Screw Osteointegration—Increasing Biomechanical Resistance to Pull-Out Effect
Abstract
1. Introduction
2. Spinal Fixation Devices
2.1. Plates
2.2. Cages
2.3. Rods
2.4. Screws
2.5. Overview
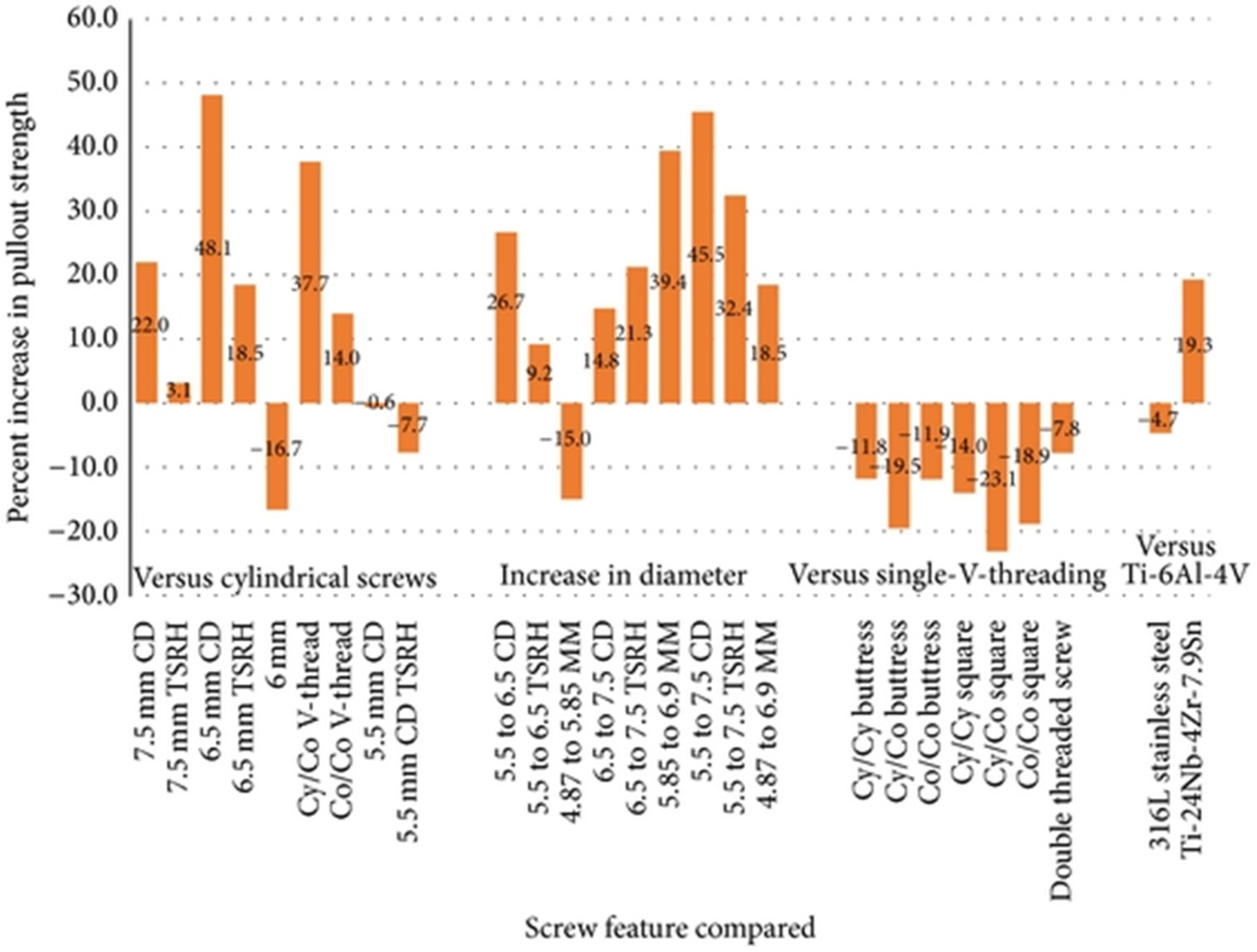
3. Pull-Out Effect
4. Osteointegration
5. Material Optimization
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smit, T.H.; Helder, M.N. 20—In vivo models of regenerative medicine in the spine. In Biomaterials for Spinal Surgery; Ambrosio, L., Tanner, E., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 582–607. [Google Scholar]
- Li, L.; Shi, J.; Zhang, K.; Yang, L.; Yu, F.; Zhu, L.; Liang, H.; Wang, X.; Jiang, Q. Early osteointegration evaluation of porous Ti6Al4V scaffolds designed based on triply periodic minimal surface models. J. Orthop. Transl. 2019, 19, 94–105. [Google Scholar] [CrossRef]
- Martín-Fernández, M.; López-Herradón, A.; Piñera, A.; Tomé-Bermejo, F.; Duart, J.; Vlad, M.D.; Rodríguez-Arguisjuela, M.; Alvarez-Galovich, L. Potential risks of using cement-augmented screws for spinal fusion in patients with low bone quality. Spine J. 2017, 17, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Khan, M.R.; Ngie, D.C.S.; Lim, S.F.; Lim, B.H. Selection of suitable pedicle screw for degenerated cortical and cancellous bone of human lumbar spine: A finite element study. Int. J. Artif. Organs 2020, 44, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Shega, F.D.; Zhang, H.; Manini, D.R.; Tang, M.; Liu, S. Comparison of Effectiveness between Cobalt Chromium Rods versus Titanium Rods for Treatment of Patients with Spinal Deformity: A Systematic Review and Meta-Analysis. Adv. Orthop. 2020, 2020, 8475910. [Google Scholar] [CrossRef] [PubMed]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Son, H.J.; Choi, S.H.; Heo, D.R.; Kook, I.; Lee, M.K.; Ahn, H.S.; Kang, C.-N. Outcomes of the use of cement-augmented cannulated pedicle screws in lumbar spinal fusion. Spine J. 2021, 21, 1857–1865. [Google Scholar] [CrossRef]
- Raj, K.H.; Gnanavel, S.; Ramalingam, S. Investigation of 3D printed biodegradable PLA orthopedic screw and surface modified with nanocomposites (Ti–Zr) for biocompatibility. Ceram. Int. 2023, 49, 7299–7307. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Mutsuzaki, H.; Hara, Y.; Nagashima, K.; Okano, E.; Yanagisawa, Y.; Noguchi, H.; Sankai, T.; Yamazaki, M. Safety and Osteointegration of Titanium Screws Coated with a Fibroblast Growth Factor-2–Calcium Phosphate Composite Layer in Non-Human Primates: A Pilot Study. J. Funct. Biomater. 2023, 14, 261. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Ma, Y.; Liang, T.; Yu, C.; Lu, Z.; Xu, G.; Wang, Z.; Chen, J.; Jiang, J.; et al. Predicting the Failure Risk of Internal Fixation Devices in Chinese Patients Undergoing Spinal Internal Fixation Surgery: Development and Assessment of a New Predictive Nomogram. BioMed Res. Int. 2021, 2021, 8840107. [Google Scholar] [CrossRef]
- DeWald, C.J.; Stanley, T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: Surgical considerations and treatment options in patients with poor bone quality. Spine 2006, 31, S144–S151. [Google Scholar]
- Lehman, R.A., Jr.; Kang, D.G.; Wagner, S.C. Management of osteoporosis in spine surgery. JAAOS-J. Am. Acad. Orthop. Surg. 2015, 23, 253–263. [Google Scholar] [CrossRef]
- Eltorai, A.E. On-demand antibiotic-eluting microchip for implanted spinal screws. J. Orthop. 2017, 14, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Nemade, A.; Shikalgar, A.; Sancheti, S.; Wadkar, S.P. Biomechanical analysis of spinal pedicle screws under static compression and tensile bending. Mater. Today Proc. 2021, 47, 4778–4785. [Google Scholar] [CrossRef]
- Cho, P.G.; Ji, G.Y.; Park, S.H.; Shin, D.A. Biomechanical Analysis of Biodegradable Cervical Plates Developed for Anterior Cervical Discectomy and Fusion. Asian Spine J. 2018, 12, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lü, G.; Wang, B.; Li, L.; Kuang, L. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking stand-alone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: A retrospective study with 2-year follow-up. Eur. Spine J. 2016, 25, 2255–2262. [Google Scholar] [CrossRef]
- Jain, S.; Eltorai, A.E.M.; Ruttiman, R.; Daniels, A.H. Advances in Spinal Interbody Cages. Orthop. Surg. 2016, 8, 278–284. [Google Scholar] [CrossRef]
- Tan, J.-H.; Cheong, C.K.; Hey, H.W.D. Titanium (Ti) cages may be superior to polyetheretherketone (PEEK) cages in lumbar interbody fusion: A systematic review and meta-analysis of clinical and radiological outcomes of spinal interbody fusions using Ti versus PEEK cages. Eur. Spine J. 2021, 30, 1285–1295. [Google Scholar] [CrossRef]
- Suh, P.B.; Puttlitz, C.; Lewis, C.; Bal, B.S.; McGilvray, K. The effect of cervical interbody cage morphology, material composition, and substrate density on cage subsidence. JAAOS-J. Am. Acad. Orthop. Surg. 2017, 25, 160–168. [Google Scholar]
- Seaman, S.; Kerezoudis, P.; Bydon, M.; Torner, J.C.; Hitchon, P.W. Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J. Clin. Neurosci. 2017, 44, 23–29. [Google Scholar] [CrossRef]
- Yoshihara, H. Rods in spinal surgery: A review of the literature. Spine J. 2013, 13, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, A.; Berti, F.; Villa, T.; Pennati, G.; La Barbera, L. Computational and Experimental Fatigue Analysis of Contoured Spinal Rods. J. Biomech. Eng. 2019, 141, 044505. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Titanium spinal-fixation implants. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 347–369. [Google Scholar]
- Nakai, M.; Niinomi, M.; Zhao, X.; Zhao, X. Self-adjustment of Young’s modulus in biomedical titanium alloys during orthopaedic operation. Mater. Lett. 2011, 65, 688–690. [Google Scholar] [CrossRef]
- Narita, K.; Niinomi, M.; Nakai, M.; Hieda, J.; Oribe, K. Specific characteristics of mechanically and biologically compatible titanium alloy rods for use in spinal fixation applications. Mater. Lett. 2012, 86, 178–181. [Google Scholar] [CrossRef]
- Joyce, T.J.; Smith, S.L.; Rushton, P.R.P.; Bowey, A.J.; Gibson, M.J. Analysis of Explanted Magnetically Controlled Growing Rods from Seven UK Spinal Centers. Spine 2018, 43, E16–E22. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.-L.; Xiao, Z.-Z.; Xiao, T.; Zhong, N.-S.; Huang, S.-H.; Liu, J.-M.; Liu, Z.-L. Development and biomechanical test of a new pedicle screw for thoracolumbar spinal surgery. Med. Eng. Phys. 2022, 104, 103808. [Google Scholar] [CrossRef]
- Yahiro, M.A. Comprehensive literature review: Pedicle screw fixation devices. Spine 1994, 19, 2274S–2278S. [Google Scholar]
- Christodoulou, E.; Chinthakunta, S.; Reddy, D.; Khalil, S.; Apostolou, T.; Drees, P.; Kafchitsas, K. Axial pullout strength comparison of different screw designs: Fenestrated screw, dual outer diameter screw and standard pedicle screw. Scoliosis 2015, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Wang, J.; Gong, S. Mechanical Analysis of Posterior Pedicle Screw System Placement and Internal Fixation in the Treatment of Lumbar Fractures. Comput. Math. Methods Med. 2022, 2022, 6497754. [Google Scholar] [CrossRef]
- Luo, J.; Gong, M.; Gao, M.; Huang, S.; Yu, T.; Zou, X. Both unilateral and bilateral pedicle screw fixation are effective for lumbar spinal fusion—A meta-analysis-based systematic review. J. Orthop. Transl. 2014, 2, 66–74. [Google Scholar] [CrossRef]
- Bianco, R.-J.; Arnoux, P.-J.; Wagnac, E.; Mac-Thiong, J.-M.; Aubin, C.-É. Minimizing Pedicle Screw Pullout Risks: A Detailed Biomechanical Analysis of Screw Design and Placement. Clin. Spine Surg. 2017, 30, E226–E232. [Google Scholar]
- McDonough, P.W.; Davis, R.; Tribus, C.; Zdeblick, T.A. The Management of Acute Thoracolumbar Burst Fractures with Anterior Corpectomy and Z-Plate Fixation. Spine 2004, 29, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, E.E.; Tarplett, L.J.; Davies, E.M.; Harley, J.M.; King, L.J. Lumbar Spine Fusion and Stabilization: Hardware, Techniques, and Imaging Appearances. Radiographics 2007, 27, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Echenique, A.; Cegoñino, J.; del Palomar, A.P. Is there any advantage of using stand-alone cages? A numerical approach. Biomed. Eng. Online 2019, 18, 63. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Vottis, C.; Triantafyllopoulos, G.; Papagelopoulos, P.J.; Pneumaticos, S.G. PEEK rod systems for the spine. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 111–116. [Google Scholar] [CrossRef]
- Nowak, B. Experimental study on the loosening of pedicle screws implanted to synthetic bone vertebra models and under non-pull-out mechanical loads. J. Mech. Behav. Biomed. Mater. 2019, 98, 200–204. [Google Scholar] [CrossRef]
- Zdero, R.; Aziz, M.S.R.; Nicayenzi, B. Chapter 8—Pullout Force Testing of Cortical and Cancellous Screws in Whole Bone. In Experimental Methods in Orthopaedic Biomechanics; Zdero, R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 117–132. [Google Scholar]
- Chapman, J.R.; Harrington, R.M.; Lee, K.M.; Anderson, P.A.; Tencer, A.F.; Kowalski, D. Factors Affecting the Pullout Strength of Cancellous Bone Screws. J. Biomech. Eng. 1996, 118, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kiyak, G.; Balikci, T.; Heydar, A.M.; Bezer, M. Comparison of the Pullout Strength of Different Pedicle Screw Designs and Augmentation Techniques in an Osteoporotic Bone Model. Asian Spine J. 2018, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.; Franke, J.; Kalidindi, K.K.V. Advancements in osteoporotic spine fixation. J. Clin. Orthop. Trauma 2020, 11, 778–785. [Google Scholar] [CrossRef]
- Xu, M.; Yang, J.; Lieberman, I.; Haddas, R. Stress distribution in vertebral bone and pedicle screw and screw–bone load transfers among various fixation methods for lumbar spine surgical alignment: A finite element study. Med. Eng. Phys. 2018, 63, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Loenen, A.C.; Noriega, D.C.; Wills, C.R.; Noailly, J.; Nunley, P.D.; Kirchner, R.; Ito, K.; van Rietbergen, B. Misaligned spinal rods can induce high internal forces consistent with those observed to cause screw pullout and disc degeneration. Spine J. 2020, 21, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Cho, S.K.; Wu, C. The biomechanics of pedicle screw-based instrumentation. J. Bone Jt. Surg. 2010, 92, 1061–1065. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Tan, S.H.; Chou, S.M. Effects of bone materials on the screw pull-out strength in human spine. Med. Eng. Phys. 2006, 28, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Rahyussalim, A.J.; Kurniawati, T.; Besri, N.N.; Hukmi, K. Osteoporotic pedicle screw: Review of various types of pedicle screw and cement augmentation. AIP Conf. Proc. 2019, 2193, 020003. [Google Scholar] [CrossRef]
- Patel, P.S.; Shepherd, D.E.; Hukins, D.W. The effect of screw insertion angle and thread type on the pullout strength of bone screws in normal and osteoporotic cancellous bone models. Med. Eng. Phys. 2010, 32, 822–828. [Google Scholar] [CrossRef]
- Karakaşlı, A.; Acar, N.; Özcanhan, M.; Ertem, F. Biomechanical comparison of pullout strengths of five cortical screw types: An innovative measurement method. Jt. Dis. Relat. Surg. 2016, 27, 138–145. [Google Scholar]
- Varghese, V.; Krishnan, V.; Kumar, G.S. Comparison of pullout strength of pedicle screws following revision using larger diameter screws. Med. Eng. Phys. 2019, 74, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Onoda, Y.; Hashimoto, K.; Aizawa, T.; Ozawa, H. Innovation of Surgical Techniques for Screw Fixation in Patients with Osteoporotic Spine. J. Clin. Med. 2022, 11, 2577. [Google Scholar] [CrossRef]
- Conrad, B.P.; Cordista, A.G.; Horodyski, M.; Rechtine, G.R. Biomechanical Evaluation of the Pullout Strength of Cervical Screws. J. Spinal Disord. Tech. 2005, 18, 506–510. [Google Scholar] [CrossRef]
- Jain, P.; Rana, M.; Biswas, J.K.; Khan, M.R. Biomechanics of spinal implants—A review. Biomed. Phys. Eng. Express 2020, 6, 042002. [Google Scholar]
- Chao, C.-K.; Hsu, C.-C.; Wang, J.-L.; Lin, J. Increasing Bending Strength and Pullout Strength in Conical Pedicle Screws: Biomechanical Tests and Finite Element Analyses. J. Spinal Disord. Tech. 2008, 21, 130–138. [Google Scholar] [CrossRef]
- Amaritsakul, Y.; Chao, C.-K.; Lin, J. Comparison study of the pullout strength of conventional spinal pedicle screws and a novel design in full and backed-out insertions using mechanical tests. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 250–257. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Choi, W.-S.; Rhyu, K.-W. Assessment of pedicle screw pullout strength based on various screw designs and bone densities—An ex vivo biomechanical study. Spine J. 2012, 12, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Shea, T.M.; Laun, J.; Gonzalez-Blohm, S.A.; Doulgeris, J.J.; Lee, W.E.; Aghayev, K.; Vrionis, F.D. Designs and Techniques That Improve the Pullout Strength of Pedicle Screws in Osteoporotic Vertebrae: Current Status. BioMed Res. Int. 2014, 2014, 748393. [Google Scholar] [CrossRef] [PubMed]
- Vishnubhotla, S.; McGarry, W.B.; Mahar, A.T.; Gelb, D.E. A titanium expandable pedicle screw improves initial pullout strength as compared with standard pedicle screws. Spine J. 2011, 11, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, D.; Bhardwaj, J.S.; Kumar, N.; Chanda, S. Expandable pedicle screw may have better fixation than normal pedicle screw: Preclinical investigation on instrumented L4-L5 vertebrae based on various physiological movements. Med. Biol. Eng. Comput. 2022, 60, 2501–2519. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.-Q.; Chen, H.-F.; Su, Y.-Y.; Zhu, M.; Zhu, C.-T. Percutaneous kyphoplasty combined with the posterior screw-rod system in treatment of osteoporotic thoracolumbar fractures. Indian J. Orthop. 2013, 47, 230–233. [Google Scholar] [CrossRef]
- Frankel, B.M.; D’Agostino, S.; Wang, C. A biomechanical cadaveric analysis of polymethylmethacrylate-augmented pedicle screw fixation. J. Neurosurg. Spine 2007, 7, 47–53. [Google Scholar] [CrossRef]
- Saadeh, Y.S.; Swong, K.N.; Yee, T.J.; Strong, M.J.; Kashlan, O.N.; Szerlip, N.J.; Oppenlander, M.E.; Park, P. Effect of Fenestrated Pedicle Screws with Cement Augmentation in Osteoporotic Patients Undergoing Spinal Fusion. World Neurosurg. 2020, 143, e351–e361. [Google Scholar] [CrossRef]
- Sarker, A.; Leary, M.; Fox, K. Metallic additive manufacturing for bone-interfacing implants. Biointerphases 2020, 15, 050801. [Google Scholar] [CrossRef]
- Borcherding, K.; Schmidmaier, G.; Hofmann, G.O.; Wildemann, B. The rationale behind implant coatings to promote osteointegration, bone healing or regeneration. Injury 2021, 52, S106–S111. [Google Scholar] [CrossRef]
- Katsuura, Y.; Wright-Chisem, J.; Wright-Chisem, A.; Virk, S.; McAnany, S. The Importance of Surface Technology in Spinal Fusion. HSS J. 2020, 16, 113–116. [Google Scholar] [CrossRef]
- Rani, V.D.; Vinoth-Kumar, L.; Anitha, V.; Manzoor, K.; Deepthy, M.; Shantikumar, V.N. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Sikidar, A.; Kalyanasundaram, D. Design of Polymeric Orthopedic Screws with Variable Stiffness and Multi-Objective Optimization of Injection Molding Process. Int. J. Precis. Eng. Manuf. 2023, 24, 629–643. [Google Scholar] [CrossRef]
- Ringel, F.; Ryang, Y.-M.; Kirschke, J.S.; Müller, B.S.; Wilkens, J.J.; Brodard, J.; Combs, S.E.; Meyer, B. Radiolucent Carbon Fiber–Reinforced Pedicle Screws for Treatment of Spinal Tumors: Advantages for Radiation Planning and Follow-Up Imaging. World Neurosurg. 2017, 105, 294–301. [Google Scholar] [CrossRef]
- Moroni, A.; Faldini, C.; Chilò, V.; Rocca, M.; Stea, S.; Giannini, S. The Effect of Surface Material and Roughness on Bone Screw Stability. J. Orthop. Trauma 1999, 13, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Huang, S.-S.; Yu, W.-X.; Hsu, Y.-W.; Hsu, F.-Y. Collagen Scaffolds Containing Hydroxyapatite-CaO Fiber Fragments for Bone Tissue Engineering. Polymers 2020, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Bădilă, A.E.; Rădulescu, D.M.; Ilie, A.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef]
- Ponnusamy, K.E.; Iyer, S.; Gupta, G.; Khanna, A.J. Instrumentation of the osteoporotic spine: Biomechanical and clinical considerations. Spine J. 2011, 11, 54–63. [Google Scholar] [CrossRef]
- Słota, D.; Głąb, M.; Tyliszczak, B.; Douglas, T.E.L.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-Kupiec, A. Composites Based on Hydroxyapatite and Whey Protein Isolate for Applications in Bone Regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef]
- Jang, S.H.; Lee, J.H.; Cho, J.Y.; Lee, H.-Y.; Lee, S.-H. The Efficacy of Hydroxyapatite for Screw Augmentation in Osteoporotic Patients. Neurol. Med.-Chir. 2013, 53, 875–881. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Czechowska, J.; Cichoń, E.; Zima, A. New Hybrid Bioactive Composites for Bone Substitution. Processes 2020, 8, 335. [Google Scholar] [CrossRef]
- Müller, V.; Djurado, E. Microstructural designed S58 bioactive glass/ hydroxyapatite composites for enhancing osteointegration of Ti6Al4V-based implants. Ceram. Int. 2022, 48, 35365–35375. [Google Scholar] [CrossRef]
- Honda, M.; Matsumoto, M.; Aizawa, M. Potential Application of Protamine for Antimicrobial Biomaterials in Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 4368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Li, L.; Huang, C.; Meng, X.; Liu, J.; Bai, X.; Ren, L.; Wang, X.; Yang, K.; et al. Promoting osteointegration effect of Cu-alloyed titanium in ovariectomized rats. Regen. Biomater. 2022, 9, rbac011. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Joerger, A.-K.; Seitz, S.; Lange, N.; Ryang, Y.-M.; Bernhardt, D.; Combs, S.; Gempt, J.; Meyer, B. Early results, complication and revision rates following CFR-PEEK pedicle screw instrumentation for spinal metastases and spinal primary bone tumors. Brain Spine 2022, 2, 101465. [Google Scholar] [CrossRef]
- Cucinotta, F.; Guglielmino, E.; Longo, G.; Risitano, G.; Santonocito, D.; Sfravara, F. Topology Optimization Additive Manufacturing-Oriented for a Biomedical Application. In Advances on Mechanics, Design Engineering and Manufacturing II; Springer: Berlin/Heidelberg, Germany, 2019; pp. 184–193. [Google Scholar] [CrossRef]
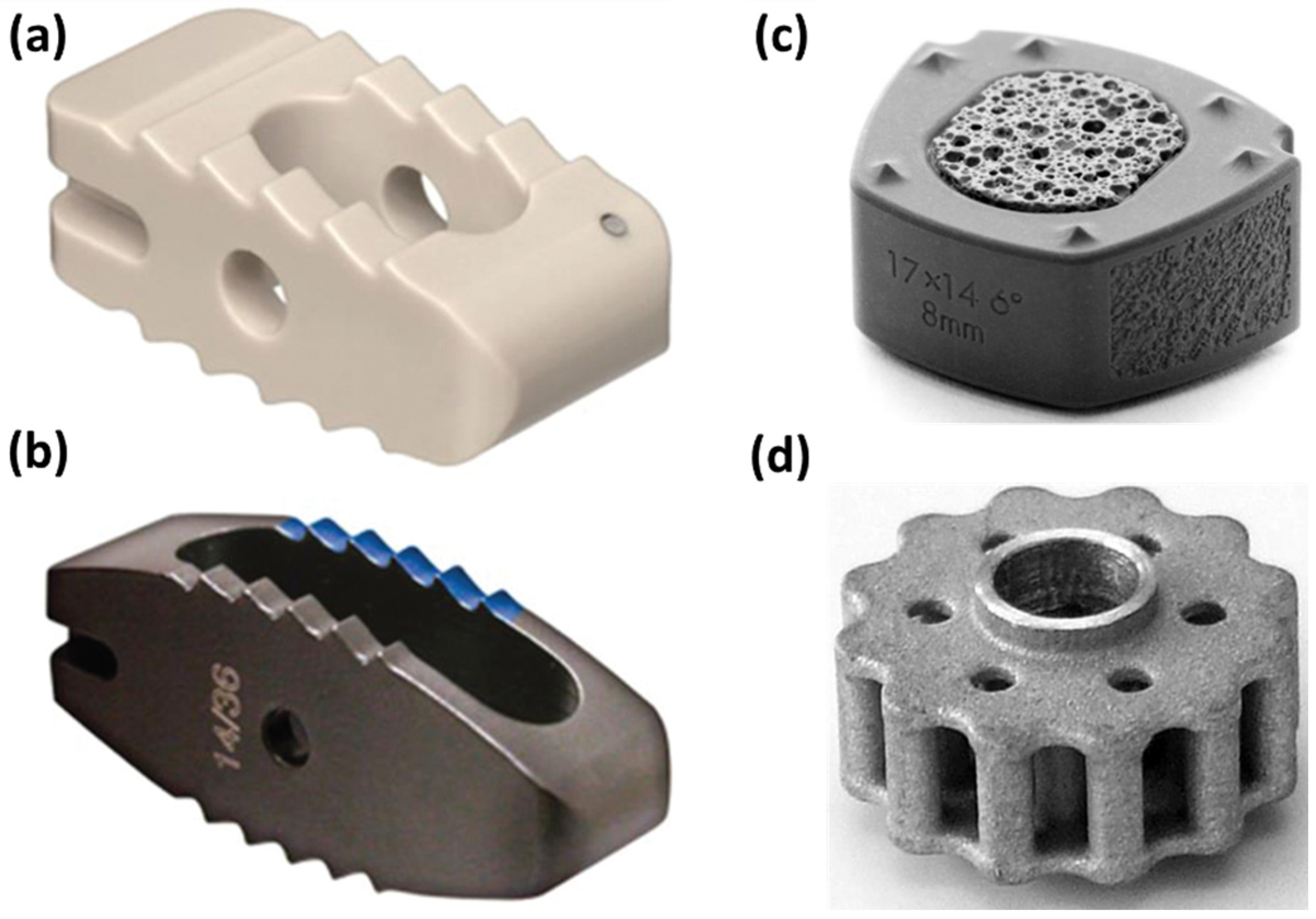



| Devices | Applications | Standard Materials | Advantages/Benefits | Disadvantages/Problems | Refs. |
|---|---|---|---|---|---|
| Plates | Spinal stabilization | Titanium | Increased spinal stability Direct decompression of the spinal cord High rate of neurologic improvement Low complication rate | Not preferred for multilevel fusion Not a stand-alone fixation method | [6,33,34] |
| Cages | Anterior/posterior interbody fusion (restore the height of collapsed disc from injury, degenerative disc disease, or scoliosis) | Titanium PEEK Ceramic Acrylic | Increased spinal stability Preserved facet joints Minimal destruction of the posterior and facet joint ligaments and of the endplates | Fibrosis occurrence Increased minimal principal stresses Maintenance of disc heights and lordotic alignment not achieved in the long term | [6,35] |
| Rods | Spinal fusion (add stability to a spinal implant; used for scoliosis correctional surgery) | Titanium CoCr PEEK Stainless steel Nitinol | Increased spinal stability High fusion rates Increased load sharing Preferred over plates for multilevel fusion | Not a stand-alone fixation method | [6,34,36] |
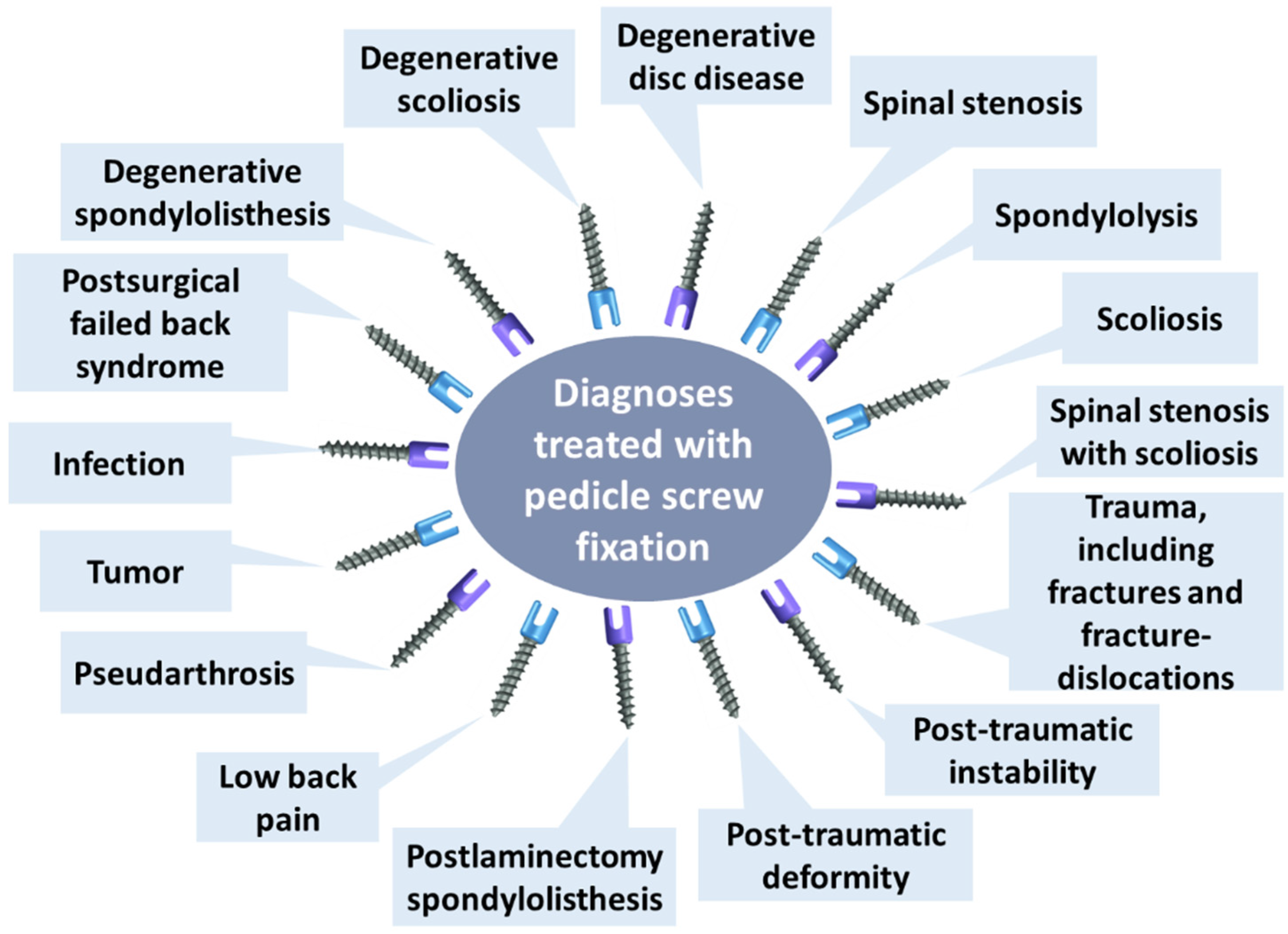
| Screws | Pedicle screw fixation (holds vertebrae together to attach plates and rods) | Titanium (Ti6Al4V) doped with: | Increased spinal stability Firm fixation Complete decompression and strain relief on the spinal nerves Early patient discharge | Loss of fixation Improper placement Fatigue and bending failure Dural tears Cerebral spinal fluid leaks Nerve root injury Infection | [6,30,31] |
|
| Proposed Strategy | Device Specifications | Observations | Ref. |
|---|---|---|---|
| Screws with metallic core (i.e., Ti6Al4V) and polymeric shell (i.e., poly L-lactic acid) | Pull-out force: 150–182 N Bending force: 574–614 N | Improved structural rigidity Quick healing property for the bones attributed to the outer bioabsorbable material Minimization of the loading on the bone during dynamic activities | [67] |
| Biomicroconcretes containing porous hydroxyapatite–chitosan granules and α-tricalcium phosphate-based bone cement | Total open porosity: 45 ± 5 vol.% (Series A) and 50 ± 5 vol.% (Series B) Pore size distribution: ranging from 0.005 µm to 40 µm Mechanical strength of Series A composites: ranging from 5.4 ± 0.8 MPa to 6.2 ± 1.0 MPa [similar to compressive strength of trabecular bone (4–12 MPa)] | Mechanical strength is influenced by the amount of chitosan in hybrid HAp–CTS granules Higher compressive strength was noticed for biomicroconcretes containing granules with a lower amount of chitosan (17 wt.%) | [75] |
| Bioactive glass/hydroxyapatite composites for Ti6Al4V implant improvement | Morphology: highly porous coral-like coating Bioactive glass thickness: ranging from 6 μm to 30 μm Nanocrystalline hydroxyapatite layer thickness: 150 nm | The presence of the bioactive glass-topcoat layer significantly improves the reactivity in terms of mineralization response compared to single-layered hydroxyapatite coating | [76] |
| Antimicrobial protamine-loaded hydroxyapatite coating | Disc diameter: 15 mm Disc thickness: 1–2 mm Antimicrobial powder amount: 0.30 g | Bactericidal properties against Escherichia coli and Staphylococcus aureus Osteoconductivity and biocompatibility proven through in vitro and in vivo tests | [77] |
| Copper–titanium alloy screws | Tensile strength: 597 ± 3.1 MPa Elongation: 26% ± 3.5% Yield strength: 457 ± 7.0 MPa Vickers hardness: 215 ± 8.5 HV Total length: 7 mm Upper width: 4 mm Thread width: 2 mm Thread length: 3 mm | Excellent mechanical properties and bio-functionalization Corrosion resistance and antibacterial performance Improved fixation stability stimulates the vascular network reconstruction around the implant Promotes the proliferation and differentiation of osteoblasts, mineralization, and deposition of collagens | [78] |
| Radiolucent carbon fiber-reinforced PEEK pedicle screw coated with titanium | Carbon-fiber amount: 55% volume Length: 100 mm Diameter: 5.5 mm | Increased turnout resistance Less material fatigue than standard titanium alloy devices Postoperative artifact–reduced imaging | [68] |
| Porous PEKK implants | Morphology: interconnected macropores and micro/nano topography Pore size: >200 μm Maximum push-out force: 97.6 ± 9.4 N | More than double the amount of newly formed bone than in PEEK implants Better osteointegration and mechanical stability than PEEK devices | [79] |
| Biodegradable poly(lactic acid) implants surface-functionalized with TiO2 + ZrO2 nanocomposites | Crystal size of Ti–Zr nanocomposites: 19.2 nm Nanocomposite layer weight: ~62 mg Weight of apatite layer deposited on the 18th day: 65 mg | Improved osteointegration Enhanced mechanical properties | [8] |
| Metallic titanium implants with nanoleafy surface pattern | Surface morphology: a network of vertically aligned, non-periodic, leaf-like structures with thickness in the nanoscale Critical load to adhesive failure: 0.44 ± 0.023 N | Good biocompatibility A higher increase in osteoblast cell proliferation, alkaline phosphatase activity, and collagen synthesis than other nanomorphologies A higher percentage of bone contact with no inflammatory cytokine production | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costăchescu, B.; Niculescu, A.-G.; Grumezescu, A.M.; Teleanu, D.M. Screw Osteointegration—Increasing Biomechanical Resistance to Pull-Out Effect. Materials 2023, 16, 5582. https://doi.org/10.3390/ma16165582
Costăchescu B, Niculescu A-G, Grumezescu AM, Teleanu DM. Screw Osteointegration—Increasing Biomechanical Resistance to Pull-Out Effect. Materials. 2023; 16(16):5582. https://doi.org/10.3390/ma16165582
Chicago/Turabian StyleCostăchescu, Bogdan, Adelina-Gabriela Niculescu, Alexandru Mihai Grumezescu, and Daniel Mihai Teleanu. 2023. "Screw Osteointegration—Increasing Biomechanical Resistance to Pull-Out Effect" Materials 16, no. 16: 5582. https://doi.org/10.3390/ma16165582
APA StyleCostăchescu, B., Niculescu, A.-G., Grumezescu, A. M., & Teleanu, D. M. (2023). Screw Osteointegration—Increasing Biomechanical Resistance to Pull-Out Effect. Materials, 16(16), 5582. https://doi.org/10.3390/ma16165582









