1. Introduction
Angiogenesis is closely interconnected with tissue regeneration, as it provides the necessary vascular network for metabolically active tissue [
1,
2,
3,
4,
5]. Even more so during bone regeneration, in which the lack of functional vascularization represents the main cause of failure [
6,
7,
8,
9]. However, successful bone regeneration does not only depend on sufficient vascularization but also on the appropriate cocktail of signals triggering incoming mesenchymal stem cells to differentiate into the osteogenic lineage [
4]. As such, the interplay between ingrowing vessels and bone tissue has become a topic of scientific interest revealing the existence of a coupling between osteoblasts (OB) and endothelial cells (EC). OBs secrete vascular endothelial growth factor (VEGF) to promote angiogenesis [
10]. In turn, ECs release several osteogenic factors such as fibroblast growth factors (FGF), platelet-derived growth factors (PDGFs), or bone morphogenetic proteins (BMPs) [
11]. ECs are highly heterogeneous and specialized with respect to a function and a local microenvironment [
12]. Recently, type-H vessels were discovered. Their main characteristic is a level of CD31 and Endomucin (EMCN) expression which is higher than that of other blood vessels. They are defined and analyzed on the basis of positive CD31 and EMCN expression. In osteogenic niches from the bone vascular growth front and the endosteum, type-H vessels were identified together with mesenchymal stem cells and osteoprogenitors (OP) [
11]. A correlation exists between the concentration of OP and the presence of H-endothelial cells (HEC) and this particular type of vessel was shown to decrease with aging and loss of bone mass [
11,
13].
Humans display two independent mechanisms of bone growth and remodeling. Whereas endochondral ossification is responsible for long bone growth and regeneration [
14], intramembranous ossification occurs in flat bones [
15].
During endochondral ossification, chondrocytes stop proliferating to become hypertrophic, thereby forming a primary ossification center. Within this primary ossification center, secreted proangiogenic factors are responsible for inducing a vascular network invasion accompanied by ossification of the structure [
14]. Type-H vessels are abundant in these osteogenic areas [
11,
16,
17]. They are directly linked to arteriole columns distally interconnected [
11] and surrounded by dense populations of osteoprogenitors [
11]. H vessels are subsequently connected to the sinusoidal vasculature formed by another vessel subtype, termed L-type, poor in perivascular osteoprogenitors [
18,
19]. Co-expression of CD31 and Endomucin (EMCN) at high levels are characteristic of type-H endothelial cells, whereas expression levels remain low in L-type ECs [
11].
This particular vascular network was not described precisely in the flat bones’ physiology, except in a single study that suggests the presence of type-H vessels in a calvarial bone regeneration model [
20]. A vascular network resembling type-H capillary and spatiotemporally organized was also described in the calvarium but in a bioengineered model enriched in bone marrow stromal cells [
21]. Intramembranous ossification shares mechanistic similarities with the endochondral process. Rather than chondrocytes condensation, a concentration of mesenchymal stem cells is observed to form an ossification center that creates at first a proangiogenic environment attracting the vascular network [
15]. Such similitudes strongly suggest that H vessels, together with osteoprogenitors, could also play a central role in the development and regeneration of flat bones.
A better understanding of bone regeneration is key for improving bone scaffolds’ materials and designs.
In the present study, we postulated that type-H-like capillaries may be present at sites of intramembranous bone development and participate in the control of osteogenesis. We employed a calvarial bone augmentation model in the rabbit and aimed to control and visualize the entire process of bone growth over time. The formation of type-H-like capillaries was monitored over time and correlated with the presence of osteogenic precursors using systematic semi-quantitative mRNA hybridization for CD31, EMCN, and Osterix.
2. Materials and Methods
2.1. Experimental Design
The research described herein is part of a larger study characterizing several bone substitutes for which 37 animals were employed over a period of 12 weeks. We report here the results acquired with a single bone substitute at 2 and 4 weeks, which were obtained from 12 animals out of 37.
Four cylinders were screwed on rabbit skulls and filled with bone scaffolds. Two and four weeks after implantation, animals were sacrificed, samples were block sectioned before being processed for histology. Two staining protocols were performed on each biopsy (eosin hematoxylin and Masson’s trichrome) as well as in situ mRNA hybridization specific for CD31, Endomucin, and Osterix. Total bone synthesis, vascular characterization, and osteogenic precursors identification were performed with respect to time and height within the scaffolds (
Figure 1A).
The following allocation table was used for the entire study: the substitutes were positioned at least once in each of the 4 cylinders placed on rabbits’ skulls at each time point. Furthermore, each animal received 4 different conditions.
The results we describe here were obtained with Bio-Oss (BO: Bio-Oss
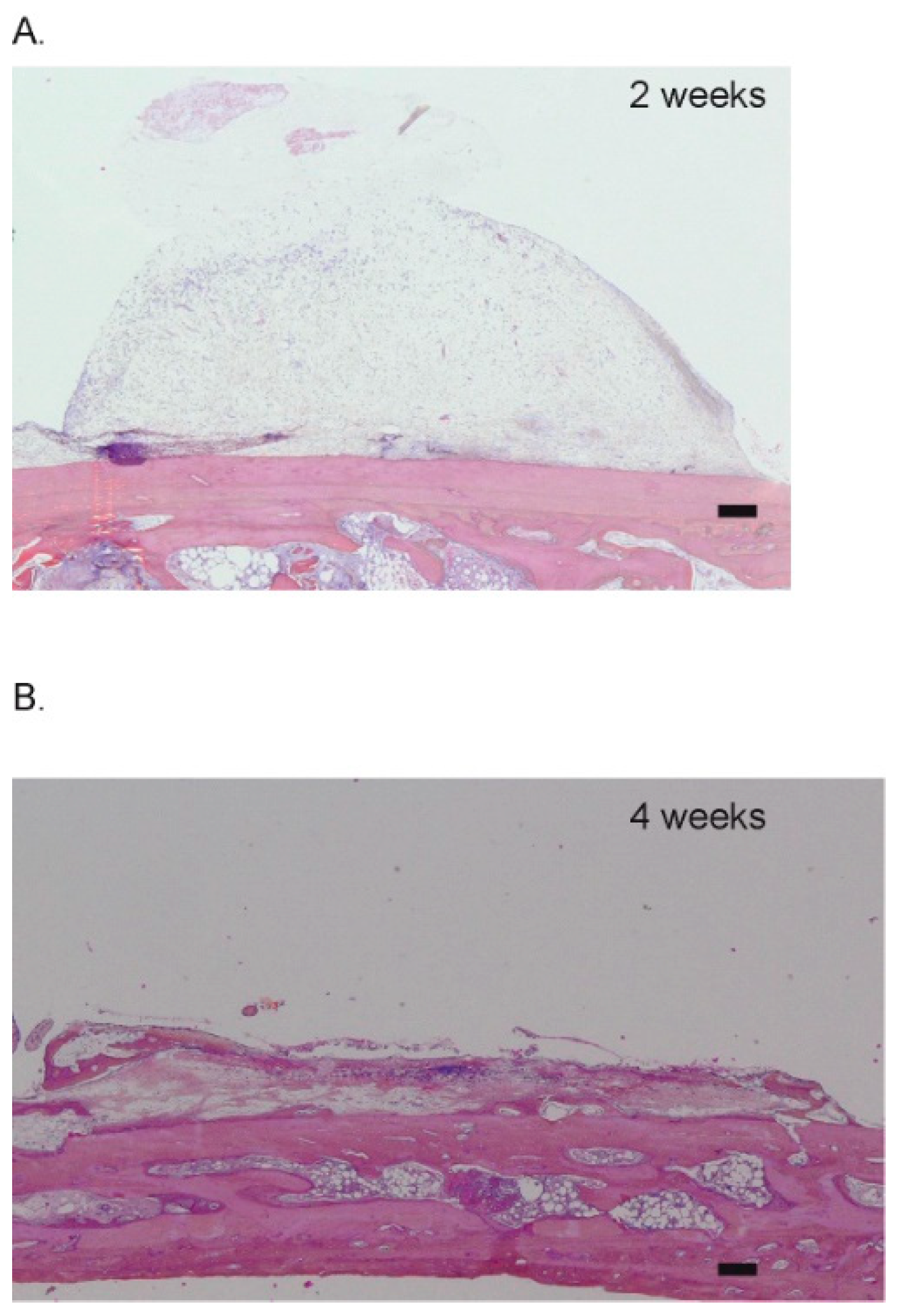
®, 250–1000 µm, 60% porosity, Geistlich Pharma AG, Wolhusen, Switzerland) at 2 and 4 weeks. Six biopsies were analyzed at both 2 and 4 weeks that represent a total of 12 biopsies obtained from 12 rabbits, according to the allocation table. In addition, we included controls for which the cylinders were filled with coagulated rabbit blood. Four controls were performed at 2 and 4 weeks, for a total of 8 biopsies (
Table 1). These control samples were added to verify the pertinence of the model, i.e., that no ectopic bone growth could be observed without scaffold (
Figure 2C,
Appendix A,
Figure A1).
2.2. Animals and Ethics
The protocol was approved by the local academic committee and the cantonal and federal veterinary agencies (authorizations GE/100/18).
Thirty-seven New Zealand white rabbits (male or female, 2.5–3 kg, older than 3 months, UNIGE breeding, Arare, Switzerland) were used in total. Twelve rabbits served in achieving data that we report in that study.
2.3. Surgeries
Surgical procedures were performed as previously described by our group [
22,
23]. Briefly, animals were left for one-week acclimation in the facility and received a prophylactic broad-spectrum antibiotic coverage (2 h preoperative, 3 days postoperative, enrofloxacin Baytril 10%, 5–10 mg/kg PO, Bayer, Leverkusen, Germany). On the day of the surgeries, they were pre-anesthetized by an injection of ketamine (25 mg/kg, 50 mg/mL, 0.5 mL/kg IM, Pfizer, New York, NY, USA) plus xylazine (3 mg/kg, 20 mg/mL, 0.15 mL/kg IM, Bayer). Deep anesthesia was induced by propofol 2% IV (Braun, Sempach, Switzerland) and maintained with sevoflurane 3% (Abbvie, Chicago, IL, USA) in pure oxygen. A remifentanil IV perfusion (Bichsel, Unterseen, Switzerland) (ear vein, 0.008–0.5 um/kg/min, 5 g/mL) ensured analgesia during the procedure. A midsagittal incision and periosteum elevation were then performed before screwing 4 PEEK cylinders and drilling 5 intramedullary holes (diameter 0.8 mm, depth ca. 1 mm) in the bone bed of each cylinder. After filling the cylinders with bone substitutes and closing (
Figure 1B), the surgical site was sutured with intermittent non-resorbable sutures (Prolene 4.0, Ethicon, Somerville, NJ, USA). Animal received buprenorphine hydrochloride (Reckitt Benckiser, Slough, UK), every 6 h and up to 3 days (0.02 mg/kg, 0.03 mg/mL, 0.67 mL/kg SC) as postoperative analgesia. Sutures were removed after ca. 10 days.
Poly ether ether ketone (PEEK) cylinders and caps were produced by Boutyplast, Leyment, FR and fixed with CpTi Gr5 microscrews (diameter 1.2 mm, length 4 mm) from Global D, Brignais, FR. Bio-Oss was previously soaked in blood before placement, ca. 75 mg of particles were used per cylinder.
Animals were sacrificed at 2 and 4 weeks, the cylinders were removed, and the biopsies were fixed in formalin 4%. One month of decalcification (Osteosoft, Sigma, St Louis, MO, USA) was required before embedding the biopsies in paraffin. To ensure a thorough assessment of each biopsy, 3 levels of cuts were made from the middle of the biopsies, with a 150 µm offset. The sections were 4 µm thick.
2.4. Histological Analysis
Hematoxylin-eosin staining was employed to describe the general aspect of the section, of tissue and scaffold structure, and for cell identification and discrimination.
Masson–Goldner staining, which specifically labels collagen fibers green/blue, was employed to further discriminate cells from the surrounding connective tissue and for the semi-quantitation of newly formed bone. Observation was performed on a stereo video microscope (VHX-5000, Keyence, Itasca, IL, USA) following a procedure previously described by our group [
22].
2.5. mRNA Hybridization and Image Analysis
With the aim of distinguishing capillaries subpopulations, mRNA in situ hybridization was used (ViewRNATM Tissue Assay Core Kit (Thermofisher, Watham, MA, USA) and the complementary blue module) according to manufacturer’s instructions. This assay is highly sensitive when compared to traditional fluorescent in situ hybridization (FISH). Twenty base pairs probes are used combined with a sequential branched DNA amplification technique to reach a single-copy sensitivity at single-cell resolution so that semi-quantitative analysis is allowed.
Three different probes were used: CD31 (PECAM1 type1, ref VF1-4240612, Thermofisher), Endomucin (EMCN type 6, ref VPKA3CN, Affymetrix), and Osterix (OSX type 6, Sp7 ref VF6-4237545, Affymetrix).
Two mRNA targets were detected by using simultaneously the type 1 and type 6 probe sets, that is CD31–EMCN and CD31–OSX. These 2 co-hybridizations were always performed on directly adjacent sections (4 µm thick) to allow subsequent digital merging of images thanks to morphological landmarks. The relative expression of CD31, EMCN, and OSX was therefore systematically analyzed on the same histological regions.
Briefly, sections were first deparaffinized, heated at 85 °C for 10 min, and digested with a protease solution (dil. 1/100) for 20 min at 40 °C. Once pretreated, the sections were re-fixed in 10% neutral buffer formalin for 5 min at room temperature. Probe sets were then applied for 3 h at 40 °C (CD31 (PECAM type 1)—Osterix (Osx type 6) and CD31 (PECAM type 1)—Osterix (Osx type 6)) to a final dilution of 1/80. As a negative control, probe set diluent was applied without any probe. Slices were rinsed vigorously several times before signal pre-amplifier and amplifier hybridization at 40 °C. Label probe (type 6) hybridization was then performed (dil. 1/1000, 15 min at room temperature) before the fast blue substrate was added (darkness, room temperature for 30 min). After rinsing, Label probe (type 1) hybridization was operated (dil. 1/1000, 15 min at 40 °C) and the fast red substrate was added for 60 min at room temperature. Slices were finally coverslipped.
Images were recorded under a fluorescence microscope (Zeiss Axio Imager 2, Oberkochen, Germany) at a 20-fold magnification, with the same intensity and exposure time. Each biopsy, (i.e., the whole cylinder content) was analyzed entirely, yielding an average of 80 captures digitally assembled. ViewRNATMFastRed (λexcitation = 553 nm and λemission = 568 nm) was used to detect CD31, ViewRNATMFastBlue (λexcitation = 653 nm and λemission = 668 nm) to detect EMCN and OSX.
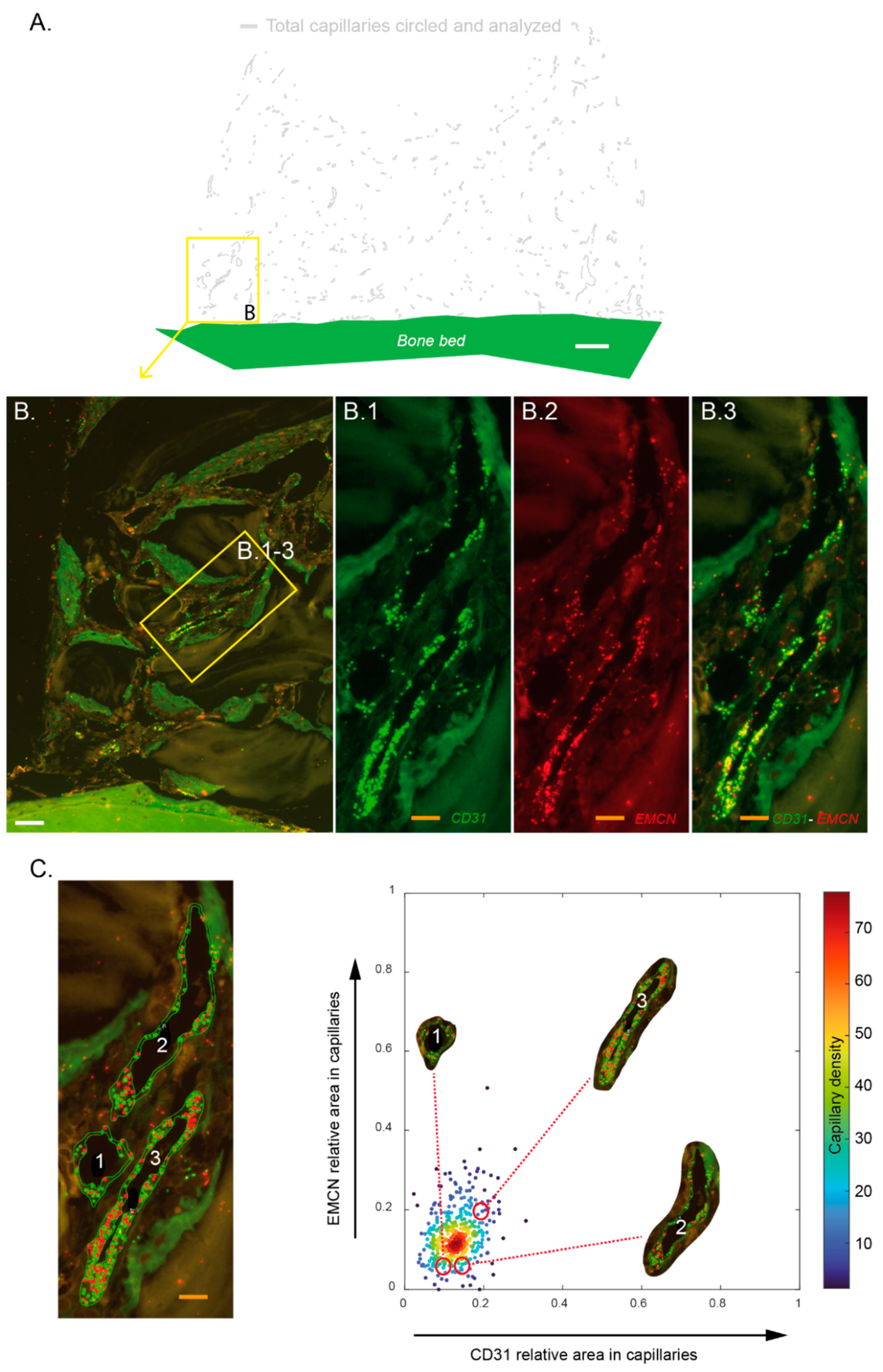
Images of fluorescent mRNA hybridization staining of CD31 and EMCN were analyzed with QuPath 0.3.0 [
24]. Each capillary was circled on a morphological basis combined with CD31 expression. The bone bed was also tagged to serve as the base for distance measurements. These identifications were always made at the same magnification, with an identical “selection brush” width. For each biopsy, 2 slides (in the center of the biopsy) were systematically analyzed twice by 2 operators. In total, 7255 capillaries were analyzed, i.e., an average of 269 per section. Within these delimited surfaces, mRNA punctate signals in each channel (green for CD31, red for EMCN) were segmented with a trained pixel classifier based on an artificial neural network. All these measurements were compiled in a custom-made analysis framework with a graphical user interface written in Matlab 2021b. Among other features, capillaries were characterized by their relative CD31 and EMCN areas and by their minimal distance to the bone bed. Thresholds to define high versus low CD31 and EMCN relative areas in capillaries were user-defined per sample. They were set in order to have most of the capillary density in the “CD31 Low/EMCN Low” category. Practically, a cross delimiting the different categories of capillaries with respect to their CD31–EMCN expression was placed on scatter plots. Capillaries’ density was color-coded, and the cross was always placed at the limit of the denser core, i.e., the red–orange–yellow capillaries’ population.
For the sake of clarity, we set the colors on our figures as follows: green for CD31 and red for EMCN in the images where only these two labels were visualized, green for CD31 and red for OSX in the images where only these two labels were visualized and finally green for CD31, red for EMCN and blue for OSX when the three labels were visualized on the same image.
2.6. Statistical Analysis
New bone volume data were checked for normal distribution and equivalence of variances. Unpaired t-tests were used to compare bone filling at 2 and 4 weeks. Significance levels were set to p ≤ 0.05.
Capillaries’ distance distribution was compared using a two-sample Kolmogorov–Smirnov test. Significance levels were set to p ≤ 0.05.
4. Discussion
In this study, we employed a vertical bone augmentation model that allowed spatiotemporal control over the osteogenesis process. This model enabled the analysis of the entire spectrum of stages throughout bone growth on the same sample over time. Furthermore, mRNA in situ hybridization demonstrated that CD31- and EMCN-expressing capillaries, typical of type-H vessels found during long bone growth, are also present during intramembranous osteogenesis.
In the bone regeneration model employed herein, several identical cylinders are screwed onto the skull of an animal and filled with a scaffold [
22,
23]. The scaffold is necessary for bone growth, as it triggers a vertical “ectopic” osteogenesis that extends above the native calvarial bone plateau. The regenerative cells need to be guided and supported for new bone tissue to be synthetized. The choice of the scaffold is therefore crucial, both in its biochemical and architectural nature. This model may therefore be considered as an extension of the bone system in which the tissue growth is controlled and may advantageously be visualized.
In the present study, the sham samples were filled with coagulated blood only. In these controls, no bone synthesis was observed apart from the healing of intramedullary perforations created to attract osteogenic precursors and vascularization. This is in line with previous studies that also relied on such an ectopic augmentation model [
22,
23,
25,
26]. The scaffold employed consisted of 0.25–1 mm bovine bone particles that have represented a gold standard in orofacial bone regeneration for several decades. The particles are compacted to create an interconnected scaffold that displays around 60% porosity, which is ideal for vascular and bone growth [
27].
Additionally, the choice of the animal model is also important. We relied on rabbit skulls, a model that allows the simultaneous assessment of four cylinders [
22,
23,
28], and thus the testing of four simultaneous conditions on the same animal. Such optimization of the workflow constitutes an important ethical argument.
Finally, the morphology and bone metabolism of this model are similar to humans [
29], which makes it a clinically relevant model, used in ca. 80% of calvarial studies of vertical augmentation. The calvarial model may be clinically translated as a “one-wall defect”, such as a class IV defect in the jaw. The stringency of this model permits the accurate assessment of the vertical osteoconduction of the materials that are being evaluated. Herein, we advantageously employed the model’s characteristics to scrutinize the cellular and vascular mechanisms at play during the regeneration process.
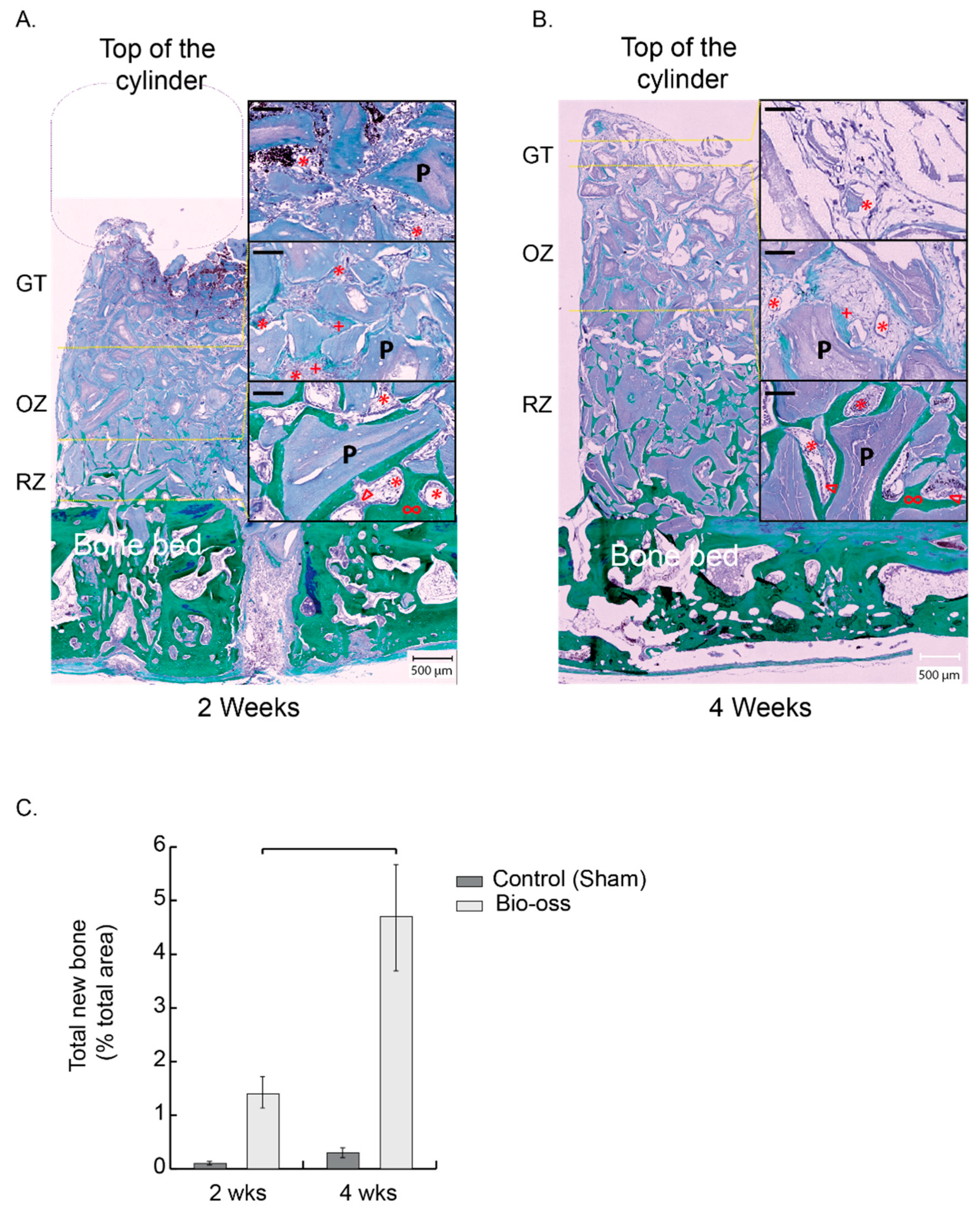
The process of bone augmentation reproduces the mechanisms of bone growth and regeneration. The site is first colonized by inflammatory cells, particularly neutrophils and macrophages, which will first form a matrix within the coagulum that will evolve into granulation tissue. This tissue attracts vascular and osteogenic cells by chemotaxis. A new bone matrix will therefore be synthesized, mineralized, and remodeled. Our calvarial model advantageously allows the concurrent analysis of these stages on a single biopsy. We have thus described three successive zones starting from the bone bed: first, a remodeling zone, second, an osteogenic zone, and third, a granulation tissue. These zones shift over time to ultimately fill the entire volume with mature bone. Using a hybrid collagen–particulate bone substitute in the same model, we have previously shown that the filling was satisfactory and almost complete after 12 weeks [
22]. In the present study, we used a different scaffold, i.e., the gold standard Bio-Oss
®, and we purposely limited the harvesting time points to the first 4 weeks of the process, during which osteogenesis is most important. The bone filling was ensured to arrive at 4 weeks at a level of 2.4 mm average height and a filling of 4.7%. These measures fit with data from previous studies [
26] and with our own data [
22], where ca. 12% bone filling over the entire scaffold was reached after 12 weeks. With 50% filling at 4 weeks and about 5% new bone, we can extrapolate to 10–12% at 12 weeks with a complete cylinder filling and consider the bone growth process in accordance with the model we used.
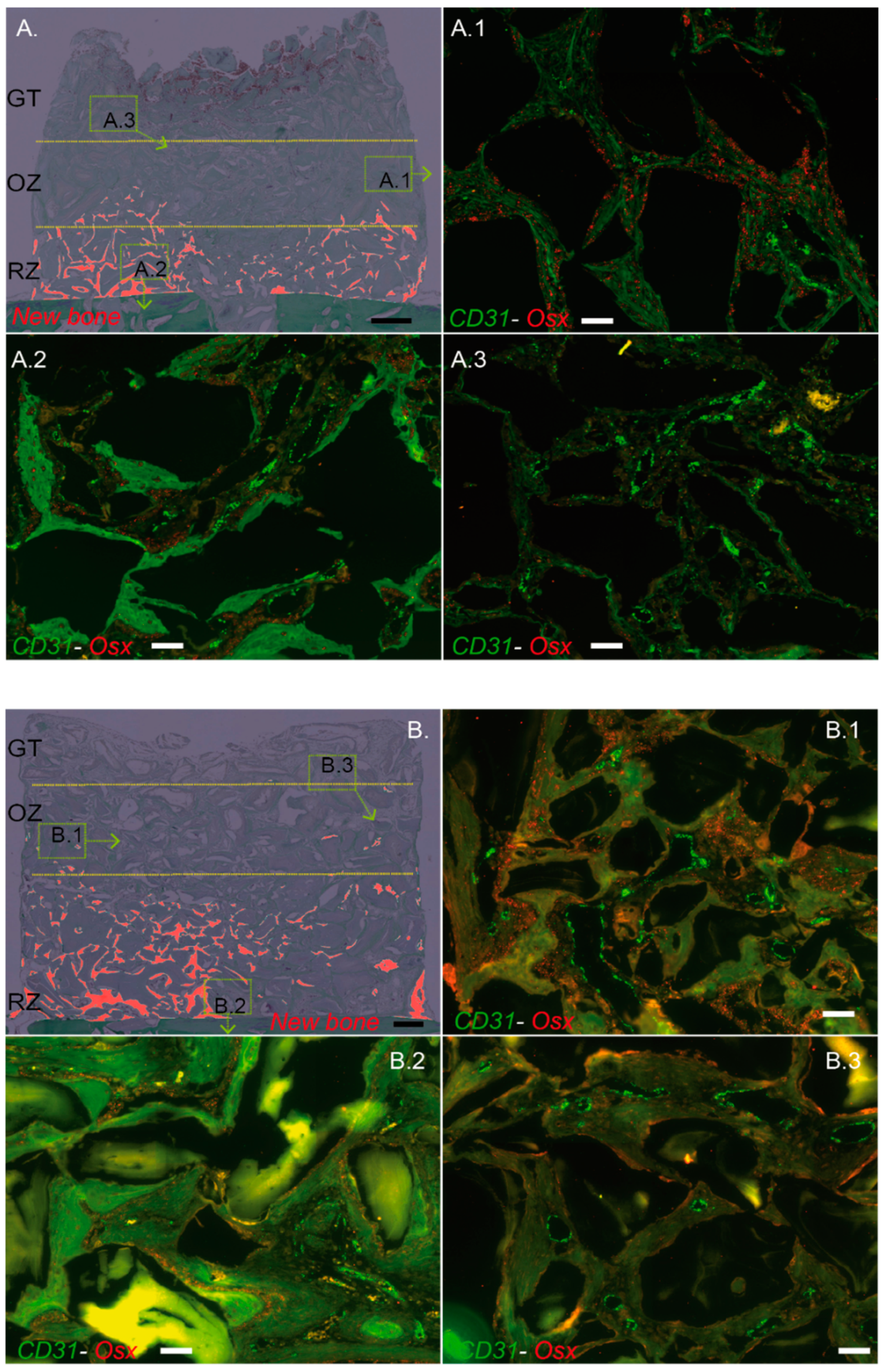
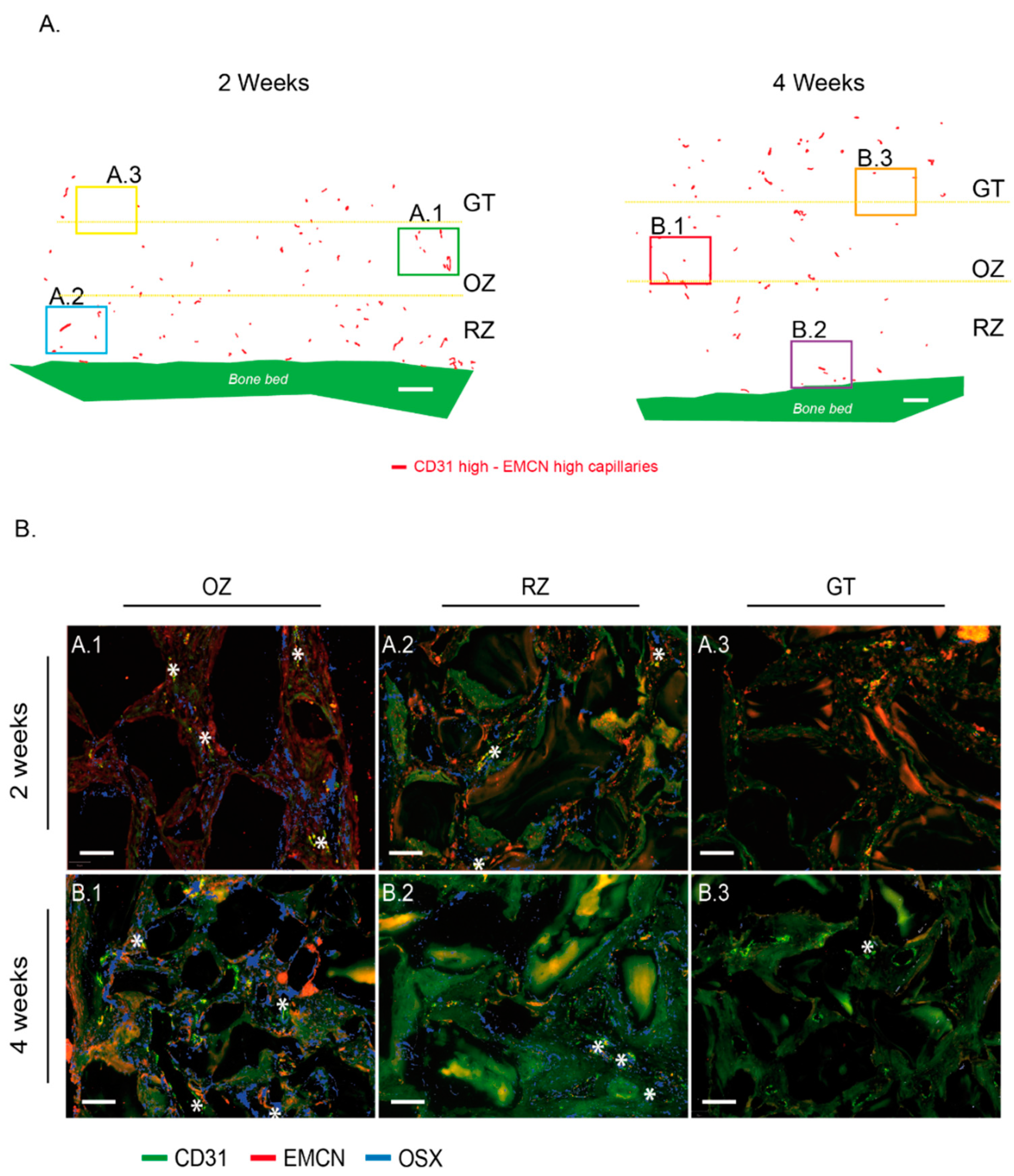
The remodeling zone increased in size from 2 to 4 weeks as bone filling augmented. At the same time, the osteogenic zone also migrated upwards, attracted by the granulation tissue that reached the top of the scaffold. The osteogenic zone maintained its dimensions in height, approximately 2 mm, while shifting towards the top of the scaffold. It is worth mentioning that the characterization of this osteogenic zone, which is essentially rich in osteogenic precursors, was not only performed on a histological and morphological basis, but also relied on the molecular identification of Osterix using the mRNA in situ hybridization technique that we developed. In addition, the three zones were richly vascularized.
Considering all these observations, this model is a dynamic model in which bone growth, and thus osteogenesis, is controlled in space and time.
We further developed and validated an in situ mRNA hybridization technique to evaluate the expression of CD31 and EMCN in capillaries and Osterix in osteogenic precursors. It was technically impossible to perform a triple CD31–EMCN–OSTERIX hybridization. Nevertheless, we were able to systematically perform CD31–EMCN and CD31–Osterix co-hybridizations on directly adjacent sections (4 µm thickness), so that photomicrographs could easily be superimposed thanks to morphological landmarks, (e.g., the particles). We were thus able to validate the presence of certain capillaries surrounded by osteogenic precursors.
From a technical standpoint, each section was analyzed by two specialists who circled the capillaries following the same methodology. A significant number of capillaries were identified and analyzed for each biopsy. Finally, we developed an analytical tool that enabled us to demonstrate that the capillaries identified in our biopsies express CD31 and EMCN (we verified the translation of the CD31 and EMCN glycoproteins in tissues by immunofluorescence (data not shown)). Moreover, the technique was sensitive enough to show variations in the expression of these two markers, and thus to analyze subpopulations, notably the “CD31 High/EMCN High” capillaries.
In the long bone development, the process of bone growth is termed endochondral. During this process, type-H capillaries that strongly express CD31 and EMCN are described in direct contact with osteogenic precursor concentrations [
11,
16,
17]. In the intramembranous bone growth process encountered in flat bones from the orofacial area, mesenchymal stem cells concentrate and differentiate into bone cells instead of chondrocytes in the endochondral process. Irrespective of the process, the osteogenic precursors are therefore concentrated in foci ultimately leading to bone synthesis. In view of these similarities, it is therefore highly likely that the intramembranous process has a vascular system similar to that found in the endochondral process for maintaining osteogenic niches.
Our first observation showed that all the tagged capillaries expressed CD31 and EMCN and were distributed over the entire surface analyzed, that is in all tissue areas. We then focused our analyses on the capillaries that expressed the highest levels of CD31 and EMCN. By mapping these “CD31 High/EMCN High” capillaries, we observed that they were present in all the regions at 2 weeks, with a preferential distribution above the new bone tissue, in a zone that we qualified as osteogenic since i. it concentrated a high proportion of osteogenic precursors and ii. its collagenous matrix was beginning to mineralize. The further away from the bone bed and the remodeling zone, the more these capillaries were present. This profile was also observed at 4 weeks, but shifted upwards, as the remodeling zone had grown in height. The main population of capillaries expressed the lowest levels of CD31 and EMCN and was labeled “CD31 Low/EMCN Low”. Their distribution displayed an inverted profile when compared to “CD31 High/EMCN High” capillaries, with a majority of capillaries present in the remodeling zone.
Around the “CD31 High/EMCN High” capillaries of the osteogenic zone, foci of osteogenic precursors were concentrated, both at 2 and 4 weeks. Interestingly, we could also observe osteogenic precursors in the remodeling zone, albeit in a lower proportion and more diffusely distributed than in the osteogenic zone. Specifically, these precursors were localized in the fibroconnective tissue, where “CD31 High/EMCN High” were also observed, and not in direct contact with trabeculae or newly synthesized osteons. Within the remodeling zone itself, it seems that osteogenic precursors could still be slightly mobilized in the regions not yet mineralized, in the vicinity of “CD31 High/EMCN High” capillaries.
In light of these analyses, there appears to be a population of H-like capillaries strongly expressing CD31–EMCN and promoting the migration and concentration of osteogenic precursors in areas adjacent to active bone growth.
The resemblance between the endochondral and intramembranous systems does not end with this observation. Indeed, in the endochondral system, it was shown that type-H capillaries decrease with age, especially in embryonic bone development, as ossification increases [
11,
13]. Our spatiotemporal model of bone growth depicts a time-dependent vertical migration of tissue zones that reproduces the temporal evolution of bone development. Type-H-like capillaries decrease proportionally with time in mineralized areas to increase in adjacent as yet unmineralized areas. Biopsies were not analyzed over periods longer than 4 weeks herein. That said, current results may further suggest that the “CD31 High/EMCN High” capillary population should decrease over the entire height of the cylinder and that the relative CD31–EMCN expression dot plots would be more homogeneous, with a population centered on the dense “CD31 Low/EMCN Low” core.
It is further interesting to note that the expression of EMCN in the main capillary population increases slightly in time, unlike CD31 expression. EMCN is strongly involved in neovascularization [
30], while CD31 plays an important role in intercellular junctions and the mechanisms of leukocyte transendothelial migration [
31,
32], especially for neutrophils and monocytes. When CD31 decreases, the migration of these cells through the capillaries to the tissues decreases [
33]. We observed that “CD31 Low/EMCN Low” are essentially present in the remodeling zone, where angiogenesis is extremely important, and neutrophils and macrophages are much less active than in granulation tissue and osteogenic zones. Increased expression for EMCN and decreased expression for CD31 could therefore be explained by the physiological and metabolic stage of the tissues in which these capillaries are found.
In conclusion, we identified and mapped capillaries that express high levels of CD31 and EMCN in an intramembranous bone growth model. Because these capillaries highly resemble the type-H vessels typical of endochondral bone development, we labeled them herein as type-H-like vessels. Such type-H-like vessels appeared preferentially located in osteogenic zones adjacent to bone growth and remodeling areas and were surrounded by foci of osteogenic precursors. Whereas further histological and molecular characterization is warranted, these capillaries may represent the central structure of the osteogenic niche of developing or regenerating flat bones. In time, it may be possible to qualitatively and quantitatively improve bone growth by controlling and promoting the development of these capillaries. One key factor for the control of this capillary system may lie in the fine-tuning of the architecture and composition of bone substitutes.