Effect of Tempering Temperature on Hydrogen Embrittlement of SCM440 Tempered Martensitic Steel
Abstract
:1. Introduction
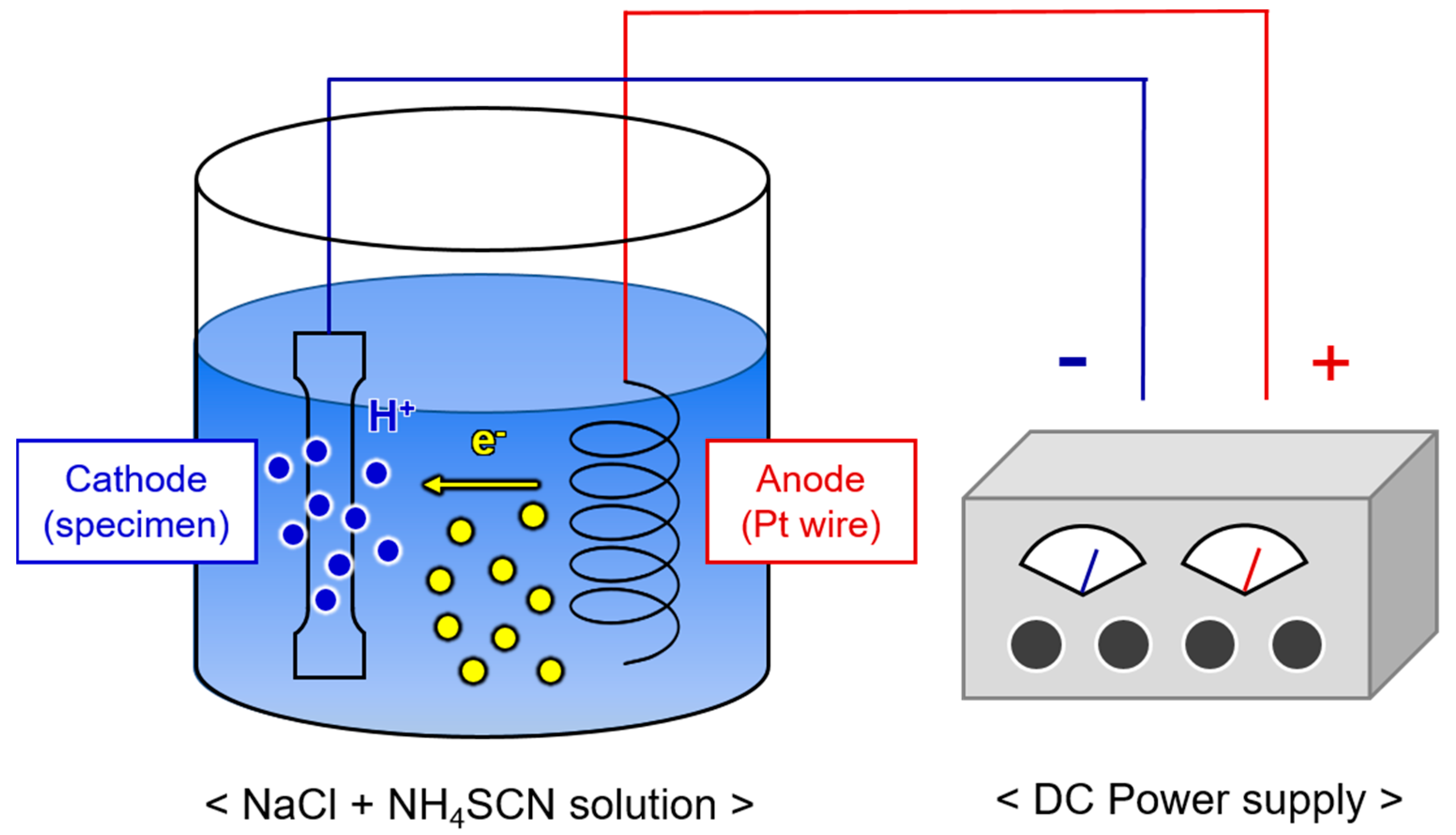
2. Materials and Methods
3. Results and Discussion
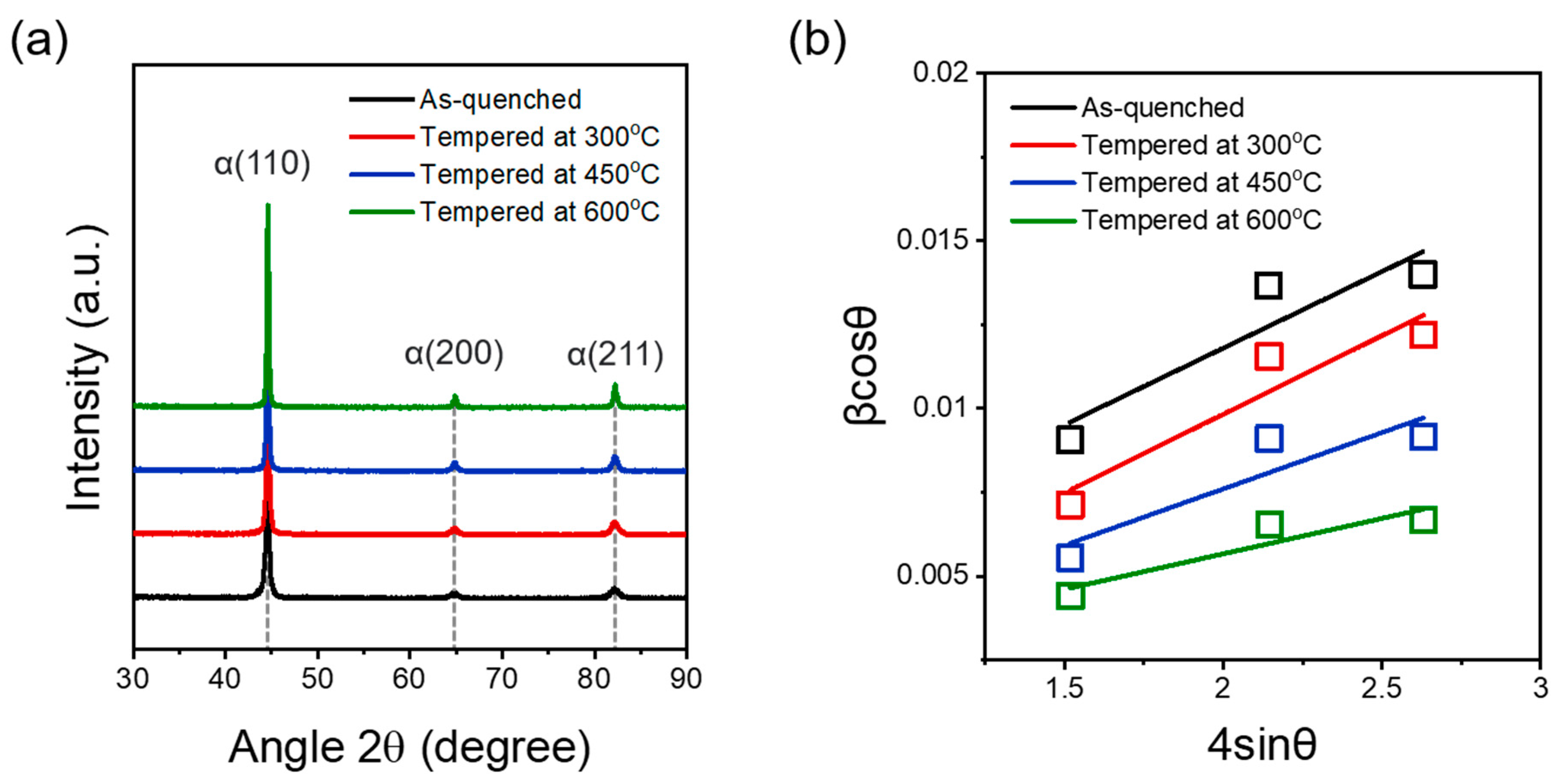
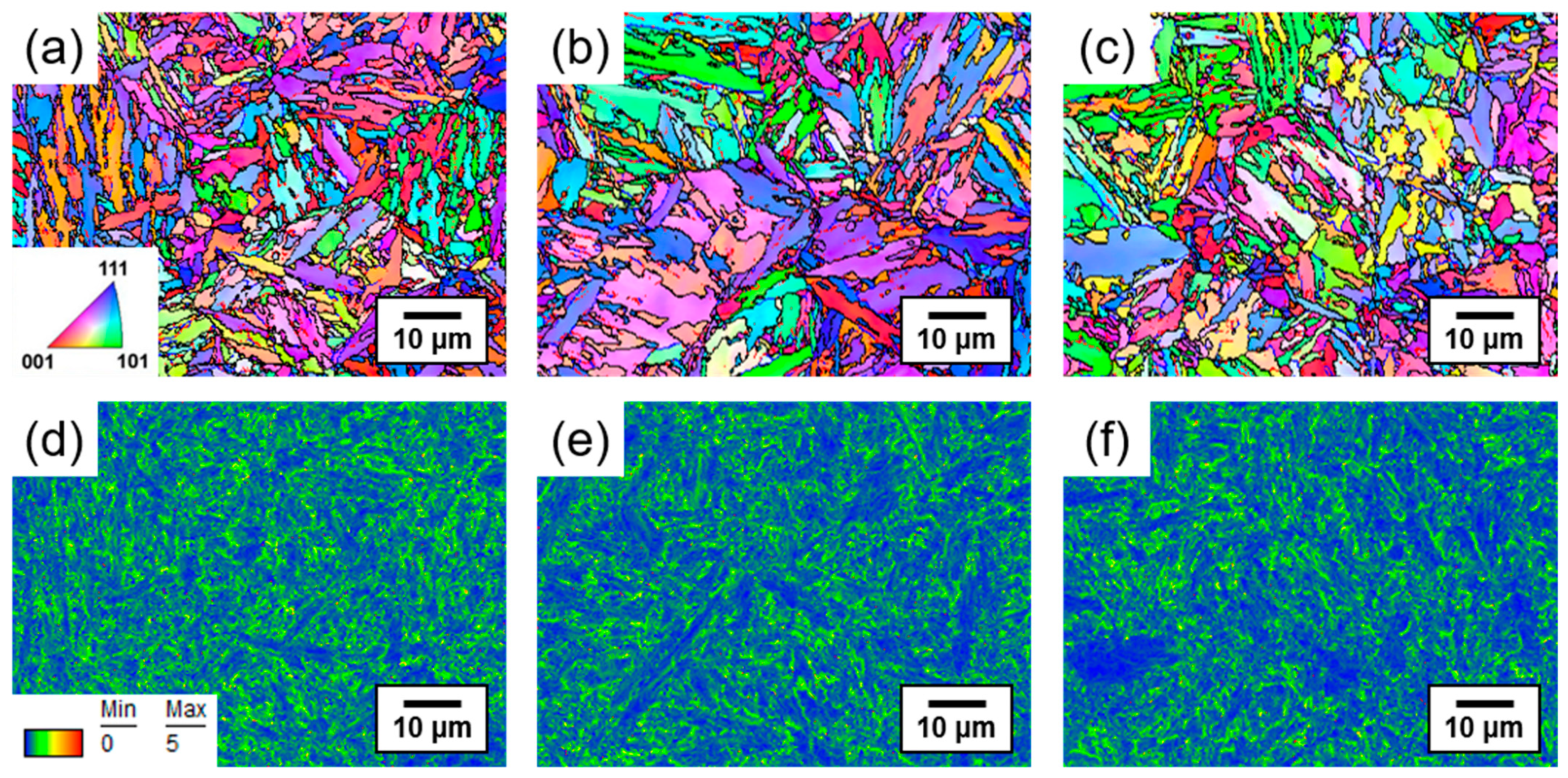
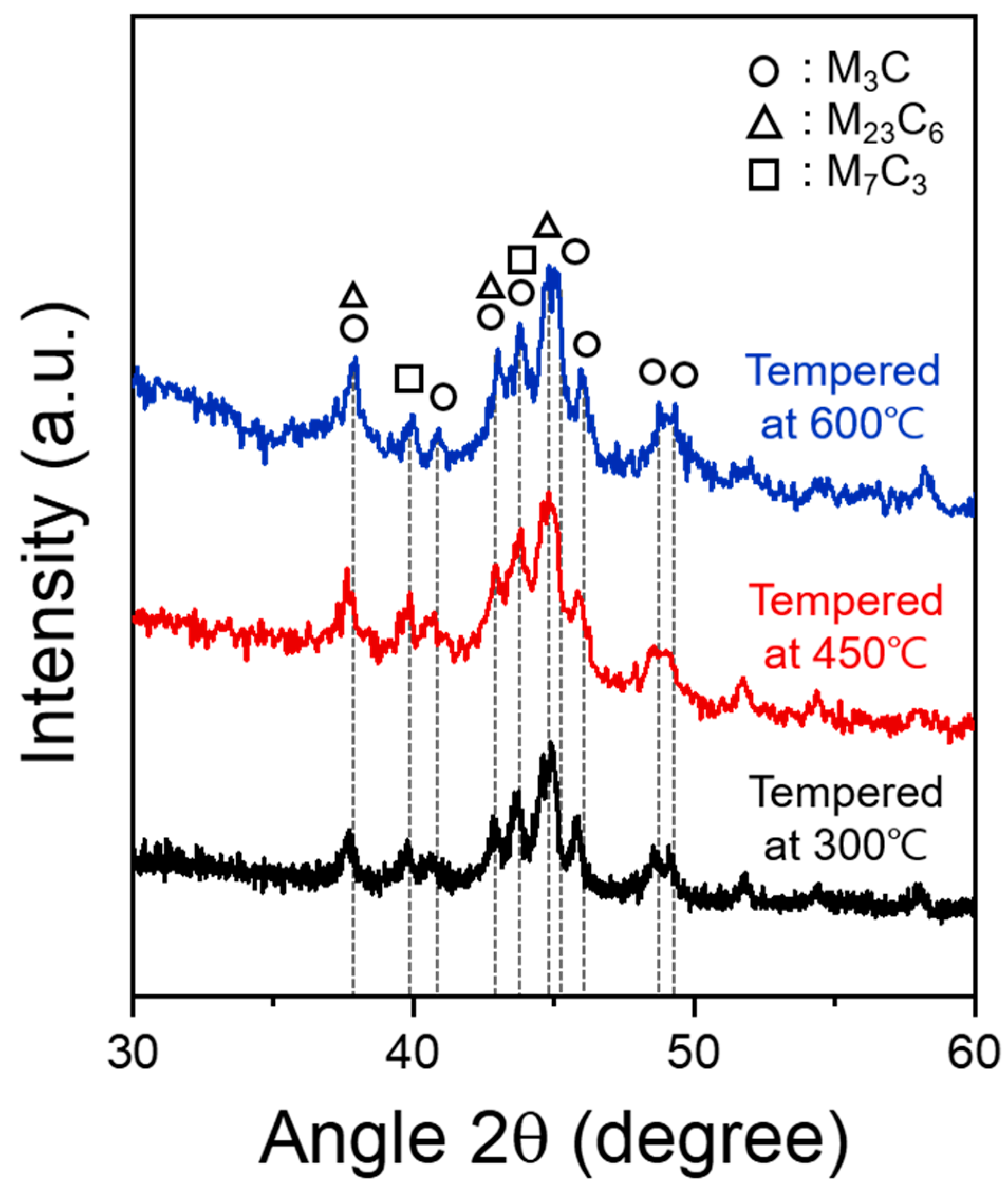
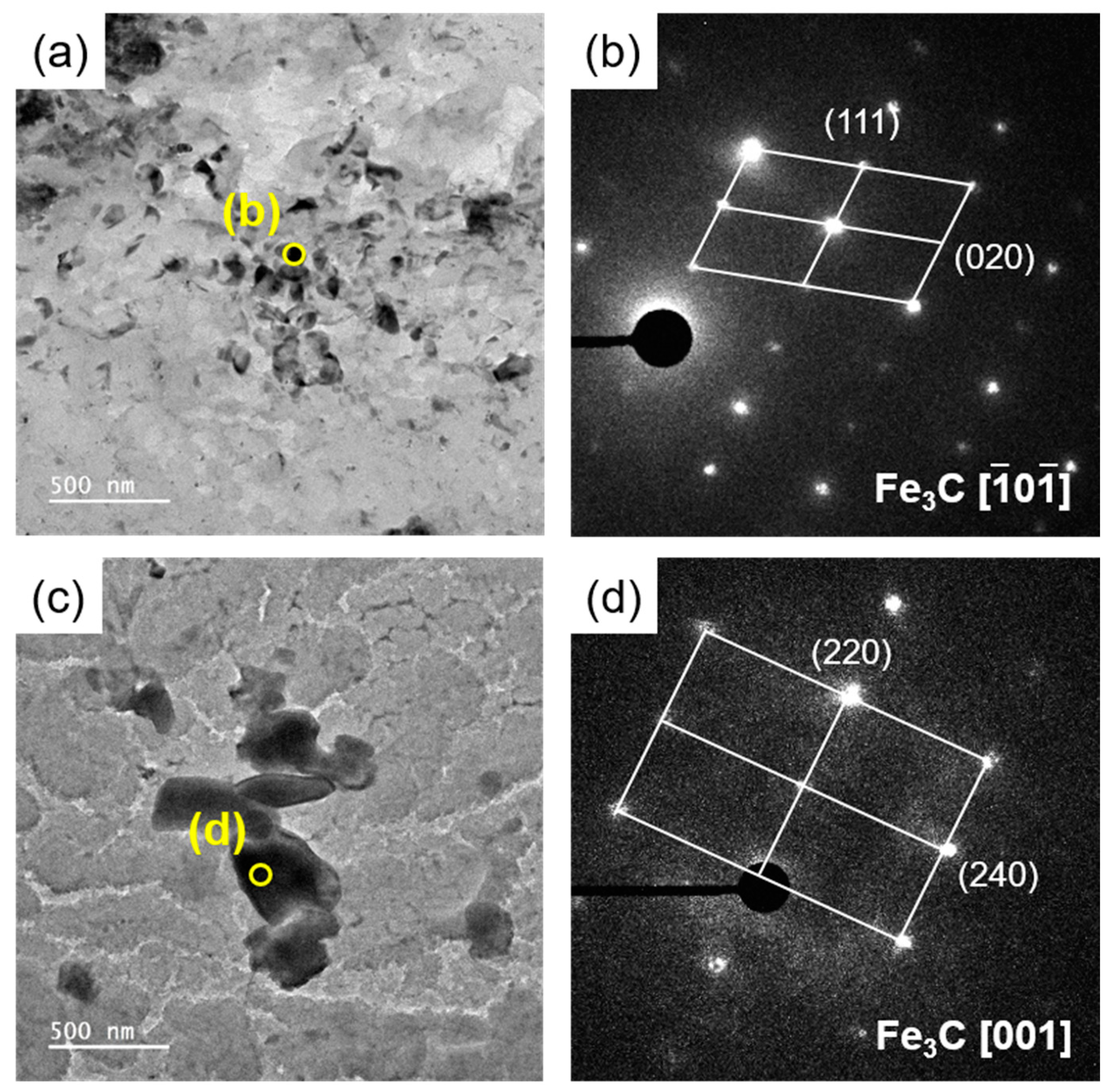
3.1. Influence of Tempering Temperature on Microstructure
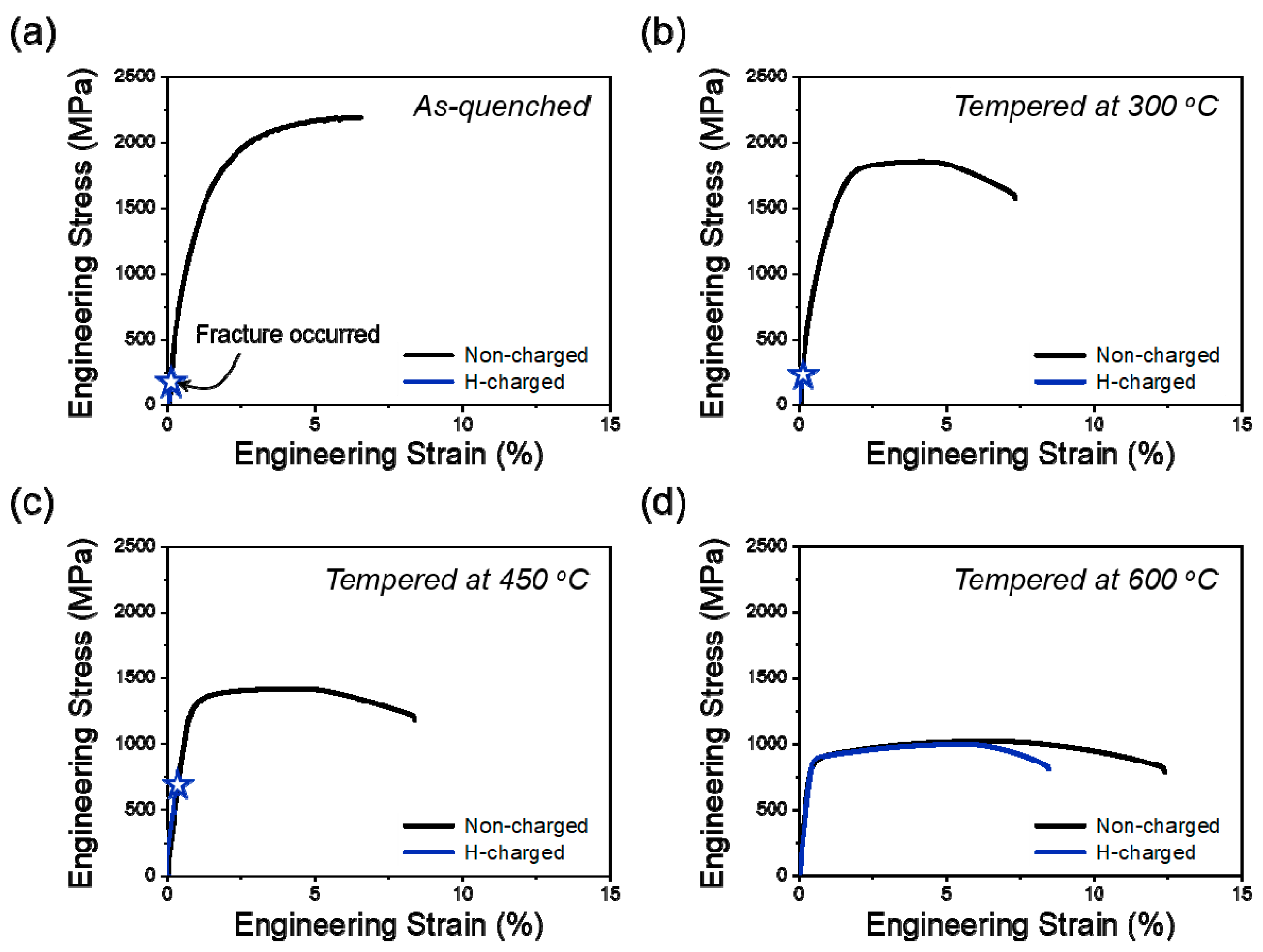
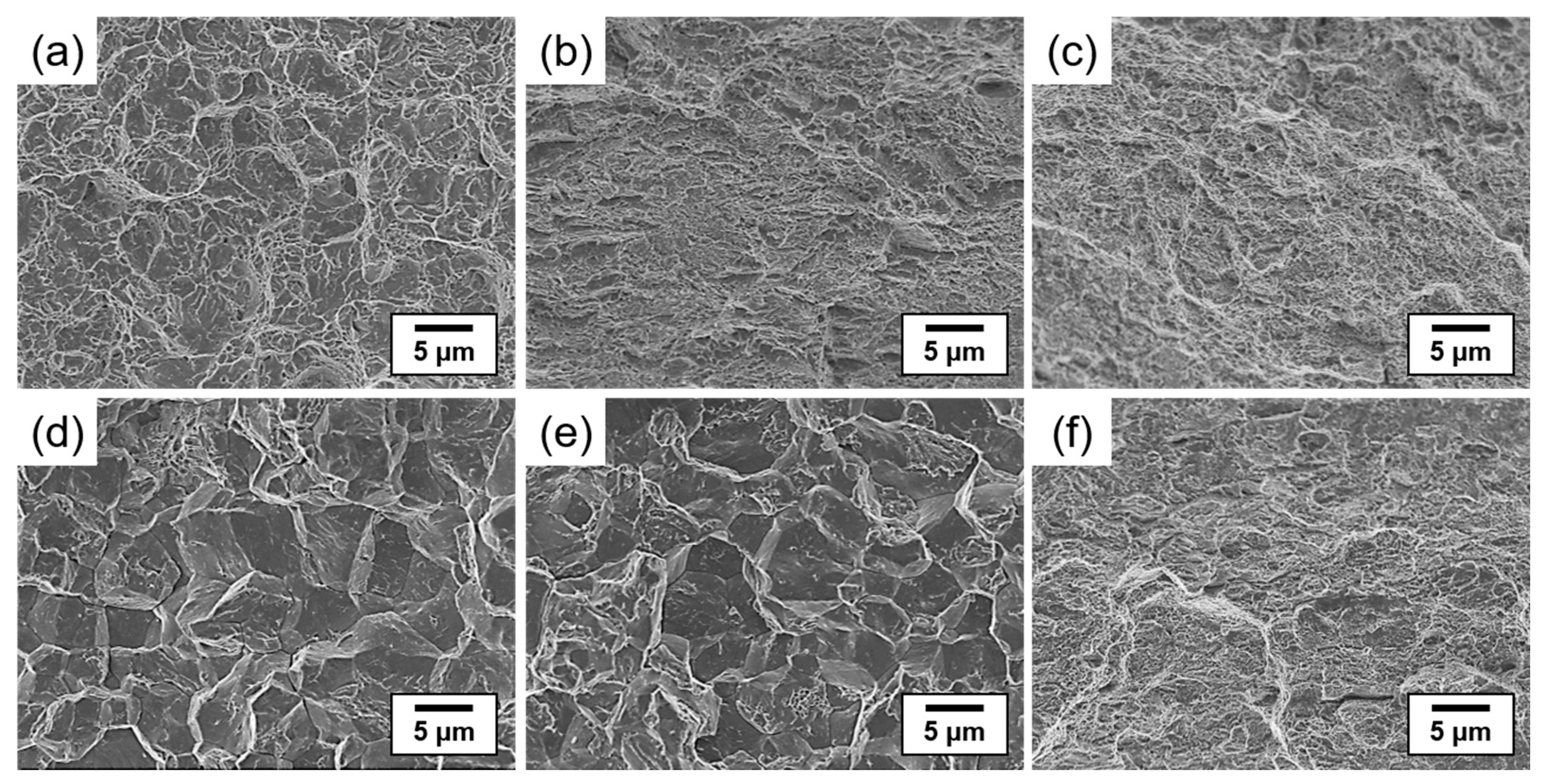
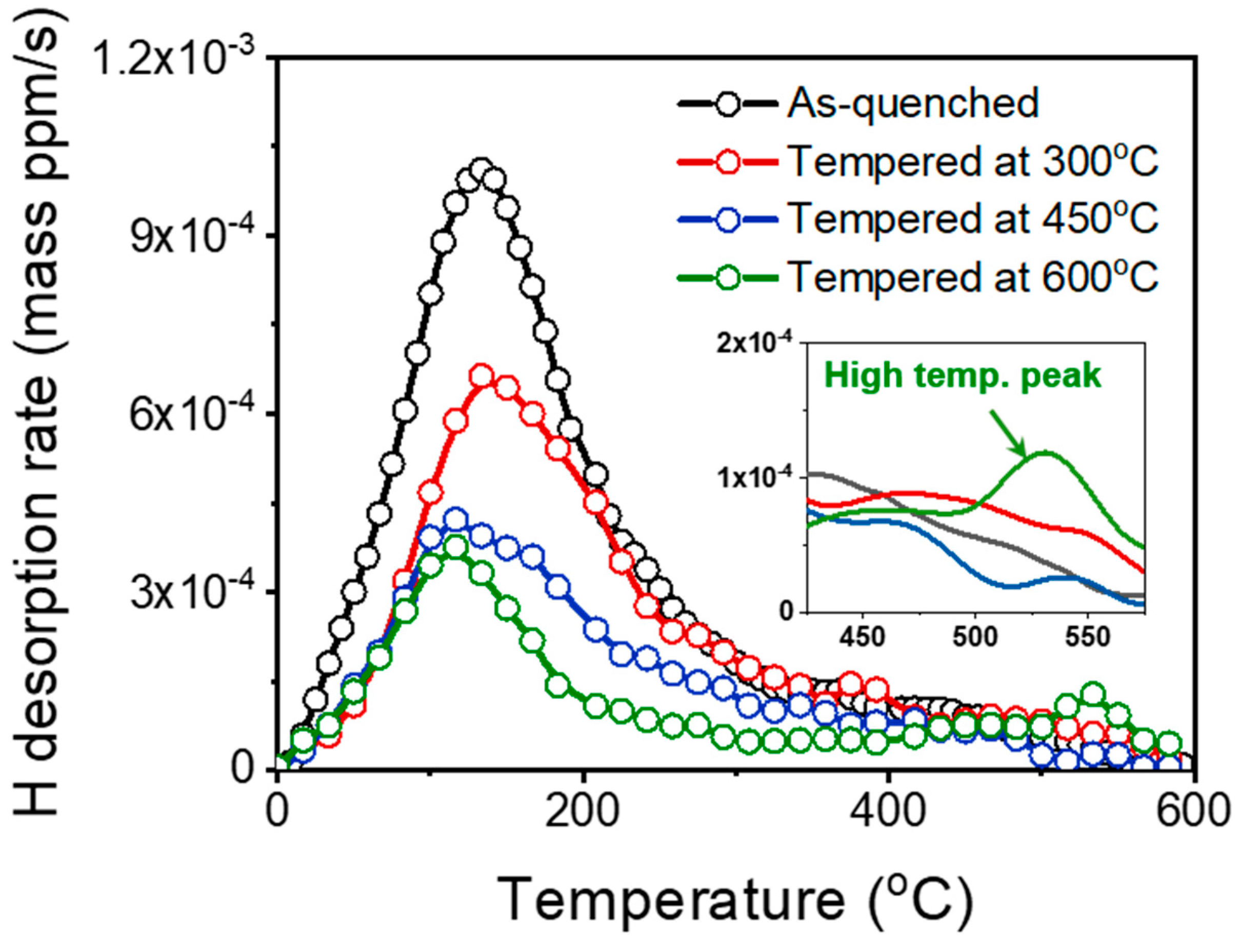
3.2. Influence of Tempering Temperature on Tensile Properties and Hydrogen Embrittlement
4. Conclusions
- SCM440 steels fabricated by quenching and tempering at various temperatures exhibited a microstructure composed of tempered martensite and cementite, whereas the as-quenched SCM440 steel had a fully lath-like martensitic structure. As the tempering temperature increased, the morphology of cementite changed from a long lamellar shape to a segmented short-rod shape via gradual spheroidization to reduce the surface energy of the cementite.
- The hydrogen embrittlement resistance of SCM440 tempered martensitic steels increased with an increasing tempering temperature, and this was mainly attributed to the decrease in the fraction of low-angle grain boundaries and dislocation density, which act as reversible hydrogen trap sites.
- In the as-quenched steel and steel specimen tempered at 300 °C with a relatively high dislocation density after electrochemical hydrogen charging, intergranular and quasi-cleavage fracture modes were observed, and hydrogen-assisted cracks were initiated from the grain boundaries. The mechanism of hydrogen-assisted crack formation can be attributed to the combined effect of the HELP and HEDE mechanisms.
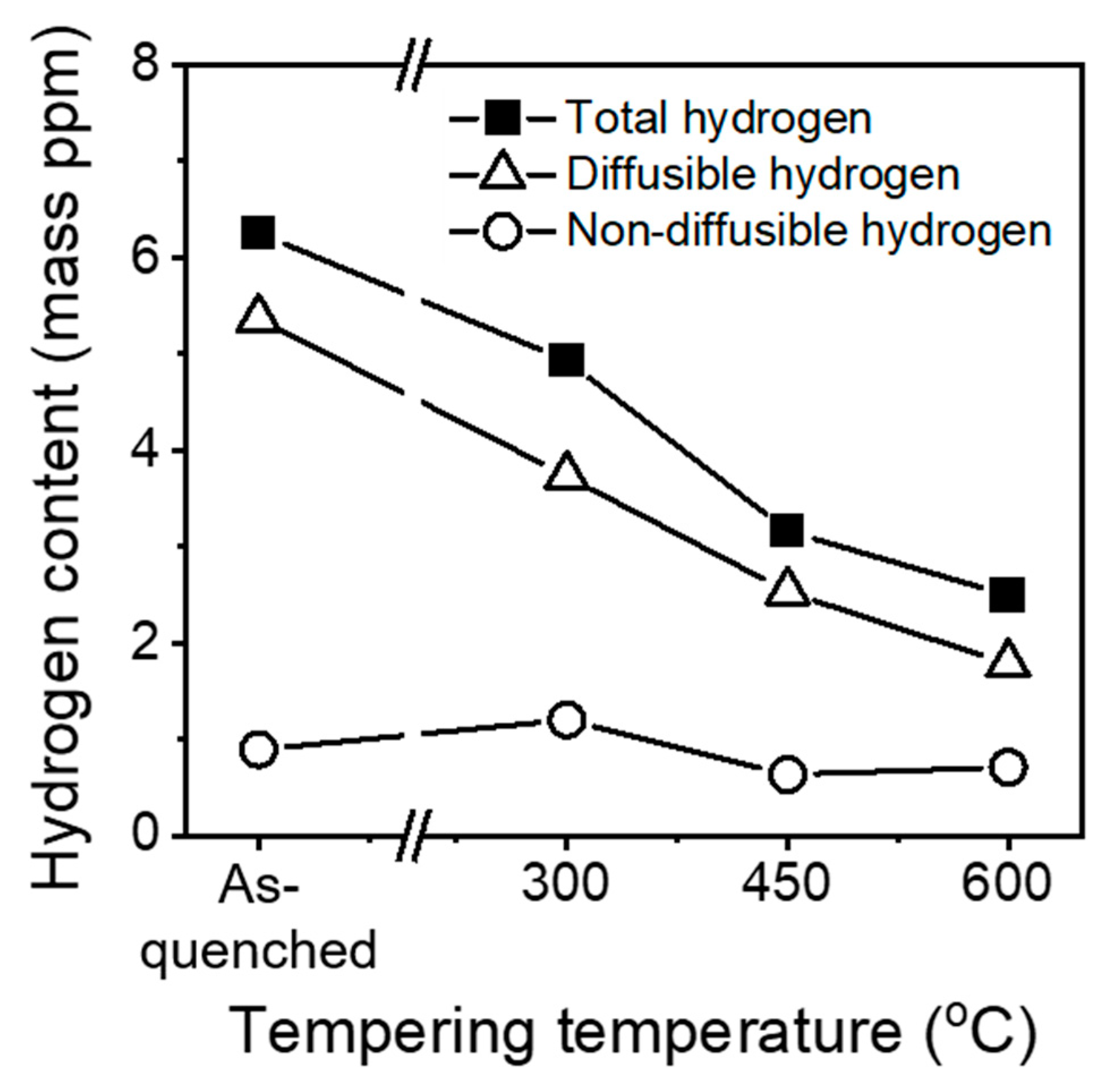
- Thermal desorption analysis results revealed that the diffusible hydrogen content decreased with an increasing tempering temperature. The hydrogen embrittlement resistance increased with decreasing the fraction of the diffusible hydrogen because the hydrogen embrittlement was mainly governed by the amount of diffusible hydrogen.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venezuela, J.; Liu, Q.; Zhang, M.; Zhou, Q.; Atrens, A. A review of hydrogen embrittlement of martensitic advanced high-strength steels. Corros. Rev. 2016, 34, 153–186. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.H.; Lee, D.L.; Park, K.T.; Lee, C.S. Microstructural influences on hydrogen delayed fracture of high strength steels. Mater. Sci. Eng. A 2009, 505, 105–110. [Google Scholar] [CrossRef]
- Hirata, K.; Iikubo, S.; Koyama, M.; Tsuzaki, K.; Ohtani, H. First-Principles Study on Hydrogen Diffusivity in BCC, FCC, and HCP Iron. Metall. Mater. Trans. A 2018, 49, 5015–5022. [Google Scholar] [CrossRef]
- Takai, K.; Watanuki, R. Hydrogen in Trapping States Innocuous to Environmental Degradation of High-strength Steels. ISIJ Int. 2003, 43, 520–526. [Google Scholar] [CrossRef]
- Louthan, M.R.; Caskey, G.R.; Donovan, J.A.; Rawl, D.E. Hydrogen embrittlement of metals. Mater. Sci. Eng. A 1972, 10, 357–368. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Lee, Y.K.; Raabe, D.; Tsuzaki, K. Overview of hydrogen embrittlement in high-Mn steels. Int. J. Hydrogen Energy 2017, 42, 12706–12723. [Google Scholar] [CrossRef]
- Pfeil, L.B. The effect of occluded hydrogen on the tensile strength of iron. Proc. R Soc. 1926, 112, 182–195. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Metall. Trans. 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Nagumo, M. Function of Hydrogen in Embrittlement of High-strength Steels. ISIJ Int. 2001, 41, 590–598. [Google Scholar] [CrossRef]
- Golovanenko, S.A.; Zikeev, V.N.; Serebryanaya, E.B.; Popova, L.V. The influence of alloy elements and structure on the resistance of constructional steels to hydrogen embrittlement. Met. Term Obrab Met. 1978, 1, 2–14. [Google Scholar]
- Yoo, J.; Jo, M.C.; Kim, S.; Oh, J.; Bian, J.; Sohn, S.S.; Lee, S. Effects of Ti alloying on resistance to hydrogen embrittlement in (Nb+Mo)-alloyed ultra-high-strength hot-stamping steels. Mater. Sci. Eng. A 2020, 791, 139763. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Hsu, T.Y.; Zhou, S.; Wang, L.; Jin, X. Improved resistance to hydrogen embrittlement in a high-strength steel by quenching–partitioning–tempering treatment. Scr. Mater. 2015, 97, 21–24. [Google Scholar] [CrossRef]
- Nagao, A.; Hayashi, K.; Oi, K.; Mitao, S. Effect of Uniform Distribution of Fine Cementite on Hydrogen Embrittlement of Low Carbon Martensitic Steel Plates. ISIJ Int. 2012, 52, 213–221. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.; Mun, D.J.; Bae, C.M.; Lee, C.S. Comparative study on the effects of Cr, V, and Mo carbides for hydrogen-embrittlement resistance of tempered martensitic steel. Sci. Rep. 2019, 9, 5219. [Google Scholar] [CrossRef]
- Peral, L.B.; Zafra, A.; Blason, S.; Rodriguez, C.; Belzunce, J. Effect of hydrogen on the fatigue crack growth rate of quenched and tempered CrMo and CrMoV steels. Int. J. Fatigue 2019, 120, 201–214. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Jiang, C.; Xiao, J. Effects of Niobium and Vanadium on Hydrogen-Induced Delayed Fracture in High Strength Spring Steel. J. Iron Steel Res. Int. 2011, 18, 49–53. [Google Scholar] [CrossRef]
- Zafra, A.; Peral, L.B.; Belzunce, J.; Rodríguez, C. Effects of hydrogen on the fracture toughness of 42CrMo4 steel quenched and tempered at different temperatures. Int. J. Press. Vessels Pip. 2019, 171, 34–50. [Google Scholar] [CrossRef]
- Kang, H.J.; Yoo, J.S.; Park, J.T.; Ahn, S.T.; Kang, N.; Cho, K.M. Effect of nano-carbide formation on hydrogen-delayed fracture for quenching and tempering steels during high-frequency induction heat treatment. Mater. Sci. Eng. A 2012, 543, 6–11. [Google Scholar] [CrossRef]
- Shi, X.; Yan, W.; Wang, W.; Shan, Y.; Yang, K. Novel Cu-bearing high-strength pipeline steels with excellent resistance to hydrogen-induced cracking. Mater. Des. 2016, 92, 300–305. [Google Scholar] [CrossRef]
- Kimura, Y.; Sakai, Y.; Hara, T.; Belyakov, A.; Tsuzaki, K. Hydrogen induced delayed fracture of ultrafine grained 0.6% O steel with dispersed oxide particles. Scr. Mater. 2003, 49, 1111–1116. [Google Scholar] [CrossRef]
- ASTM Standards. In Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2008.
- Saastamoinen, A.; Kaijalainen, A.; Heikkala, J.; Porter, D.; Suikkanen, P. The effect of tempering temperature on microstructure, mechanical properties and bendability of direct-quenched low-alloy strip steel. Mater. Sci. Eng. A 2018, 730, 284–294. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray debye-scherrer spectrum. Philos. Mag. A 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.; Nix, W.D.; Hutchinson, J.W. Mechanism-based strain gradient plasticity—I. Theory. J. Mech. Phys. Solids 1999, 47, 1239–1263. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Han, L.; Gu, J. Effect of Tempering Temperature on Microstructure Evolution and Hardness of 9Cr1. 5Mo1CoB(FB2) Steel. Steel Res. Int. 2021, 92, 2000519. [Google Scholar] [CrossRef]
- Badji, R.; Chauveau, T.; Bacroix, B. Texture, misorientation and mechanical anisotropy in a deformed dual phase stainless steel weld joint. Mater. Sci. Eng. A 2013, 575, 94–103. [Google Scholar] [CrossRef]
- Masoumi, M.; Silva, C.C.; Abreu, H.F.G. Effect of crystallographic orientations on the hydrogen-induced cracking resistance improvement of API 5L X70 pipeline steel under various thermomechanical processing. Corros. Sci. 2016, 111, 121–131. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Conforto, E.; Berziou, C.; Savall, C.; Feaugas, X. Grain size and grain-boundary effects on diffusion and trapping of hydrogen in pure nickel. Acta Mater. 2012, 60, 6814–6828. [Google Scholar] [CrossRef]
- Mine, Y.; Tachibana, K.; Horita, Z. Grain-boundary diffusion and precipitate trapping of hydrogen in ultrafine-grained austenitic stainless steels processed by high-pressure torsion. Mater. Sci. Eng. A 2011, 528, 8100–8105. [Google Scholar] [CrossRef]
- Takano, N. Hydrogen diffusion and embrittlement in 7075 aluminum alloy. Mater. Sci. Eng. A 2006, 483–484, 336–339. [Google Scholar] [CrossRef]
- Mine, Y.; Tsumagari, T.; Horita, Z. Hydrogen trapping on lattice defects produced by high-pressure torsion in Fe—0.01 mass% C alloy. Scr. Mater. 2010, 63, 552–555. [Google Scholar] [CrossRef]
- Xu, H.; Xia, X.; Hua, L.; Sun, Y.; Dai, Y. Evaluation of hydrogen embrittlement susceptibility of temper embrittled 2.25Cr-1Mo steel by SSRT method. Eng. Fail. Anal. 2012, 19, 43–50. [Google Scholar] [CrossRef]
- Dutta, R.K.; Petrov, R.H.; Delhez, R.; Hermans, M.J.M.; Richardson, I.M.; Bottger, A.J. The effect of tensile deformation by in situ ultrasonic treatment on the microstructure of low-carbon steel. Acta Mater. 2013, 61, 1592–1602. [Google Scholar] [CrossRef]
- Lin, Y.T.; Yi, H.L.; Chang, Z.Y.; Lin, H.C.; Yen, H.W. Role of vanadium carbide in hydrogen embrittlement of press-hardened steels: Strategy from 1500 to 2000 MPa. Front. Mater. 2021, 7, 611390. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.Y.; Peng, H.; Zhao, P.W.; Cai, Z.X. Effect of tempering temperature on hydrogen embrittlement in V-containing low alloy high strength steel. Mater. Lett. 2021, 302, 130327. [Google Scholar] [CrossRef]
- Rehrl, J.; Mraczek, K.; Pichler, A.; Werner, E. Mechanical properties and fracture behavior of hydrogen charged AHSS/UHSS grades at high- and low strain rate tests. Mater. Sci. Eng. A 2014, 590, 360–367. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, D.; Chiang, M.H.; Cheng, G.J.; Lin, H.C.; Yen, H.W. Response of Hydrogen Desorption and Hydrogen Embrittlement to Precipitation of Nanometer-Sized Copper in Tempered Martensitic Low-Carbon Steel. JOM 2019, 71, 1349–1356. [Google Scholar] [CrossRef]
- Wei, F.G.; Tsuzaki, K. Response of hydrogen trapping capability to microstructural change in tempered Fe—0.2C martensite. Scr. Mater. 2005, 52, 467–472. [Google Scholar] [CrossRef]
- Doshida, T.; Takai, K. Dependence of hydrogen-induced lattice defects and hydrogen embrittlement of cold-drawn pearlitic steels on hydrogen trap state, temperature, strain rate and hydrogen content. Acta Mater. 2014, 79, 93–107. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The effect of TiC on the hydrogen induced ductility loss and trapping behavior of Fe-C-Ti alloys. Corros. Sci. 2016, 112, 308–326. [Google Scholar] [CrossRef]

| Steel | Grain Boundary Fraction (1) (%) | Geometrically Necessary Dislocation (GND) Density (1) (1014/m2) | Dislocation Density (2) (1014/m2) | |
|---|---|---|---|---|
| Low-Angle Grain Boundary (<15°) | High-Angle Grain Boundary (>15°) | |||
| As-quenched | 24.8 | 75.2 | 4.59 | 4.81 |
| Tempered at 300 °C | 21.3 | 78.7 | 4.61 | 5.24 |
| Tempered at 450 °C | 21.3 | 78.7 | 4.29 | 2.60 |
| Tempered at 600 °C | 19.4 | 80.6 | 3.96 | 1.03 |
| Steel | Tensile Properties | Hydrogen Embrittlement Resistance | ||||
|---|---|---|---|---|---|---|
| Yield Strength (MPa) | Tensile Strength (MPa) | Total Elongation (%) | Relative Reduction Area (RRA) | Relative Elongation (RE) | ||
| As-quenched | Non-charged | 1421 ± 6 | 2190 ± 6 | 6.5 ± 0.4 | 0.03 | 0.01 |
| H-charged | 111 ± 17 | 111 ± 12 | 0.08 ± 0.0 | |||
| Tempered at 300 °C | Non-charged | 1379 ± 11 | 1857 ± 14 | 7.3 ± 0.5 | 0.05 | 0.01 |
| H-charged | 180 ± 7 | 180 ± 8 | 0.07 ± 0.0 | |||
| Tempered at 450 °C | Non-charged | 1204 ± 5 | 1422 ± 12 | 8.3 ± 0.1 | 0.09 | 0.04 |
| H-charged | 628 ± 4 | 628 ± 8 | 0.3 ± 0.0 | |||
| Tempered at 600 °C | Non-charged | 893 ± 5 | 1021 ± 7 | 12.4 ± 0.2 | 0.69 | 0.71 |
| H-charged | 894 ± 6 | 1000 ± 6 | 8.5 ± 0.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-G.; Kim, J.-Y.; Hwang, B. Effect of Tempering Temperature on Hydrogen Embrittlement of SCM440 Tempered Martensitic Steel. Materials 2023, 16, 5709. https://doi.org/10.3390/ma16165709
Kim S-G, Kim J-Y, Hwang B. Effect of Tempering Temperature on Hydrogen Embrittlement of SCM440 Tempered Martensitic Steel. Materials. 2023; 16(16):5709. https://doi.org/10.3390/ma16165709
Chicago/Turabian StyleKim, Sang-Gyu, Jae-Yun Kim, and Byoungchul Hwang. 2023. "Effect of Tempering Temperature on Hydrogen Embrittlement of SCM440 Tempered Martensitic Steel" Materials 16, no. 16: 5709. https://doi.org/10.3390/ma16165709
APA StyleKim, S.-G., Kim, J.-Y., & Hwang, B. (2023). Effect of Tempering Temperature on Hydrogen Embrittlement of SCM440 Tempered Martensitic Steel. Materials, 16(16), 5709. https://doi.org/10.3390/ma16165709






