In Silico Guided Design of Metal/Semiconductor Photocatalysts: A Case of Cu-Modified TiO2 for Ciprofloxacin Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. DFT Calculations
2.2. Preparation of TiO2
2.3. Preparation of Cu/TiO2
2.4. Photodegradation of the Ciprofloxacin
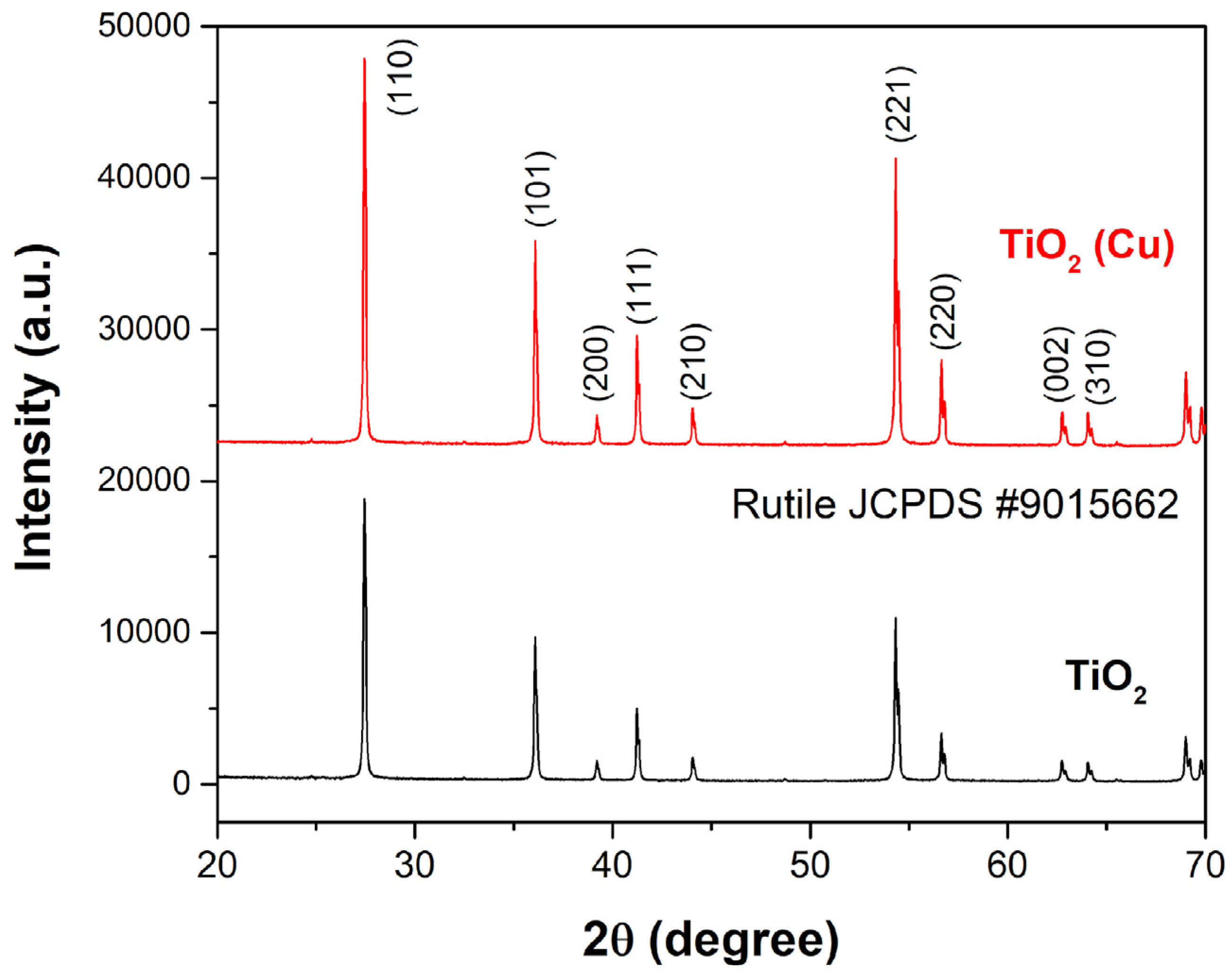
2.5. XRD Analysis
2.6. SEM Analysis
2.7. TOC Analysis
3. Results
3.1. DFT Screening of d-Metal Adhesion
3.2. Characterization of Prepared Photocatalysts
3.3. Photodegradation of Ciprofloxacin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akter, S.; Islam, M.S.; Kabir, M.H.; Shaikh, M.A.A.; Gafur, M.A. UV/TiO2 photodegradation of metronidazole, ciprofloxacin and sulfamethoxazole in aqueous solution: An optimization and kinetic study. Arab. J. Chem. 2022, 15, 103900. [Google Scholar] [CrossRef]
- Costa, L.N.; Nobre, F.X.; Lobo, A.O.; de Matos, J.M.E. Photodegradation of ciprofloxacin using Z-scheme TiO2/SnO2 nanostructures as photocatalyst. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100466. [Google Scholar] [CrossRef]
- Barbara Ambrosetti, L.C.; Palmisano, R. Photo-Degradation of Amoxicillin, Streptomycin, Erythromycin and Ciprofloxacin by UV and UV/TiO2 Processes. Evaluation of Toxicity Changes Using a Respirometric Biosensor. J. Environ. Anal. Chem. 2015, 2, 1000143. [Google Scholar] [CrossRef]
- Abellán, M.N.; Giménez, J.; Esplugas, S. Photocatalytic degradation of antibiotics: The case of sulfamethoxazole and trimethoprim. Catal. Today 2009, 144, 131–136. [Google Scholar] [CrossRef]
- Pecquet, S.; Ravoire, S.; Andremont, A. Faecal excretion of ciprofloxacin after a single oral dose and its effect on faecal bacteria in healthy volunteers. J. Antimicrob. Chemother. 1990, 26, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, G.; Jayaseelan, A.; Farnood, R. Photocatalytic reactive oxygen species generation and their mechanisms of action in pollutant removal with biochar supported photocatalysts: A review. J. Clean. Prod. 2022, 346, 131155. [Google Scholar] [CrossRef]
- Qutob, M.; Hussein, M.A.; Alamry, K.A.; Rafatullah, M. A review on the degradation of acetaminophen by advanced oxidation process: Pathway, by-products, biotoxicity, and density functional theory calculation. RSC Adv. 2022, 12, 18373–18396. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Ohno, T.; Andou, Y. Recent Progress in Photocatalytic Efficiency of Hybrid Three-Dimensional (3D) Graphene Architectures for Pollution Remediation. Top. Catal. 2022, 65, 1634–1647. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Abdullah, C.A.C.; Chung, E.L.T.; Andou, Y. Recent progress in visible light-doped ZnO photocatalyst for pollution control. Int. J. Environ. Sci. Technol. 2022, 20, 5753–5772. [Google Scholar] [CrossRef]
- Shafiee, A.; Rabiee, N.; Ahmadi, S.; Baneshi, M.; Khatami, M.; Iravani, S.; Varma, R.S. Core–Shell Nanophotocatalysts: Review of Materials and Applications. ACS Appl. Nano Mater. 2022, 5, 55–86. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1694. [Google Scholar] [CrossRef] [PubMed]
- Goktas, A.; Modanlı, S.; Tumbul, A.; Kilic, A. Facile synthesis and characterization of ZnO, ZnO:Co, and ZnO/ZnO:Co nano rod-like homojunction thin films: Role of crystallite/grain size and microstrain in photocatalytic performance. J. Alloys Compd. 2022, 893, 162334. [Google Scholar] [CrossRef]
- Žerjav, G.; Arshad, M.S.; Djinović, P.; Junkar, I.; Kovač, J.; Zavašnik, J.; Pintar, A. Improved electron–hole separation and migration in anatase TiO2 nanorod/reduced graphene oxide composites and their influence on photocatalytic performance. Nanoscale 2017, 9, 4578–4592. [Google Scholar] [CrossRef]
- Al-Namshah, K.S.; Shkir, M.; Ibrahim, F.A.; Hamdy, M.S. Auto combustion synthesis and characterization of Co doped ZnO nanoparticles with boosted photocatalytic performance. Phys. B Condens. Matter 2022, 625, 413459. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2016, 7, 34–59. [Google Scholar] [CrossRef]
- Shao, C.; Lin, L.; Duan, L.; Jiang, Y.; Shao, Q.; Cao, H. Nickel-enhanced electrochemical activities of shape-tailored TiO2{001} nanocrystals for water treatment: A combined experimental and DFT studies. Electrochim. Acta 2021, 376, 138066. [Google Scholar] [CrossRef]
- Hossain, M.N.; Choueiri, R.M.; Abner, S.; Chen, L.D.; Chen, A. Electrochemical Reduction of Carbon Dioxide at TiO2/Au Nanocomposites. ACS Appl. Mater. Interfaces 2022, 14, 51889–51899. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Zhang, W.; Zhang, R.; Wang, B.; Ji, C.; Pei, Z.; Sang, S. Tuning Schottky Barrier and Contact Type of Metal–Semiconductor in Ti3C2T2/MoS2 (T = F, O, OH) by Strain: A First-Principles Study. J. Phys. Chem. C 2021, 125, 16200–16210. [Google Scholar] [CrossRef]
- Biswas, A.; Nandi, S.; Kamboj, N.; Pan, J.; Bhowmik, A.; Dey, R.S. Alteration of Electronic Band Structure via a Metal-Semiconductor Interfacial Effect Enables High Faradaic Efficiency for Electrochemical Nitrogen Fixation. ACS Nano 2021, 15, 20364–20376. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lv, P. DFT insight into the effect of Cu atoms on adsorption and dissociation of CO2 over a Pd8/TiO2(101) surface. RSC Adv. 2021, 11, 17391–17398. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The Work Function of TiO2. Surfaces 2018, 1, 73–89. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Yang, L.; Sharman, E.; Jiang, J. Material descriptors for photocatalyst/catalyst design. WIREs Comput. Mol. Sci. 2018, 8, e1369. [Google Scholar] [CrossRef]
- Badalov, S.V.; Bocchini, A.; Wilhelm, R.; Kozub, A.L.; Gerstmann, U.; Schmidt, W.G. Rutile, anatase, brookite and titania thin film from Hubbard corrected and hybrid DFT. Mater. Res. Express 2023, 10, 075501. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter Inst. Phys. J. 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Cococcioni, M.; de Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in theLDA+Umethod. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Batalović, K.; Radaković, J.; Bundaleski, N.; Rakočević, Z.; Pašti, I.; Skorodumova, N.V.; Rangel, C.M. Origin of photocatalytic activity enhancement in Pd/Pt-deposited anatase N-TiO2–Experimental insights and DFT study of the (001) surface. Phys. Chem. Chem. Phys. 2020, 22, 18536–18547. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kokalj, A. XCrySDen—A new program for displaying crystalline structures and electron densities. J. Mol. Graph. Model. 1999, 17, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Viana, M.M.; Soares, V.F.; Mohallem, N.D.S. Synthesis and characterization of TiO2 nanoparticles. Ceram. Int. 2010, 36, 2047–2053. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Mohammadi Ziarani, G. Photo and Chemical Reduction of Copper onto Anatase-Type TiO2 Nanoparticles with Enhanced Surface Hydroxyl Groups as Efficient Visible Light Photocatalysts. Photochem. Photobiol. 2015, 91, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Introduction to Solid State Physics: International Edition, 8th ed.; John Wiley Sons (WIE): Hoboken, NJ, USA, 2005. [Google Scholar]
- De Angelis, F.; Di Valentin, C.; Fantacci, S.; Vittadini, A.; Selloni, A. Theoretical studies on anatase and less common TiO2 phases: Bulk, surfaces, and nanomaterials. Chem. Rev. 2014, 114, 9708–9753. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Kangawa, Y.; Shiraishi, K. Atomic Structures and Electronic Properties of Semiconductor Interfaces. In Comprehensive Semiconductor Science and Technology; Bhattacharya, P., Fornari, R., Kamimura, H., Eds.; Elsevier: Amsterdam, The Netherland, 2011; pp. 113–174. [Google Scholar]
- Ortega, Y.; Hernandez, N.C.; Menendez-Proupin, E.; Graciani, J.; Sanz, J.F. Nitrogen/gold codoping of the TiO2(101) anatase surface. A theoretical study based on DFT calculations. Phys. Chem. Chem. Phys. PCCP 2011, 13, 11340–11350. [Google Scholar] [CrossRef]
- Jeon, M.-K.; Park, J.-W.; Kang, M. Hydrogen Production from Methanol/Water Decomposition in a Liquid Photosystem Using the Anatase and Rutile Forms of Cu-TiO2. J. Ind. Eng. Chem 2007, 13, 84–91. [Google Scholar]
- Long, F.; Cao, X.; Liu, P.; Jiang, X.; Jiang, J.; Zhang, X.; Xu, J. Tuning charge transfer of Pt cluster by support defects in Pt/TiO2 for photocatalytic conversion of fatty acid into diesel-like alkane. J. Clean. Prod. 2022, 375, 133975. [Google Scholar] [CrossRef]
- Triquet, T.; Tendero, C.; Latapie, L.; Manero, M.-H.; Richard, R.; Andriantsiferana, C. TiO2 MOCVD coating for photocatalytic degradation of ciprofloxacin using 365 nm UV LEDs-kinetics and mechanisms. J. Environ. Chem. Eng. 2020, 8, 104544. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, S.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO(2) nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, N.A.; Khasri, A.; Ridzuan, M.J.M.; Salleh, N.H.M.; Chaijak, P. Synergistic Adsorption/Photodegradation of Ciprofloxacin by UV Light-Driven Nanocomposite Photocatalyst of Cu Doped AC/TiO2: Experimental Design via RSM-CCD; Research Square: Durham, NC, USA, 2023. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S. Photocatalytic degradation of ciprofloxacin by synthesized TiO2 nanoparticles on montmorillonite: Effect of operation parameters and artificial neural network modeling. J. Mol. Catal. A Chem. 2015, 409, 149–161. [Google Scholar] [CrossRef]
- Thai, T.; Salisbury, B.H.; Zito, P.M. Ciprofloxacin. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]

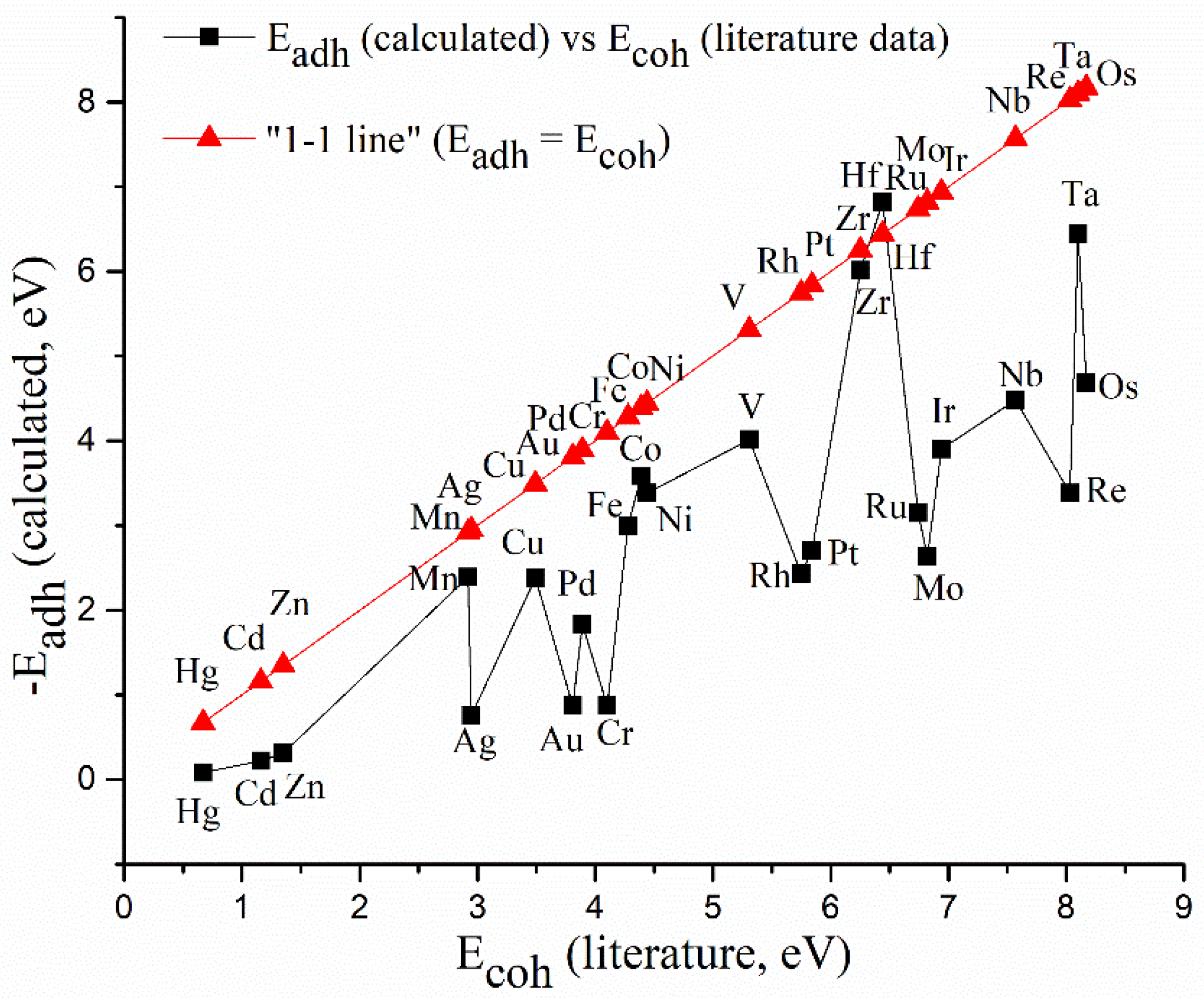
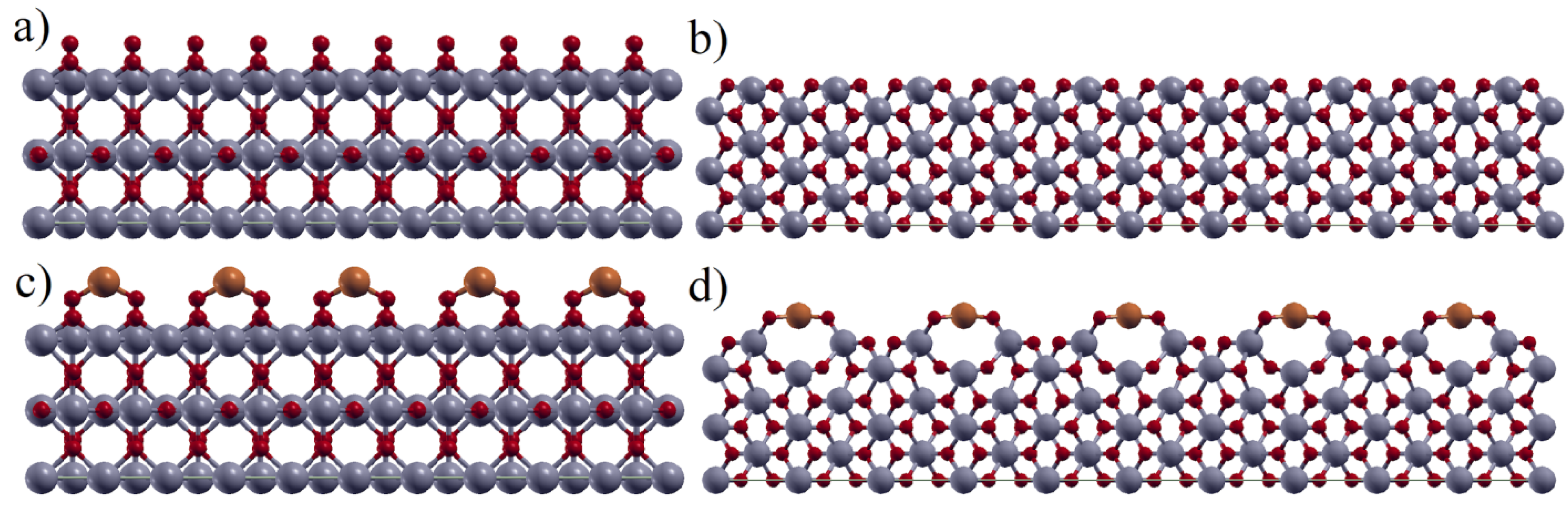
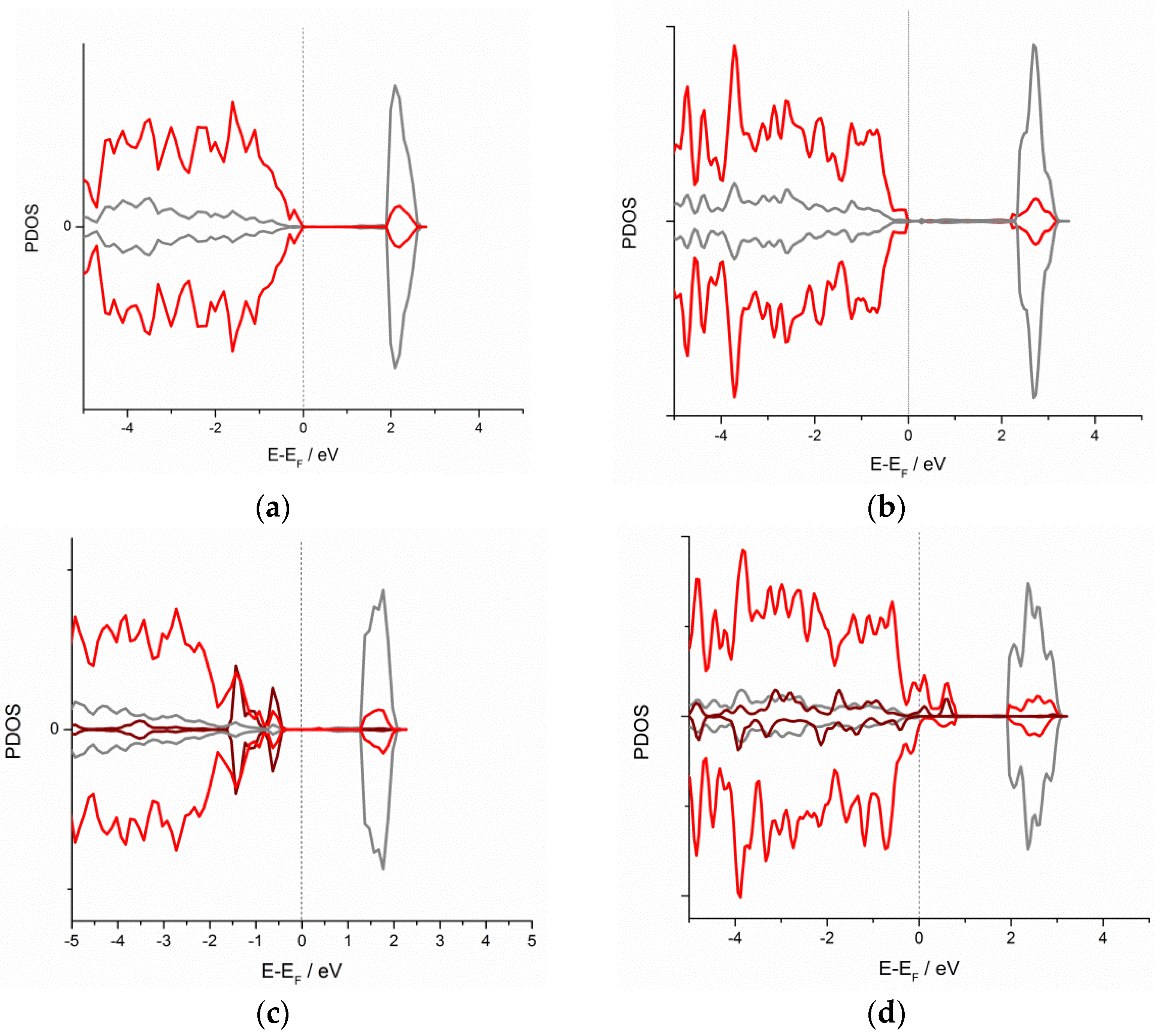
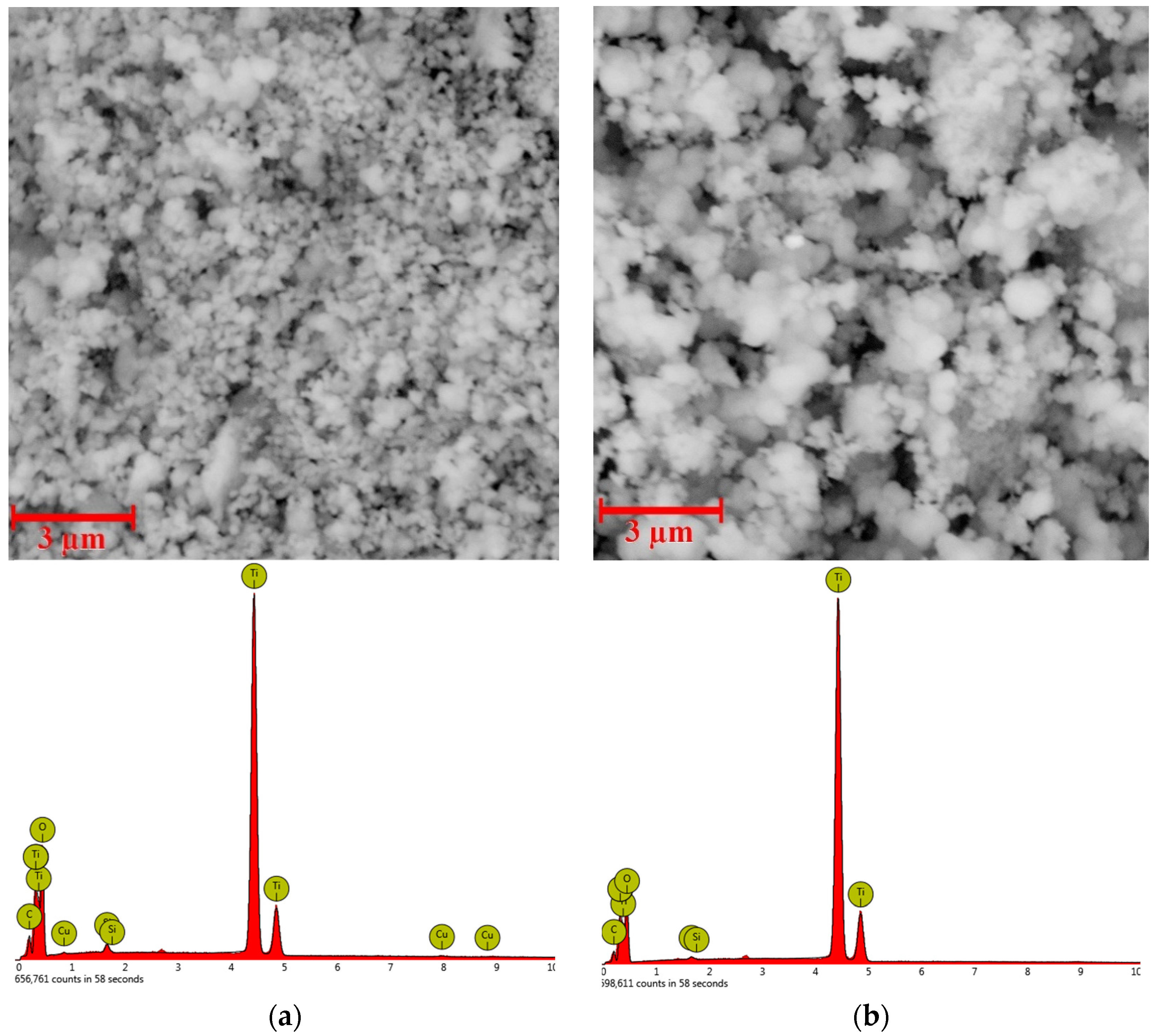
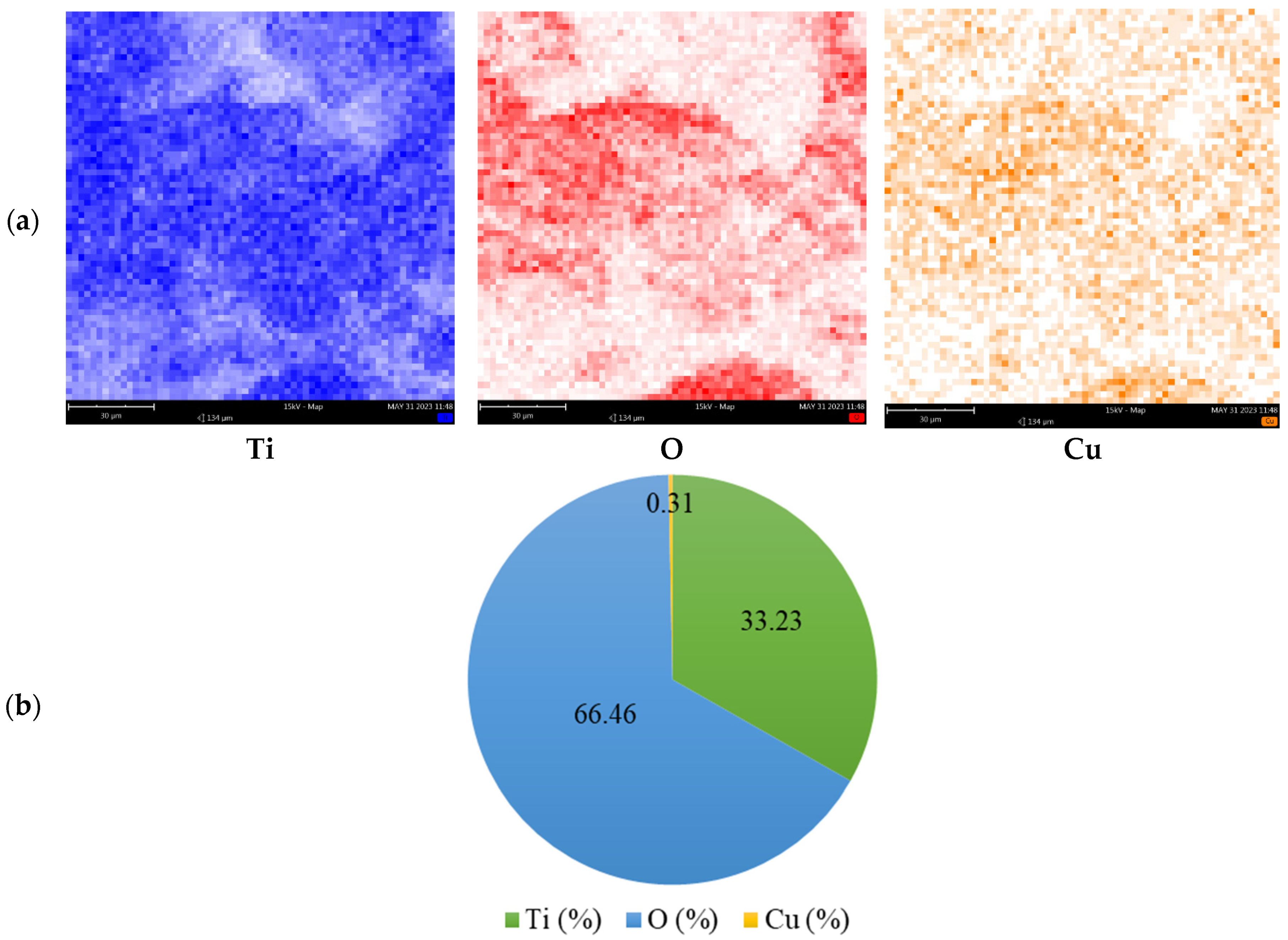
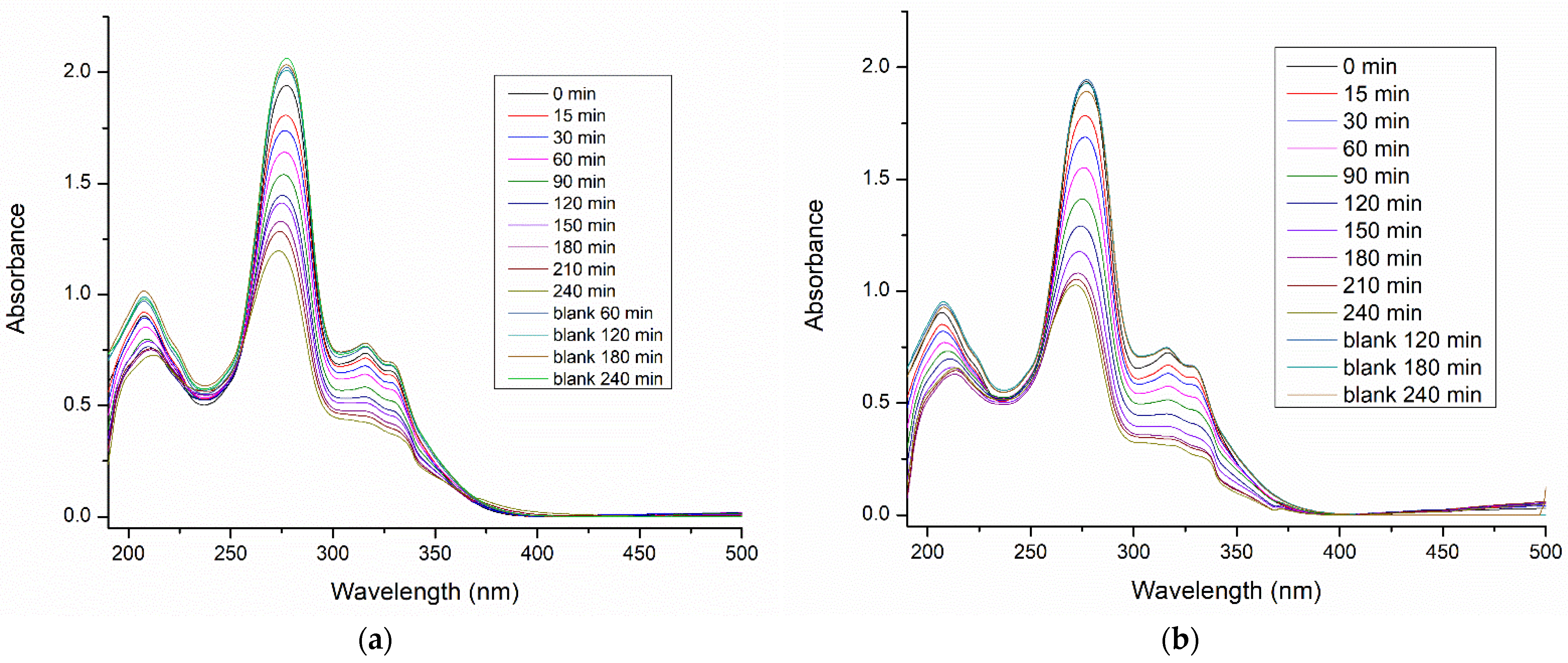
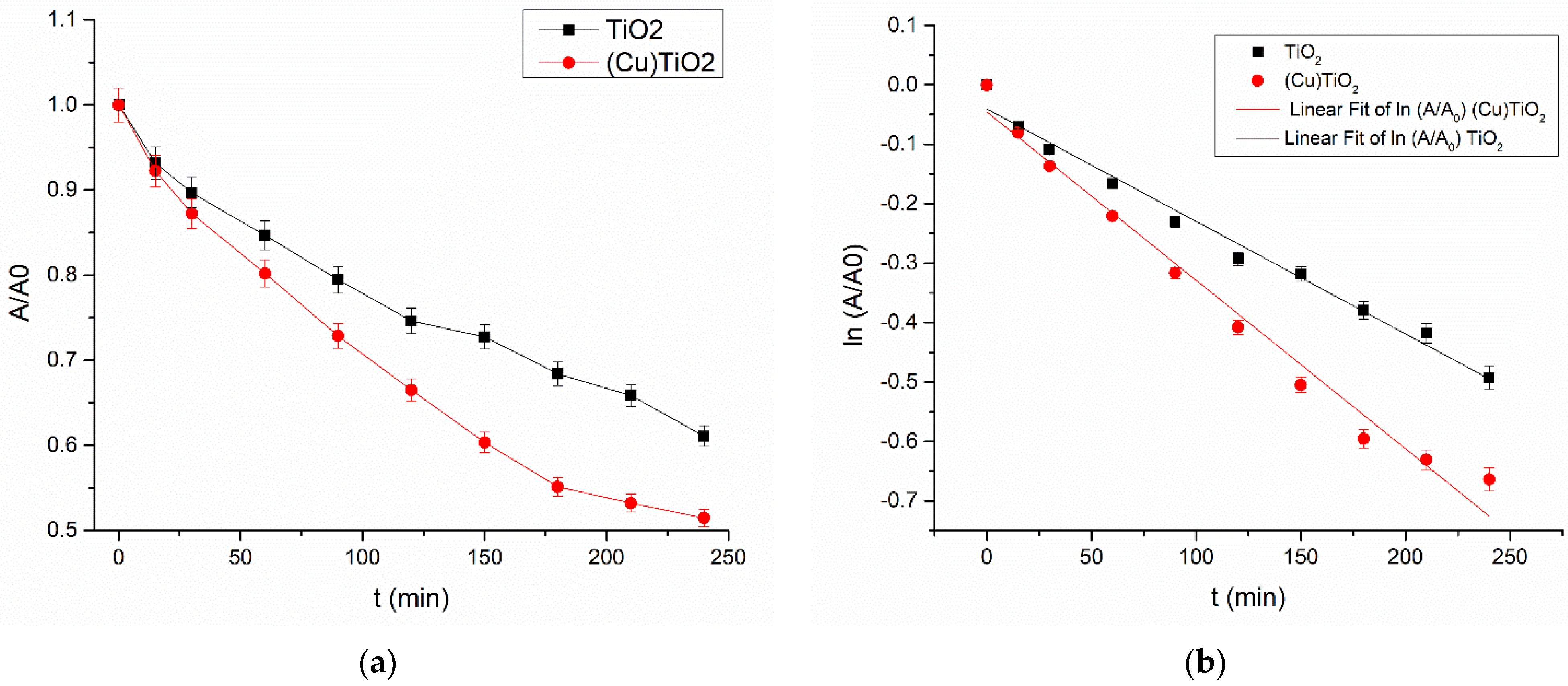
| Metal/Ads. Site | Bridge (eV) | Hollow (eV) | Top (eV) | Metal/Ads. Site | Bridge (eV) | Hollow (eV) | Top (eV) |
|---|---|---|---|---|---|---|---|
| Ag | −0.868 | −0.690 | −0.274 | Nb | −4.476 | −4.416 | −0.604 |
| Au | −0.680 | −0.757 | −0.523 | Ni | −3.376 | −2.967 | n.s. |
| Cd | −0.220 | n.s. | −0.050 | Os | −4.675 | −3.902 | −0.675 |
| Co | −3.581 | −2.967 | −0.422 | Pd | −1.398 | −1.830 | −0.806 |
| Cr | −0.870 | n.s. | −0.115 | Pt | −1.962 | −2.698 | −0.939 |
| Cu | −2.376 | −1.206 | −0.270 | Re | −2.700 | −3.382 | −0.259 |
| Fe | −2.492 | −2.994 | −0.431 | Rh | −2.429 | −1.802 | −0.799 |
| Hf | −6.817 | −2.695 | −1.106 | Ru | −2.805 | −3.145 | −0.860 |
| Hg | −0.076 | −0.048 | −0.041 | Ta | −4.805 | −6.435 | −0.769 |
| Ir | −2.887 | −3.897 | −0.979 | V | −4.006 | −2.348 | −0.342 |
| Mn | −2.385 | −0.931 | −0.268 | Zn | −0.314 | −0.077 | −0.053 |
| Mo | −2.628 | −2.598 | −0.141 | Zr | −6.014 | −5.503 | −1.170 |
| Eadh (eV) | Electrons Transferred from Cu to TiO2 | |
|---|---|---|
| (Cu)TiO2(001) | −2.61 | 0.65 |
| (Cu)TiO2(110) | −6.71 | 1.05 |
| Sample | Crystallite Size (Å) | Lattice Strain (%) | Lattice Parameter (Å) |
|---|---|---|---|
| TiO2(Cu) | 751(19) | 0.01(3) | a = 4.59332(8) b = 4.59332(8) c = 2.95910(7) |
| TiO2 | 685(20) | 0 | a = 4.59274(14) b = 4.59274(14) c = 2.95958(12) |
| k (min−1) | R2 | |
|---|---|---|
| (Cu)TiO2 | 0.0028 ± 0.0002 | 0.97942 |
| TiO2 | 0.00189 ± 0.00009 | 0.98365 |
| Initial TOC (t = 0) mg/L | Final TOC (t = 240 min) mg/L | TOC Removal (%) | |
|---|---|---|---|
| TiO2 | 14.8 | 13.4 | 9.5 |
| (Cu)TiO2 | 14.8 | 13.0 | 12.2 |
| Catalyst | Catalyst Dosage (mg/L) | Initial Concentration (mg/L) | UV-Irradiation Parameters | Time (min) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Cu/TiO2 | 20 | 20 | 15 W λ = 254 nm | 240 | 50 | Present study |
| TiO2 | 700 | 80 | 24 W λ = 254 nm | 600 | 89 | [1] |
| TiO2 | 120 | 20 | Irradiance, 100 Wm−2 λ = 365 nm | 60 | 75 | [42] |
| GMC-TiO2 composite | 350 | 15 | 14 W λ = 254 nm | 90 | 100 | [43] |
| Cu-doped AC/TiO2 | 320 | 100 | 7 W λ = 254 nm | 120 | 95 | [44] |
| TiO2/MMT | 100 | 20 | 16 W UV-C | 120 | 62 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, M.; Živković, S.; Ognjanović, M.; Momčilović, M.; Relić, D.; Vasić Anićijević, D. In Silico Guided Design of Metal/Semiconductor Photocatalysts: A Case of Cu-Modified TiO2 for Ciprofloxacin Degradation. Materials 2023, 16, 5708. https://doi.org/10.3390/ma16165708
Kovačević M, Živković S, Ognjanović M, Momčilović M, Relić D, Vasić Anićijević D. In Silico Guided Design of Metal/Semiconductor Photocatalysts: A Case of Cu-Modified TiO2 for Ciprofloxacin Degradation. Materials. 2023; 16(16):5708. https://doi.org/10.3390/ma16165708
Chicago/Turabian StyleKovačević, Marija, Sanja Živković, Miloš Ognjanović, Miloš Momčilović, Dubravka Relić, and Dragana Vasić Anićijević. 2023. "In Silico Guided Design of Metal/Semiconductor Photocatalysts: A Case of Cu-Modified TiO2 for Ciprofloxacin Degradation" Materials 16, no. 16: 5708. https://doi.org/10.3390/ma16165708
APA StyleKovačević, M., Živković, S., Ognjanović, M., Momčilović, M., Relić, D., & Vasić Anićijević, D. (2023). In Silico Guided Design of Metal/Semiconductor Photocatalysts: A Case of Cu-Modified TiO2 for Ciprofloxacin Degradation. Materials, 16(16), 5708. https://doi.org/10.3390/ma16165708







