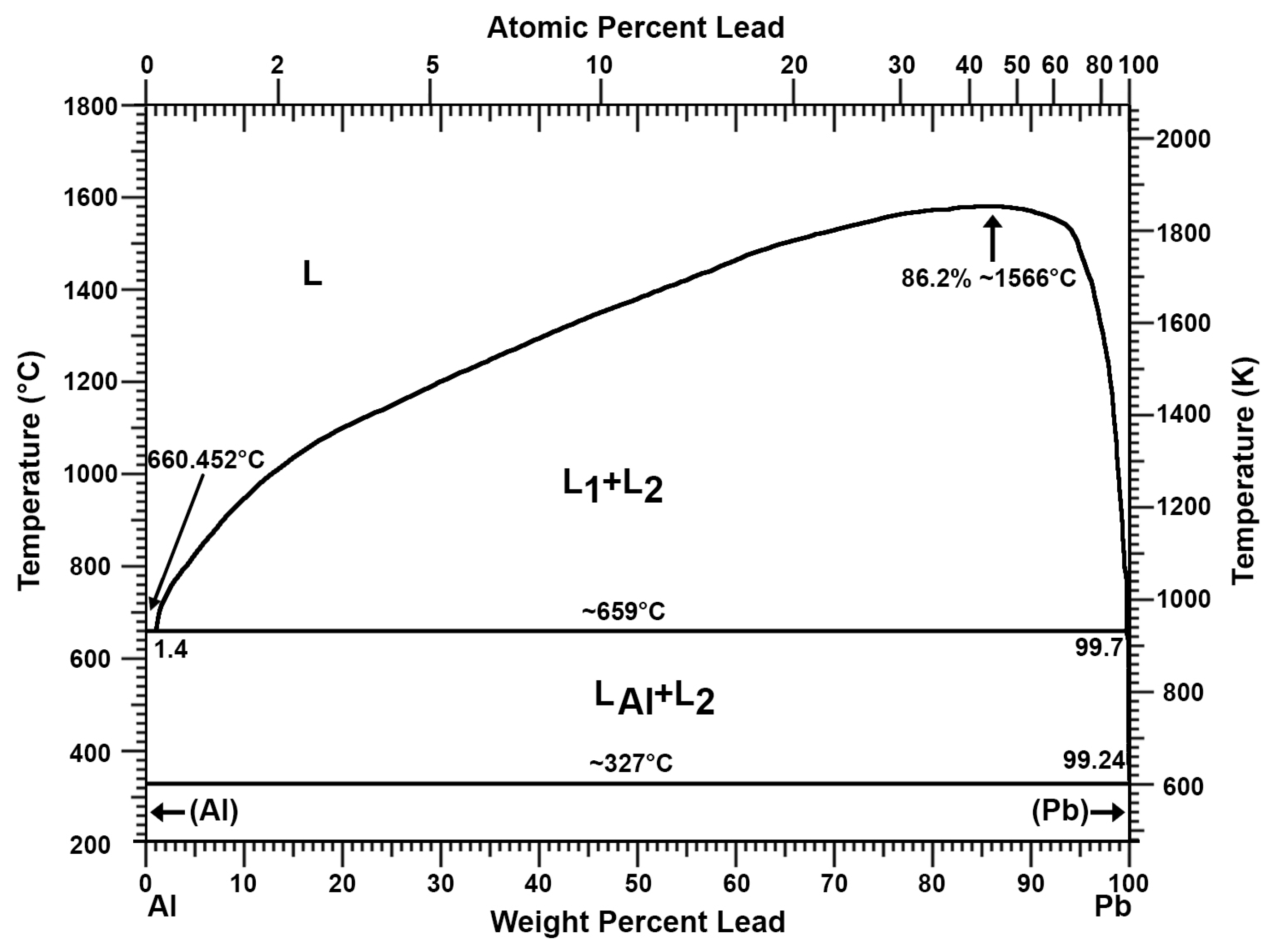
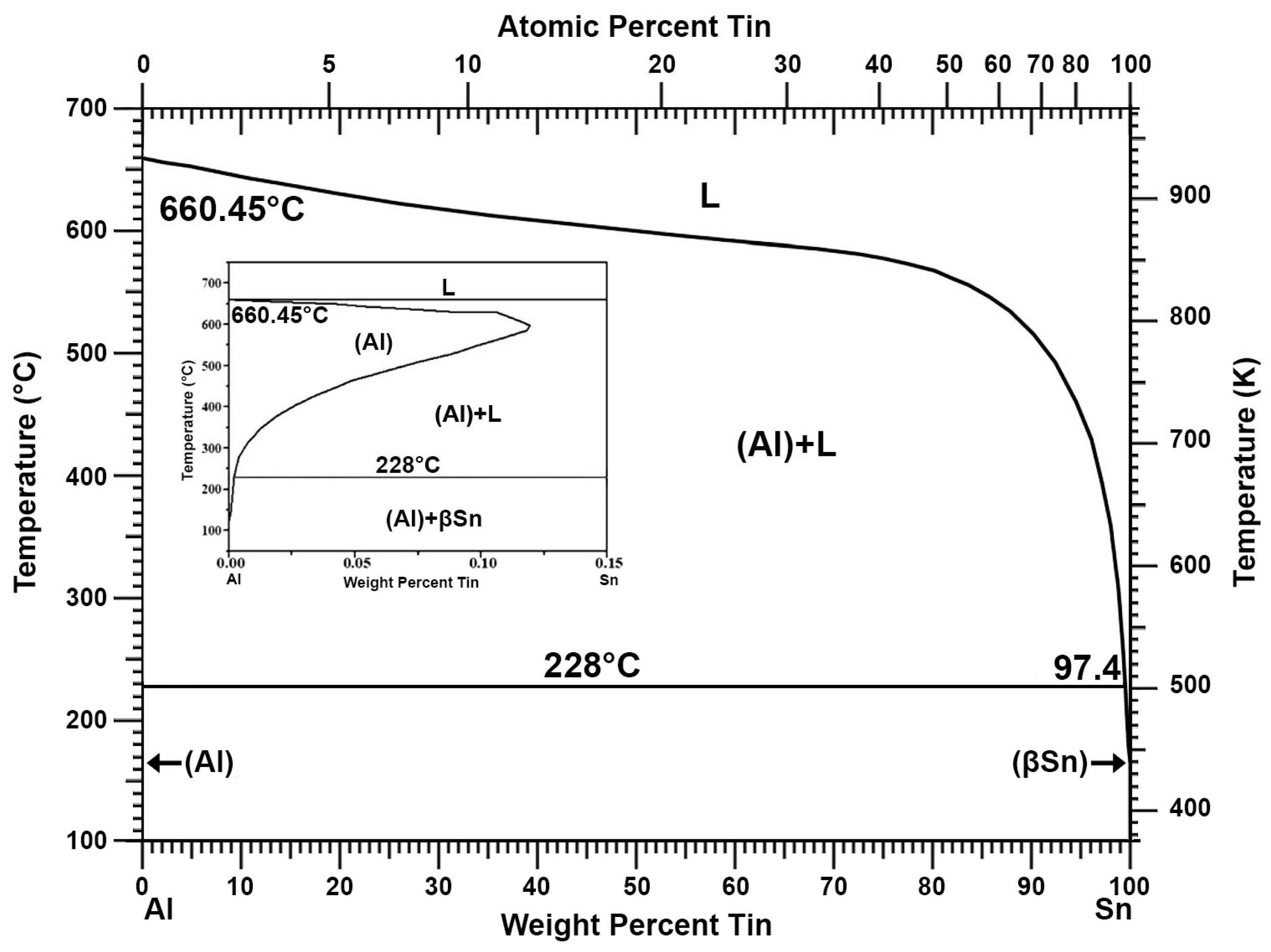
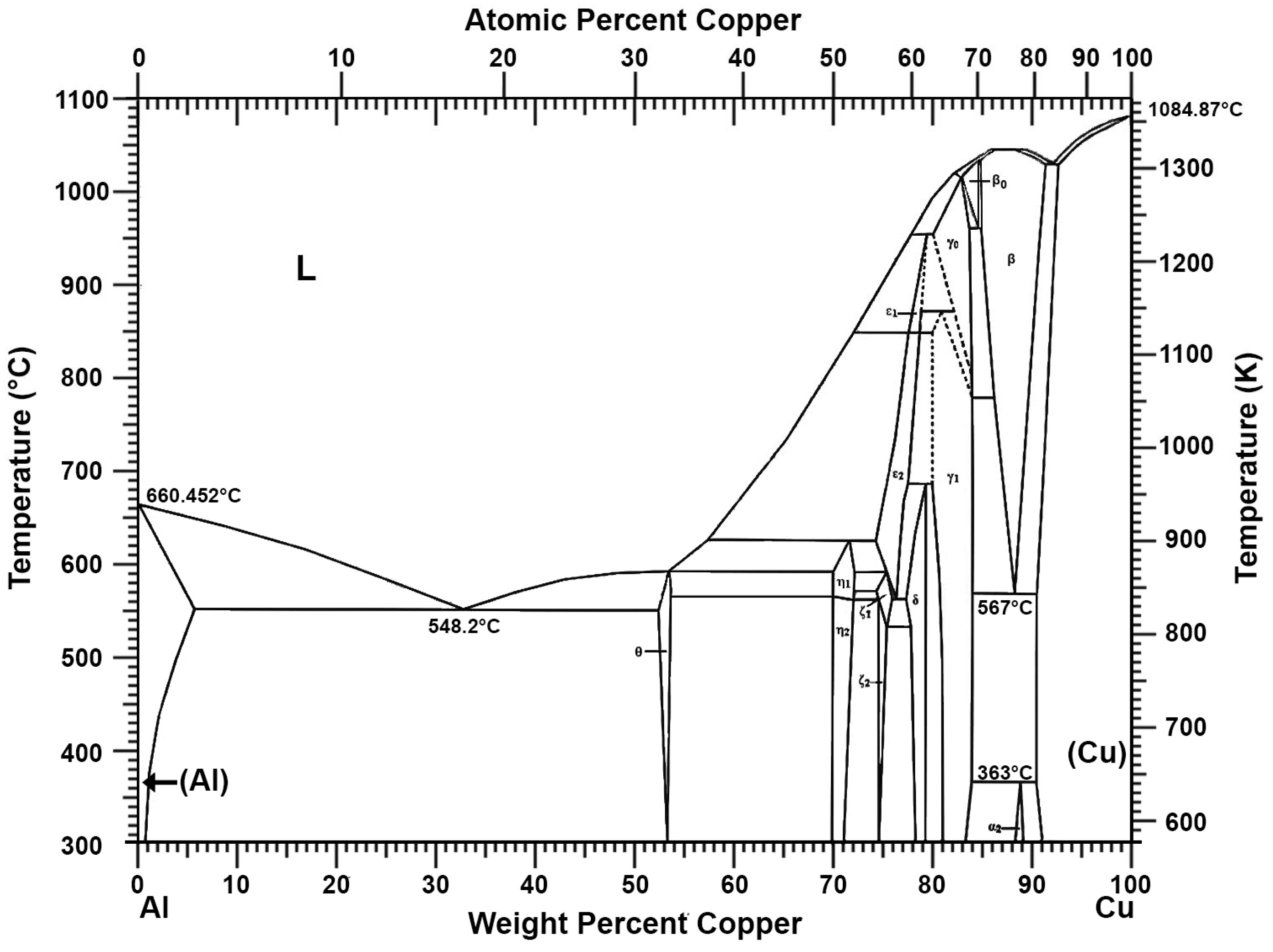
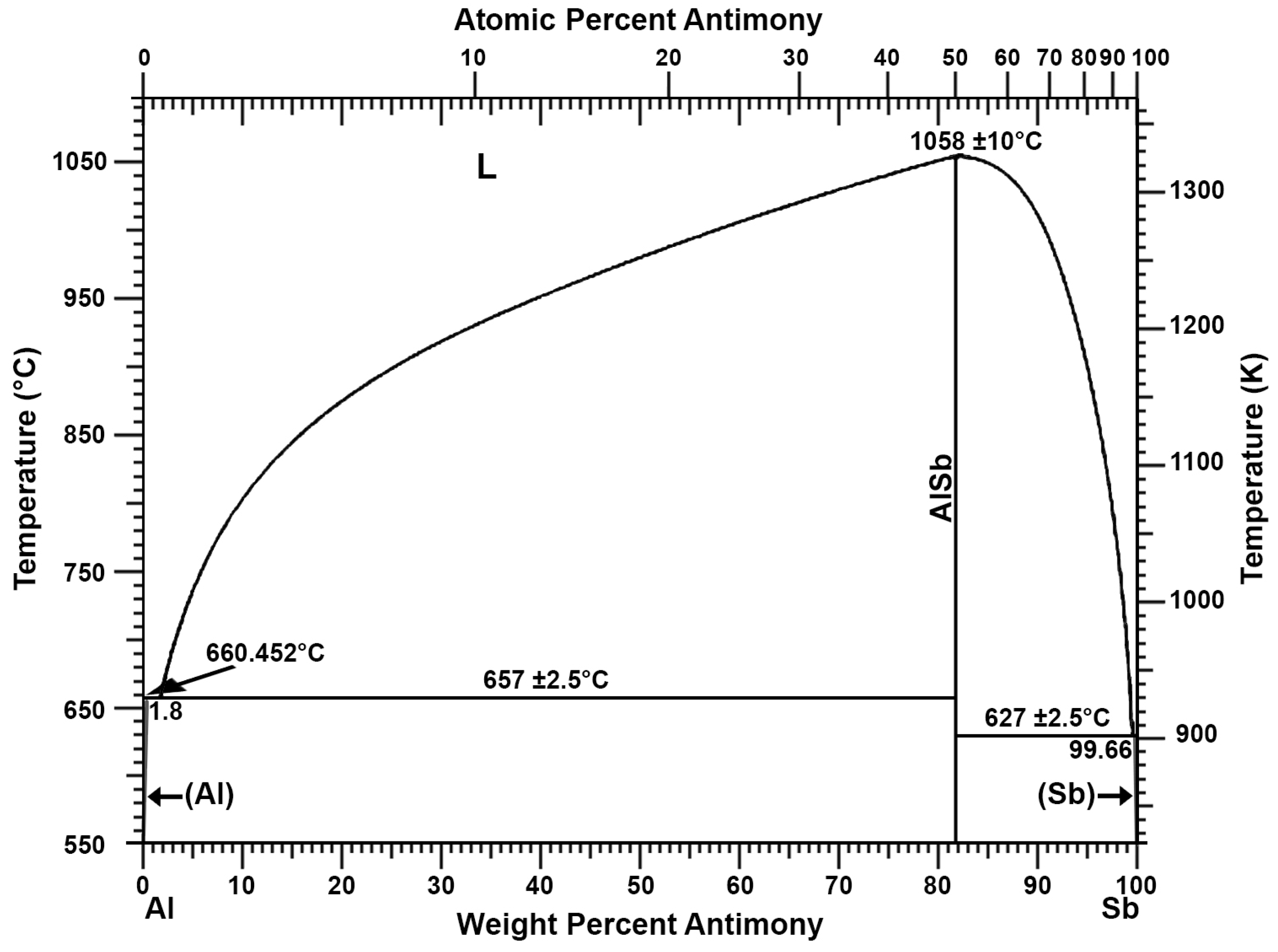
Recovery of Pure Lead-Tin Alloy from Recycling Spent Lead-Acid Batteries
Abstract
:1. Introduction
2. Materials and Methods
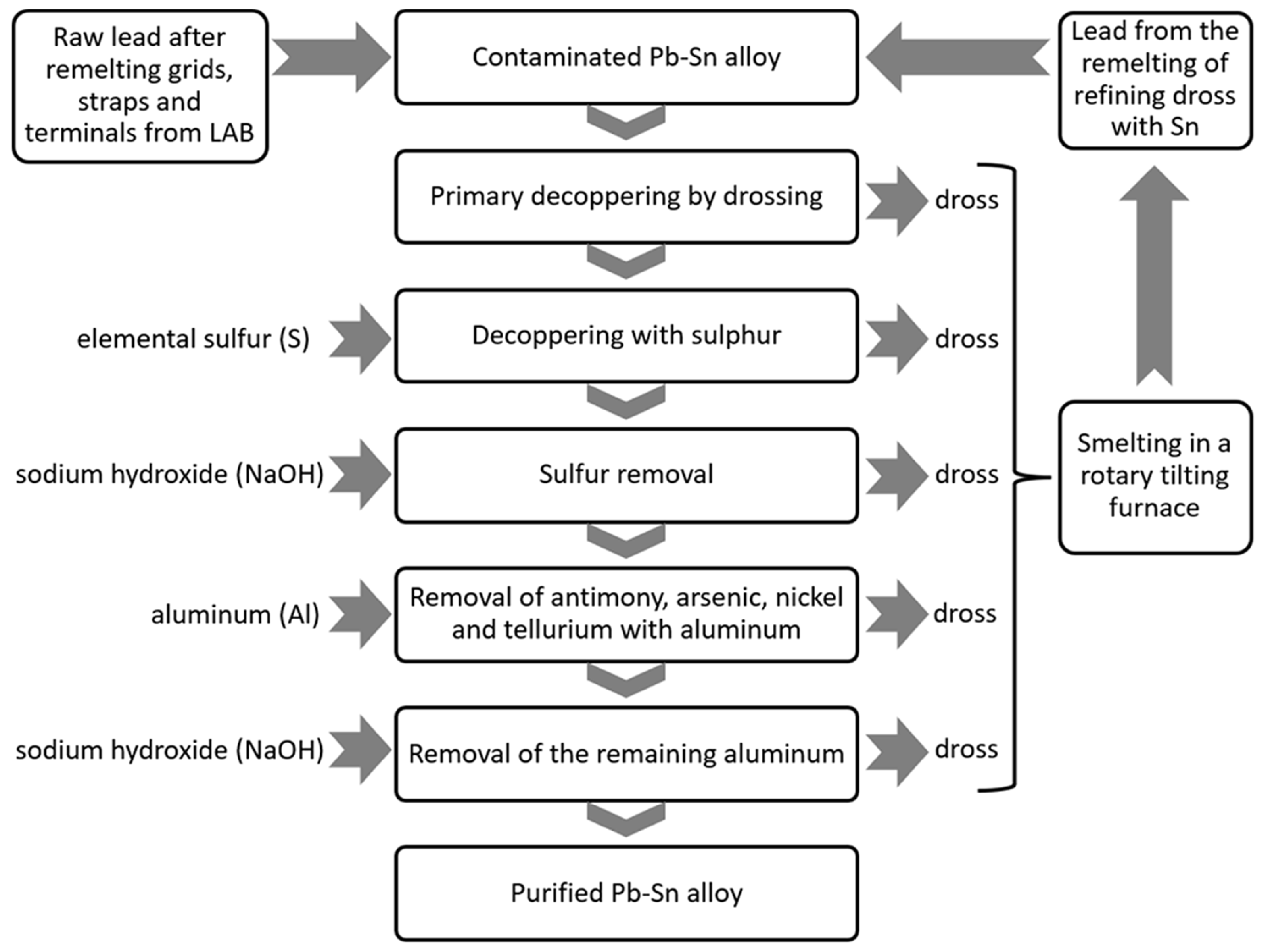
2.1. Refining of Secondary Lead Containing Tin—Description of the Process
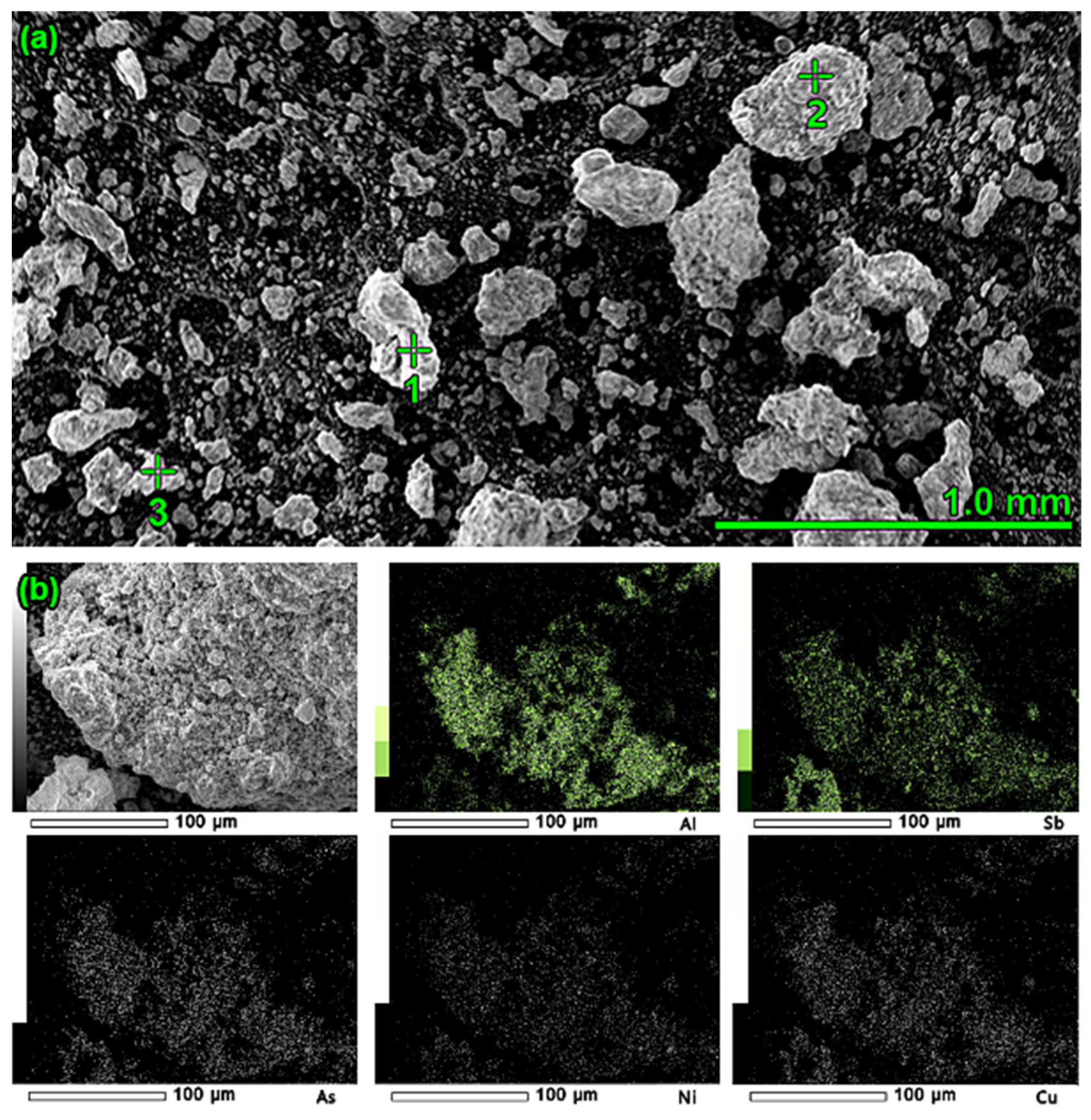
2.2. Origin and Characteristics of the Material
2.3. Place and Method of Testing
2.4. Chemical Analysis
3. Results and Discussion
3.1. Preliminary Information
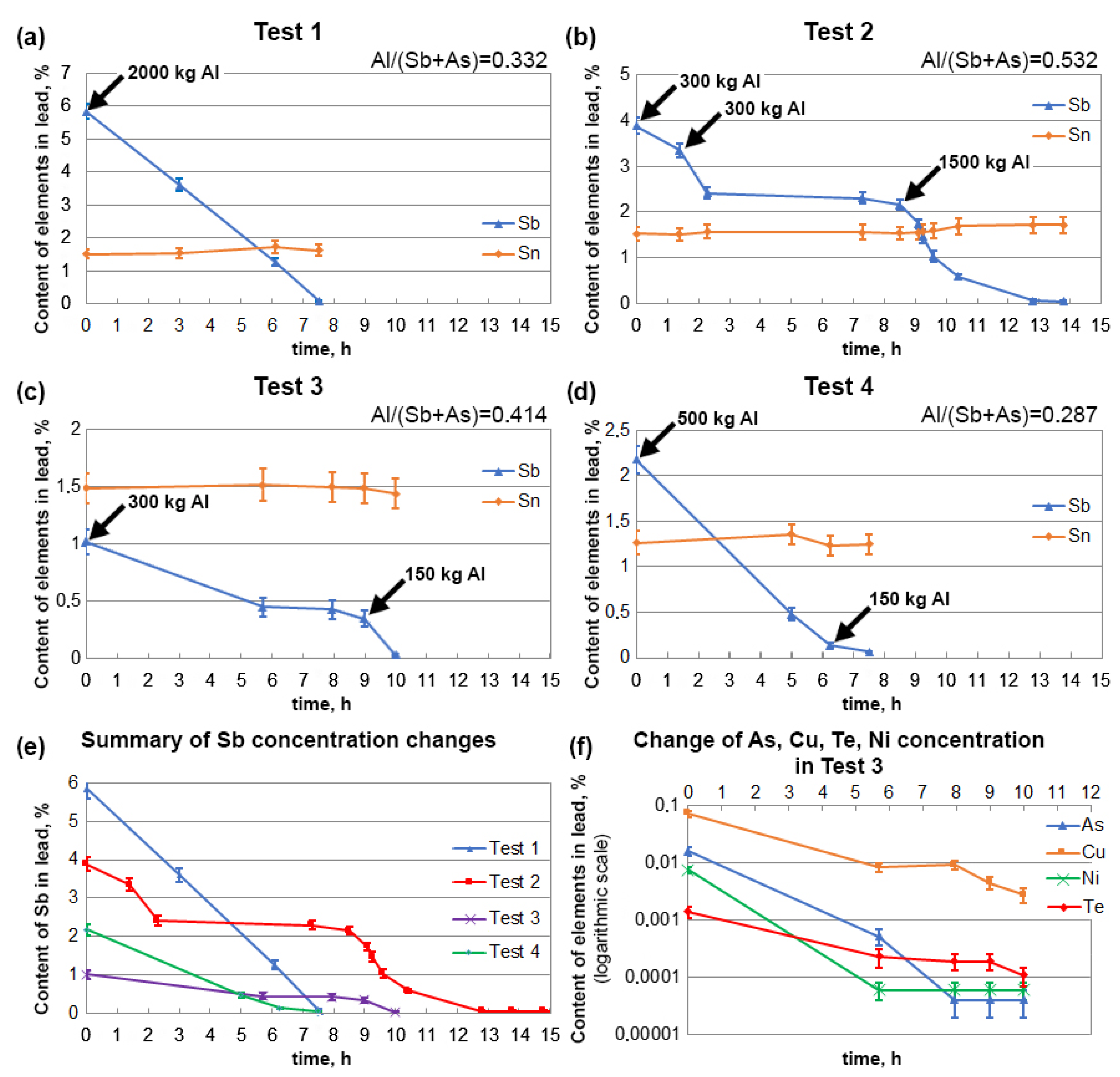
3.2. Refining of Alloys with Low Tin Content
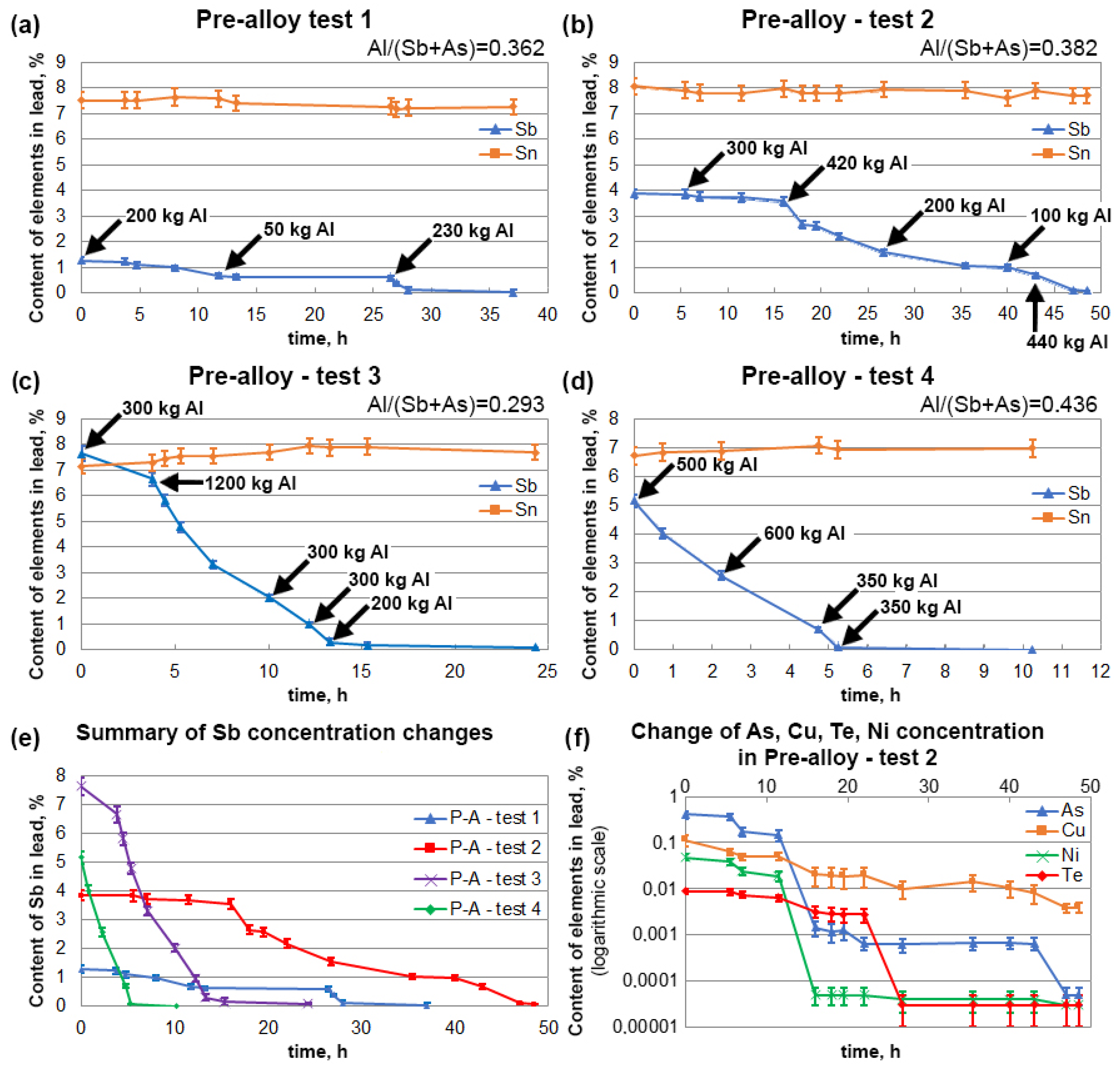
3.3. Refining of Alloys with High Tin Content
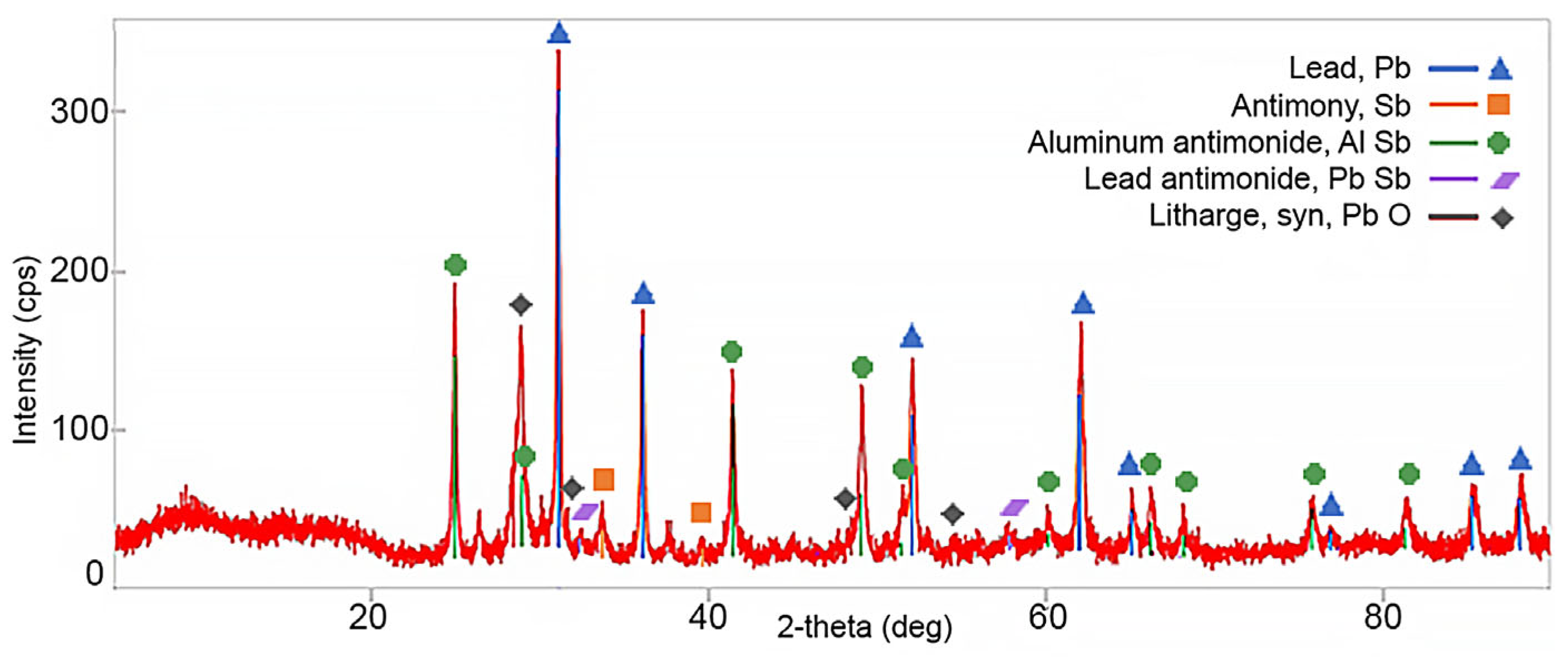
3.4. Dross Resulting from the Refining Process with Aluminium
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szela, R.; Małecki, S.; Gargul, K. Lead Oxide Production in Barton Reactor—Effect of Increased Air Humidity on Lead Oxide Production Parameters. Materials 2022, 15, 4941. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, J.; Liang, S.; Hou, H.; Hu, J.; Liu, B.; Kumar, R.V. Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products. J. Power Sources 2019, 436, 226853. [Google Scholar] [CrossRef]
- Kavaliauskas, Ž.; Šajev, I.; Blažiūnas, G.; Gecevičius, G. Electronic Life Cycle Monitoring System for Various Types of Lead Acid Batteries. Appl. Sci. 2023, 13, 4746. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, P.; Cao, J.; Zhang, J.; Huang, Y.; Chen, B. Analysis of a more sustainable method for recycling waste lead batteries: Surface renewal promotes desulfurization agent regeneration. Waste Manag. 2022, 137, 319–328. [Google Scholar] [CrossRef]
- Suzuki, Y.; Imamura, Y.; Katsube, D.; Kogure, A.; Hirai, N.; Kimura, M. Visualization of Electrolyte Reaction Field Near the Negative Electrode of a Lead Acid Battery by Means of Amplitude/Frequency Modulation Atomic Force Microscopy. Materials 2023, 16, 2146. [Google Scholar] [CrossRef]
- Rada, S.; Pintea, A.; Opre, R.; Unguresan, M.; Popa, A. Recycled and Nickel- or Cobalt-Doped Lead Materials from Lead Acid Battery: Voltammetric and Spectroscopic Studies. Materials 2023, 16, 4507. [Google Scholar] [CrossRef]
- Prengaman, R.D. Chapter 2—Lead Alloys for Valve-Regulated Lead-Acid Batteries. In Valve-Regulated Lead-Acid Batteries; Rand, D.A.J., Garche, J., Moseley, P.T., Parker, C.D., Eds.; Elsevier: Oxford, UK, 2004; pp. 15–35. [Google Scholar] [CrossRef]
- Cartigny, Y.; Fiorani, J.M.; Maître, A.; Vilasi, M. Thermodynamic assessment on the Pb–Ca–Sn ternary system. Intermet. 2003, 11, 1205–1210. [Google Scholar] [CrossRef]
- Tian, X.; Wu, Y.; Hou, P.; Liang, S.; Qu, S.; Xu, M.; Zuo, T. Environmental impact and economic assessment of secondary lead production: Comparison of main spent lead-acid battery recycling processes in China. J. Clean. Prod. 2017, 144, 142–148. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Wu, X.; Hu, Y.; Yu, W.; Wang, J.; Dong, J.; Li, M.; Liang, S.; Hu, J.; et al. A critical review on secondary lead recycling technology and its prospect. Renew. Sustain. Energy Rev. 2016, 61, 108–122. [Google Scholar] [CrossRef]
- Huang, K.; Liu, H.; Dong, H.; Lin, M.; Ruan, J. A novel approach to recover lead oxide from spent lead acid batteries by desulfurization and crystallization in sodium hydroxide solution after sulfation. Resour. Conserv. Recycl. 2021, 167, 105385. [Google Scholar] [CrossRef]
- Chen, B.; Cao, J.; Ge, F.; Zhang, J.; Huang, Y. An innovative synergistic recycling route of spent lead paste and lead grid based on sodium nitrate reuse. J. Environ. Chem. Eng. 2022, 10, 108454. [Google Scholar] [CrossRef]
- Blanpain, B.; Arnout, S.; Chintinne, M.; Swinbourne, D.R. Chapter 8—Lead Recycling. In Handbook of Recycling; Worrell, E., Reuter, M.A., Eds.; Elsevier: Oxford, UK, 2014; pp. 95–111. [Google Scholar] [CrossRef]
- Pan, H.; Geng, Y.; Dong, H.; Ali, M.; Xiao, S. Sustainability evaluation of secondary lead production from spent lead acid batteries recycling. Resour. Conserv. Recycl. 2019, 140, 13–22. [Google Scholar] [CrossRef]
- Liu, T.; Bao, Z.; Qiu, K. Recycling of lead from spent lead-acid battery by vacuum reduction-separation of Pb-Sb alloy coupling technology. Waste Manag. 2020, 103, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Hou, H.; Li, W.; Hu, J.; Li, M.; Yu, W.; Wang, Z.; Liang, S.; Xiao, K.; et al. A green strategy to synthesize two-dimensional lead halide perovskite via direct recovery of spent lead-acid battery. Resour. Conserv. Recycl. 2021, 169, 105463. [Google Scholar] [CrossRef]
- Yu, W.; Li, M.; Liang, S.; Xu, Q.; Zhang, P.; Hou, H.; Hu, J.; Yang, J. Novel PbO@C composite material directly derived from spent lead-acid batteries by one-step spray pyrolysis process. Waste Manag. 2023, 165, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, W.; Luo, L.; Xie, X. Recycling lead from waste lead-acid batteries by the combination of low temperature alkaline and bath smelting. Sep. Purif. Technol. 2023, 310, 123156. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Liang, S.; Hou, H.; Wang, J.; Hu, G.; Li, Z.; Hu, J.; Xiao, K.; Liu, B. Ammonia chloride assisted air-chlorination recovery of tin from pyrometallurgical slag of spent lead-acid battery. Resour. Conserv. Recycl. 2021, 170, 105611. [Google Scholar] [CrossRef]
- Li, F.; Wei, X.; Chen, Y.; Zhu, N.; Zhao, Y.; Cui, B.; Wu, P. Efficient recovery of lead and iron from disposal residues of spent lead-acid batteries. Resour. Conserv. Recycl. 2022, 187, 106614. [Google Scholar] [CrossRef]
- Pan, D.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. Resour. Conserv. Recycl. 2019, 146, 140–155. [Google Scholar] [CrossRef]
- Kim, E.; Horckmans, L.; Spooren, J.; Vrancken, K.C.; Quaghebeur, M.; Broos, K. Selective leaching of Pb, Cu, Ni and Zn from secondary lead smelting residues. Hydrometall. 2017, 169, 372–381. [Google Scholar] [CrossRef]
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997; Volume 2, pp. 581–714. [Google Scholar]
- Kim, W.J.; Seo, S.; Lee, S.I.; Kim, D.W.; Kim, M.J. A study on pyro-hydrometallurgical process for selective recovery of Pb, Sn and Sb from lead dross. J. Hazard. Mater. 2021, 417, 126071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, W.; Liu, Q.; Zhang, Y.; Li, Y.; Chen, X.; Yang, B.; Xu, B. Clean and efficient separation process for high-antimony crude tin. Vacuum 2023, 212, 111986. [Google Scholar] [CrossRef]
- Kern, E.F. Method of Purifying Lead, Tin, and Lead-Tin Alloys. U.S. Patent US1957837A, 28 June 1927. [Google Scholar]
- Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. ASM Handbook Alloy Phase Diagrams; ASM International: Cleveland, OH, USA, 2016; Volume 3. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Drees, S.; Ratke, L. Strip casting of Al-Pb alloys—A numerical analysis. Mater. Sci. Eng. A 2000, 282, 262–269. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Q.; Li, X.; Meng, C.; Ye, B.; Gou, B. Low-temperature synthesis of Ti3Al(Sn)C2 solid solution using replacement reaction. J. Mater. Sci. Mater. Electron. 2020, 31, 20601–20610. [Google Scholar] [CrossRef]
- Morachevskii, A.G.; Butukhanova, T.V. Interaction of aluminum with admixtures dissolved in liquid lead. Russ. J. Appl. Chem. 2009, 82, 1317–1320. [Google Scholar] [CrossRef]
- Morachevskii, A.G. Physicochemical studies of utilization of lead batteries. Russ. J. Appl. Chem. 2014, 87, 241–257. [Google Scholar] [CrossRef]
- Vajsgant, Z.I.; Moraczewski, A.G.; Khabachev, M.N. Method of Refining Lead to Remove Antimony, Tin, and Arsenic Impurities. Patent RU2219265 (C2), 9 August 2001. [Google Scholar]
- Ellis, T.W.; Mirza, A.H. The refining of secondary lead for use in advanced lead-acid batteries. J. Power Sources 2010, 195, 4525–4529. [Google Scholar] [CrossRef]
- Cybulski, A.; Prajsnar, R. Method of Decoppering Lead with Aluminium and Alloy Al-Zn. Patent PL395342A1, 20 June 2011. [Google Scholar]
- Murray, J.L. The aluminium-copper system. Int. Mater. Rev. 1985, 30, 211–234. [Google Scholar] [CrossRef]
- Cybulski, A.; Prajsnar, R. Decoppering lead with aluminium and Al-Zn alloy. Rudy I Met. Nieżelazne 2011, 56, 503–510. [Google Scholar]
- Yang, C. The Process of High Tin-Lead Calcium Alloy Is Produced Using Lead-Acid Accumulator Scrap Lead Aperture Plate. Patent CN108728648A, 25 May 2018. [Google Scholar]
- Yang, C. The Process of Low Tin-Lead Calcium Alloy Is Produced Using Lead-Acid Accumulator Scrap Lead Aperture Plate. Patent CN108588453A, 25 May 2018. [Google Scholar]
- Yang, C. Technique for Producing Lead-Calcium Alloy Using Waste Lead Grids of Waste Lead-Acid Storage Batteries. Patent WO2019223560A1, 12 May 2019. [Google Scholar]
- Wie, Z.; Liu, D.; Wei, Y. Process Method for Producing Low-Tin Lead-Calcium Alloy by Using Waste Lead-Acid Storage Battery Waste Lead Grid. Patent CN113178636A, 27 April 2021. [Google Scholar]
- Li, H.; Qin, W.; Li, J.; Tian, Z.; Jiao, F.; Yang, C. Tracing the global tin flow network: Highly concentrated production and consumption. Resour. Conserv. Recycl. 2021, 169, 105495. [Google Scholar] [CrossRef]
- Eurostat. Waste Statistics—Recycling of Batteries and Accumulators. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_recycling_of_batteries_and_accumulators (accessed on 13 June 2023).
- Sohn, H.Y.; Olivas-Martinez, M. Chapter 2.3—Lead and Zinc Production. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Oxford, UK, 2014; pp. 671–700. [Google Scholar] [CrossRef]
- Sharma, R.C.; Chang, Y.A. The Al-S (Aluminum-Sulfur) system. J. Ph. Equilib. 1987, 8, 128–131. [Google Scholar] [CrossRef]
- Makuei, F.M.; Senanayake, G. Extraction of tellurium from lead and copper bearing feed materials and interim metallurgical products—A short review. Miner. Eng. 2018, 115, 79–87. [Google Scholar] [CrossRef]
- Szyndler, T.; Gnida, R.; Malecha, D.K.; Małecki, S.; Jarosz, P. Patent Application: A Method of Recovering Lead and Tin from Recycled Batteries or Lead Dross. Patent PL439991, 27 December 2021. [Google Scholar]
- Szyndler, T.; Gnida, R.; Malecha, D.K.; Małecki, S.; Jarosz, P. Patent Application: Method of Refining a Lead-Tin Alloy. Patent EP23157067.2, 16 February 2023. [Google Scholar]
- Prengaman, R.D. Current-collectors for lead-acid batteries. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Oxford, UK, 2017; pp. 269–299. [Google Scholar] [CrossRef]
- Prengaman, R.D. Secondary Batteries-Lead-Acid Systems—Lead Alloys. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Oxford, UK, 2009; pp. 648–654. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- IEC 60584-1:2013; Thermocouples—Part 1: EMF Specifications and Tolerances. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2013.
- IEC 60584-2:1982; Thermocouples. Part 2: Tolerances. International Electrotechnical Commission (IEC): Geneva, Switzerland, 1982.
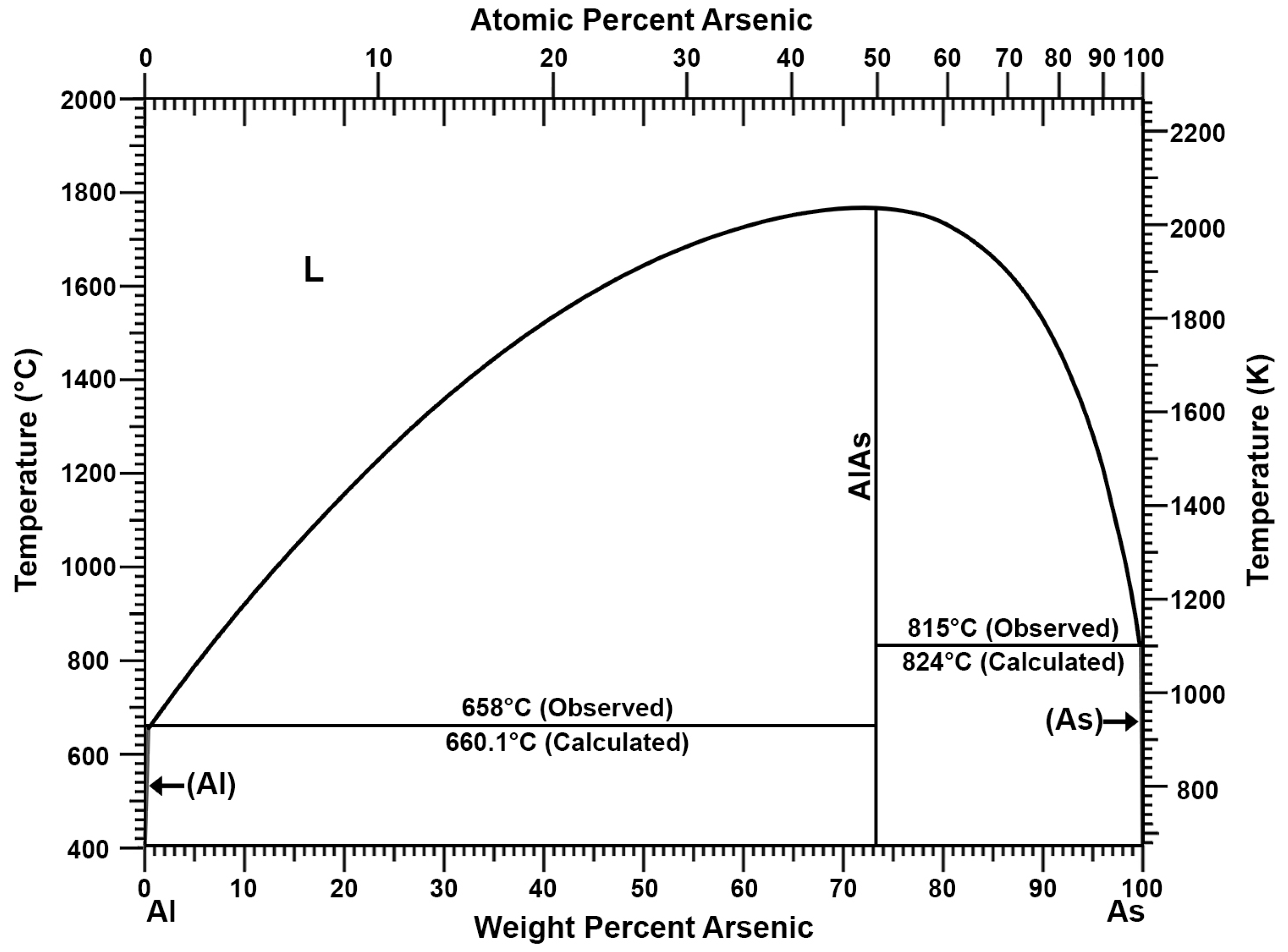
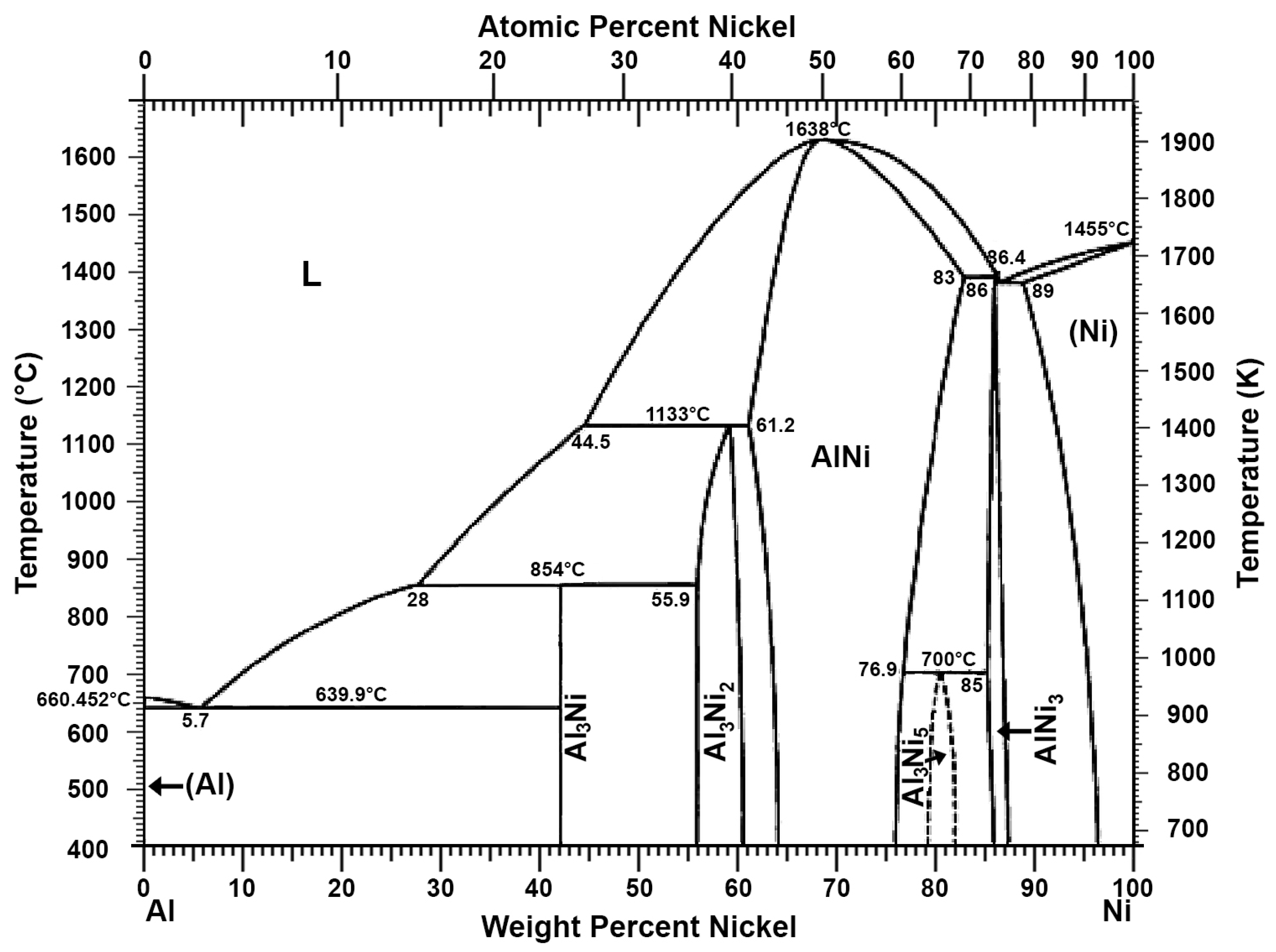
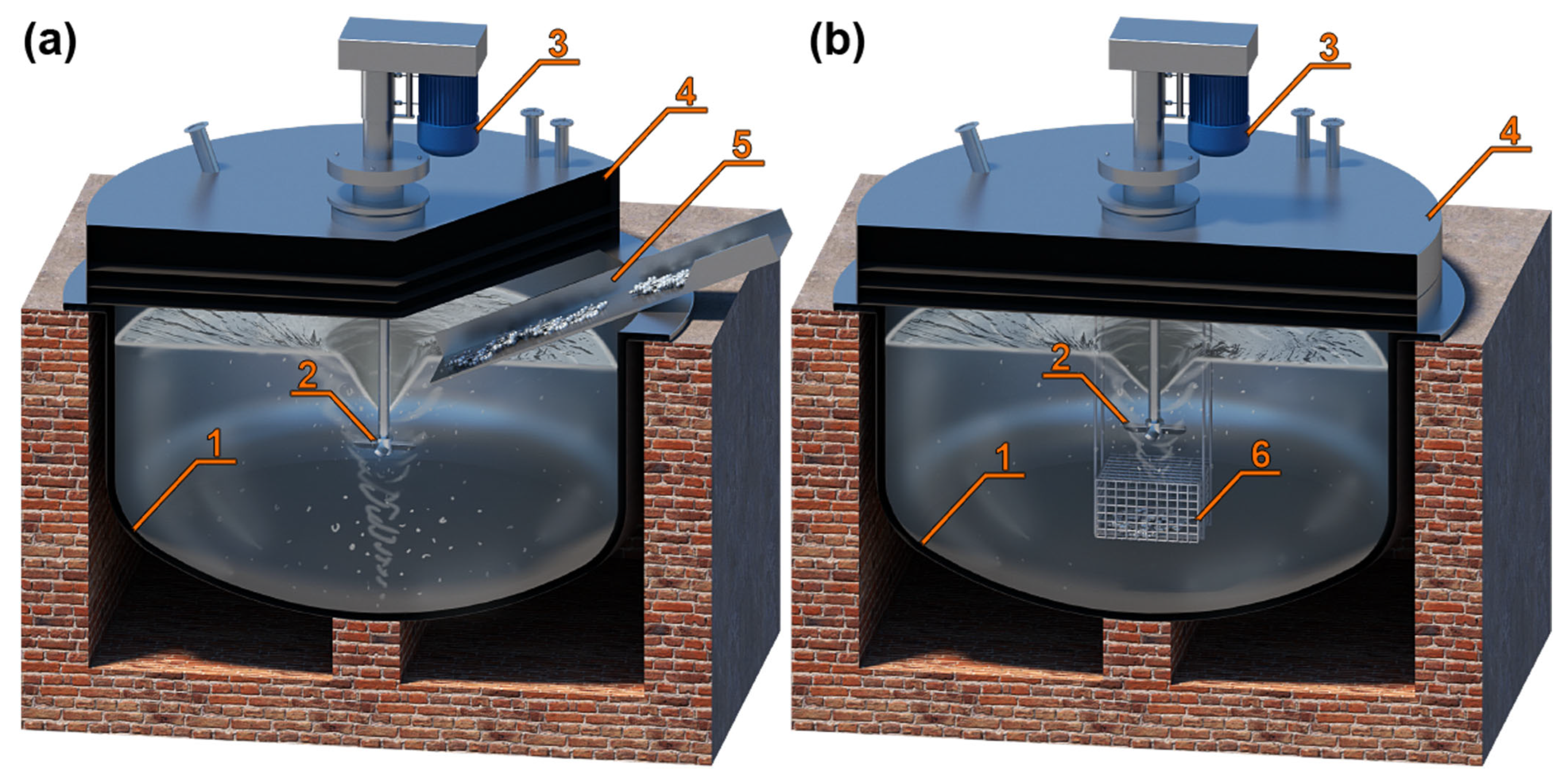
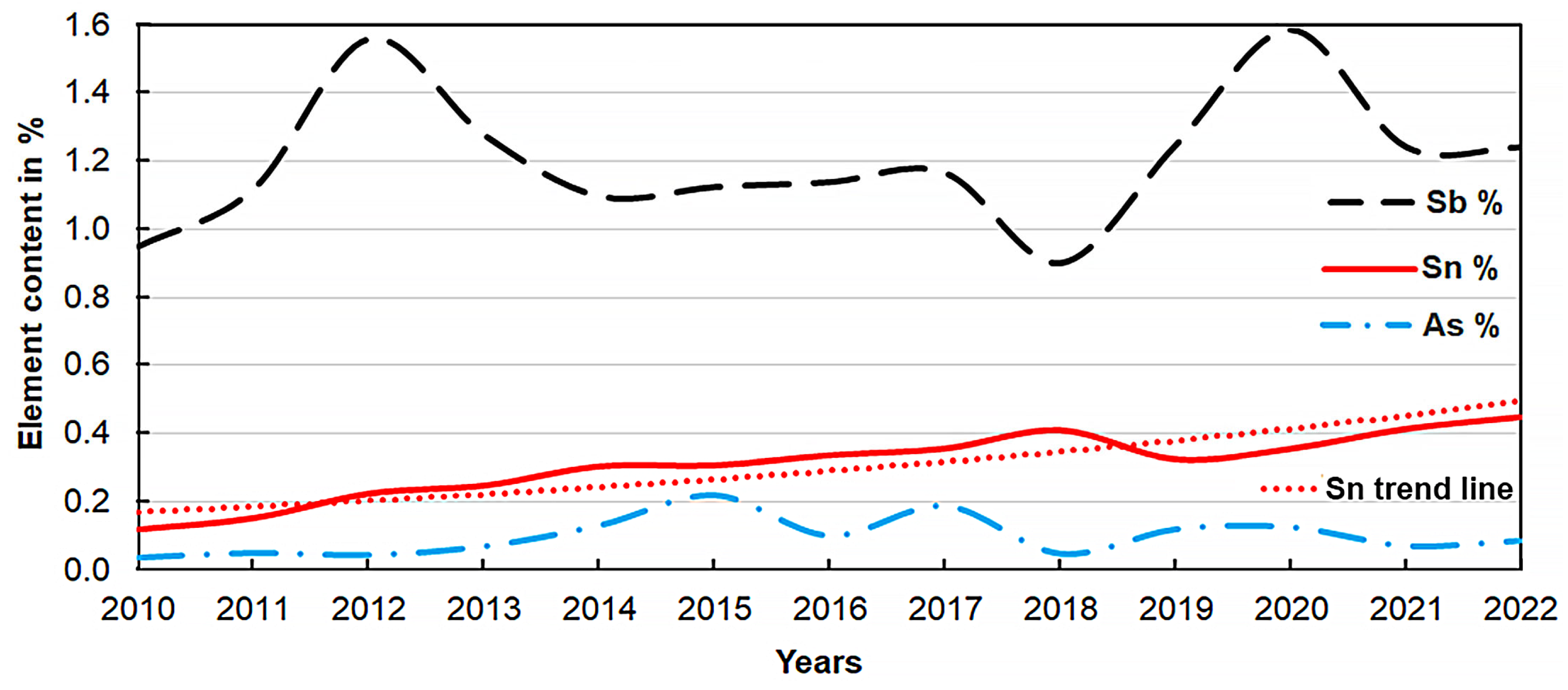

| Description | Al% | Fe% | Zn% | Si% | Cu% | Cr% | Sn% |
|---|---|---|---|---|---|---|---|
| Aluminium scrap | 98.1 ± 1.8 | 0.50 ± 0.09 | 0.49 ± 0.09 | 0.39 ± 0.06 | 0.18 ± 0.04 | 0.028 ± 0.009 | 0.008 ± 0.003 |
| Number | Desc. | Sb% | As% | Sn% | Cu% | Ni% | Te% |
|---|---|---|---|---|---|---|---|
| Test 1 | Before | 5.83 ± 0.23 | 0.188 ± 0.024 | 1.51 ± 0.14 | 0.012 ± 0.003 | 0.0005 ± 0.0001 | 0.0007 ± 0.0001 |
| After | 0.096 ± 0.007 | 0.0003 ± 0.0001 | 1.61 ± 0.17 | 0.0021 ± 0.0005 | 0.00003 ± 0.00002 | 0.00025 ± 0.00005 | |
| Test 2 | Before | 3.88 ± 0.18 | 0.062 ± 0.005 | 1.53 ± 0.14 | 0.017 ± 0.003 | 0.0024 ± 0.0003 | 0.0007 ± 0.0001 |
| After | 0.0097 ± 0.0004 | 0.0002 ± 0.0001 | 1.66 ± 0.18 | 0.0016 ± 0.0004 | 0.00003 ± 0.00002 | 0.00007 ± 0. 00002 | |
| Test 3 | Before | 1.02 ± 0.11 | 0.016 ± 0.003 | 1.48 ± 0.13 | 0.073 ± 0.009 | 0.0076 ± 0.0009 | 0.0014 ± 0.0003 |
| After | 0.030 ± 0.009 | 0.00004 ± 0.00002 | 1.42 ± 0.13 | 0.0028 ± 0.0008 | 0.00006 ± 0.00002 | 0.00011 ± 0.00006 | |
| Test 4 | Before | 2.18 ± 0.15 | 0.0004 ± 0.0001 | 1.26 ± 0.11 | 0.032 ± 0.006 | 0.00013 ± 0.00006 | 0.0010 ± 0.0002 |
| After | 0.062 ± 0.005 | 0.00013 ± 0.00009 | 1.23 ± 0.11 | 0.0025 ± 0.0007 | 0.00005 ± 0.00002 | 0.0004 ± 0.00007 |
| Number | Desc. | Sb% | As% | Sn% | Cu% | Ni% | Te% |
|---|---|---|---|---|---|---|---|
| P-A Test 1 | Before | 1.28 ± 0.13 | 0.038 ± 0.004 | 7.53 ± 0.37 | 0.065 ± 0.009 | 0.0004 ± 0.0001 | 0.0006 ± 0.0001 |
| After | 0.0082 ± 0.0008 | 0.00004 ± 0.00002 | 7.35 ± 0.33 | 0.0023 ± 0.0005 | 0.00002 ± 0.00002 | 0.00009 ± 0.00003 | |
| P-A Test 2 | Before | 3.84 ± 0.18 | 0.407 ± 0.072 | 8.04 ± 0.39 | 0.11 ± 0.03 | 0.049 ± 0.007 | 0.0088 ± 0.0012 |
| After | 0.063 ± 0.007 | 0.00005 ± 0.00002 | 7.64 ± 0.34 | 0.0039 ± 0.0009 | 0.00003 ± 0.00002 | 0.00003 ± 0.00002 | |
| P-A Test 3 | Before | 7.63 ± 0.29 | 0.205 ± 0.038 | 7.15 ± 0.32 | 0.14 ± 0.04 | 0.0089 ± 0.0008 | 0.0018 ± 0.0003 |
| After | 0.091 ± 0.009 | 0.00008 ± 0.00003 | 7.69 ± 0.34 | 0.0021 ± 0.0005 | 0.00002 ± 0.00002 | 0.00017 ± 0.00007 | |
| P-A Test 4 | Before | 5.16 ± 0.23 | 0.0006 ± 0.0001 | 6.73 ± 0.31 | 0.025 ± 0.005 | 0.0005 ± 0.0001 | 0.0008 ± 0.0002 |
| After | 0.0056 ± 0.0005 | 0.00003 ± 0.00002 | 6.98 ± 0.31 | 0.0015 ± 0.0004 | 0.00002 ± 0.00002 | 0.00014 ± 0.00005 |
| Pb% | Sb% | Al% | Sn% | As% | Cu% | Fe% | Ni% | Mg% | Si% | |
|---|---|---|---|---|---|---|---|---|---|---|
| First | 58.9 ± 4.3 | 23.3 ± 2.1 | 6.83 ± 0.57 | 3.30 ± 0.38 | 0.33 ± 0.09 | 0.22 ± 0.07 | 0.17 ± 0.04 | 0.073 ± 0.014 | 0.04 ± 0.01 | 0.007 ± 0.001 |
| Second | 36.9 ± 3.1 | 44.6 ± 4.2 | 12.0 ± 1.1 | 2.43 ± 0.31 | 0.02 ± 0.01 | 0.08 ± 0.02 | 0.20 ± 0.05 | 0.006 ± 0.002 | 0.05 ± 0.01 | 0.12 ± 0.09 |
| Average | 47.9 | 33.95 | 9.42 | 2.87 | 0.18 | 0.15 | 0.19 | 0.040 | 0.045 | 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malecha, D.; Małecki, S.; Jarosz, P.; Kowalik, R.; Żabiński, P. Recovery of Pure Lead-Tin Alloy from Recycling Spent Lead-Acid Batteries. Materials 2023, 16, 5882. https://doi.org/10.3390/ma16175882
Malecha D, Małecki S, Jarosz P, Kowalik R, Żabiński P. Recovery of Pure Lead-Tin Alloy from Recycling Spent Lead-Acid Batteries. Materials. 2023; 16(17):5882. https://doi.org/10.3390/ma16175882
Chicago/Turabian StyleMalecha, Daniel, Stanisław Małecki, Piotr Jarosz, Remigiusz Kowalik, and Piotr Żabiński. 2023. "Recovery of Pure Lead-Tin Alloy from Recycling Spent Lead-Acid Batteries" Materials 16, no. 17: 5882. https://doi.org/10.3390/ma16175882
APA StyleMalecha, D., Małecki, S., Jarosz, P., Kowalik, R., & Żabiński, P. (2023). Recovery of Pure Lead-Tin Alloy from Recycling Spent Lead-Acid Batteries. Materials, 16(17), 5882. https://doi.org/10.3390/ma16175882