Kinetic and Thermodynamic Analyses of Co-Pyrolysis of Nylon-Polyethylene Pouch Wastes
Abstract
:1. Introduction
2. Materials and Analysis Methods
2.1. Materials
2.2. TGA Analysis
2.3. Theoretical Basis for Kinetic Analysis
2.3.1. Determination of Ea Based on Isoconversional Methods
2.3.2. Determination of lnk0 and f(a) Based on Compensation Effect
2.3.3. Determination of Thermodynamic Parameters
3. Results and Discussion
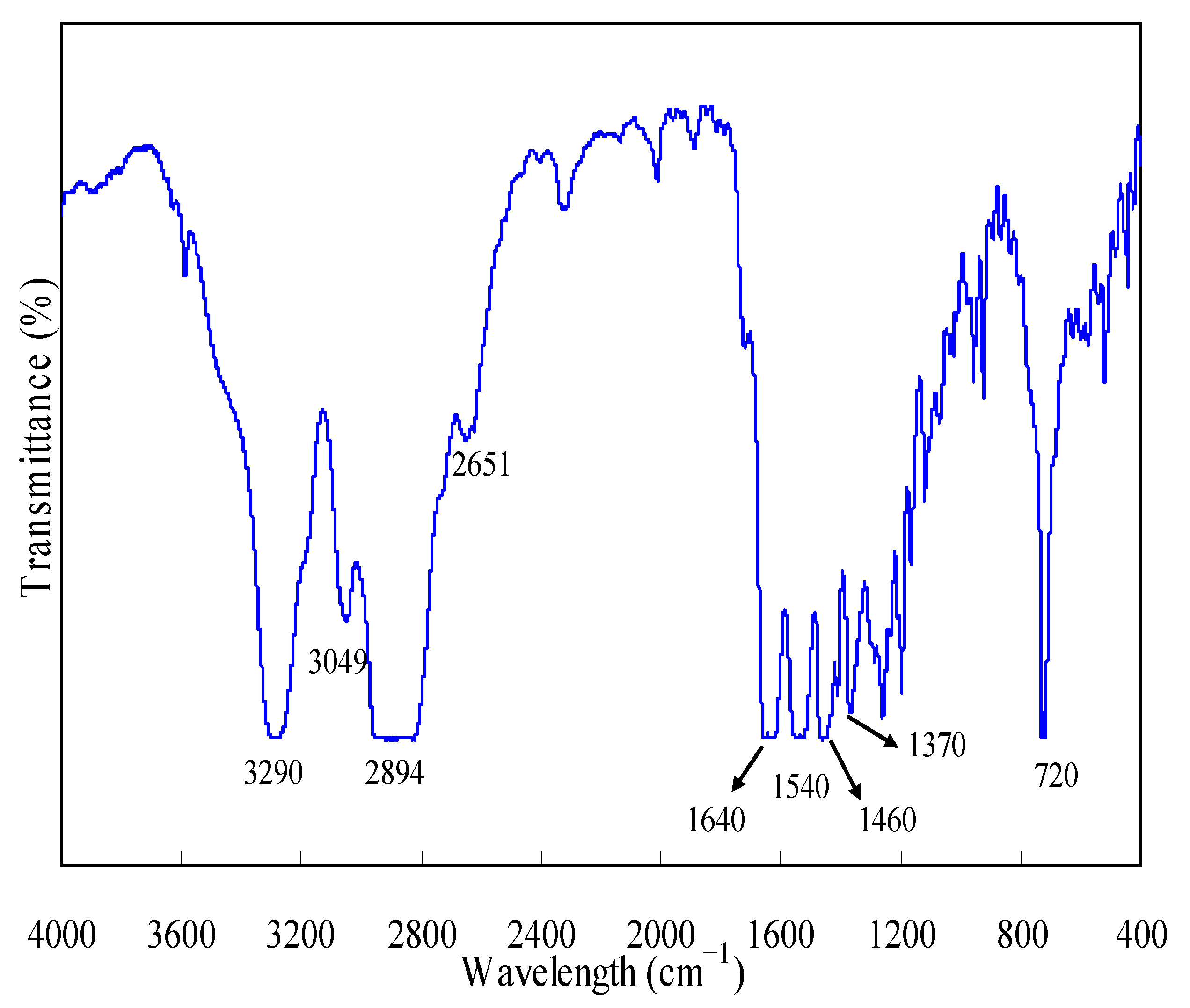
3.1. FTIR Examination
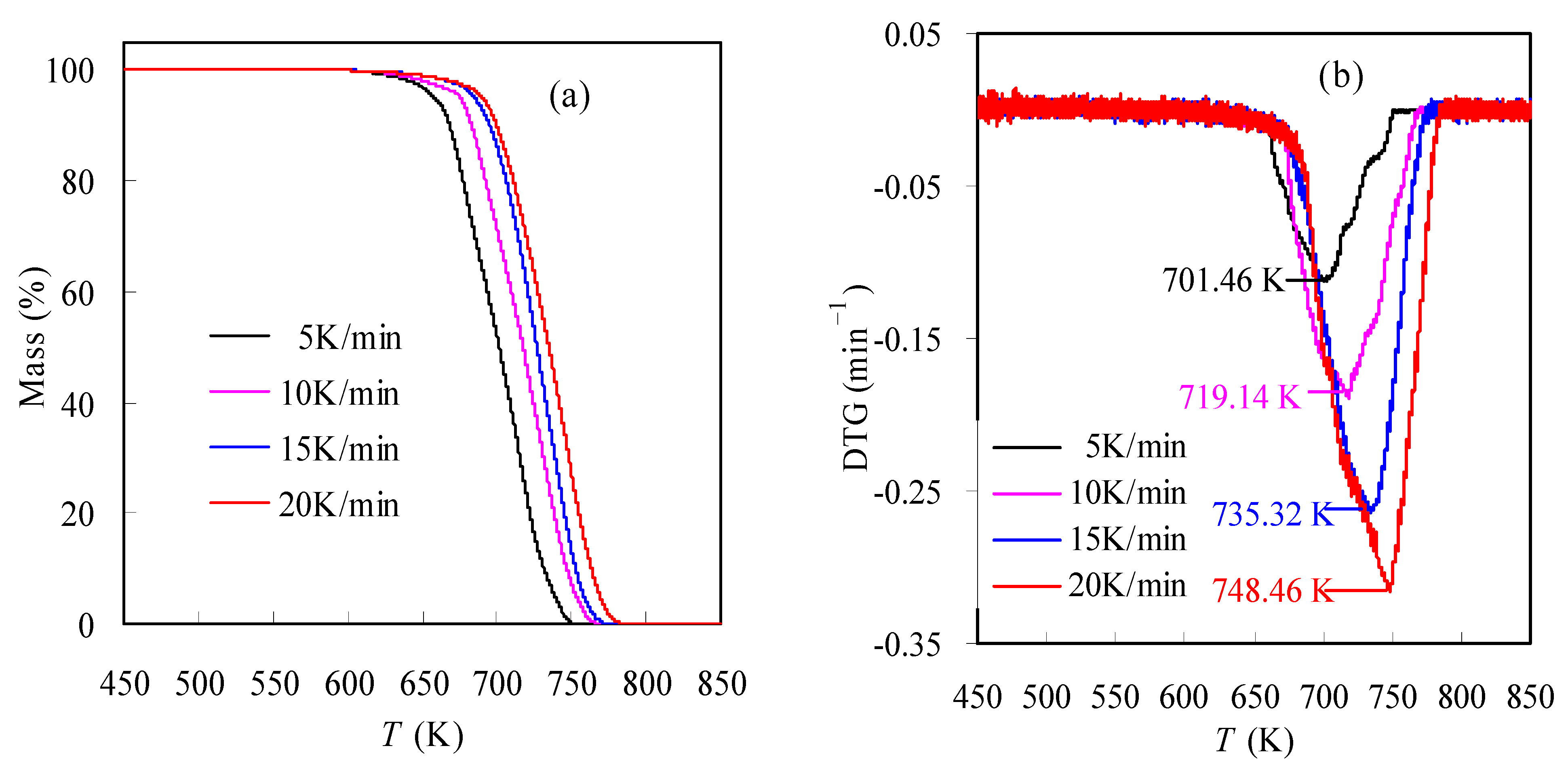
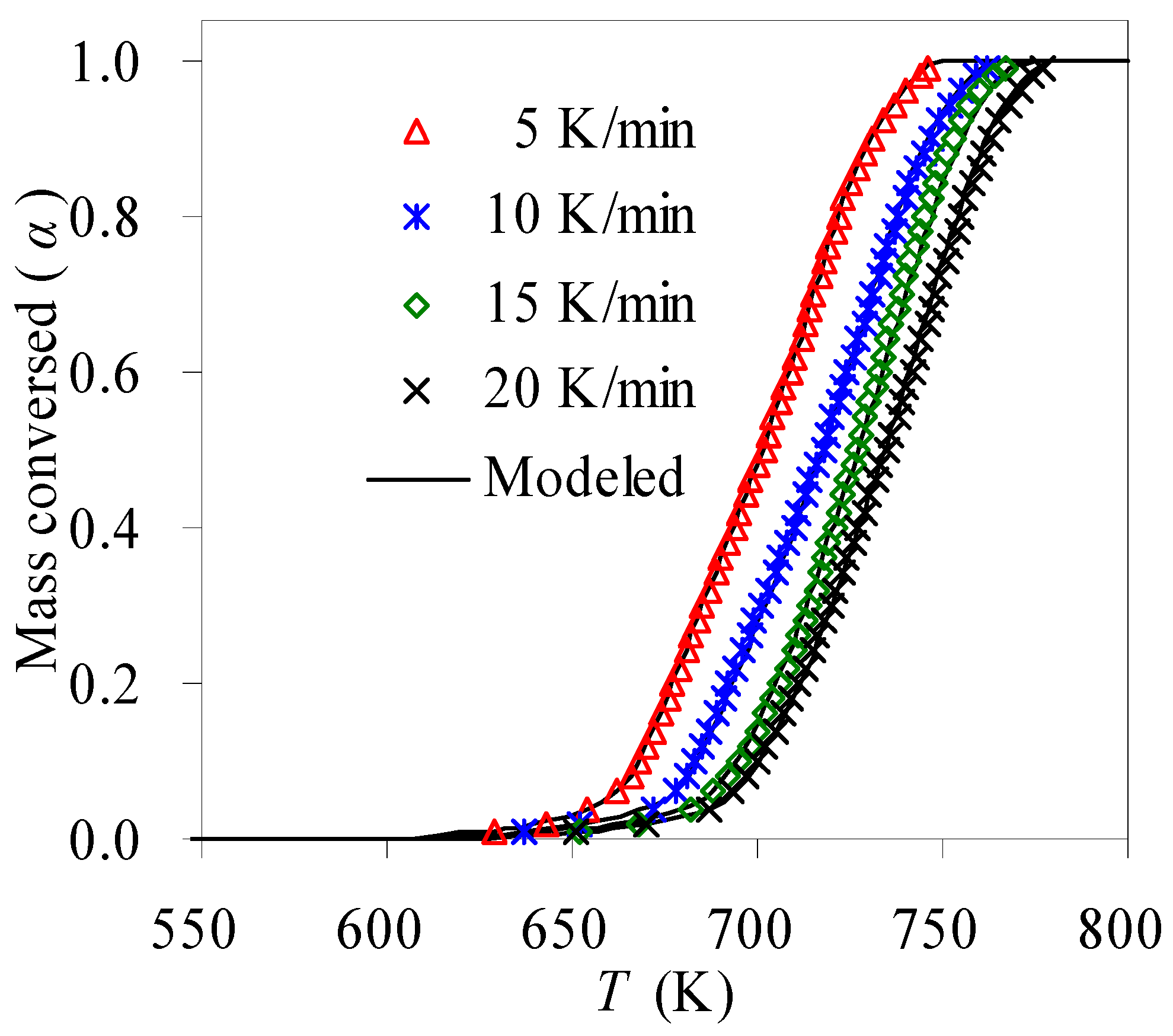
3.2. Pyrolysis Features of N-PE Waste
3.3. Kinetics Analysis of Thermal Degradation
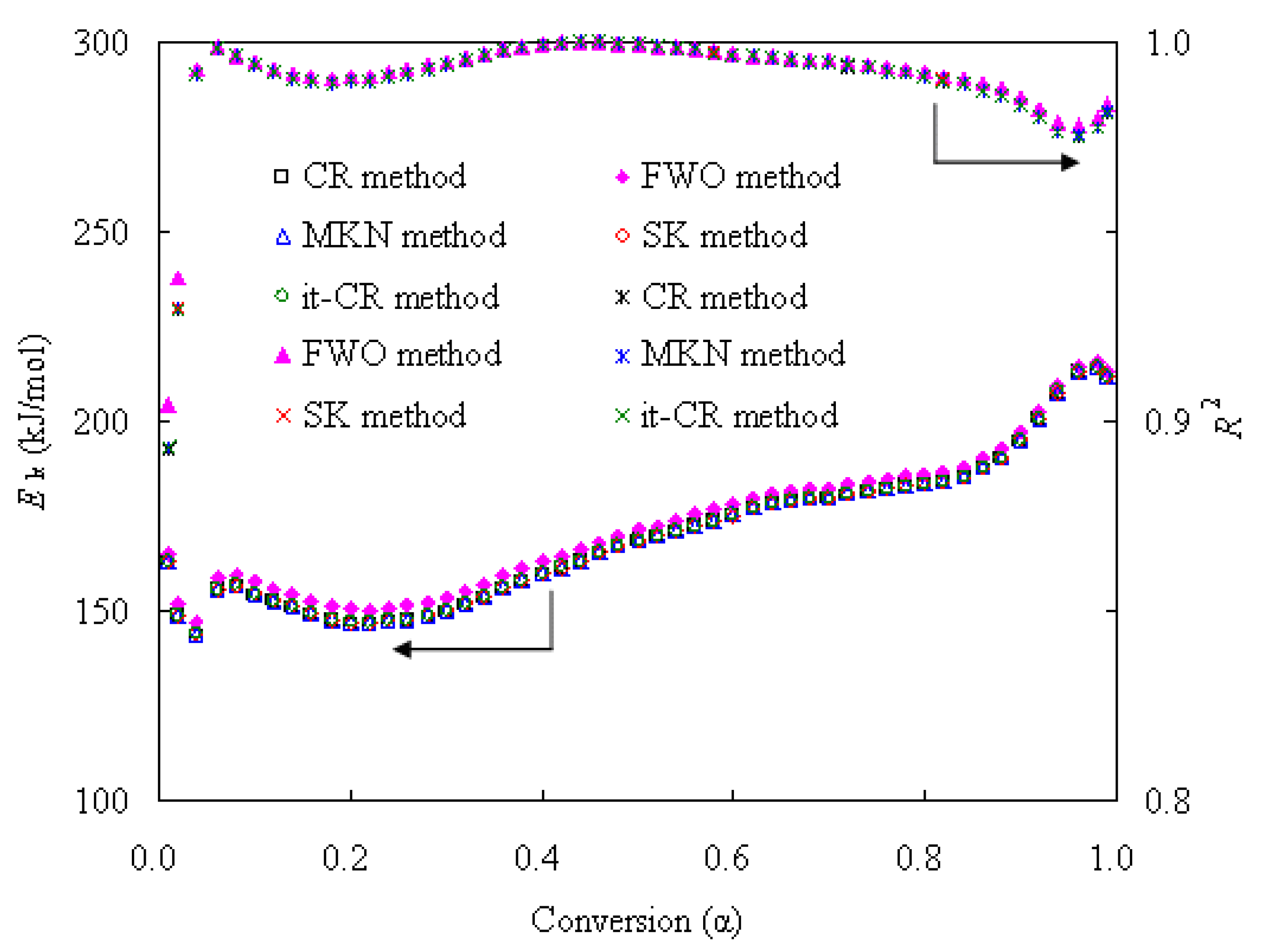
3.3.1. Determination of Ek with Various Kinetic Methods
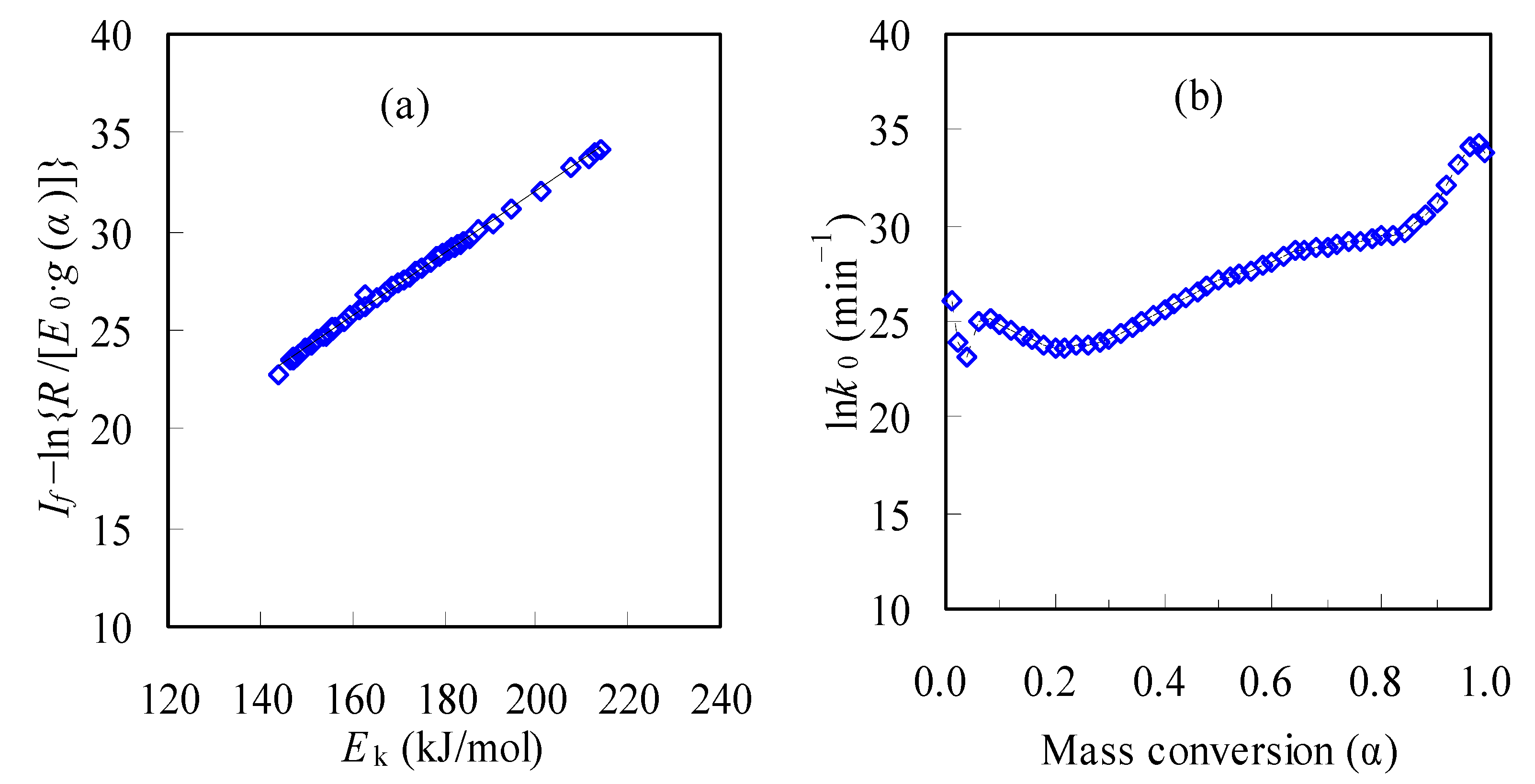
3.3.2. Determination of g(α) and lnk0 Based on Compensation Effect
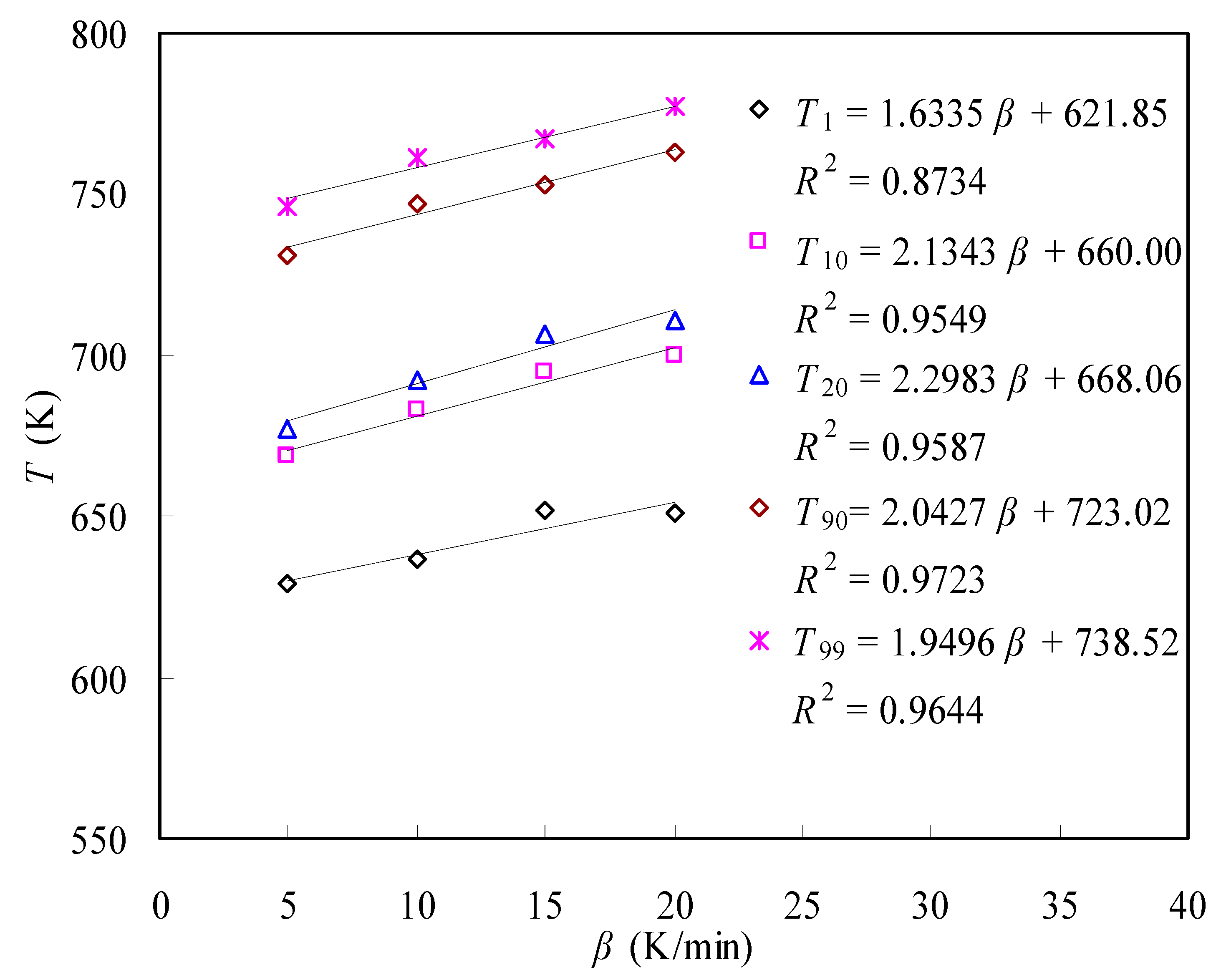
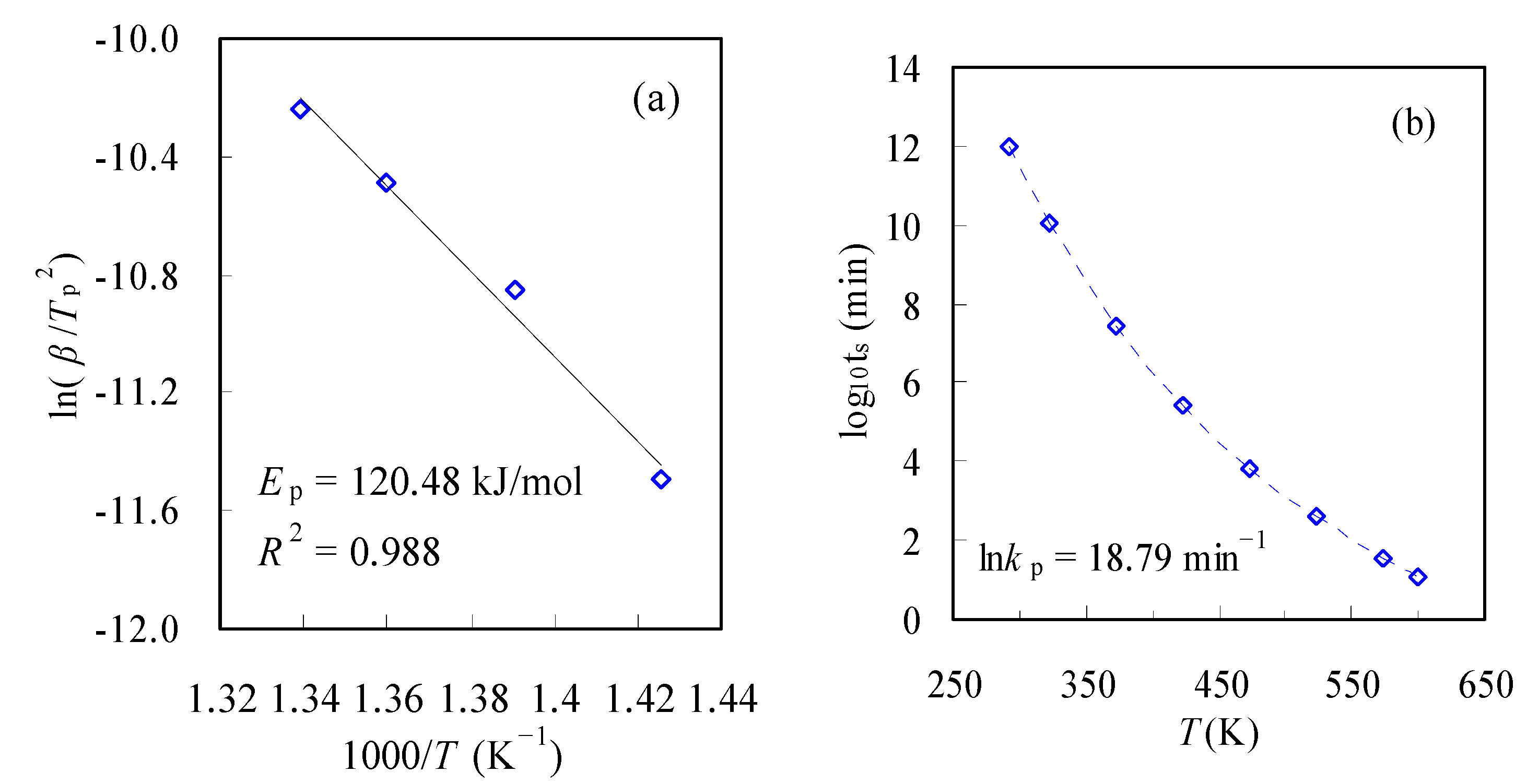
3.3.3. Service Life Prediction
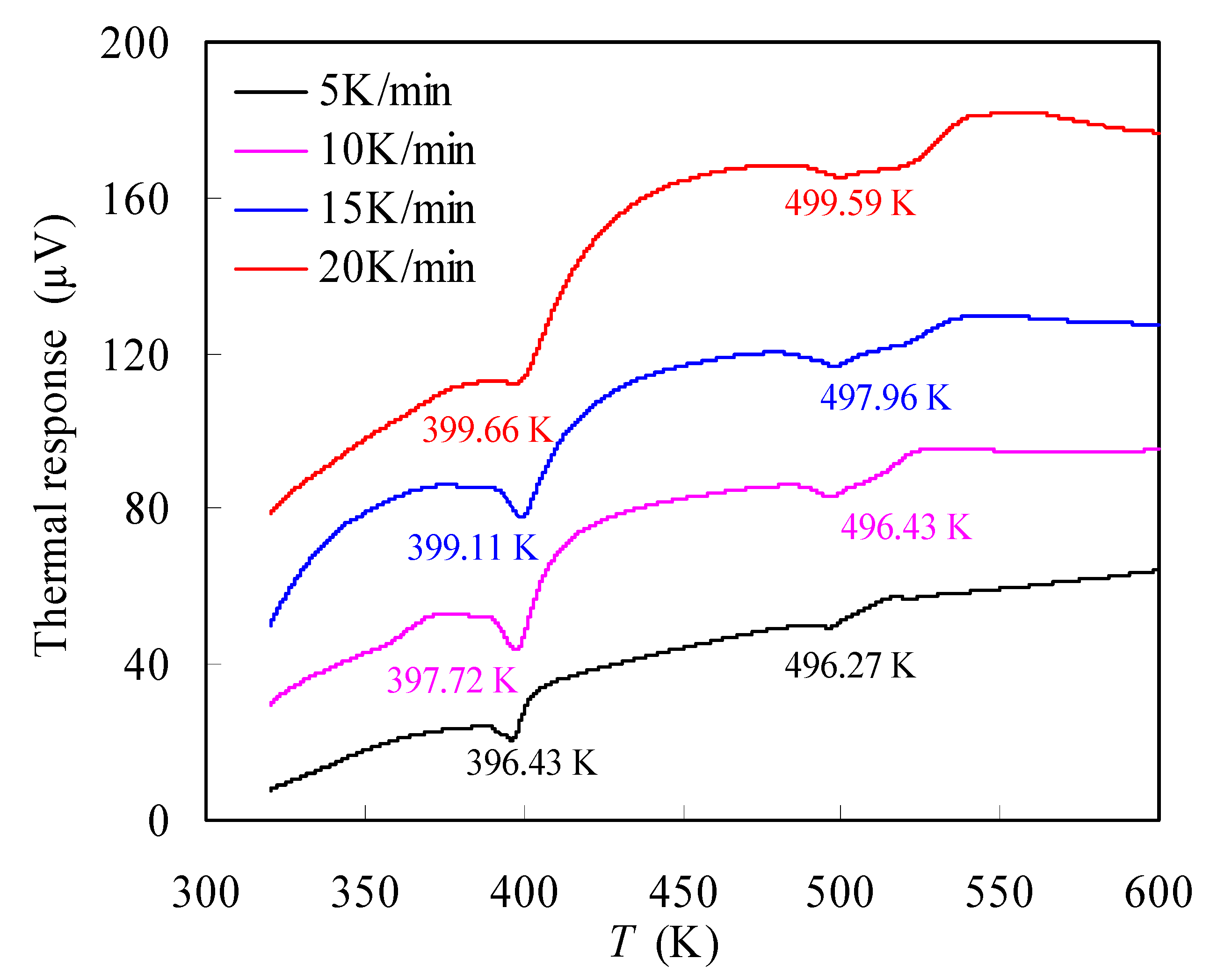
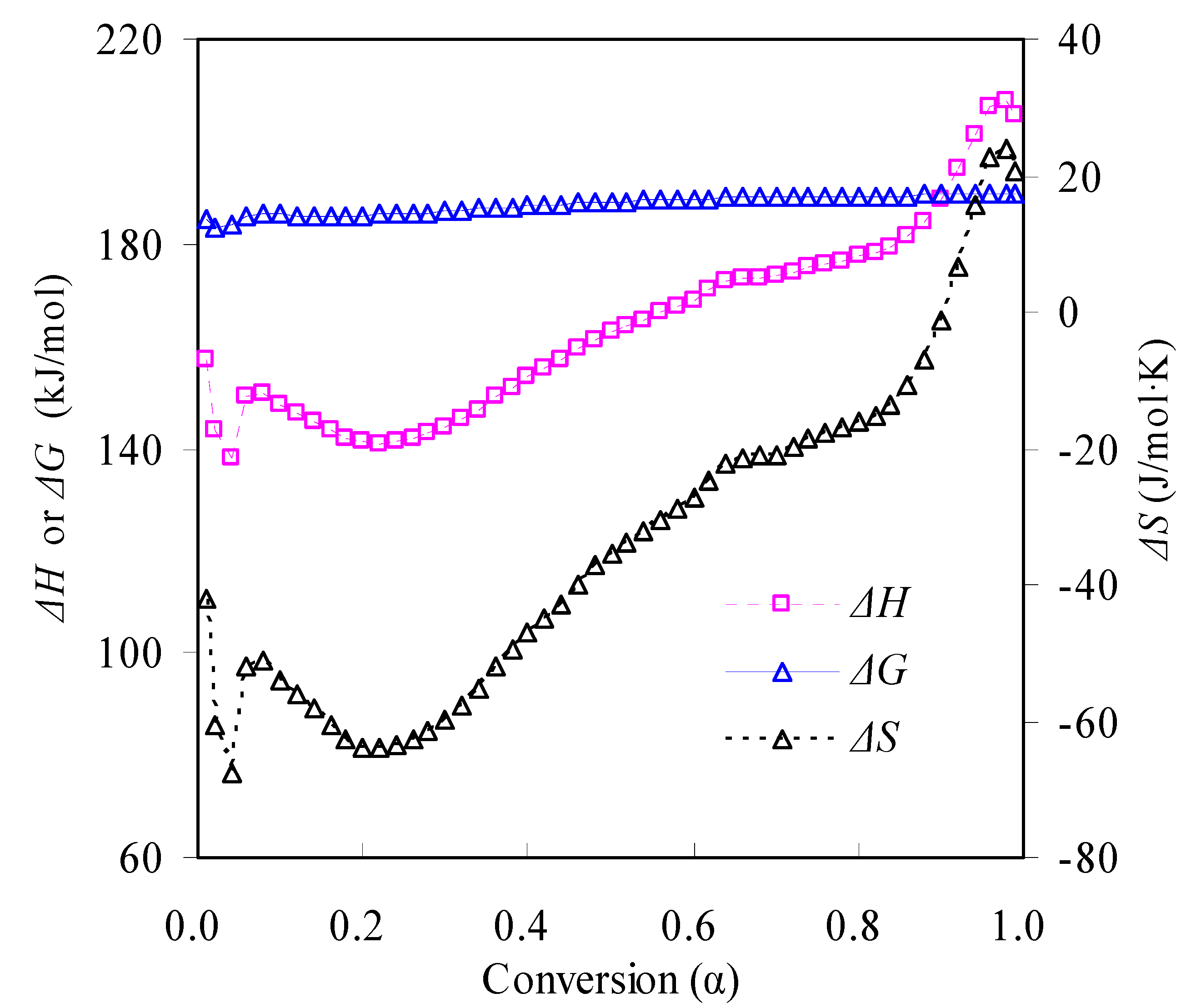
3.3.4. Thermodynamic Parameters
4. Conclusions
- TGA results show that the co-pyrolysis of the N-PE pouch waste underwent one decomposition stage, indicating that the respective pyrolyses of nylon-6 and PE may overlap with each other. In the meantime, all specific temperatures of T1, T5, T30, Tp and T99 and pyrolysis performance parameters CPI and HRI are all found to increase with the heating rate.
- The co-pyrolysis of the N-PE pouch waste was kinetically analyzed, and the Ek values from the isoconversional CR, MKN, SK, it-CR and it-FWO methods are very similar to each other, whereas the ones from the FWO method are fairly higher. For all these methods, the Ek values within 143~215 kJ/mol demonstrated strictly the same α-dependence.
- The methodology of combining model fitting with the compensation effect was tried for seeking the pyrolysis mechanism together with the pre-exponential factor, resulting in the A3/2 model of g(α) = [−ln(1 − α)]2/3, or f(α) = 3/2 (1 − α)·[−ln(1 − α)]1/3 with the k0 range of 1.12 × 1010~7.56 × 1014 min−1 for the pyrolysis of the N-PE waste. With Ek, k0 and g(α) thus obtained, the α-T curves were calculated and then demonstrated very satisfactory correlations against experimental results.
- Thermodynamic parameters in terms of ΔG, ΔH and ΔS and the service lifetime predictions were estimated concerning the pyrolysis decomposition process of the N-PE waste, and the information provided helpful knowledge for applying polymer-based products and future waste disposal.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bugatti, V.; Zuppardi, F.; Viscusi, G.; Gorrasi, G. Active packaging based on coupled nylon/PE pouches filled with active nano-hybrid: Effect on the shelf life of fresh milk. Nanomaterials 2021, 11, 1881. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jeon, Y.J.; Min, S.C. Effects of packaging parameters on the microbial decontamination of Korean steamed rice cakes using in-package atmospheric cold plasma treatment. Food Sci. Biotechnol. 2021, 30, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of plastic films for modified atmosphere packaging of fruits and vegetables: A review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Harmon, R.E.; SriBala, G.; Broadbelt, L.J.; Burnham, A.K. Insight into polyethylene and polypropylene pyrolysis: Global and mechanistic models. Energy Fuels 2021, 35, 6765–6775. [Google Scholar] [CrossRef]
- Xu, F.F.; Wang, B.; Yang, D.; Hao, J.H.; Qiao, Y.Y.; Tian, Y.Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Dubdub, I.; Al-Yaari, M. Thermal behavior of mixed plastics at different heating rates: I. Pyrolysis kinetics. Polymers 2021, 13, 3413. [Google Scholar] [CrossRef]
- Budrugeac, P.; Cucos, A.; Dascãlu, R.; Atkinson, I.; Osiceanu, P. Application of model-free and multivariate nonlinear regression methods for evaluation of the kinetic scheme and kinetic parameters of thermal decomposition of low density polyethylene. Thermochim. Acta 2022, 708, 179138. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Perez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee recommendations for analysis of thermal decomposition kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2022, 689, 178597. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Vyazovkin, S. The status of pyrolysis kinetics studies by thermal analysis: Quality is not as good as it should and can readily be. Thermo 2022, 2, 435–452. [Google Scholar] [CrossRef]
- Kim, S.S.; Jeon, J.K.; Park, Y.K.; Kim, S. Thermal pyrolysis of fresh and waste fishing nets. Waste Manag. 2005, 25, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Eimontas, J.; Yousef, S.; Striūgas, N.; Abdelnaby, M.A. Catalytic pyrolysis kinetic behaviour and TG-FTIR-GC–MS analysis of waste fishing nets over ZSM–5 zeolite catalyst for caprolactam recovery. Renew. Energy 2021, 179, 1385–1403. [Google Scholar] [CrossRef]
- Pannase, A.M.; Singh, R.K.; Ruj, B.; Gupta, P. Decomposition of polyamide via slow pyrolysis: Effect of heating rate and operating temperature on product yield and composition. J. Anal. Appl. Pyrolysis 2020, 151, 104886. [Google Scholar] [CrossRef]
- Bockhorn, H.; Donner, S.; Gernsbeck, M.; Hornung, A.; Hornung, U. Pyrolysis of polyamide 6 under catalytic conditions and its application to reutilization of carpets. J. Anal. Appl. Pyrolysis 2001, 58–59, 79–94. [Google Scholar] [CrossRef]
- Sinfrônio, F.S.M.; Santos, J.C.O.; Pereira, L.G.; Souza, A.G.; Conceicão, M.M.; Fernandes, V.J., Jr.; Fonseca, V.M. Kinetic of thermal degradation of low-density and high-density polyethylene by non-isothermal thermogravimetry. J. Thermal Anal. Calorim. 2005, 79, 393–399. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F. Pyrolysis of synthetic polymers and plastic wastes. Fuel Process. Technol. 2008, 89, 678–687. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Thermal degradation behaviours of polyethylene and propylene. Part I: Pyrolysis and mechanism. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Ning, X.; Xie, T.; Wang, J. Thermal degradation properties of LDPE insulation for new and aged fine wires. J. Thermal Anal. Calorim. 2019, 137, 461–471. [Google Scholar] [CrossRef]
- Chen, R.Y.; Li, Q.W.; Xu, X.K.; Zhang, D.D. Pyrolysis kinetics and reaction mechanism of representative non-charring polymer waste with micron particle size. Energy Convers. Manag. 2019, 198, 111923. [Google Scholar] [CrossRef]
- Trache, D.; Abdelaziz, A.; Siouani, B. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Thermal Anal. Calorim. 2017, 128, 335–348. [Google Scholar] [CrossRef]
- Sygula, E.; Swiechowski, K.; Hejna, M.; Kunaszyk, I.; Bialowiec, A. Municipal solid waste thermal analysis-Pyrolysis kinetics and decomposition reactions. Energies 2021, 14, 4510. [Google Scholar] [CrossRef]
- Komandur, J.; Kumar, A.; Para, P.; Mohanty, K. Kinetic parameters estimation of thermal and co-pyrolysis of groundnut de-oiled cake and polyethylene terephthalate (PET) waste. Energies 2022, 15, 7502. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef]
- Madhysudanan, P.M.; Krishnan, K.; Ninan, K.N. New equations for kinetic analysis of non-isothermal reactions. Thermochim. Acta 1993, 221, 13–21. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Gao, Z.M.; Nakada, M.; Amasaki, I. A consideration of errors and accuracy in the isoconversional methods. Thermochim. Acta 2001, 369, 137–142. [Google Scholar] [CrossRef]
- Vyazovkin, S. Determining pre-exponential factor in model-free kinetic methods: How and why? Molecules 2021, 26, 3077. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvedova, D.; Vlaev, L. Non-isothermal kinetics of thermal degradation of chitin. J. Thermal Anal. Calorim. 2013, 111, 763–771. [Google Scholar] [CrossRef]
- Hao, Y.H.; Huang, Z.; Ye, Q.Q.; Wang, J.W.; Yang, X.Y.; Fan, X.Y.; Li, Y.L.; Peng, Y.W. A comparison study on non-isothermal decomposition kinetics of chitosan with different analysis methods. J. Thermal Anal. Calorim. 2017, 128, 1077–1091. [Google Scholar] [CrossRef]
- Wan, H.B.; Huang, Z. Pyrolysis and oxidative thermal decomposition investigations of tennis ball rubber wastes through kinetic and thermodynamic evaluations. Materials 2023, 16, 2328. [Google Scholar] [CrossRef] [PubMed]
- Porubská, M.; Szöllős, O.; Kóňová, A.; Janigová, I.; Jašková, M.; Jomová, K.; Chodák, I. FTIR spectroscopy study of polyamide-6 irradiated by electron and proton beams. Polym. Degrad. Stab. 2012, 97, 523–531. [Google Scholar] [CrossRef]
- Duque, J.V.F.; Martins, M.F.; Bittencourt, F.L.F.; Debenest, G.; Orlando, M.T.D.; Profeti, L.P.R.; Profeti, D. Recovering wax from polyethylene waste using C-DPyR. Energy 2023, 272, 127135. [Google Scholar] [CrossRef]
- Jaiswal, P.B.; Pushkar, B.K.; Maikap, K.; Mahanwar, P.A. Abiotic aging assisted bio-oxidation and degradation of LLDPE/LDPE packaging polyethylene film by stimulated enrichment culture. Polym. Degrad. Stab. 2022, 206, 110156. [Google Scholar] [CrossRef]
- Gu, J.W.; Yang, X.T.; Li, C.M.; Kou, K.C. Synthesis of cyanate ester microcapsules via solvent evaporation technique and its application in epoxy resins as a healing agent. Ind. Eng. Chem. Res. 2016, 55, 10941–10946. [Google Scholar] [CrossRef]
- Fang, S.W.; Yu, Z.S.; Lin, Y.S.; Hu, S.C.; Liao, Y.F.; Ma, X.Q. Thermogravimetric analysis of the co-pyrolysis of paper sludge and municipal solid waste. Energy Convers. Manag. 2015, 101, 626–631. [Google Scholar] [CrossRef]
- Wan, H.B.; Huang, Z. Pyrolysis evaluation of tennis string polyurethane and water-borne polyurethane wastes through isoconversional kinetic analysis. Polymers 2022, 14, 1501. [Google Scholar] [CrossRef]
- Wan, H.B.; Huang, Z. Kinetic analysis of pyrolysis and thermo-oxidative decomposition of tennis string nylon wastes. Materials 2021, 14, 7564. [Google Scholar] [CrossRef]
- Yu, J.C.; Gröbner, G.; Tonpheng, B.; Andersson, O. Microstructure, nucleation and thermal properties of high-pressure crystallized MWCNT/nylon–6 composites. Polymer 2011, 52, 5521–5527. [Google Scholar] [CrossRef]
- Bateni, F.; Motlagh, G.H. Electrospun polyamide/graphene oxide nanofibers as fillers for polyethylene: Preparation and characterization. J. Appl. Polym. Sci. 2021, e51506. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescud, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
| Parameters | β (K/min) | |||
|---|---|---|---|---|
| 5 | 10 | 15 | 20 | |
| T5 (K) | 658.43 | 675.94 | 685.10 | 690.82 |
| T30 (K) | 685.38 | 701.12 | 714.17 | 719.59 |
| HRI (K) | 330.55 | 338.61 | 344.25 | 346.96 |
| Ti (K) | 600.61 | 602.75 | 601.72 | 597.74 |
| Tp (K) | 701.46 | 719.14 | 735.32 | 748.46 |
| DTGp (min−1) | −0.113 | −0.189 | −0.264 | −0.315 |
| DTGm (min−1) | −0.016 | −0.030 | −0.038 | −0.051 |
| ΔT (K) | 51.97 | 62.81 | 57.04 | 68.90 |
| CPI (×1010 min−2·K−3) | 0.82 | 2.07 | 4.02 | 5.27 |
| No. | Symbol | g(α) Function | lnk0 = a·Ek + b | ||
|---|---|---|---|---|---|
| a | b | R2 | |||
| Chemical reaction equation | |||||
| 1 | F1/3 | g(α) = 1 − (1 − α)2/3 | 0.1631 | −1.3526 | 0.9931 |
| 2 | F1/2 | g(α) = 1 − (1 − α)1/2 | 0.1660 | −2.0653 | 0.9934 |
| 3 | F2/3 | g(α) = 1 − (1 − α)1/3 | 0.1693 | −2.9472 | 0.9938 |
| 4 | F3/4 | g(α) = 1 − (1 − α)1/4 | 0.1710 | −3.4938 | 0.9940 |
| 5 | F3/2 | g(α) = (1 − α)−1/2 − 1 | 0.1909 | −5.7914 | 0.9953 |
| 6 | F2 | g(α) = (1 − α)−1 − 1 | 0.2080 | −7.6834 | 0.9938 |
| 7 | F3 | g(α) = (1 − α)−2 − 1 | 0.2477 | −13.0338 | 0.9848 |
| 8 | F1 | g(α) = −ln(1 − α) | 0.1768 | −2.9720 | 0.9947 |
| 9 | G1 | g(α) = 1 − (1 − α)2 | 0.1481 | 1.8709 | 0.9932 |
| 10 | G2 | g(α) = 1 − (1 − α)3 | 0.1421 | 3.0684 | 0.9943 |
| 11 | G3 | g(α) = 1 − (1 − α)4 | 0.1381 | 3.8420 | 0.9951 |
| Sigmoidal rate equation | |||||
| 12 | A1/3 | g(α) = [−ln(1 − α)]3 | 0.2879 | −23.0267 | 0.9080 |
| 13 | A1/2 | g(α) = [−ln(1 − α)]2 | 0.2323 | −12.9993 | 0.9528 |
| 14 | A2/3 | g(α) = [−ln(1 − α)]3/2 | 0.2046 | −7.9856 | 0.9763 |
| 15 | A3/4 | g(α) = [−ln(1 − α)]4/3 | 0.1953 | −6.3144 | 0.9834 |
| 16 | A3/2 | g(α) = [−ln(1 − α)]2/3 | 0.1583 | 0.3705 | 0.9981 |
| 17 | A5/2 | g(α) = [−ln(1 − α)]2/5 | 0.1435 | 3.0445 | 0.9900 |
| 18 | A2 | g(α) = [−ln(1 − α)]1/2 | 0.1491 | 2.0417 | 0.9946 |
| 19 | A3 | g(α) = [−ln(1 − α)]1/3 | 0.1398 | 3.7129 | 0.9856 |
| 20 | A4 | g(α) = [−ln(1 − α)]1/4 | 0.1352 | 4.5486 | 0.9785 |
| Other mechanism equation | |||||
| 21 | P1/2 | g(α) = α1/2 | 0.1398 | 3.4122 | 0.9914 |
| 22 | P1/3 | g(α) = α1/3 | 0.1336 | 4.6266 | 0.9821 |
| 23 | G7 | g(α) = [1 − (1 − α)1/2]1/2 | 0.1437 | 2.4951 | 0.9929 |
| 24 | G8 | g(α) = [1 − (1 − α)1/3]1/2 | 0.1453 | 2.0541 | 0.9935 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.-B.; Huang, Z. Kinetic and Thermodynamic Analyses of Co-Pyrolysis of Nylon-Polyethylene Pouch Wastes. Materials 2023, 16, 5738. https://doi.org/10.3390/ma16175738
Wan H-B, Huang Z. Kinetic and Thermodynamic Analyses of Co-Pyrolysis of Nylon-Polyethylene Pouch Wastes. Materials. 2023; 16(17):5738. https://doi.org/10.3390/ma16175738
Chicago/Turabian StyleWan, Hai-Bo, and Zhen Huang. 2023. "Kinetic and Thermodynamic Analyses of Co-Pyrolysis of Nylon-Polyethylene Pouch Wastes" Materials 16, no. 17: 5738. https://doi.org/10.3390/ma16175738
APA StyleWan, H.-B., & Huang, Z. (2023). Kinetic and Thermodynamic Analyses of Co-Pyrolysis of Nylon-Polyethylene Pouch Wastes. Materials, 16(17), 5738. https://doi.org/10.3390/ma16175738






