Potential Role of GGBS and ACBFS Blast Furnace Slag at 90 Days for Application in Rigid Concrete Pavements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Mortar Mixes
2.1.2. Concrete Mixes
2.2. Methods
2.2.1. The Influence of HAI of GGBS on the Composition of Mortars
2.2.2. Characterization of the Microstructure of Standard Mortar and Mortars with GGBS
X-ray Diffraction on Mortars
Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX) Measurements
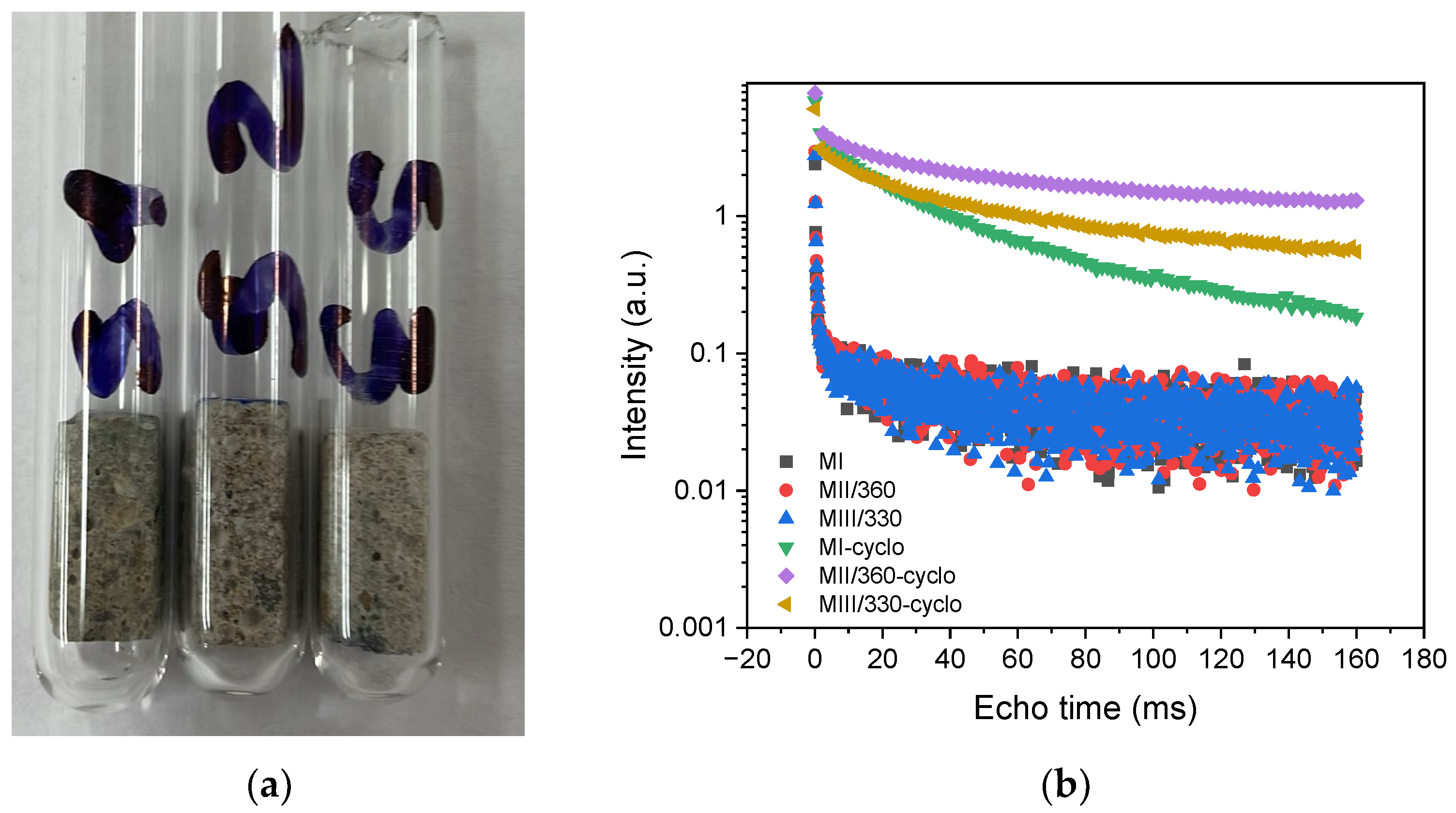
2.2.3. NMR Relaxometry Measurements of the Prepared Mortars
2.2.4. Road Concrete Mixes with Incorporated Slag (GGBS and ACBFS)
- D—Migration coefficient in non-equilibrium state (×10−12 m2/s);
- V—Applied voltage (V);
- T—Mean value between initial and final temperature in the anolytic solution, (°C);
- L—Sample thickness (mm);
- Xd—Average chlorine penetration depth (mm).
3. Results and Discussion
3.1. Mortar Mixes with GGBS
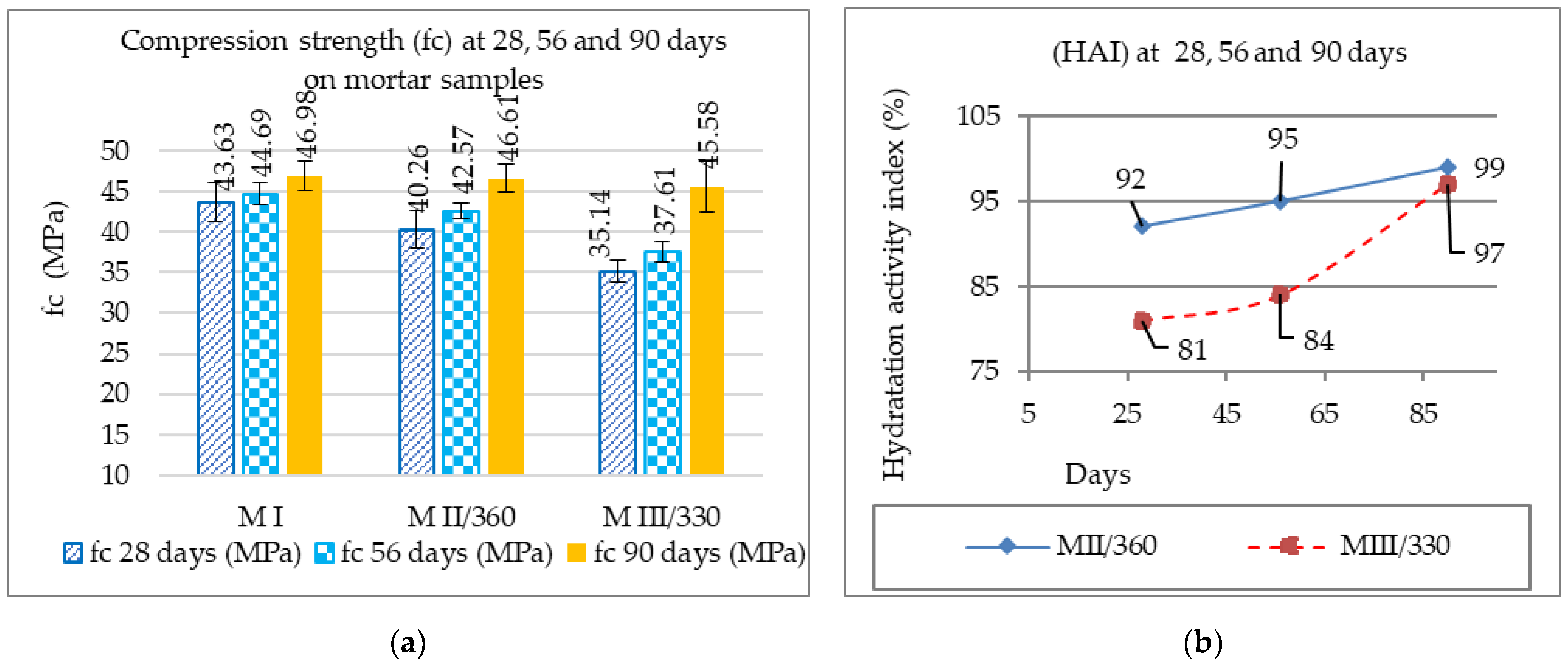
3.1.1. The Influence of HAI of GGBS on the Composition of Mortars
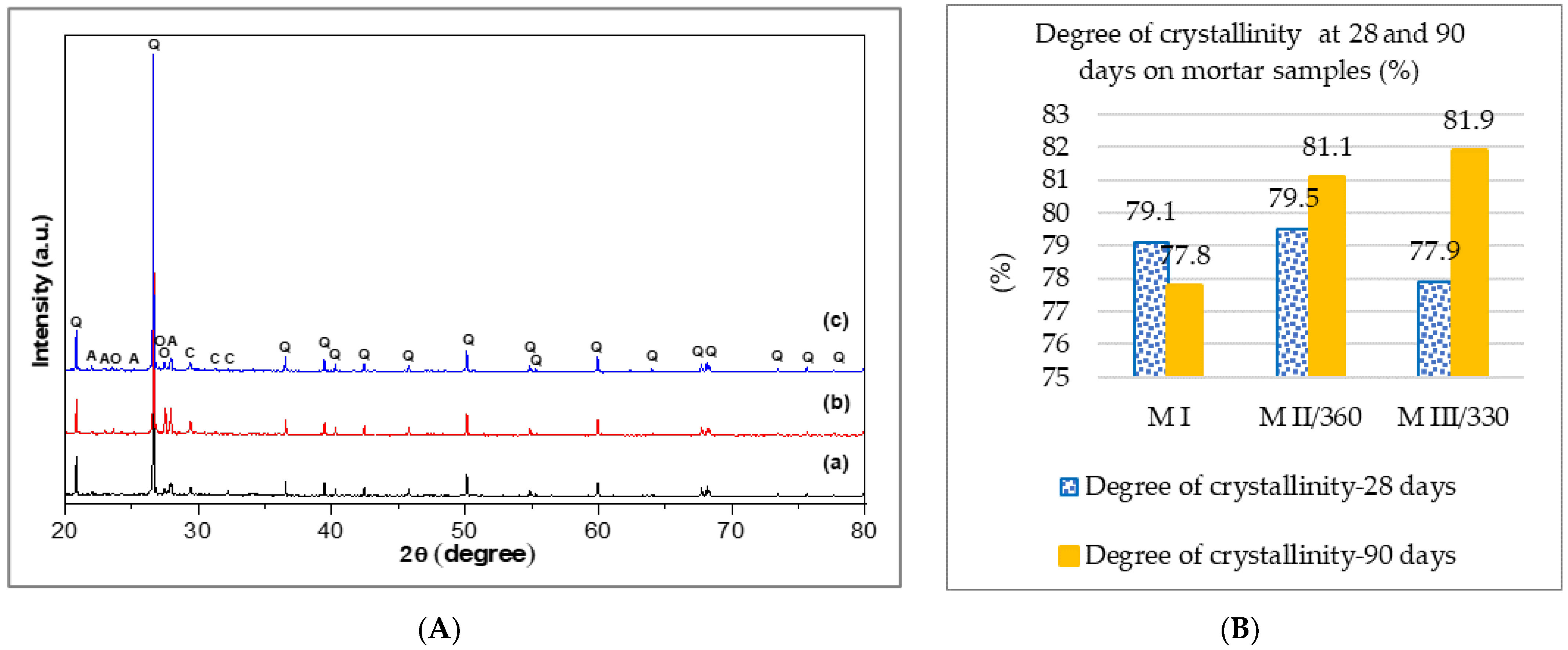
3.1.2. X-ray Diffraction
3.1.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX) Measurements
3.1.4. NMR Relaxometry Measurements
3.2. Road Concrete Mixes with Incorporated Slag (GGBS and ACBFS) Tested at 90 Days
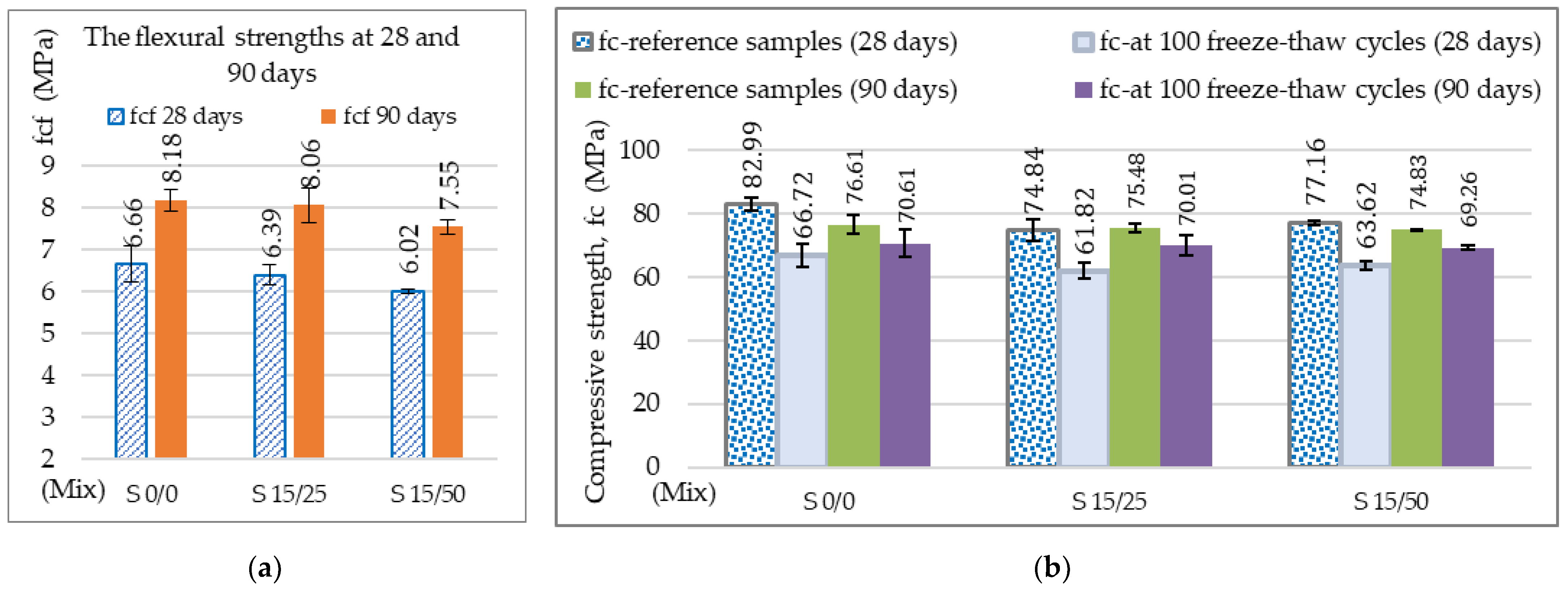
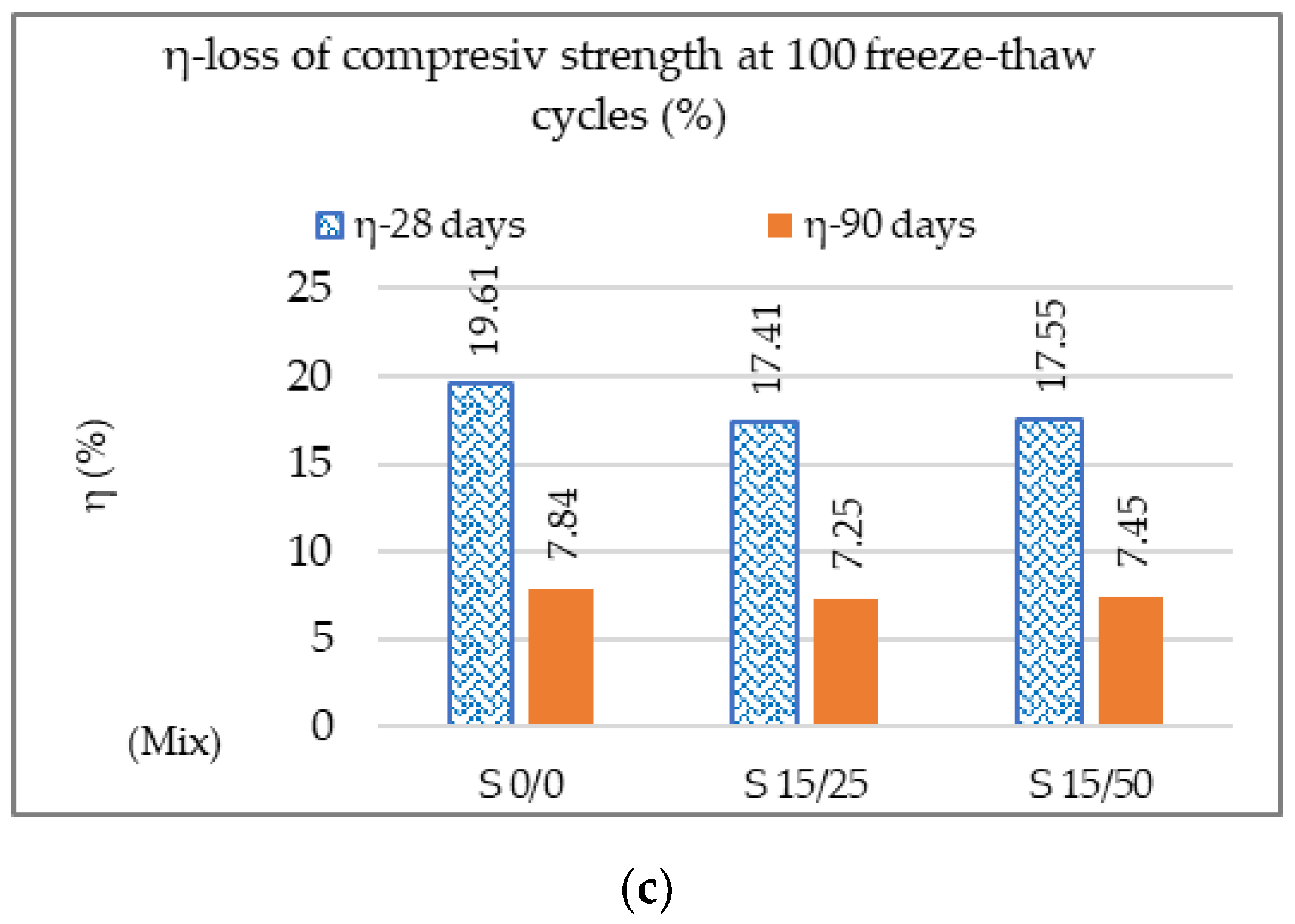
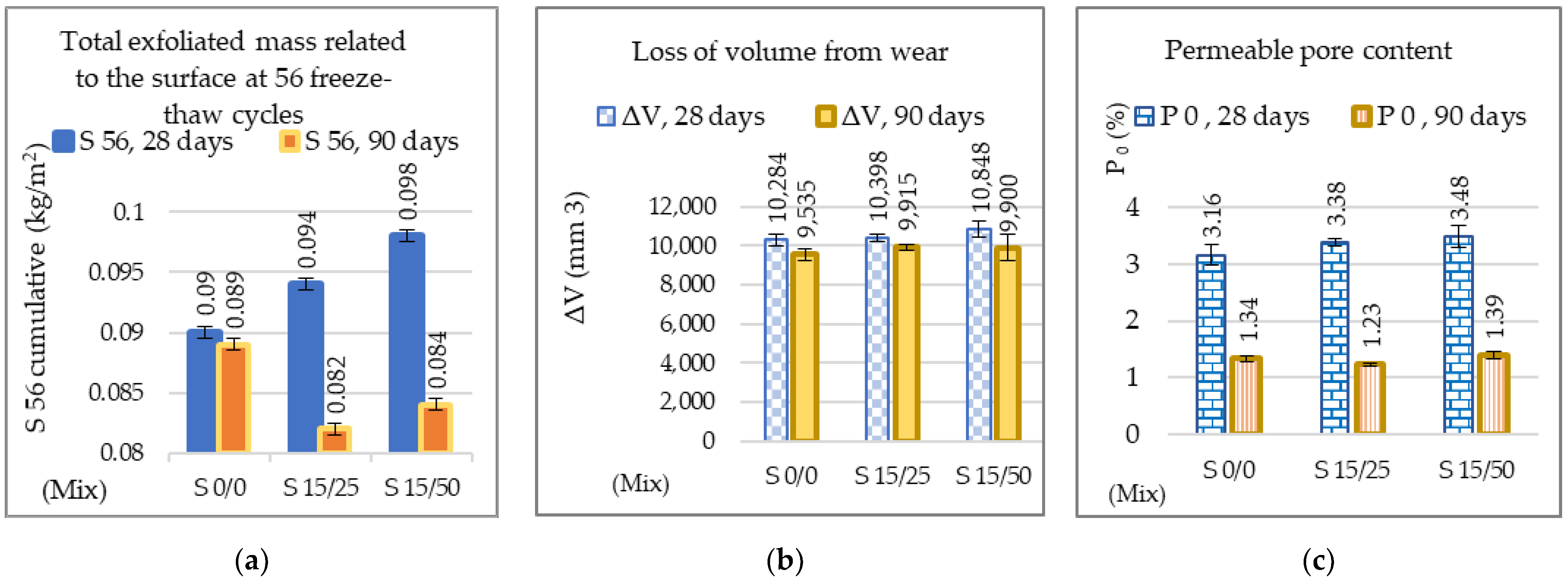
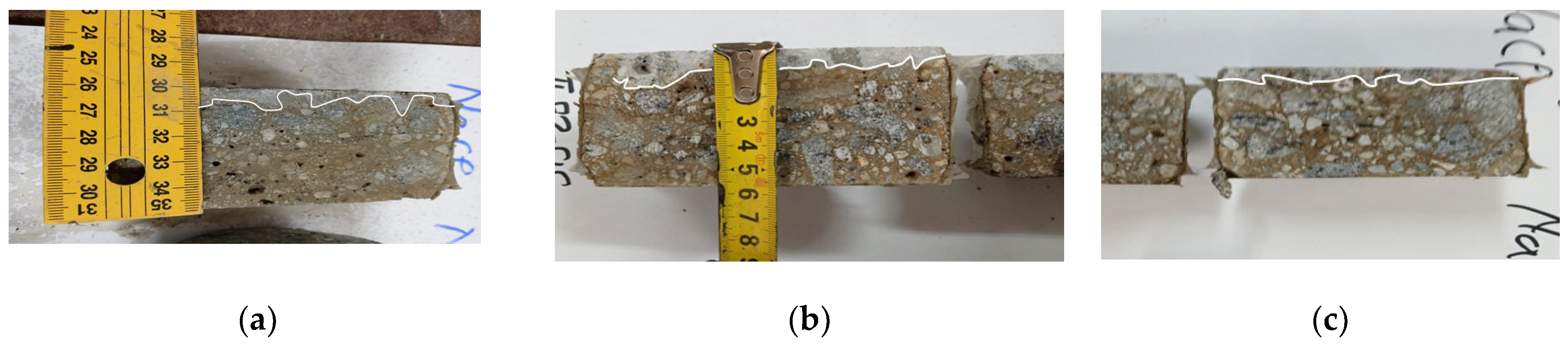
3.2.1. Mechanical Resistances, Mechanical Wear, Freeze–Thaw, and Permeable Pore Content
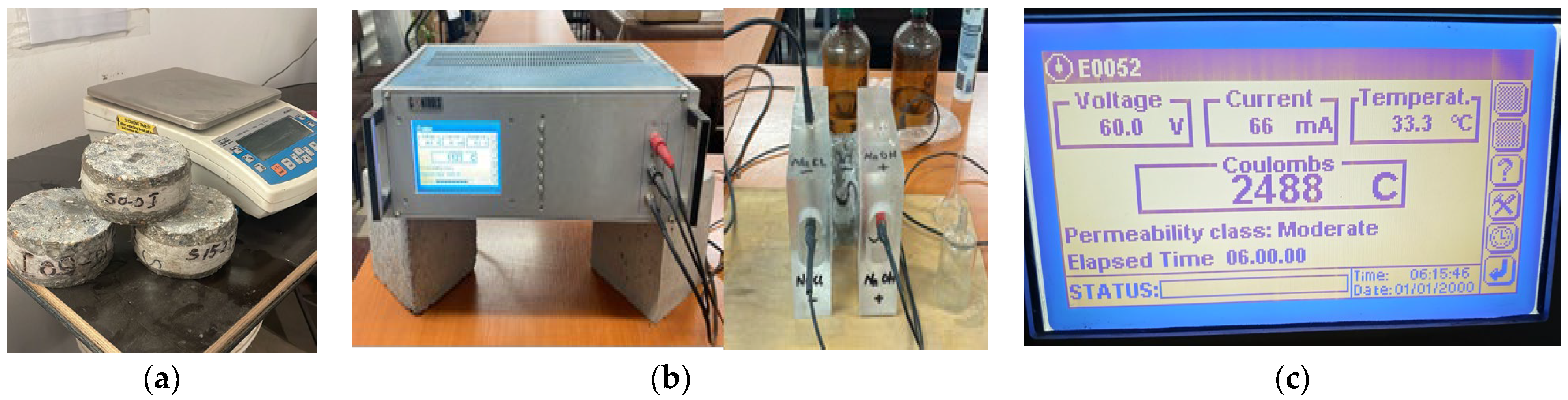
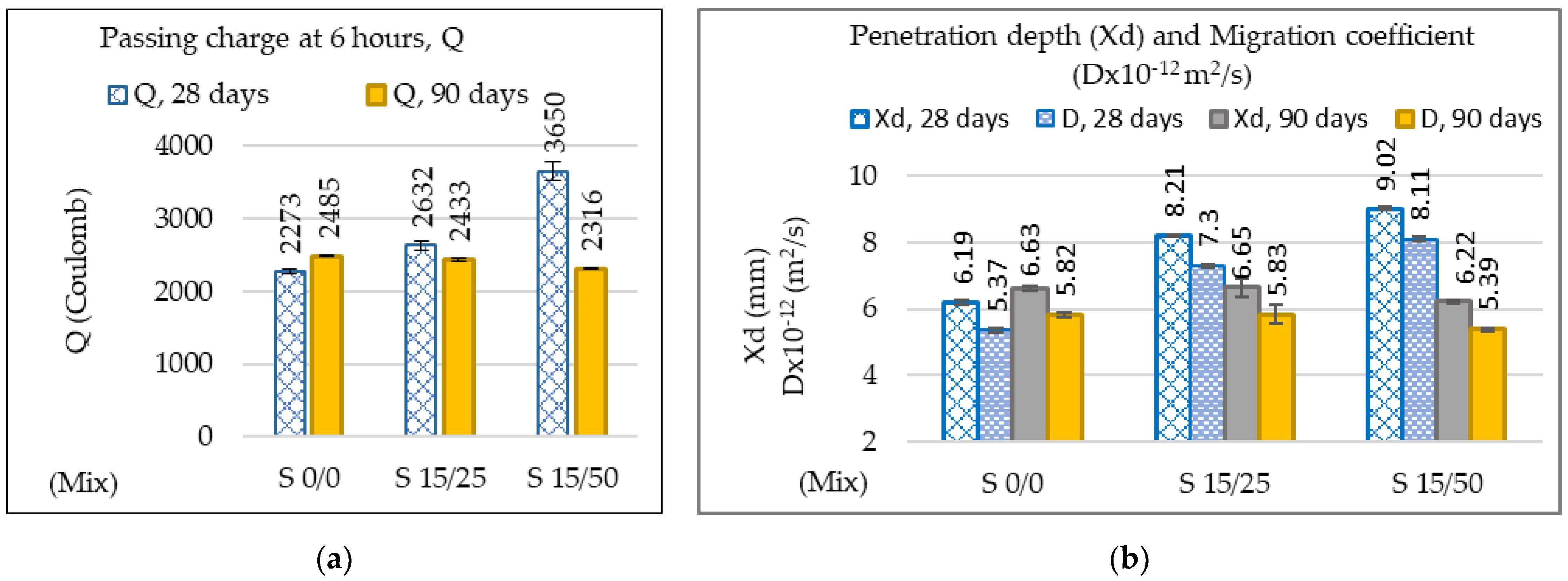
3.2.2. Resistance to Corrosion from Chlorides and from Carbonation at 90 Days
3.2.3. Carbonation Corrosion Resistance at 90 Days
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahasenan, N.; Smith, S.; Humphreys, K. The cement industry and global climate change: Current and potential future cement industry CO2 emissions. In Greenhouse Gas Control Technologies-6th International Conference; Elsevier: Amsterdam, The Netherlands, 2003; pp. 995–1000. [Google Scholar]
- Ahmed, H.U.; Mohammed, A.A.; Rafiq, S.; Mohammed, A.S.; Mosavi, A.; Sor, N.H.; Qaidi, S.M.A. Compressive strength of sustainable geopolymer concrete composites: A state-of-the-art review. Sustainability 2021, 13, 13502. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Mejeoumov, G.G. Improved Cement Quality and Grinding Efficiency by Means of Closed Mill Circuit Modeling; Texas A&M University: College Station, TX, USA, 2007; ISBN 0549415645. [Google Scholar]
- Ober, J.A. Mineral Commodity Summaries 2018; US Geological Survey: Reston, VA, USA, 2018.
- Lam, T.V.; Nguyen, M.H. Incorporating Industrial By-Products into Geopolymer Mortar: Effects on Strength and Durability. Materials 2023, 16, 4406. [Google Scholar] [CrossRef] [PubMed]
- Peceño, B.; Bakit, J.; Cortes, N.; Alonso-Fariñas, B.; Bonilla, E.; Leiva, C. Assessing Durability Properties and Economic Potential of Shellfish Aquaculture Waste in the Construction Industry: A Circular Economy Perspective. Sustainability 2022, 14, 8383. [Google Scholar] [CrossRef]
- Luna-Galiano, Y.; Leiva Fernández, C.; Villegas Sánchez, R.; Fernández-Pereira, C. Development of Geopolymer Mortars Using Air-Cooled Blast Furnace Slag and Biomass Bottom Ashes as Fine Aggregates. Processes 2023, 11, 1597. [Google Scholar] [CrossRef]
- Alonso-Fariñas, B.; Rodríguez-Galán, M.; Arenas, C.; Torralvo, F.A.; Leiva, C. Sustainable management of spent fluid catalytic cracking catalyst from a circular economy approach. Waste Manag. 2020, 110, 10–19. [Google Scholar] [CrossRef]
- Shalini, A.; Gurunarayanan, G.; Sakthivel, S. Performance of rice husk ash in geopolymer concrete. Int. J. Innov. Res. Sci. Tech 2016, 2, 73–77. [Google Scholar]
- Luna Galiano, Y.; Fernández Pereira, C.; Pérez, C.M.; Suarez, P. Influence of BFS content in the mechanical properties and acid attack resistance of fly ash based geopolymers. Key Eng. Mater. 2016, 663, 50–61. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Yan, C.; Yang, J.; Wu, Z. Design and Properties of Coal Gangue-Based Geopolymer Mortar. Buildings 2022, 12, 1932. [Google Scholar] [CrossRef]
- Dimulescu, C.; Burlacu, A. Industrial Waste Materials as Alternative Fillers in Asphalt Mixtures. Sustainability 2021, 13, 8068. [Google Scholar] [CrossRef]
- Popescu, D.; Burlacu, A. Considerations on the Benefits of Using Recyclable Materials for Road Construction. Rom. J. Transp. Infrastruct. 2017, 6, 43–53. [Google Scholar] [CrossRef][Green Version]
- Forton, A.; Mangiafico, S.; Sauzéat, C.; Di Benedetto, H.; Marc, P. Behaviour of binder blends: Experimental results and modelling from LVE properties of pure binder, RAP binder and rejuvenator. Road Mater. Pavement Des. 2021, 22, S197–S213. [Google Scholar] [CrossRef]
- Cadar, R.D.; Boitor, R.M.; Dragomir, M.L. An Analysis of Reclaimed Asphalt Pavement from a Single Source—Case Study: A Secondary Road in Romania. Sustainability 2022, 14, 7075. [Google Scholar] [CrossRef]
- Forton, A.; Mangiafico, S.; Sauzéat, C.; Di Benedetto, H.; Marc, P. Properties of blends of fresh and RAP binders with rejuvenator: Experimental and estimated results. Constr. Build. Mater. 2020, 236, 117555. [Google Scholar] [CrossRef]
- Shi, J.; Tan, J.; Liu, B.; Chen, J.; Dai, J.; He, Z. Experimental study on full-volume slag alkali-activated mortars: Air-cooled blast furnace slag versus machine-made sand as fine aggregates. J. Hazard. Mater. 2021, 403, 123983. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Santhanam, M.; Rakesh, S.; Kumar, A.; Gupta, A.K.; Kumar, R.; Sen, S.; Ramna, R.V. Utilization of Air-Cooled Blast Furnace Slag As a 100% Replacement of River Sand in Mortar and Concrete. Indian Concr. J. 2022, 96, 6–21. [Google Scholar]
- Smith, K.D.; Morian, D.A.; Van Dam, T.J. Use of Air-Cooled Blast Furnace Slag as Coarse Aggregate in Concrete Pavements—A Guide to Best Practice; Report No. FHWA-HIF-12-009; Federal Highway Administration: Washington, DC, USA, 2012.
- Abdel-Ghani, N.T.; El-Sayed, H.A.; El-Habak, A.A. Utilization of by-pass cement kiln dust and air-cooled blast-furnace steel slag in the production of some “green” cement products. HBRC J. 2018, 14, 408–414. [Google Scholar] [CrossRef]
- Golias, M.; Castro, J.; Weiss, J. The influence of the initial moisture content of lightweight aggregate on internal curing. Constr. Build. Mater. 2012, 35, 52–62. [Google Scholar] [CrossRef]
- Abadel, A.A. Physical, Mechanical, and Microstructure Characteristics of Ultra-High-Performance Concrete Containing Lightweight Aggregates. Materials 2023, 16, 4883. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Q.; Xie, C.; Xue, Y.; Duan, Y.; Cui, X. The Effect of Fineness on the Hydration Activity Index of Ground Granulated Blast Furnace Slag. Materials 2019, 12, 2984. [Google Scholar] [CrossRef]
- Samudrala, M.; Mujeeb, S.; Lanjewar, B.A.; Chippagiri, R.; Kamath, M.; Ralegaonkar, R. V 3D-Printable Concrete for Energy-Efficient Buildings. Energies 2023, 16, 4234. [Google Scholar] [CrossRef]
- Liu, Z.; Takasu, K.; Suyama, H.; Koyamada, H.; Liu, S.; Hao, Q. The Effect of Cementitious Materials on the Engineering Properties and Pore Structure of Concrete with Recycled Fine Aggregate. Materials 2023, 16, 305. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, X.; Zhang, G.; Li, L. Study the Mechanical Properties of Geopolymer under Different Curing Conditions. Minerals 2023, 13, 690. [Google Scholar] [CrossRef]
- Prusty, J.K.; Pradhan, B. Investigation on effect of precursor materials and sand-to-binder ratio on strength development, acid resistance and microstructure evolution of geopolymer mortar. Constr. Build. Mater. 2022, 346, 128501. [Google Scholar] [CrossRef]
- Smarzewski, P. Fresh and Mechanical Properties of High-Performance Self-Compacting Concrete Containing Ground Granulated Blast Furnace Slag and Polypropylene Fibres. Appl. Sci. 2023, 13, 1975. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Najm, O.F. Compressive strength and stability of sustainable self-consolidating concrete containing fly ash, silica fume, and GGBS. Front. Struct. Civ. Eng. 2017, 11, 406–411. [Google Scholar] [CrossRef]
- Pranav, S.; Aggarwal, S.; Yang, E.-H.; Sarkar, A.K.; Singh, A.P.; Lahoti, M. Alternative materials for wearing course of concrete pavements: A critical review. Constr. Build. Mater. 2020, 236, 117609. [Google Scholar] [CrossRef]
- Pop, A.; Bede, A.; Dudescu, M.C.; Popa, F.; Ardelean, I. Monitoring the influence of aminosilane on cement hydration via low-field NMR relaxometry. Appl. Magn. Reson. 2016, 47, 191–199. [Google Scholar] [CrossRef]
- Bede, A.; Scurtu, A.; Ardelean, I. NMR relaxation of molecules confined inside the cement paste pores under partially saturated conditions. Cem. Concr. Res. 2016, 89, 56–62. [Google Scholar] [CrossRef]
- Corbu, O.; Ioani, A.M.; Al Bakri, A.M.; Meiţă, V.; Szilagyi, H.; Sandu, A.V. Sandu the Pozzoolanic Activity Level of Powder Waste Glass in Comparisons with other Powders. Key Eng. Mater. 2015, 660, 237–243. [Google Scholar] [CrossRef]
- SR EN 197; Standard Cement—Part 1: Composition, Specification, and Conformity Criteria Common Cements. ASRO: Bucharest, Romania, 2011.
- SR EN 15167; Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout Part 1: Definitions, Specifications and Conformity Criteria. ASRO: Bucharest, Romania, 2007.
- Ahmad, J.; Kontoleon, K.J.; Majdi, A.; Naqash, M.T.; Deifalla, A.F.; Ben Kahla, N.; Isleem, H.F.; Qaidi, S.M.A. A Comprehensive Review on the Ground Granulated Blast Furnace Slag (GGBS) in Concrete Production. Sustainability 2022, 14, 8783. [Google Scholar] [CrossRef]
- D6868 Standard; Specification for Biodegradable Plastics Used as Coatings on Paper and Other Compostable Substrates. ASTM International: West Conshohocken, PA, USA, 2017.
- Nkinamubanzi, P.C.; Baalbaki, M.; Bickley, J.; Aitcin, P.C. The Use of Slag for Making High Performance Concrete. In Proceedings of the 6th NCB International Seminar on Cement and Bulding Materials, New Delhi, India, 24–27 November 1998. [Google Scholar]
- SR EN 12620+A1; Aggregates for Concrete. ASRO: Bucharest, Romania, 2008.
- IS 383; Coarse and Fine Aggregate for Concrete—Specification. Bureau of Indian Standards: New Delhi, India, 2016.
- SR EN 1097; Testing of Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption. ASRO: Bucharest, Romania, 2022.
- Nicula, L.M.; Manea, D.L.; Simedru, D.; Dragomir, M.L. Investigations Related to the Opportunity of Using Furnace Slag in the Composition of Road Cement Concrete. In Proceedings of the International Conference on Innovative Research Iasi, Iasi, Romania, 11–12 May 2023; p. 85. [Google Scholar]
- SR 667; Natural Aggregates and Processed Stone for Roads. ASRO: Bucharest, Romania, 2001.
- SR EN 196; Cement Test Methods. Part 6: Determination of Fineness. ASRO: Bucharest, Romania, 2019.
- SR EN 196-1; Methods of Testing Cement Part 1: Determination of Strength. ASRO: Bucharest, Romania, 2016.
- Phoo-Ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- SR EN 934-2+A1; Concrete Additives. ASRO: Bucharest, Romania, 2002.
- SR EN 1008; Mixing Water for Concrete. ASRO: Bucharest, Romania, 2003.
- Sosa, M.E.; Villagrán Zaccardi, Y.A.; Zega, C.J. A critical review of the resulting effective water-to-cement ratio of fine recycled aggregate concrete. Constr. Build. Mater. 2021, 313, 125536. [Google Scholar] [CrossRef]
- NE 014-2002; The Norm for the Execution of Cement Concrete Road Pavements in a Fixed and Sliding Formwork System. Matrix ROM: Bucharest, Romania, 2007; ISBN 978-973-755-185-6.
- SR EN 206; Standard for Concrete-Part 1: Specification, Performance, Production and Conformity. ASRO: Bucharest, Romania, 2021.
- Jenkins, R.; Snyder, R.L. Introduction to X-ray Powder Diffractometry; Wiley Online Library: Hoboken, NJ, USA, 1996; Volume 138. [Google Scholar]
- Venkataramanan, L.; Song, Y.-Q.; Hurlimann, M.D. Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions. IEEE Trans. Signal Process. 2002, 50, 1017–1026. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- SR EN 12390; Test on Hardened Concrete. Part 5: Bending Tensile Strength of Specimens. ASRO: Bucharest, Romania, 2019.
- SR EN 12390; Standard for Test-Hardened Concrete—Part 3: Compressive Strength of Test Specimens. ASRO: Bucharest, Romania, 2019.
- SR 3518; Tests on Concrete: Determination of the Freeze-Tawing Resistance by Measuring the Variations of the Resistance Strength and/or of the Dynamic Relative Elastics Modulus. ASRO: Bucharest, Romania, 2009.
- SR CEN/TS 12390; Test on Hardened Concrete. Part 9: Resistance to Freeze-Thaw Using De-Icing Salts. Exfoliating. ASRO: Bucharest, Romania, 2017.
- NT BUILD 492; Chloride Migration Coefficient from Nonsteady-State Migration Experiments. Nord. Finl: Oslo, Norway, 1999. Available online: https://www.betonconsultingeng.com/services/concrete-testing/nt-build-492/ (accessed on 28 June 2023).
- SR EN 1338; Concrete Pavers. Test Conditions and Methods. ASRO: Bucharest, Romania, 2006.
- ASTM C 642; Standard Test Method for Density, Absorption and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2006.
- SR CR 12793; Determination of the Depth of the Carbonation Layer of Hardened Concrete. ASRO: Bucharest, Romania, 2002.
- SS 13 72 44; Concrete Testing—Hardened Concrete—Scaling at Freezing. Swedish Institute for Standards: Stockholm, Sweden, 2005.
- Nicula, L.M.; Corbu, O.; Iliescu, M. Methods for assessing the frost-thaw resistance of road concrete used in our country and at European level. IOP Conf. Ser. Mater. Sci. Eng. 2020, 877, 012025. [Google Scholar] [CrossRef]
- Ne 012; Normative for the Production of Concrete and the Execution of Works in Concrete, Reinforced Concrete and Prestressed Concrete Part 1: Production of Concrete. Ministry of Development, Public Works and Administration: Bucharest, Romania, 2022.
- ASTM C 1202; Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM International: West Conshohocken, PA, USA, 2000.
- Bassuoni, M.T.; Nehdi, M.L.; Greenough, T.R. Enhancing the Reliability of Evaluating Chloride Ingress in Concrete Using the ASTM C 1202 Rapid Chloride Penetrability Test. J. ASTM Int. 2006, 3, 3. [Google Scholar]
- Liliana Maria, N.; Daniela Lucia, M.; Dorina, S.; Oana, C.; Ioan, A.; Mihai Liviu, D. The Advantages on Using GGBS and ACBFS Aggregate to Obtain an Ecological Road Concrete. Coatings 2023, 13, 1368. [Google Scholar] [CrossRef]
- ACI-233R-03; Slag Cement in Concrete and Mortar. American Concrete Institute: Farmington Hills, MI, USA, 2003.
- ASTM C989/C989M; Standard Specification for Slag Cement for Use in Concrete and Mortars. Available online: https://img.antpedia.com/standard/files/pdfs_ora/20210202/ASTM%20C989-18a.pdf (accessed on 24 August 2023).
- Liu, S.; Li, L. Influence of fineness on the cementitious properties of steel slag. J. Therm. Anal. Calorim. 2014, 117, 629–634. [Google Scholar] [CrossRef]
- Khan, K.; Amin, M.N. Influence of fineness of volcanic ash and its blends with quarry dust and slag on compressive strength of mortar under different curing temperatures. Constr. Build. Mater. 2017, 154, 514–528. [Google Scholar] [CrossRef]
- Badr, A.; Ashour, A.F.; Platten, A.K. Statistical variations in impact resistance of polypropylene fibre-reinforced concrete. Int. J. Impact Eng. 2006, 32, 1907–1920. [Google Scholar] [CrossRef]
- Day, K.W. Concrete Mix Design, Quality Control and Specification, 2nd ed.; E&FN Spon: London, UK, 1999. [Google Scholar]
- Swamy, R.N.; Stavrides, H. Some statistical considerations of steel fiber composites. Cem. Concr. Res. 1976, 6, 201–216. [Google Scholar] [CrossRef]
- Jakob, C.; Jansen, D.; Ukrainczyk, N.; Koenders, E.; Pott, U.; Stephan, D.; Neubauer, J. Relating Ettringite Formation and Rheological Changes during the Initial Cement Hydration. Materials 2019, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, C.; Destefanis, E.; Pastero, L.; Bernasconi, D.; Bonadiman, C.; Pavese, A. MSWI Fly Ash Multiple Washing: Kinetics of Dissolution in Water, as Function of Time, Temperature and Dilution. Minerals 2022, 12, 742. [Google Scholar] [CrossRef]
- Wang, B.; Feng, H.; Huang, H.; Guo, A.; Zheng, Y.; Wang, Y. Bonding Properties between Fly Ash/Slag-Based Engineering Geopolymer Composites and Concrete. Materials 2023, 16, 4232. [Google Scholar] [CrossRef]
- Teoreanu, I. Basics of Binder Technology; Bucharest Technical Publishing House: Bucharest, Romania, 1975. [Google Scholar]
- Matalkah, F.; Soroushian, P.; Ul Abideen, S.; Peyvandi, A. Use of non-wood biomass combustion ash in development of alkali-activated concrete. Constr. Build. Mater. 2016, 121, 491–500. [Google Scholar] [CrossRef]
- Megat Johari, M.A.; Brooks, J.J.; Kabir, S.; Rivard, P. Influence of supplementary cementitious materials on engineering properties of high strength concrete. Constr. Build. Mater. 2011, 25, 2639–2648. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Ganesh, P.; Murthy, A.R. Tensile behaviour and durability aspects of sustainable ultra-high performance concrete incorporated with GGBS as cementitious material. Constr. Build. Mater. 2019, 197, 667–680. [Google Scholar] [CrossRef]
- Liu, L.; He, Z.; Cai, X.; Fu, S. Application of Low-Field NMR to the Pore Structure of Concrete. Appl. Magn. Reson. 2021, 52, 15–31. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, J.; Bian, Z.; Dai, G. Pore Structure Characterization of Hardened Cement Paste by Multiple Methods. Adv. Mater. Sci. Eng. 2019, 2019, 3726953. [Google Scholar] [CrossRef]
- Scurtu, D.A.; Kovacs, E.; Senila, L.; Levei, E.A.; Simedru, D.; Filip, X.; Dan, M.; Roman, C.; Cadar, O.; David, L. Use of Vine Shoot Waste for Manufacturing Innovative Reinforced Cement Composites. Appl. Sci. 2023, 13, 134. [Google Scholar] [CrossRef]
- Simedru, A.F.; Becze, A.; Cadar, O.; Scurtu, D.A.; Simedru, D.; Ardelean, I. Structural Characterization of Several Cement-Based Materials Containing Chemical Additives with Potential Application in Additive Manufacturing. Int. J. Mol. Sci. 2023, 24, 7688. [Google Scholar] [CrossRef]
- Huang, J.; Li, W.; Huang, D.; Wang, L.; Chen, E.; Wu, C.; Wang, B.; Deng, H.; Tang, S.; Shi, Y.; et al. Fractal Analysis on Pore Structure and Hydration of Magnesium Oxysulfate Cements by First Principle, Thermodynamic and Microstructure-Based Methods. Fractal Fract. 2021, 5, 164. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, S.; Huang, J.; Tang, C.; Wang, L.; Liu, Y. Fractal Analysis on Pore Structure and Modeling of Hydration of Magnesium Phosphate Cement Paste. Fractal Fract. 2022, 6, 337. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-chong, T.; Dangla, P. Pore structure characterization of cement pastes blended with high-volume fly-ash. Cem. Concr. Res. 2012, 42, 194–204. [Google Scholar] [CrossRef]
- Nicula, L.M.; Manea, D.L.; Simedru, D.; Cadar, O.; Becze, A.; Dragomir, M.L. The Influence of Blast Furnace Slag on Cement Concrete Road by Microstructure Characterization and Assessment of Physical-Mechanical Resistances at 150/480 Days. Materials 2023, 16, 3332. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Lee, J.; Choi, S. Case Studies in Construction Materials Effect of replacement ratio of ferronickel slag aggregate on characteristics of cementitious mortars at different curing temperatures. Case Stud. Constr. Mater. 2023, 18, e01882. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Fall, M.; Belem, T. A contribution to understanding the hardening process of cemented pastefill. Miner. Eng. 2004, 17, 141–152. [Google Scholar] [CrossRef]
- Chu, D.C.; Kleib, J.; Amar, M.; Benzerzour, M.; Abriak, N.-E. Determination of the degree of hydration of Portland cement using three different approaches: Scanning electron microscopy (SEM-BSE) and Thermogravimetric analysis (TGA). Case Stud. Constr. Mater. 2021, 15, e00754. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York: NY, USA, 2014; ISBN 0071797874. [Google Scholar]
- Wang, L.; Guo, F.; Lin, Y.; Yang, H.; Tang, S.W. Comparison between the effects of phosphorous slag and fly ash on the CSH structure, long-term hydration heat and volume deformation of cement-based materials. Constr. Build. Mater. 2020, 250, 118807. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Cao, M.; Tang, Y.; Zhang, Z. Nanoindentation and porosity fractal dimension of calcium carbonate whisker reinforced cement paste after elevated temperatures (UP to 900 °C). Fractals 2021, 29, 2140001. [Google Scholar] [CrossRef]
- Smirnov, D.; Stepanov, S.Y.; Garipov, R.; Garayev, T.; Sungatullin, T. Influence of the porosity structure of road concrete on its durability. E3S Web Conf. 2021, 274, 04009. [Google Scholar] [CrossRef]
- Yang, K.; White, C.E. Modeling of aqueous species interaction energies prior to nucleation in cement-based gel systems. Cem. Concr. Res. 2021, 139, 106266. [Google Scholar] [CrossRef]
- Bjegović, D.; Serdar, M.; Oslaković, I.S.; Jacobs, F.; Beushausen, H.; Andrade, C.; Monteiro, A.V.; Paulini, P.; Nanukuttan, S. Test methods for concrete durability indicators. In RILEM State-of-the-Art Reports; Springer: Berlin/Heidelberg, Germany, 2016; Volume 18, pp. 51–105. [Google Scholar] [CrossRef]
- Ionescu, I.; Ispas, T. The Properties and Technology of Concrete; Bucharest Technical Publishing House: Bucharest, Romania, 1997. [Google Scholar]
- Karthika, R.B.; Vidyapriya, V.; Sri, K.V.N.; Beaula, K.M.G.; Harini, R.; Sriram, M. Experimental study on lightweight concrete using pumice aggregate. Mater. Today Proc. 2021, 43, 1606–1613. [Google Scholar] [CrossRef]
- Correia, V.; Ferreira, J.G.; Tang, L.; Lindvall, A. Effect of the addition of GGBS on the frost scaling and chloride migration resistance of concrete. Appl. Sci. 2020, 10, 3940. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Bamforth, P.B. Modelling chloride diffusion in concrete: Effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495. [Google Scholar] [CrossRef]
- Pimienta, P.; Albert, B.; Huetb, B.; Dierkens, M.; Francisco, P.; Rougeaud, P. Durability performance assessment of non-standard cementitious materials for buildings: A general method applied to the French context, Fact sheet 1—Risk of steel corrosion induced by carbonation. RILEM Tech. Lett. 2016, 1, 102–108. [Google Scholar] [CrossRef]
- Medeiros, R.A.; Lima, M.G.; Yazigi, R.; Medeiros, M.H.F. Carbonation depth in 57 years old concrete structure. Steel Compos. Struct. 2015, 19, 953–966. [Google Scholar] [CrossRef]



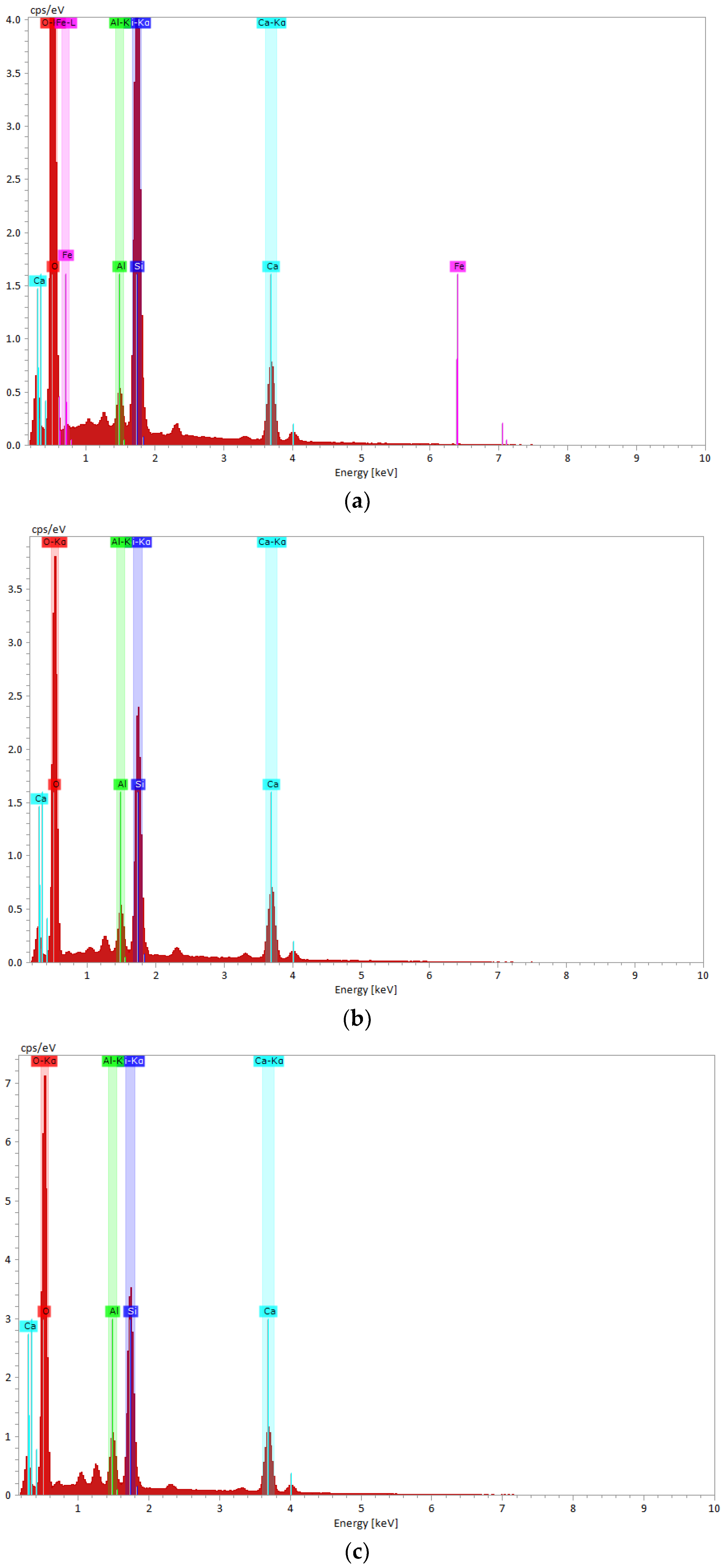




| Element | SiO2 | Al2O3 | MnO | MgO | CaO | Fe2O3 | FeO | Na2O | K2O | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| GGBS (%) | 38.10 | 9.50 | 0.23 | 8.10 | 40.80 | 0.56 | 0.30 | 0.68 | 1.73 | |
| ACBFS (%) | 39.01 | 9.14 | 0.51 | 7.57 | 41.37 | 0.58 | 0.01 | 0.64 | 1.17 |
| Materials | M I | M II/360 | M III/330 |
|---|---|---|---|
| Binder-specific surface area | 331 m2/kg | 360 m2/kg | 330 m2/kg |
| Portland cement, (g) | 450 ± 2 | 225 ± 1 | 225 ± 1 |
| (GGBS ˂ 63 µm), (g) | - | 225 ± 1 | 225 ± 1 |
| (NA_0/4 mm), (g) | 1350 ± 5 | 1350 ± 5 | 1350 ± 5 |
| Water, (mL) | 225 | 225 | 225 |
| Material (kg/m3) | S 0/0 Reference Concrete | S 15/25 (15%GGBS_25%ACBFS) | S 15/50 (15%GGBS_50%ACBFS) |
|---|---|---|---|
| Cement (C) | 370 | 314.50 | 314.50 |
| (GGBS ˂ 63 µm) | - | 55.50 | 55.50 |
| Binder (b) | 370 | 370 | 370 |
| Water (w) | 151.68 | 154.51 | 155.92 |
| w/b | 0.41 | 0.418 | 0.421 |
| (ACBFS_0/4 mm) | - | 163.44 | 326.90 |
| Natural sand (AN_0/4 mm) | 654.70 | 490.33 | 326.90 |
| Coarse aggregate (AC_4/25 mm) | 1215.88 | 1214.16 | 1214.18 |
| Aggregate total | 1870.58 | 1867.94 | 1867.97 |
| Admixtures (SP-SKY 527) | 5.55 | 6.18 | 6.29 |
| Admixtures (MA 9060) | 0.74 | 0.74 | 0.81 |
| Parameters Design | Min. Dosage Cement | (w/L) | Compaction (%) | Density (kg/m2) | Occluded Air (%) | fcm 28 Days MPa | fcf, fl 28 Days MPa |
|---|---|---|---|---|---|---|---|
| NE 014 and SR EN 206 | 360 kg/m3 | max. 0.45 | 1.15 ÷ 1.35 | 2390 ± 30 | 5.0 ÷ 6.5 | min. 50 | min. 5.5 |
| Results | 370 kg/m3 | 0.41 ÷ 0.42 | 1.26 ÷ 1.33 | 2392 ÷ 2394 | 5.1 ÷ 6.4 | - | - |
| SR EN 15167:1 | ASTM C989 (Clasa 80) | ASTM C989 (Clasa 100) | ASTM C989 (Clasa 120) |
|---|---|---|---|
| 70 | 75 | 95 | 115 |
| Frost-Thaw Resistance | Requirement SS 13 72 44 | Requirement NE 012-1 | Requirement SR 3518 |
|---|---|---|---|
| Very good | m56 < 0.10 kg/m2 | ||
| Hi | m56 < 0.20 kg/m2 | m56 < 0.50 kg/m2 | - |
| (Raised) | or m56 < 0.50 kg/m2 and m56/m28 < 2 | ||
| or m112 < 0.50 kg/m2 | |||
| Acceptable (Moderate) | m56 < 1.00 kg/m2 and m56/m28 < 2 or m112 < 1.00 kg/m2 | m56 < 1.0 kg/m2 | - |
| Unacceptable (Low) | m56 ≥ 1.00 kg/m2 and m56/m28 ≥ 2 or m112 ≥ 1.00 kg/m2 | m56 < 2.0 kg/m2 | - |
| Degree of gelling G100 | - | - | Loss of compressive strength (η) ˂ 25% |
| Class | 1 | 3 | 4 |
|---|---|---|---|
| Marcare | F | H | I |
| Criteria | No measured performance | ≤20,000 mm3/5000 mm2 | ≤18,000 mm3/5000 mm2 |
| Past Electrical Charge (Coulomb) | High | Moderate | Low | Very Low |
|---|---|---|---|---|
| Penetrability of chlorine ions | ˃4000 | 2000–4000 | 1000–2000 | 100–1000 |
| Mix | MI | MII/360 | MIII/330 |
|---|---|---|---|
| Coefficient of variation | CoV (%) | CoV (%) | CoV (%) |
| Compressive strength at 28 days-fc (MPa) | 10 | 11 | 8 |
| Compressive strength at 56 days-fc (MPa) | 6 | 4 | 7 |
| Compressive strength at 90 days-fc (MPa) | 8 | 7 | 13 |
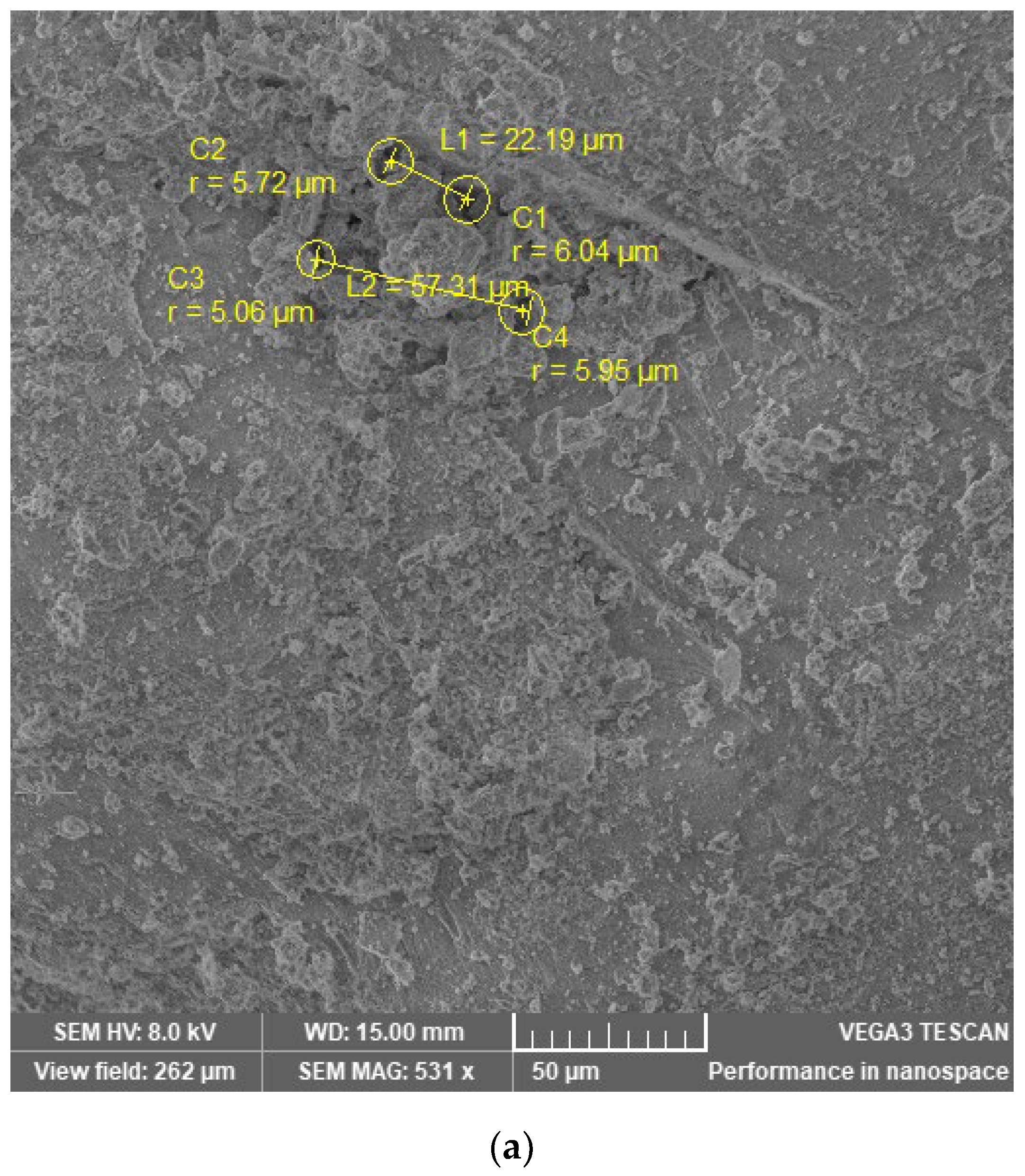
| Sample | Pore Identification Code | Pore Radius (μm) | Distance Code Li(Ci–Ci + n) | Distance (μm) |
|---|---|---|---|---|
| M I | C1 | 6.04 | L1(C1–C2) | 22.19 |
| C2 | 5.72 | |||
| C3 | 5.06 | L2(C3–C4) | 57.31 | |
| C4 | 5.95 | |||
| M II/360 | C1 | 7.40 | ||
| C2 | 10.20 | L1(C2–C3) | 26.96 | |
| C3 | 5.31 | |||
| C4 | 5.16 | L2(C4–C6) | 205.25 | |
| C5 | 7.48 | |||
| C6 | 6.66 | |||
| M III/330 | C1 | 6.57 | L2(C1–C6) | 209.19 |
| C2 | 11.58 | |||
| C3 | 6.69 | L1(C3–C4) | 32.44 | |
| C4 | 6.27 | |||
| C5 | 11.29 | |||
| C6 | 14.18 |
| Mix | S 0/0 | S 15/25 | S 15/50 |
|---|---|---|---|
| Coefficient of variation | CoV (%) | CoV (%) | CoV (%) |
| The tensile strength, -fct, fl (MPa) | 6.1 | 10.4 | 4.4 |
| Reference compressive strength, fc (MPa) | 7.0 | 4.0 | 1.0 |
| Compressive strength at 100 freeze–thaw, fc (MPa) | 12 | 9.0 | 2.0 |
| The exfoliated mass at 56 freeze–thaw cycles (kg/m2) | 8.2 | 8.8 | 13.3 |
| Loss of volume from wear (mm3) | 5.7 | 2.6 | 7.7 |
| Permeable pore content (%) | 7.3 | 3.3 | 8.9 |
| Passing charge at 6 h (Q) | 0.7 | 1.7 | 0.6 |
| Penetration depth Xd (mm) | 2.3 | 8.8 | 1.4 |
| Migration coefficient of chlorine ions (Dx (10−12 m2/s)) | 2.6 | 9.7 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicula, L.M.; Manea, D.L.; Simedru, D.; Cadar, O.; Dragomir, M.L.; Ardelean, I.; Corbu, O. Potential Role of GGBS and ACBFS Blast Furnace Slag at 90 Days for Application in Rigid Concrete Pavements. Materials 2023, 16, 5902. https://doi.org/10.3390/ma16175902
Nicula LM, Manea DL, Simedru D, Cadar O, Dragomir ML, Ardelean I, Corbu O. Potential Role of GGBS and ACBFS Blast Furnace Slag at 90 Days for Application in Rigid Concrete Pavements. Materials. 2023; 16(17):5902. https://doi.org/10.3390/ma16175902
Chicago/Turabian StyleNicula, Liliana Maria, Daniela Lucia Manea, Dorina Simedru, Oana Cadar, Mihai Liviu Dragomir, Ioan Ardelean, and Ofelia Corbu. 2023. "Potential Role of GGBS and ACBFS Blast Furnace Slag at 90 Days for Application in Rigid Concrete Pavements" Materials 16, no. 17: 5902. https://doi.org/10.3390/ma16175902
APA StyleNicula, L. M., Manea, D. L., Simedru, D., Cadar, O., Dragomir, M. L., Ardelean, I., & Corbu, O. (2023). Potential Role of GGBS and ACBFS Blast Furnace Slag at 90 Days for Application in Rigid Concrete Pavements. Materials, 16(17), 5902. https://doi.org/10.3390/ma16175902











