bulai, G.; Dobromir, M.; Doroshkevich, A.; Mardare, D. ZrO2 for photocatalytic applications. UPB Sci. Bull. Ser. A 2023, 85, 165–176. [Google Scholar]
- Wang, Y.; Zhang, Y.; Lu, H.; Chen, Y.; Liu, Z.; Su, S.; Xue, Y.; Yao, J.; Zengm, H. Novel N-doped ZrO2 with enhanced visible-light photocatalytic activity for hydrogen production and degradation of organic dyes. RSC Adv. 2018, 8, 6752–6758. [Google Scholar] [CrossRef]
- Renuka, L.; Anantharaju, K.S.; Sharma, S.C.; Nagaswarupa, H.; Vidya, Y.S.; Nagabhushana, H.P.; Prashantha, S.C. A comparative study on the structural, optical, electrochemical and photocatalytic properties of ZrO2 nanooxide synthesized by different routes. J. Alloys Compd. 2017, 695, 382–395. [Google Scholar] [CrossRef]
- Navio, J.A.; Hidalgo, M.C.; Colon, G.; Botta, S.G.; Litter, M.I. Preparation and Physicochemical Properties of ZrO2 and Fe/ZrO2 Prepared by a Sol-Gel Technique. Langmuir 2001, 17, 202–210. [Google Scholar] [CrossRef]
- IARC Group. Solar and ultraviolet radiation. IARC Monogr. Eval. Carcinog. Risks Hum. 1992, 55, 1–316. [Google Scholar]
- Lan, X.; Wang, Y.; Peng, J.; Si, Y.; Ren, J.; Ding, B.; Li, B. Designing heat transfer pathways for advanced thermoregulatory textiles. Mater. Today Phys. 2021, 17, 100342. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Najafabadi, A.H.; Zehtabi, F.; Kouchehbaghi, N.H.; Sharma, S.; Markowska-Szczupak, A.; Kowalska, E. Apatite-coated Ag/AgBr/TiO2 nanocomposites: Insights into the antimicrobial mechanism in the dark and under visible-light irradiation. Appl. Surf. Sci. 2023, 617, 156574. [Google Scholar] [CrossRef]
- Han, C.; Zhu, X.; San Martin, J.; Lin, Y.; Spears, S.; Yan, Y. Recent Progress in Engineering Metal Halide Perovskites for Efficient Visible-Light-Driven Photocatalysis. ChemSusChem 2020, 13, 4005–4025. [Google Scholar] [CrossRef] [PubMed]
- Shimosako, N.; Sakama, H. Basic photocatalytic activity of ZrO2 thin films fabricated by a sol-gel method under UV-C irradiation. Thin Solid Films 2021, 732, 138786. [Google Scholar] [CrossRef]
- Xu, J.-P.; Zhang, R.-J.; Zhang, Y.; Wang, Z.-Y.; Chen, L.; Huang, Q.-H.; Lu, H.-L.; Wang, S.-Y.; Zheng, Y.-X.; Chen, L.-Y. The thickness-dependent band gap and defect features of ultrathin ZrO2 films studied by spectroscopic ellipsometry. Phys. Chem. Chem. Phys. 2016, 18, 3316–3321. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Educational: London, UK, 1978; p. 102. [Google Scholar]
- Altalhi, T.; Gobouri, A.A.; Al-Harbi, L.; Refat, M.; El-Nahass, M.; Hassanien, A.; Atta, A.; Ahmed, E.M. Structural, diffuse reflectance spectroscopy and dielectric relaxation properties of zirconium (IV) dioxide. J. Mater. Res. Technol. 2021, 12, 1194–1202. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F.A. Contribution to the Optics of Pigments. Z. Technol. Phys. 1931, 12, 593–601. [Google Scholar]
- Moura, C.; Carvalho, P.; Vaz, F.; Cunha, L.; Alves, E. Raman spectra and structural analysis in ZrOxNy thin films. Thin Solid Films 2006, 515, 1132–1137. [Google Scholar] [CrossRef]
- Bazhanov, D.I.; Knizhnik, A.A.; Safonov, A.A.; Bagatur’yants, A.A.; Stoker, M.W.; Korkina, A.A. Structure and electronic properties of zirconium and hafnium nitrides and oxynitrides. J. Appl. Phys. 2005, 97, 044108. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lerch, M.; Maier, J. Nitrogen-doped zirconia: A comparison with cation stabilized zirconia. J. Solid State Chem. 2006, 179, 270–277. [Google Scholar] [CrossRef]
- Rawal, S.K.; Chawla, A.K.; Chawla, V.; Jayaganthan, R.; Chandra, R. Structural, optical and hydrophobic properties of sputter deposited zirconium oxynitride films. Mater. Sci. Eng. B 2010, 172, 259–266. [Google Scholar] [CrossRef]
- Mardare, M.; Manole, A.; Yıldız, A.; Luca, D. Photoinduced wettability of titanium oxide thin films. Chem. Eng. Commun. 2011, 198, 530–540. [Google Scholar] [CrossRef]
- Mardare, M.; Cornei, N.; Mita, C.; Florea, D.; Stancu, A.; Tiron, V.; Manole, A.; Adomnitei, C. Low temperature TiO2 based gas sensors for CO2. Ceram. Int. 2016, 42, 7353–7359. [Google Scholar] [CrossRef]
- Jeong, K.S.; Song, J.; Lim, D.; Lee, M.S.; Kim, H.; Cho, M.H. Structural evolution and defect control of yttrium-doped ZrO2 films grown by a sol-gel method. Appl. Surf. Sci. 2014, 320, 128–137. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L. Acidity, sulphur coverage and XPS analyses of ZrO2-SO4 powders by different procedures. Appl. Surf. Sci. 1999, 152, 63–69. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Y.; Zhao, Y.; Hou, M.; Yu, X.; Li, S. Visible-light photocatalytic tetracycline degradation over nanodots-assembled N-ZrO2−x nanostructures: Performance, degradation pathways and mechanistic insight. J. Alloys Compd. 2022, 895, 162582. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, D.; Dong, W.; Hou, X.; Liu, F.; Sun, N.; Ali, F.; Li, Z. Ferroelectric properties of pure ZrO2 thin films by chemical solution deposition. Ceram. Int. 2021, 47, 16845–16851. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, L.; Jiao, C.; Shao, Q.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. Hydrothermally synthesized Ti/Zr bimetallic MOFs derived N self-doped TiO2/ZrO2 composite catalysts with enhanced photocatalytic degradation of methylene blue. Colloids Surf. A Physicochem. Eng. 2021, 623, 126629. [Google Scholar] [CrossRef]
- Tabet, N.; Faiz, M.; Al-Oteibi, A. XPS study of nitrogen-implanted ZnO thin films obtained by DC-Magnetron reactive plasma. J. Electron. Spectrosc. Relat. Phenom. 2008, 163, 15–18. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S.; Sakai, H.; Takizawa, S. Rapid enhanced photocatalytic degradation of dyes using novel N-doped ZrO2. J. Environ. Manag. 2016, 165, 224–234. [Google Scholar] [CrossRef]
- Leinweber, P.; Kruse, J.; Walley, F.L.; Gillespie, A.; Eckhardt, K.-U.; Blyth, R.I.; Regier, T. Nitrogen K-edge XANES-an overview of reference compounds used to identify ‘unknown’ organic nitrogen in environmental samples. J. Synchrotron Radiat. 2007, 14, 500–511. [Google Scholar] [CrossRef]
- Wahba, M.A.; Yakout, S.M.; Mohamed, W.A.; Galal, H.R. Remarkable photocatalytic activity of Zr doped ZnO and ZrO2/ZnO nanocomposites: Structural, morphological and photoluminescence properties. Mater. Chem. Phys. 2020, 256, 123754. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.; Tryk, D. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2001, 1, 1–21. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Bico, J.; Marzolin, C.; Quéré, D. Pearl Drops. Europhys. Lett. 1999, 47, 220–226. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V.; Bulanin, K.M.; Chistyakova, L.V.; Maevskaya, M.V.; Bahnemann, D.W. Self-cleaning properties of zirconium dioxide thin films. J. Photochem. Photobiol. A Chem. 2018, 367, 397–405. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Thao, N.T.; Nga, H.T.P.; Vo, N.Q.; Nguyen, H.D.K. Advanced oxidation of rhodamine B with hydrogen peroxide over Zn single bond Cr layered double hydroxide catalysts. J. Sci. Adv. Mater. Dev. 2017, 2, 317–325. [Google Scholar]
- Yu, Z.; Zhang, L.; Watanabe, S. Facile modification of TiO2 nanoparticles with H2O2 + NH4F for enhanced visible light photodegradation of rhodamine B and methylene blue. Mater. Today Commun. 2022, 33, 104213. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Gracia-Pinilla, M.A.; Pillai, S.C.; O’Shea, K. UV and visible light-driven production of hydroxyl radicals by reduced form of N, F, and P codoped titanium dioxide. Molecules 2019, 24, 2147. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Agorku, E.S.; Pandey, A.C.; Mamba, B.B.; Mishra, A.K. Gd,C,N,S multi-doped ZrO2 for photocatalytic degradation of Indigo Carmine dye from synthetic water under simulated solar light. Mater. Today Proc. 2015, 2, 3909–3920. [Google Scholar] [CrossRef]
- Hamed, N.K.A.; Nafarizal, N.; Ahmad, M.K.; Faridah, A.B.; Makhtar, S.N.N.M.; Noor, S.F.M.; Shimomura, M. Synergistic coupling {101}-{111} facet of N-doped TiO2 film enhance spatial charge separation for methylene blue photodegradation. J. Alloys Compd. 2023, 961, 170977. [Google Scholar] [CrossRef]
- Wannapop, S.; Somdee, A.; Bovornratanaraks, T. Experimental study of thin film Fe2O3/TiO2 for photocatalytic Rhodamine B degradation. Inorg. Chem. Comm. 2021, 128, 108585. [Google Scholar] [CrossRef]

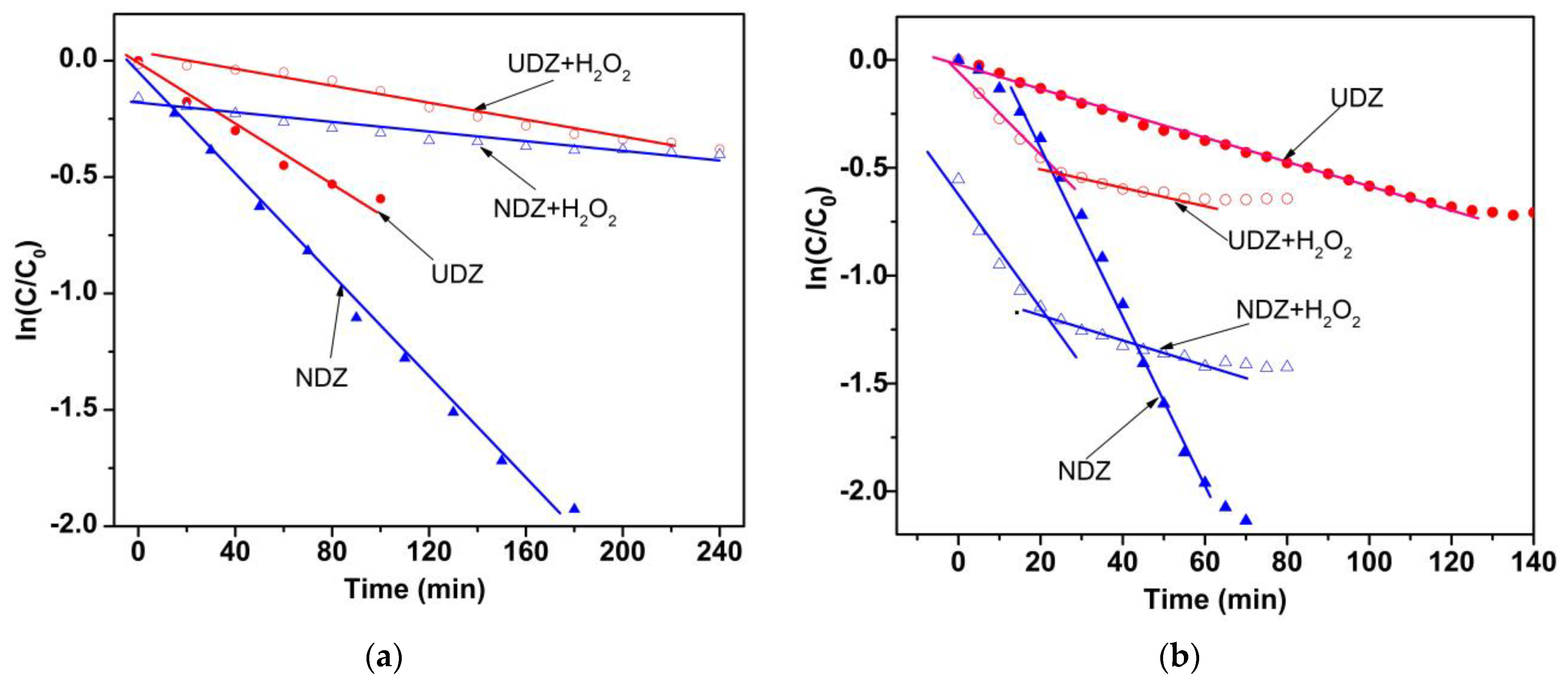
| Film | Parameter | Irradiation Method | ||||
|---|---|---|---|---|---|---|
| Indirect | Direct | |||||
| − | + | − | + | + | ||
| UDZ | kapp (min−1) | 1.06 × 10−2 | 1.24 × 10−3 | 5.52 × 10−3 | 1.82 × 10−2 | 3.52 × 10−3 |
| R2 | 0.9657 | 0.9745 | 0.9944 | 0.9592 | 0.9772 | |
| NDZ | kapp (min−1) | 5.97 × 10−3 | 1.96 × 10−3 | 1.66 × 10−2 | 1.96 × 10−2 | 3.70 × 10−3 |
| R2 | 0.9845 | 0.9703 | 0.9899 | 0.9485 | 0.9405 | |
| Photocatalyst | Preparation Method | Active Surface of the Film | Light Source | Dye Concentration/Volume | Irradiation Time/Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| ZrO2/ITO | HiPIMS * | 20 × 10 mm2 | 100 W/visible LED lamp | 3 mg·L−1 RhB/10 mL | 240 min./48% (“indirect irradiation”) | This work |
| 3 mg·L−1 RhB/5 mL | 140 min./51% (“direct irradiation”) | |||||
| N-ZrO2/ITO | HiPIMS ** | 20 × 10 mm2 | 100 W/visible LED lamp | 3 mg·L−1 RhB/10 mL | 240 min./86% (“indirect irradiation”) | This work |
| 3 mg·L−1 RhB/5 mL | 80 min./90% (“direct irradiation”) | |||||
| TiO2/FTO | Hydrothermal synthesis/120 °C/10 h | 35 × 35 mm2 | 300 W/Xenon lamp | 5 mg·L−1 MB/100 mL | 300 min./59% | |
| 0.5%N-TiO2/FTO | 300 min./95% | |||||
| 1%N-TiO2/FTO | 300 min./57% | [43] | ||||
| 2%N-TiO2/FTO | 300 min./66% | |||||
| 0.5%Fe2O3/TiO2/FTO | Hydrothermal nanorod/160 °C/2 h | 20 × 20 mm2 | 10 W/365 nm LED | 4.8 mg·L−1 RhB/20 mL | 300 min./52% | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mita, C.; Cornei, N.; Frenti, M.; Bulai, G.; Dobromir, M.; Tiron, V.; Doroshkevich, A.S.; Mardare, D. Photocatalytic Activity of N-Doped ZrO2 Thin Films Determined by Direct and Indirect Irradiation. Materials 2023, 16, 5901. https://doi.org/10.3390/ma16175901
Mita C, Cornei N, Frenti M, Bulai G, Dobromir M, Tiron V, Doroshkevich AS, Mardare D. Photocatalytic Activity of N-Doped ZrO2 Thin Films Determined by Direct and Indirect Irradiation. Materials. 2023; 16(17):5901. https://doi.org/10.3390/ma16175901
Chicago/Turabian StyleMita, Carmen, Nicoleta Cornei, Mariana Frenti, Georgiana Bulai, Marius Dobromir, Vasile Tiron, Aleksandr S. Doroshkevich, and Diana Mardare. 2023. "Photocatalytic Activity of N-Doped ZrO2 Thin Films Determined by Direct and Indirect Irradiation" Materials 16, no. 17: 5901. https://doi.org/10.3390/ma16175901
APA StyleMita, C., Cornei, N., Frenti, M., Bulai, G., Dobromir, M., Tiron, V., Doroshkevich, A. S., & Mardare, D. (2023). Photocatalytic Activity of N-Doped ZrO2 Thin Films Determined by Direct and Indirect Irradiation. Materials, 16(17), 5901. https://doi.org/10.3390/ma16175901