Characterization of SiO2 Plasma Etching with Perfluorocarbon (C4F8 and C6F6) and Hydrofluorocarbon (CHF3 and C4H2F6) Precursors for the Greenhouse Gas Emissions Reduction
Abstract
:1. Introduction
2. Experiment
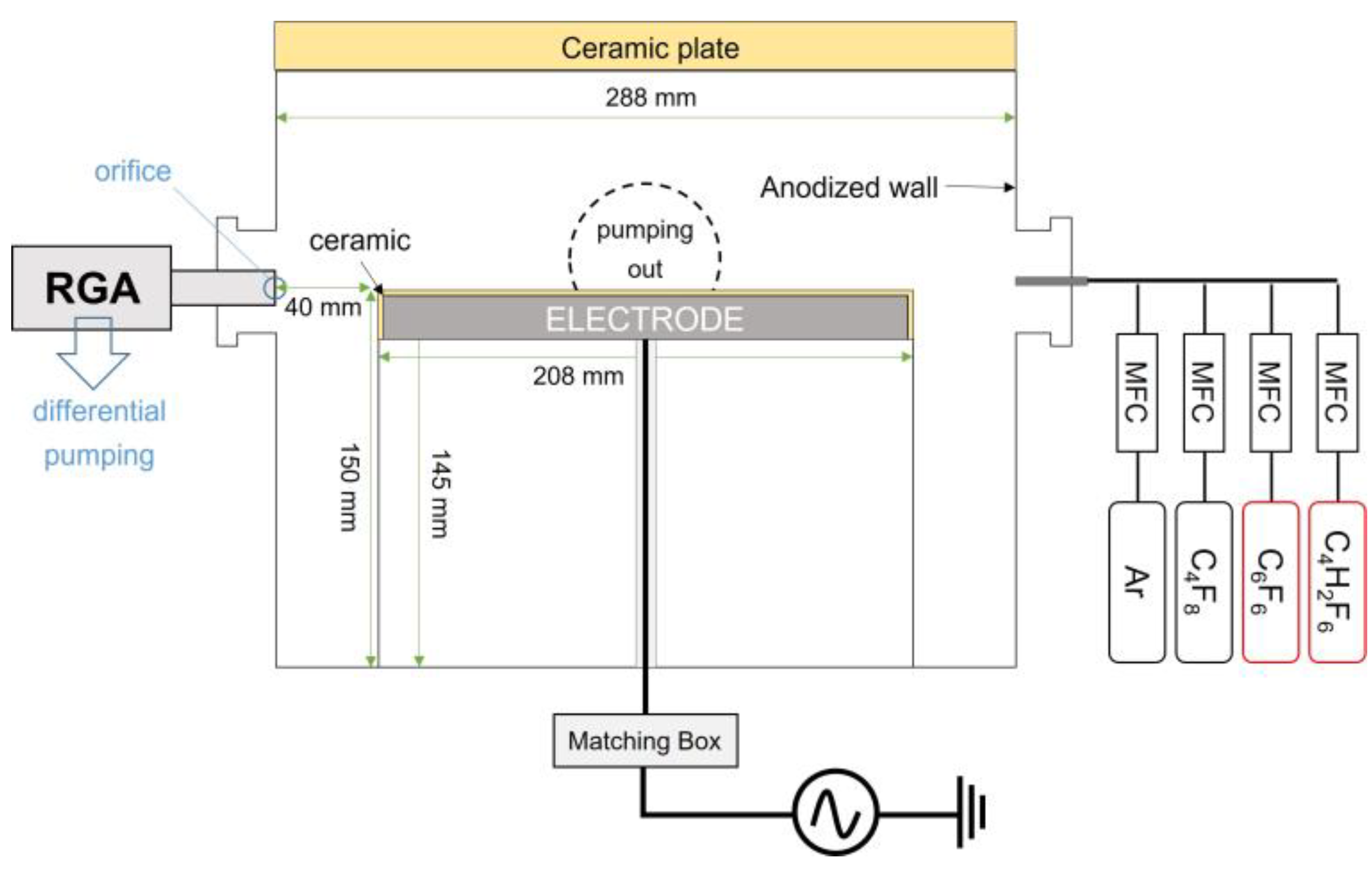
2.1. Processing Chamber Setup
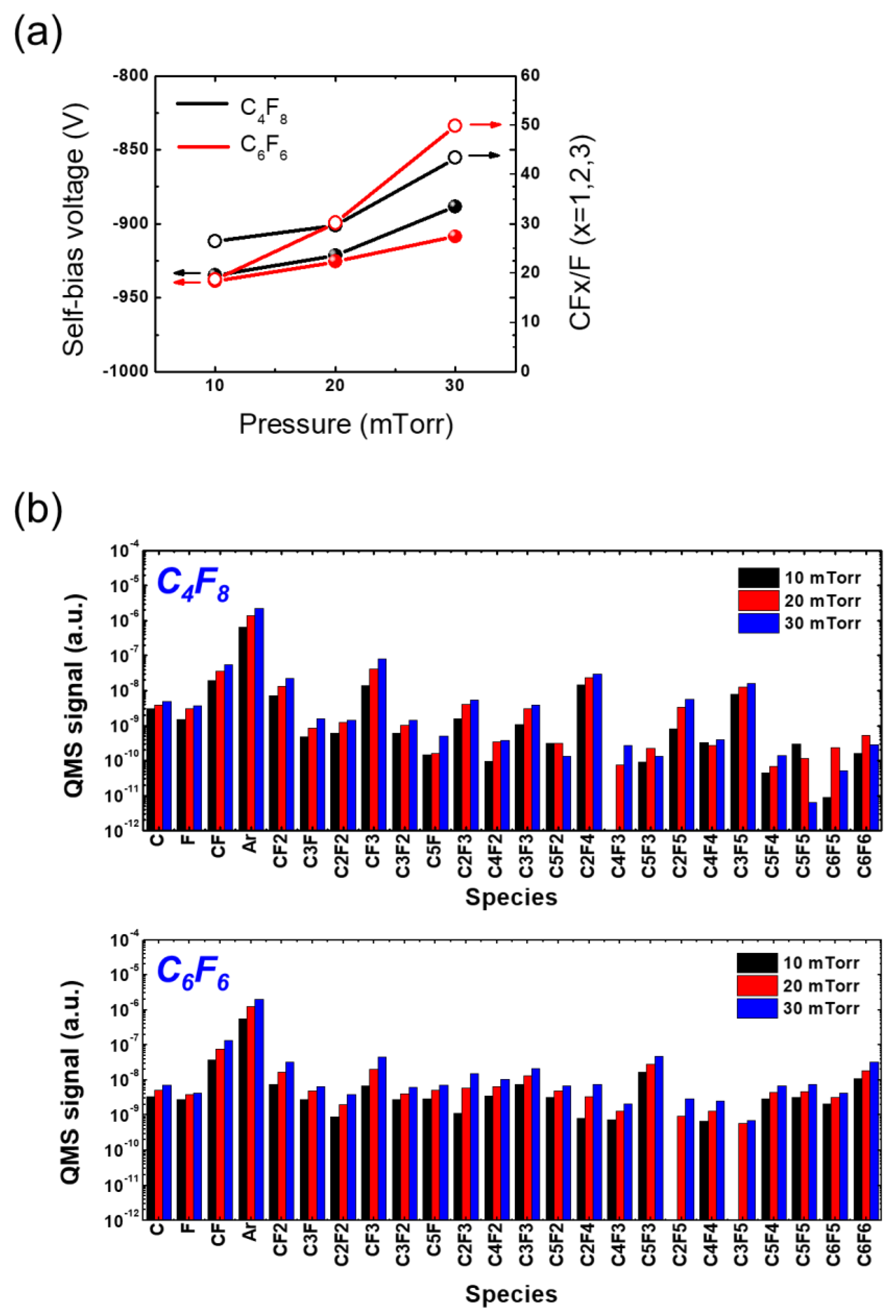
2.2. Plasma Diagnosis
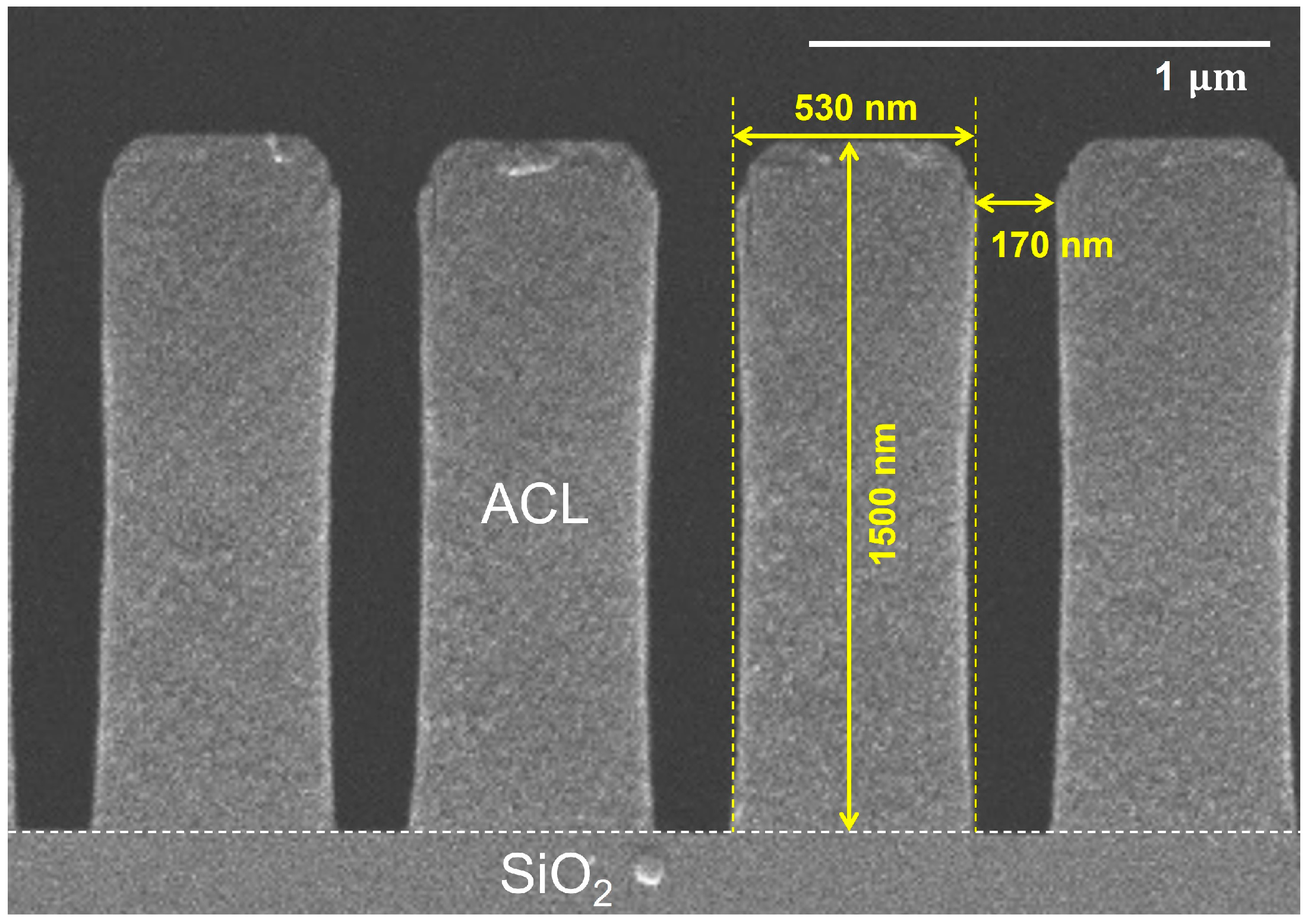
2.3. Sample Preparation
3. Results and Discussion
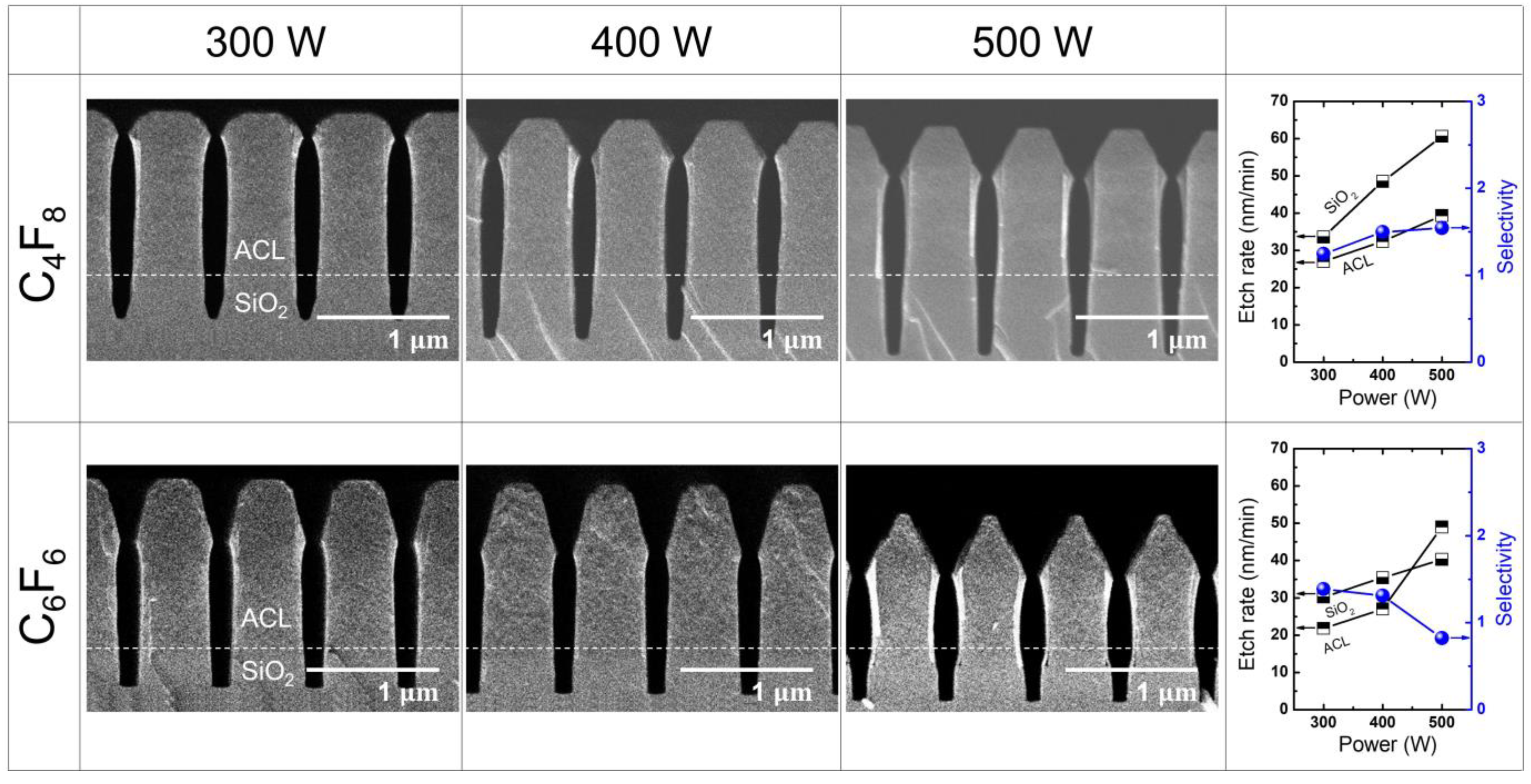
3.1. SiO2 Etching with PFC Precursors (C4F8 and C6F6)
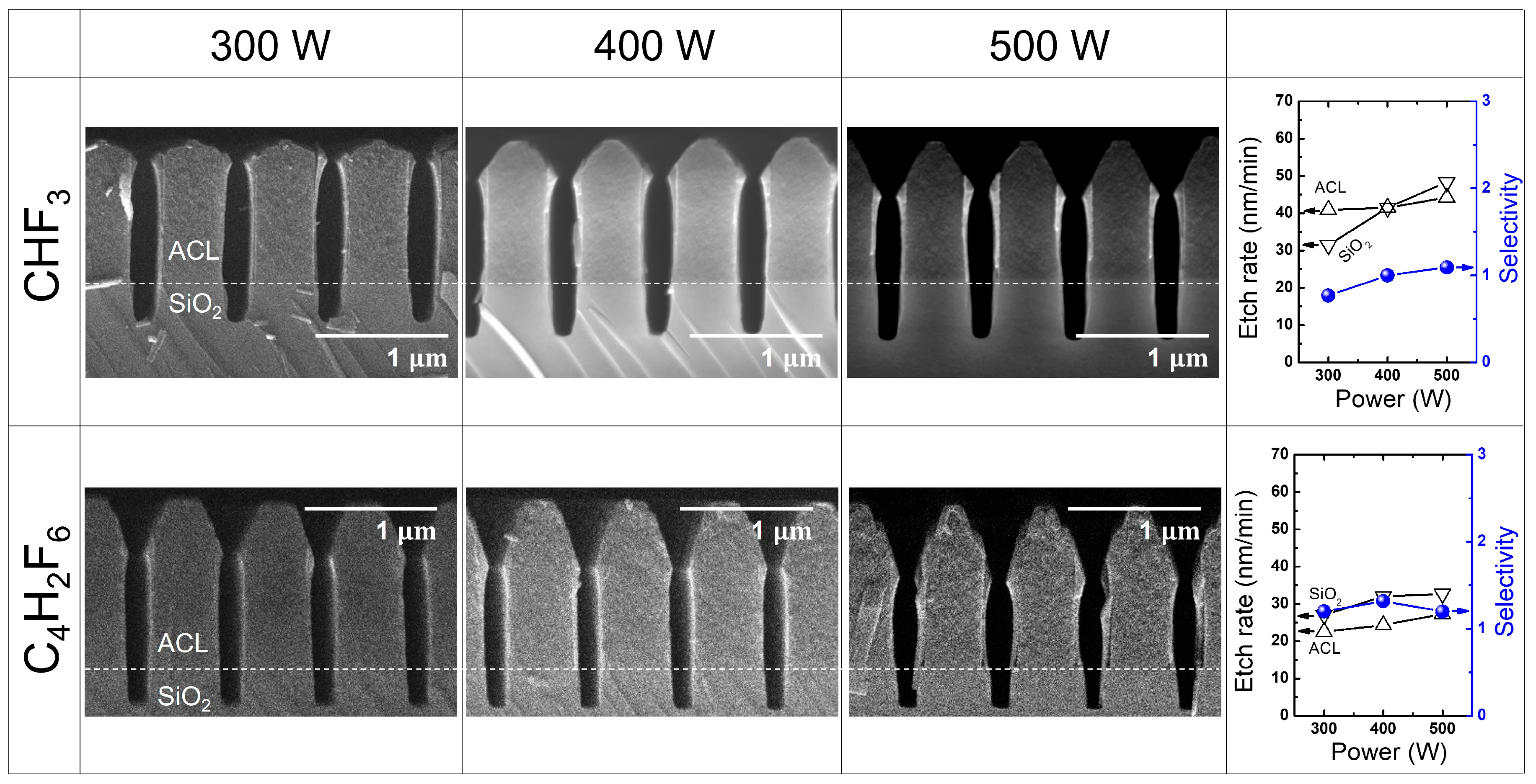
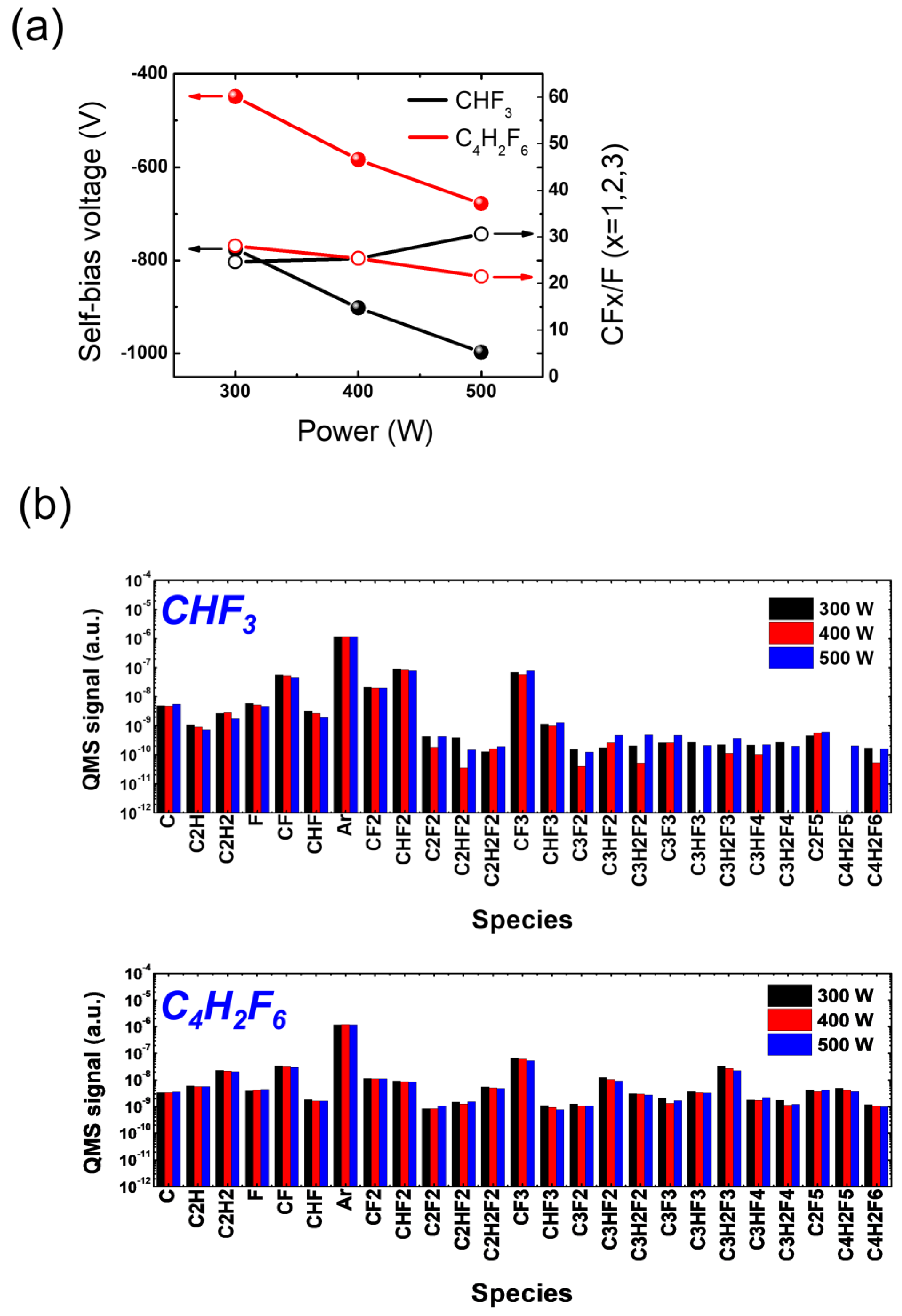
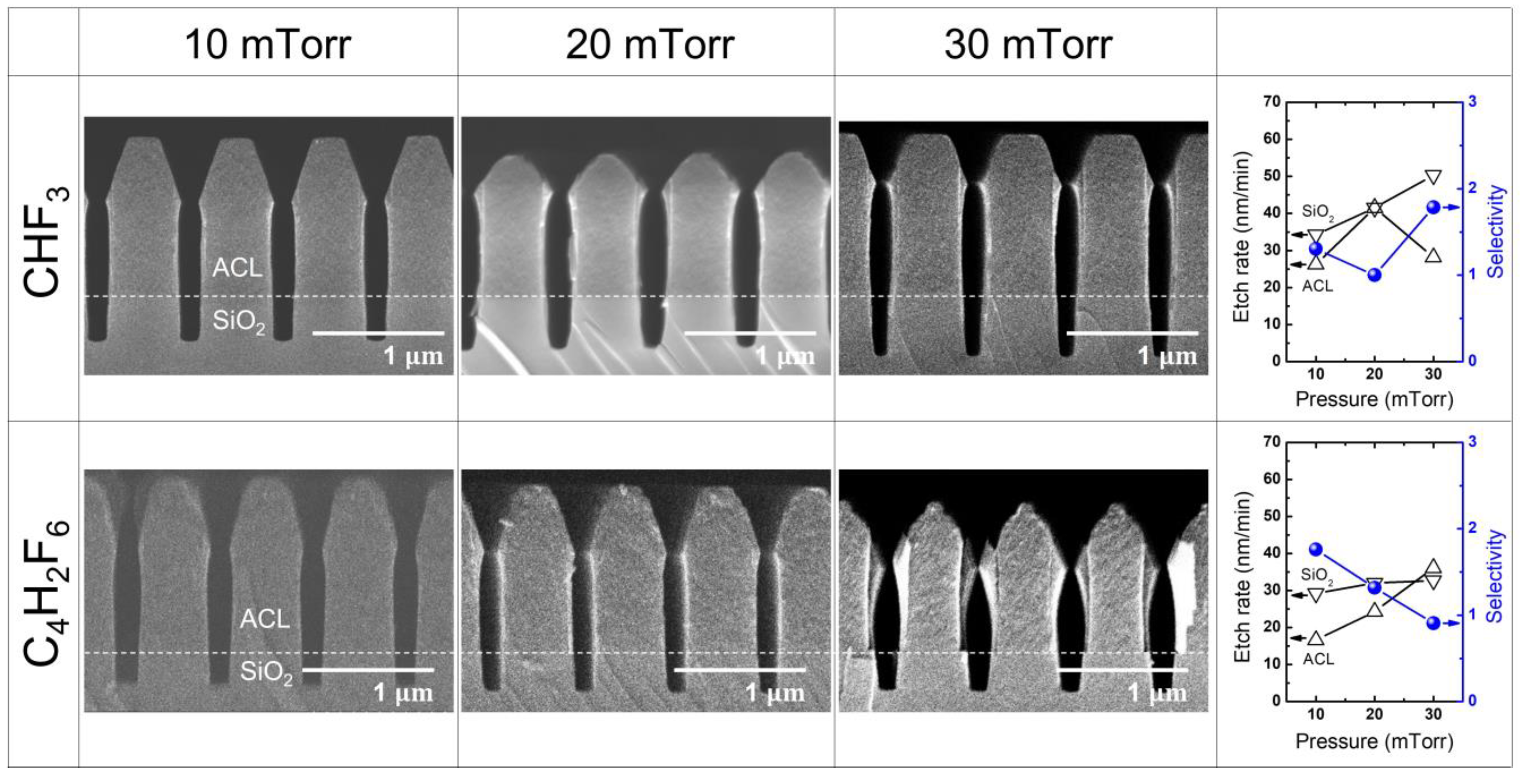
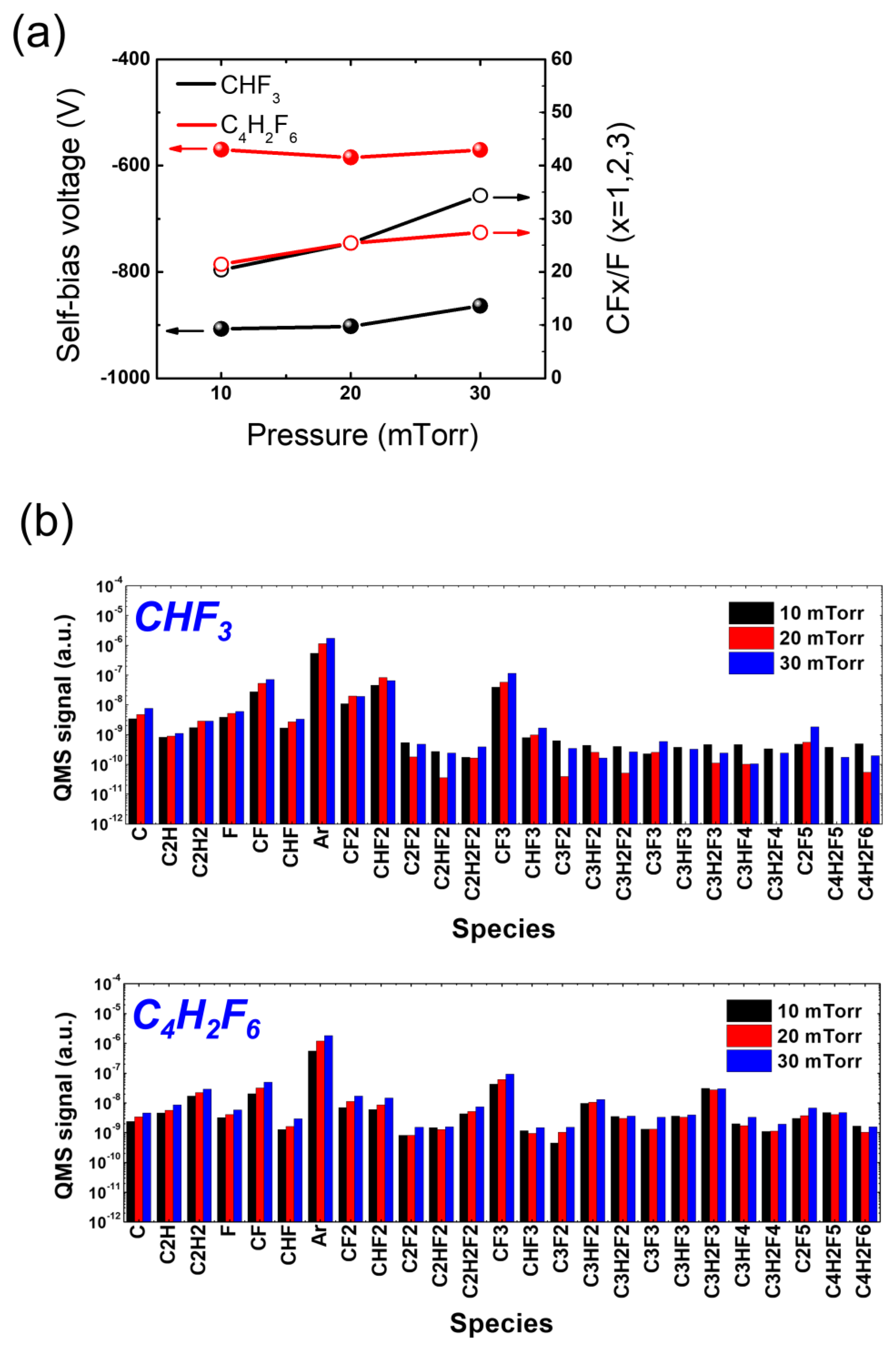
3.2. SiO2 Etching with HFC Precursors (CHF3 and C4H2F6)
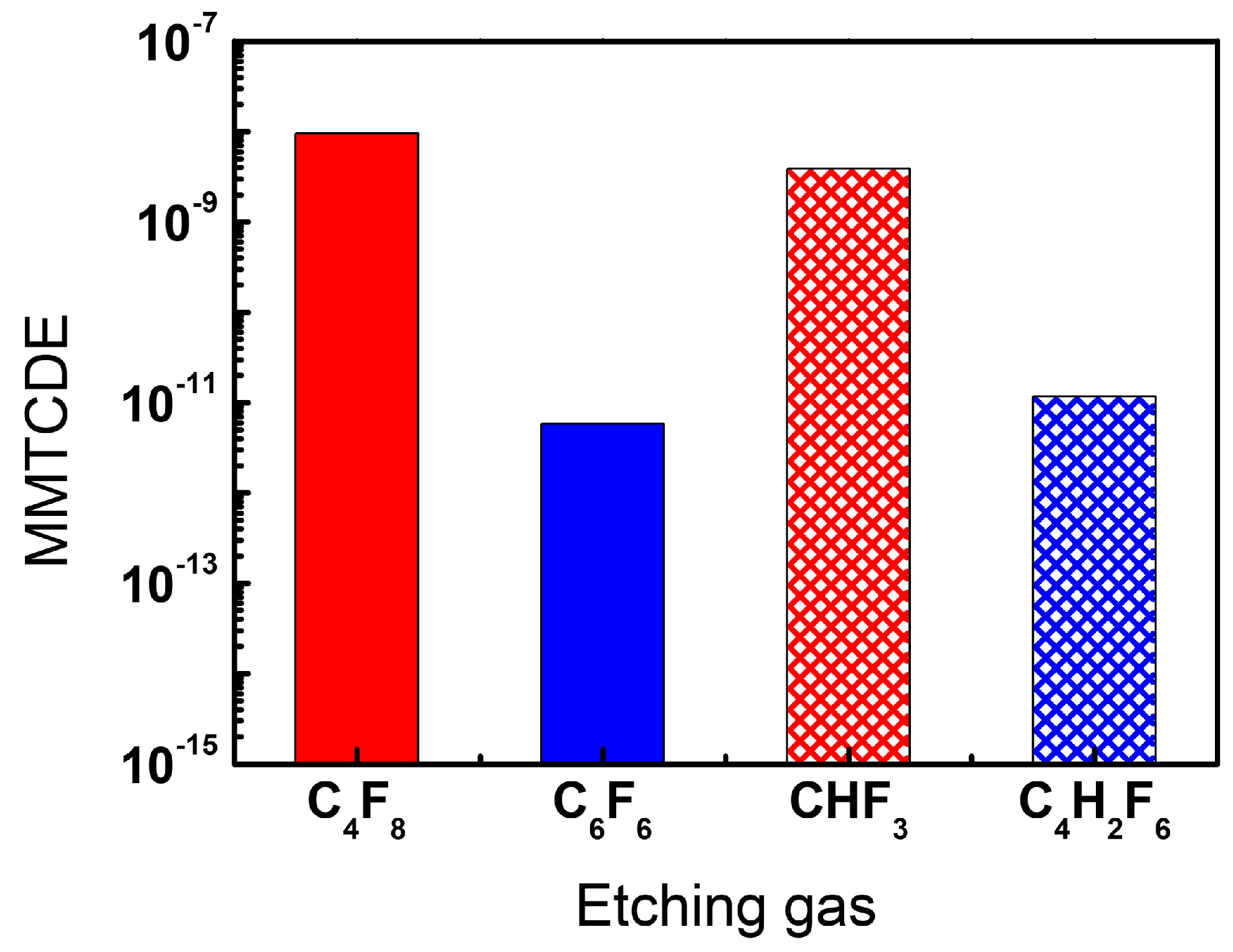
3.3. Evaluation of the Greenhouse Effect
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samukawa, S.; Hori, M.; Rauf, S.; Tachibana, K.; Bruggeman, P.; Kroesen, G.; Whitehead, J.C.; Murphy, A.B.; Gutsol, A.F.; Starikovskaia, S.; et al. The 2012 Plasma Roadmap. J. Phys. D Appl. Phys. 2012, 45, 253001. [Google Scholar] [CrossRef]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Donnelly, V.M.; Kornblit, A. Plasma etching: Yesterday, today, and tomorrow. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 31, 050825. [Google Scholar] [CrossRef] [Green Version]
- Constantine, C. Plasma etching of III–V semiconductors in CH4/H2/Ar electron cyclotron resonance discharges. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1990, 8, 596–606. [Google Scholar] [CrossRef]
- Vartuli, C.B.; Pearton, S.J.; Lee, J.W.; Hong, J.; MacKenzie, J.D.; Abernathy, C.R.; Shul, R.J. ICl/Ar electron cyclotron resonance plasma etching of III–V nitrides. Appl. Phys. Lett. 1996, 69, 1426–1428. [Google Scholar] [CrossRef]
- Tang, C.C.; Hess, D.W. Tungsten Etching in CF4 and SF6 Discharges. J. Electrochem. Soc. 1984, 131, 115–120. [Google Scholar] [CrossRef]
- Turban, A.P.a.G. Plasma Etching of Refractory Metals (W, Mo, Ta) and Silicon in SF6 and SF6-O2. An Analysis of the Reaction Products. Plasma Chem. Plasma Process. 1985, 5, 333–351. [Google Scholar]
- Osipov, A.A.; Andrianov, N.A.; Speshilova, A.B.; Gagaeva, A.E.; Risquez, S.; Vorobyev, A.; Alexandrov, S.E. Highly Selective Plasma Etching Technique for Molybdenum. Plasma Chem. Plasma Process. 2023, 43, 697–707. [Google Scholar] [CrossRef]
- Gasvoda, R.J.; Zhang, Z.; Wang, S.; Hudson, E.A.; Agarwal, S. Etch selectivity during plasma-assisted etching of SiO2 and SiNx: Transitioning from reactive ion etching to atomic layer etching. J. Vac. Sci. Technol. A 2020, 38, 050803. [Google Scholar] [CrossRef]
- Volynets, V.; Barsukov, Y.; Kim, G.; Jung, J.-E.; Nam, S.K.; Han, K.; Huang, S.; Kushner, M.J. Highly selective Si3N4/SiO2 etching using an NF3/N2/O2/H2 remote plasma. I. Plasma source and critical fluxes. J. Vac. Sci. Technol. A 2020, 38, 023007. [Google Scholar] [CrossRef]
- Oehrlein, M.S.a.G.S. A Review of SiO2 Etching Studies in Inductively Coupled Fluorocarbon Plasmas. J. Electrochem. Soc. 2001, 148, 11. [Google Scholar] [CrossRef]
- Standaert, T.E.F.M.; Hedlund, C.; Joseph, E.A.; Oehrlein, G.S.; Dalton, T.J. Role of fluorocarbon film formation in the etching of silicon, silicon dioxide, silicon nitride, and amorphous hydrogenated silicon carbide. J. Vac. Sci. Technol. A Vac. Surf. Film. 2004, 22, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Rueger, N.R.; Beulens, J.J.; Schaepkens, M.; Doemling, M.F.; Mirza, J.M.; Standaert, T.E.F.M.; Oehrlein, G.S. Role of steady state fluorocarbon films in the etching of silicon dioxide using CHF3 in an inductively coupled plasma reactor. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997, 15, 1881–1889. [Google Scholar] [CrossRef] [Green Version]
- Schaepkens, M.; Standaert, T.E.F.M.; Rueger, N.R.; Sebel, P.G.M.; Oehrlein, G.S.; Cook, J.M. Study of the SiO2-to-Si3N4 etch selectivity mechanism in inductively coupled fluorocarbon plasmas and a comparison with the SiO2-to-Si mechanism. J. Vac. Sci. Technol. A Vac. Surf. Film. 1999, 17, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Schaepkens, M.; Oehrlein, G.S.; Hedlund, C.; Jonsson, L.B.; Blom, H.-O. Selective SiO2-to-Si3N4 etching in inductively coupled fluorocarbon plasmas: Angular dependence of SiO2 and Si3N4 etching rates. J. Vac. Sci. Technol. A Vac. Surf. Film. 1998, 16, 3281–3286. [Google Scholar] [CrossRef]
- Rošková, Z.; Schneider, J.; Štengel, M. Predicted Hydrofluorocarbon (HFC) and Perfluorocarbon (PFC) Emissions for the Years 2010–2050 in the Czech Republic. Atmosphere 2023, 14, 111. [Google Scholar] [CrossRef]
- Mikhaylov, A.; Moiseev, N.; Aleshin, K.; Burkhardt, T. Global climate change and greenhouse effect. Entrep. Sustain. Issues 2020, 7, 2897–2913. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 7 August 2023).
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Kweku, D.; Bismark, O.; Maxwell, A.; Desmond, K.; Danso, K.; Oti-Mensah, E.; Quachie, A.; Adormaa, B. Greenhouse Effect: Greenhouse Gases and Their Impact on Global Warming. J. Sci. Res. Rep. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Kweku, D.W.; Bismark, O.; Maxwell, A.; Desmond, K.A.; Danso, K.B.; Oti-Mensah, E.A.; Quachie, A.T.; Adormaa, B.B. Benefits, Costs, and Cooperation in Greenhouse Gas Abatement. Clim. Chang. 2000, 47, 239–258. [Google Scholar]
- Sung, D.; Wen, L.; Tak, H.; Lee, H.; Kim, D.; Yeom, G. Investigation of SiO2 Etch Characteristics by C6F6/Ar/O2 Plasmas Generated Using Inductively Coupled Plasma and Capacitively Coupled Plasma. Materials 2022, 15, 1300. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Kang, H.; You, S.; Kim, C.; Chae, H. Low Global Warming C4H3F7O Isomers for Plasma Etching of SiO2 and Si3N4 Films. ACS Sustain. Chem. Eng. 2022, 10, 10537–10546. [Google Scholar] [CrossRef]
- Lim, N.; Choi, Y.S.; Efremov, A.; Kwon, K.H. Dry Etching Performance and Gas-Phase Parameters of C6F12O + Ar Plasma in Comparison with CF4 + Ar. Materials 2021, 14, 1595. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hori, M.; Goto, T.; Ito, M.; Ishii, N. Spatial distributions of the absolute CF and CF2 radical densities in high-density plasma employing low global warming potential fluorocarbon gases and precursors for film formation. J. Vac. Sci. Technol. A Vac. Surf. Film. 2001, 19, 2134–2141. [Google Scholar] [CrossRef]
- Lee, H.S.; Yang, K.C.; Kim, S.G.; Shin, Y.J.; Suh, D.W.; Song, H.D.; Lee, N.E.; Yeom, G.Y. SiO2 etch characteristics and environmental impact of Ar/C3F6O chemistry. J. Vac. Sci. Technol. A 2018, 36, 061306. [Google Scholar] [CrossRef]
- A N Goyette, Y.W.a.J.K.O. Inductively coupled plasmas in low global-warming-potential gases. J. Phys. D Appl. Phys. 2000, 33, 2004–2009. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Chae, M.G.; Kim, D.S.; Lee, W.O.; Song, H.D.; Choi, C.; Yeom, G.Y. In situ dry cleaning of Si wafer using OF2/NH3 remote plasma with low global warming potential. J. Phys. D Appl. Phys. 2018, 51, 445201. [Google Scholar] [CrossRef] [Green Version]
- You, S.; Lee, Y.J.; Chae, H.; Kim, C.-K. Plasma Etching of SiO2 Contact Holes Using Hexafluoroisopropanol and C4F8. Coatings 2022, 12, 679. [Google Scholar] [CrossRef]
- Papadimitriou, V.C.; Spitieri, C.S.; Papagiannakopoulos, P.; Cazaunau, M.; Lendar, M.; Daele, V.; Mellouki, A. Atmospheric chemistry of (CF3)2C=CH2: OH radicals, Cl atoms and O3 rate coefficients, oxidation end-products and IR spectra. Phys. Chem. Chem. Phys. 2015, 17, 25607–25620. [Google Scholar] [CrossRef]
- Jeong, W.N.; Lee, Y.S.; Cho, C.H.; Seong, I.H.; You, S.J. Investigation into SiO2 Etching Characteristics Using Fluorocarbon Capacitively Coupled Plasmas: Etching with Radical/Ion Flux-Controlled. Nanomaterials 2022, 12, 4457. [Google Scholar] [CrossRef]
- Seong, I.H.; Lee, J.J.; Cho, C.H.; Lee, Y.S.; Kim, S.J.; You, S.J. Characterization of SiO2 over Poly-Si Mask Etching in Ar/C4F8 Capacitively Coupled Plasma. Appl. Sci. Converg. Technol. 2021, 30, 176–182. [Google Scholar] [CrossRef]
- Cho, C.; You, K.; Kim, S.; Lee, Y.; Lee, J.; You, S. Characterization of SiO2 Etching Profiles in Pulse-Modulated Capacitively Coupled Plasmas. Materials 2021, 14, 5036. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Oh, S.; Lee, J.; Cho, C.; Kim, S.; You, S. A Quantification Method in Quadrupole Mass Spectrometer Measurement. Appl. Sci. Converg. Technol. 2021, 30, 50–53. [Google Scholar] [CrossRef]
- Yoo, S.-W.; Cho, C.; Kim, K.; Lee, H.; You, S. Characteristics of SiO2 Etching by Capacitively Coupled Plasma with Different Fluorocarbon Liquids (C7F14, C7F8) and Fluorocarbon Gas (C4F8). Appl. Sci. Converg. Technol. 2021, 30, 102–106. [Google Scholar] [CrossRef]
- Yeom, H.J.; Lee, J.J.; Kim, S.J.; Lee, Y.S.; Cho, C.h.; Kim, J.H.; Lee, H.-C.; You, S. Mass Filter Characteristic and Design Role of Quadrupole Mass Spectrometer for Radical Measurement in Low-pressure Plasmas. Appl. Sci. Converg. Technol. 2020, 29, 77–81. [Google Scholar] [CrossRef]
- Singh, H.; Coburn, J.W.; Graves, D.B. Mass spectrometric detection of reactive neutral species: Beam-to-background ratio. J. Vac. Sci. Technol. A Vac. Surf. Film. 1999, 17, 2447–2455. [Google Scholar] [CrossRef]
- Lee, Y.; Yeom, H.; Choi, D.; Kim, S.; Lee, J.; Kim, J.; Lee, H.; You, S. Database Development of SiO2 Etching with Fluorocarbon Plasmas Diluted with Various Noble Gases of Ar, Kr, and Xe. Nanomaterials 2022, 12, 3828. [Google Scholar] [CrossRef]
- Singh, H.; Coburn, J.W.; Graves, D.B. Appearance potential mass spectrometry: Discrimination of dissociative ionization products. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 299–305. [Google Scholar] [CrossRef]
- Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; p. 800. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Seong, I.; Lee, J.; Lee, S.; Cho, C.; Kim, S.; You, S. Various evolution trends of sample thickness in fluorocarbon film deposition on SiO2. J. Vac. Sci. Technol. A 2021, 40, 013001. [Google Scholar] [CrossRef]
- Coburn, J.W.; Winters, H.F. Ion- and electron-assisted gas-surface chemistry—An important effect in plasma etching. J. Appl. Phys. 1979, 50, 3189–3196. [Google Scholar] [CrossRef]
- Vasenkov, A.V.; Li, X.; Oehrlein, G.S.; Kushner, M.J. Properties of c-C4F8 inductively coupled plasmas. II. Plasma chemistry and reaction mechanism for modeling for Ar/c-C4F8/O2 discharges. J. Vac. Sci. Technol. A 2004, 22, 20. [Google Scholar] [CrossRef] [Green Version]
- Matsui, M.; Tatsumi, T.; Sekine, M. Relationship of etch reaction and reactive species flux in C4F8/Ar/O2 plasma for SiO2 selective etching over Si and Si3N4. J. Vac. Sci. Technol. A Vac. Surf. Film. 2001, 19, 2089–2096. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, S.; Lee, Y.; Cho, C.; Seong, I.; You, Y.; Choi, M.; Lee, J.; Seol, Y.; You, S. Contribution of Ion Energy and Flux on High-Aspect Ratio SiO2 Etching Characteristics in a Dual-Frequency Capacitively Coupled Ar/C4F8 Plasma: Individual Ion Energy and Flux Controlled. Materials 2023, 16, 3820. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Katsunuma, T.; Tabata, M.; Tsuji, A.; Oishi, T.; Hisamatsu, T.; Ogawa, S.; Kihara, Y. Benefits of atomic-level processing by quasi-ALE and ALD technique. J. Phys. D Appl. Phys. 2017, 50, 234002. [Google Scholar] [CrossRef]


| Name | Molecular Formula | Atmospheric Lifetime (Years) | GWP100 | Reference |
|---|---|---|---|---|
| Octafluorocyclobutane | C4F8 | 3200 | 9540 | [19] |
| Hexafluorobenzene | C6F6 | 0.23 | 7 | [23] |
| Trifluoromethane | CHF3 | 222 | 12,400 | [19] |
| Hexafluoro-isobutylene | C4H2F6 | 0.03 | 2.8 | [31] |
| Power (W) | Pressure (mTorr) | Duration (min) | Gas Flow Rate (sccm) | Electrode Temperature (°C) |
|---|---|---|---|---|
| 300–500 | 10–30 | 10 | 20 for Ar 10 for FC | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Lee, Y.; You, Y.; Cho, C.; Jeong, W.; Seong, I.; Choi, B.; Kim, S.; Seol, Y.; You, S.; et al. Characterization of SiO2 Plasma Etching with Perfluorocarbon (C4F8 and C6F6) and Hydrofluorocarbon (CHF3 and C4H2F6) Precursors for the Greenhouse Gas Emissions Reduction. Materials 2023, 16, 5624. https://doi.org/10.3390/ma16165624
Choi M, Lee Y, You Y, Cho C, Jeong W, Seong I, Choi B, Kim S, Seol Y, You S, et al. Characterization of SiO2 Plasma Etching with Perfluorocarbon (C4F8 and C6F6) and Hydrofluorocarbon (CHF3 and C4H2F6) Precursors for the Greenhouse Gas Emissions Reduction. Materials. 2023; 16(16):5624. https://doi.org/10.3390/ma16165624
Chicago/Turabian StyleChoi, Minsu, Youngseok Lee, Yebin You, Chulhee Cho, Wonnyoung Jeong, Inho Seong, Byeongyeop Choi, Sijun Kim, Youbin Seol, Shinjae You, and et al. 2023. "Characterization of SiO2 Plasma Etching with Perfluorocarbon (C4F8 and C6F6) and Hydrofluorocarbon (CHF3 and C4H2F6) Precursors for the Greenhouse Gas Emissions Reduction" Materials 16, no. 16: 5624. https://doi.org/10.3390/ma16165624
APA StyleChoi, M., Lee, Y., You, Y., Cho, C., Jeong, W., Seong, I., Choi, B., Kim, S., Seol, Y., You, S., & Yeom, G. Y. (2023). Characterization of SiO2 Plasma Etching with Perfluorocarbon (C4F8 and C6F6) and Hydrofluorocarbon (CHF3 and C4H2F6) Precursors for the Greenhouse Gas Emissions Reduction. Materials, 16(16), 5624. https://doi.org/10.3390/ma16165624







