Abstract
Bituminous coal reservoirs exhibit pronounced heterogeneity, which significantly impedes the production capacity of coalbed methane. Therefore, obtaining a thorough comprehension of the pore characteristics of bituminous coal reservoirs is essential for understanding the dynamic interaction between gas and coal, as well as ensuring the safety and efficiency of coal mine production. In this study, we conducted a comprehensive analysis of the pore structure and surface roughness of six bituminous coal samples (1.19% < Ro,max < 2.55%) using various atomic force microscopy (AFM) techniques. Firstly, we compared the microscopic morphology obtained through low-pressure nitrogen gas adsorption (LP-N2-GA) and AFM. It was observed that LP-N2-GA provides a comprehensive depiction of various pore structures, whereas AFM only allows the observation of V-shaped and wedge-shaped pores. Subsequently, the pore structure analysis of the coal samples was performed using Threshold and Chen’s algorithms at ×200 and ×4000 magnifications. Our findings indicate that Chen’s algorithm enables the observation of a greater number of pores compared to the Threshold algorithm. Moreover, the porosity obtained through the 3D algorithm is more accurate and closely aligns with the results from LP-N2-GA analysis. Regarding the effect of magnification, it was found that ×4000 magnification yielded a higher number of pores compared to ×200 magnification. The roughness values (Rq and Ra) obtained at ×200 magnification were 5–14 times greater than those at ×4000 magnification. Interestingly, despite the differences in magnification, the difference in porosity between ×200 and ×4000 was not significant. Furthermore, when comparing the results with the HP-CH4-GA experiment, it was observed that an increase in Ra and Rq values positively influenced gas adsorption, while an increase in Rsk and Rku values had an unfavorable effect on gas adsorption. This suggests that surface roughness plays a crucial role in gas adsorption behavior. Overall, the findings highlight the significant influence of different methods on the evaluation of pore structure. The 3D algorithm and ×4000 magnification provide a more accurate description of the pore structure. Additionally, the variation in 3D surface roughness was found to be related to coal rank and had a notable effect on gas adsorption.
1. Introduction
Coalbed methane (CBM) exploitation is primarily concentrated in anthracite reservoirs, with fewer occurrences in bituminous coal reservoirs [1,2,3]. This discrepancy can be attributed to the fact that bituminous coal is softer, contains more cleats, and exhibits stronger heterogeneity compared to anthracite, thereby impeding CBM production [4,5]. The pores within coal serve as the primary spaces for gas adsorption, constituting approximately 90% of the total gas content within coal pores [6]. Consequently, the desorption and migration behavior of gas in coal are closely linked to the coal pore structure [7,8]. Changes in the characteristics of bituminous coal pores are associated with the second stage of coalification. Therefore, accurately describing the changes in bituminous coal pore characteristics and analyzing the impact of alterations in coal macromolecular structure on these characteristics are essential prerequisites for efficient CBM extraction and safe coal mine production.
Due to the limitations imposed by research conditions and the inherent complexity of coal pores, accurately characterizing coal pore structure has proven to be challenging. However, advancements in technology have facilitated progress in this field. Techniques such as low-pressure gas adsorption (LP-CO2/N2-GA), He gas adsorption, scanning electron microscopy (SEM), and computed tomography (CT) have emerged as valuable tools for this purpose [9,10,11]. Among these technologies, atomic force microscopy (AFM) has been particularly impactful. It has improved the resolution down to 0.1 nm in scanning electron microscopy, allowing for direct imitation of the surface morphology and nanopores without inflicting damage to the samples [12,13]. Furthermore, AFM provides a three-dimensional (3D) image of the reservoir surface [14,15]. Liu et al. [16] found that LP-N2-GA generated a higher percentage of nanopores with a diameter < 4 nm compared to AFM, for both coal and shale samples. However, the results obtained from AFM were deemed more accurate. Combining AFM and SEM observations at the nanoscale, as demonstrated by Li et al. [17], has proven effective in revealing pore structure and mechanical properties in both 2D and 3D dimensions. AFM not only enables the measurement of pore parameters but also provides insights into the surface roughness of coal pores. Since coal pores possess a 3D structure, surface roughness plays a significant role in the adsorption capacity of coal. Nevertheless, there is little research on the relationship between coal surface roughness and coal rank, as well as the correlation between coal surface roughness and coal adsorption capacity.
Despite its utility, AFM technology is not exempt from certain limitations. The accuracy of pore analysis results is directly influenced by the segmentation of AFM images. The “grains” module of the Gwyddion software (Version 2.62) was used to segment particles in AFM images, which include the Edge Detection algorithm, Otsu’s algorithm, Segmentation algorithm, Threshold algorithm, and Watershed algorithm. Notably, the most commonly utilized algorithms, Threshold and Watershed, yield differing results in the context of pore structure analysis. Zhao et al. [15] found the Threshold algorithm to be well-suited for the characterization of pores ranging from 10 to 500 nm, while the Watershed algorithm was better optimized for pores between 1 and 200 nm. Chen et al. [18] found that because the number of AFM scanning data points in an area of any size is 512 × 512, the few data points in the region will affect the accuracy of the software, and they reconstructed the AFM three-dimensional topography to improve the accuracy of pore parameters and surface roughness. Beyond image segmentation, it should be noted that image magnification can also influence the resulting measurements [19]. Nowadays, the impact of magnification on AFM images has not been thoroughly investigated.
In this study, our objective was to gain a comprehensive understanding of the pore structure and heterogeneity of bituminous coal, and elucidate their influence on gas adsorption. To achieve this, we employed low-temperature CO2/N2 gas adsorption (LT-CO2/N2-GA) and AFM techniques to evaluate the nanopores present in six coal samples with varying ranks (Ro,max = 1.19~1.98%). Furthermore, we conducted a detailed analysis of the discrepancies in AFM pore structure calculations resulting from different algorithms and magnification levels (×200 and ×4000). The findings contribute to a better understanding of the complex relationship between coal rank and its physical properties, offering crucial knowledge for the advancement of bituminous coal bed methane exploration and utilization strategies.
2. Materials and Algorithms
2.1. Experimental Methods
The coal samples were obtained from distinct mining areas, including Liangshuijing Mine in the Shenmu mining area (LSJ), Duerping Mine in the Xishan mining area (DEP), Tunlan Mine in the Xishan mining area (TL), Dafosi Mine in the Huanglong mining area (DFS), Changping Mine in the Jincheng mining area (CP), and Gaohe Mine in the Jincheng mining area (GH). By exploring the geological characteristics of the sampling points and selectively collecting block samples from newly developed coal heading faces. All sample quantities, observations, and descriptions adhered to the respective national standards [20,21]. The proximate analysis and determination of vitrinite reflectance (Ro,max) followed the guidelines stipulated by the respective national standards [22,23]. Low-pressure N2 gas adsorption (LP-N2-GA) experiments were conducted using an automated gas sorption analyzer (Autosorb iQ-MP, Quantachrome Instruments, Boynton Beach, FL, USA), in strict accordance with the national standards [24,25].
2.2. Image Scanning and Preprocessing
2.2.1. Image Scanning
AFM experiments required the argon ion polishing of samples to attain a smooth coal surface. Owing to the brittle nature of the coal sample, it was imperative to secure it in polyester resin. The sample size did not exceed dimensions of 10 mm × 10 mm × 3 mm (see Figure 1). The AFM instrument employed in this experiment was the Bruker Dimension Icon, offering a maximum scanning range of 90 μm × 90 μm × 10 μm and a resolution of 0.15 nm in the lateral direction and 0.04 nm in the vertical direction under the Contact Mode. The 3D AFM used to observe the surface morphology and pore structure is a Dimension Icon AFM (Bruker, Santa Barbara, CA, USA) in the PeakForce QNM (Quantitative Nanoscale Mechanical Mapping) mode. The probe tip had a radius of curvature of 10 nm, and a console rigidity of 5.715 N/m. The images were captured across a magnification range of ×200 to ×4000 nm. The images were acquired over a magnification range from ×200 to ×4000 nm. The experimental procedures are as follows: (1) object sample and select the area that contains the pore structure; (2) the images are first obtained at magnification ×200 (Figure 2a), then at magnification ×4000 (Figure 2b).

Figure 1.
Coal samples.
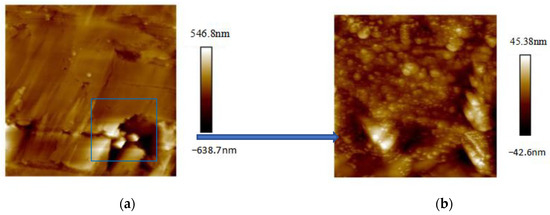
Figure 2.
The experimental procedures of the sample: (a) image at magnification ×200; (b) image at magnification ×4000.
2.2.2. Image Denoising
Image denoising aims to minimize errors in atomic force microscopy (AFM) operations. Such errors might include the skew of the sample substrate due to manual handling, the bowl-shaped deformation of the scanning surface prompted by the motion of the AFM probe, and the noise interference occurring during the AFM scanning process. The experimental procedure may introduce abnormal height values in the raw AFM data, and the AFM probe’s scanning movement may cause bowl-shaped deformations in the original data. To correct the aberrant high-value areas and bowl deformations, the Nanoscope Analysis software (Verision 1.40r1) was utilized.
2.2.3. Image Segmentation
Image segmentation determines a reasonable segmentation threshold to obtain the pore function, so as to filter out the pores in the image. It is the key to pore structure analysis, and its segmentation results directly affect accuracy.
where T is the threshold, f (x, y) is the original image, and g (x, y) is the generated binary image.
- (1)
- Threshold algorithm
Commonly used 2D segmentation algorithms mainly include the Huang, MaxEntropy, Otsu, and Yen algorithms [26,27,28,29]. The comparison of different algorithms shows that the Yen algorithm is the most accurate [19]:
where TC(T) is the total amount of correlation contributed by the pores and coal matrix and is defined as follows:
where M × N pixels is the size of f (x, y) images that are represented by m gray levels. Let G {0, 1, …, (m − l)} denote the set of gray levels and f(i), i G be the number of gray-level frequencies of the image f (x, y).
- (2)
- Chen’s algorithm
As reported by Chen et al. [18], according to the characteristics of the 3D image, when projecting the 3D sample surface in the xOy plane coordinate system, the xOy plane is used to cut the sample surface from bottom to top, the pore volume is the volume enclosed by the xOy plane, and the sample surface below the plane, which is defined by
where V is the pore volume, m3; A is the projected area, m2; h is height, m; a and b are the projected width and length, respectively, m.
2.3. 3D Roughness
Two-dimensional (2D) roughness metrics cannot comprehensively and accurately portray the morphology of coal surfaces, prompting researchers to shift from traditional 2D roughness analysis to a more exhaustive assessment using three-dimensional (3D) surface roughness parameters [30,31]. The 3D roughness parameter significantly deviates from the conventional 2D roughness single curve analysis, offering a more accurate reflection of the overall surface topography of the coal. Consequently, AFM’s 3D roughness analysis can precisely quantify the microstructure characteristics of the surface topography across different grades of metamorphic coal.
AFM images were imported into Nanoscope Analysis to calculate the average roughness (Ra), the root mean square roughness (Rq), kurtosis (Rku), and skewness (Rsk).
Ra represents the average distance of the surface deviation from the datum; the calculation formula for Ra is as follows:
where M, N are the number of data points on the X, Y (dimensionless), and Z (nm) is the height of each data point.
Rq represents the root mean square of the surface deviation from the datum; the calculation formula for Rq is as follows:
Rsk represents the degree of asymmetry of the surface height distribution; the calculation formula for Rsk is as follows [32]:
A positive Rsk value indicates that the distribution is to the right and that there are more areas where the sample surface height is lower than the average; a negative value indicates that the distribution is to the left and that there are more areas where the sample surface height is higher than the average (Figure 3a).
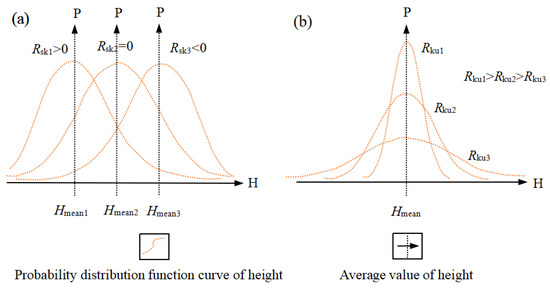
Figure 3.
Shapes of (a) surface skewness (Rsk) and (b) kurtosis coefficient (Rku).
Rku represents the probability that the surface height value is concentrated on the average height value; the calculation formula for Rku is as follows [33]:
A positive Rku value means that the waveform reaches its peak, in which the sample surface height is concentrated at the average value; a negative value means that the waveform is flat, so the surface height of the sample is distributed (Figure 3b).
2.4. Theoretical Models
2.4.1. Porosity
Porosity is the ratio of the pore volume of coal to the total volume. The total volume of coal is the sum of the skeleton volume and the pore volume. The calculation formula for porosity (%) is as follows:
where Φ is the porosity (%), VP is the pore volume (nm3) measured by the Watershed algorithm in Gwyddion, VM is the coal skeleton volume (nm3), SR is the real area represented by a single pixel (nm2), N is the number of pixels in the image, and Zmin is the minimum height of all data points on the image (nm).
2.4.2. Adsorption Experiments
The isothermal sorption curves of CH4 on different coal samples were fitted by Langmuir, Freundlich, and Spis isotherm models [34].
- (1)
- The Langmuir adsorption model is as follows:
- (2)
- The Freundlich adsorption model is as follows:
- (3)
- The Sips adsorption model is as follows:
The adsorption effective capacity was defined using the following equation [35]:
where Ci and Ce are the initial and final equilibrium concentrations.
3. Results
Table 1 presents the results of the proximate analysis and vitrinite reflectance for the coal samples. The samples include two high-volatile bituminous coals (LSJ, DFS), two medium-volatile bituminous coals (TL, GH), and two low-volatile bituminous coals (DEP, CP).

Table 1.
Proximate analysis and vitrinite reflectance results.
3.1. Characterization of Pore Structure by the LT-CO2-GA Experiment
According to the method proposed by Hudot [36], the pores of coal are divided into micropores (<2 nm), mesopores (2–50 nm), and macropores (>50 nm). Table 2 presents the micropore structure parameters derived from the NLDFT model, including specific surface area, pore volume, and minimum pore size within the range of 0.012–0.026 cm3/g, 32.027–69.924 m2/g, and 0.6–0.9 nm, respectively. Notably, it is evident that low-volatile bituminous (LVB) coal exhibits the highest pore volume (PV) and specific surface area (SSA), followed by medium-volatile bituminous (MVB) and high-volatile bituminous (HVB) coal. Furthermore, V and SSA demonstrate a polynomial relationship with increasing coal rank.

Table 2.
Micropore structure parameters of six samples.
Figure 4 illustrates the isothermal adsorption curves of CO2 for all the samples. At the same pressure, the adsorption isotherm is relationship with the pore structure of coal. As the degree of metamorphism increases, the overall CO2 adsorption exhibits an initial decrease followed by an increase. The coal sample LSJ, characterized by a moderate degree of metamorphism, exhibits the lowest adsorption capacity, while DEP and CP coal samples demonstrate higher adsorption capacities.
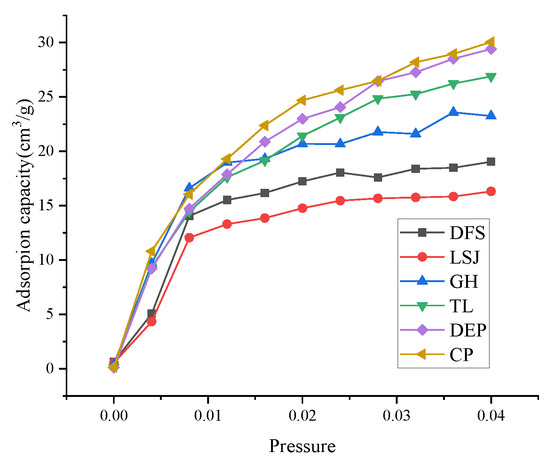
Figure 4.
Adsorption–desorption curve obtained by LT-CO2-GA.
3.2. Characterization of Pore Structure by the LT-N2-GA Experiment
The pore structure parameters obtained from the LT-N2-GA experiment are presented in Table 3. The SSA and V of the mesopores were determined using the BJH model. The mesopore SSA for all the samples fell within the range of 0.494–1.056 m2/g, with the CP coal sample exhibiting approximately twice the mesopore SSA compared to LSJ. The pore volume of BJH mesopores ranged from 2.005 × 10−3 to 4.231 × 10−3 cm3/g.

Table 3.
Mesopore and macropore structure parameters of six samples.
The coal sample adsorption isotherms are shown in Figure 5. IUPAC classification distinguishes five hysteresis loops, namely, Type H1, H2, H3, H4, and H5. The pore shapes include slit shape, ink-bottle shape, cylinder shape, etc. The adsorption isotherms of the coal samples are shown in Figure 5 [37], which are classified as Type H2 and Type H3.
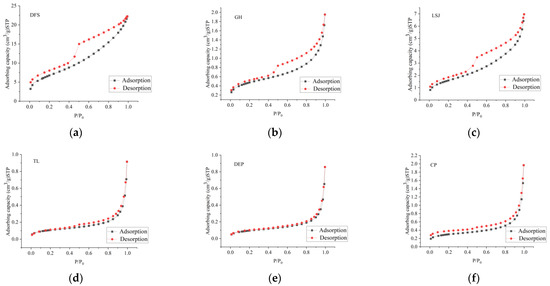
Figure 5.
Adsorption–desorption curve obtained by LT-N2-GA. (a) DFS; (b) LSJ; (c) GH; (d) TL; (e) DEP; (f) CP.
Type H2: The adsorption curves of DFS, LSJ, and GH exhibit wide-ranging adsorption loops that closely resemble Type H2 behavior. At relative pressures (P/P0) below 0.8, the adsorption and desorption curves coincide closely. However, as the pressure surpasses 0.44, distinct adsorption loops become apparent. At a relative pressure of 0.83, the adsorption capacity experiences a sudden increase, resulting in a steep curve shape with a pronounced concave appearance. When the relative pressure is close to 1, the sample is close to adsorption saturation. Then, as the relative pressure decreases, the desorption of N2 commences. Notably, when the relative pressure exceeds 0.75, the desorption capacity exhibits a steady decline. Within the relative pressure range of 0.75–0.45, the desorption curve displays a distinct inflection point characterized by a rapid decrease in desorption quantity, followed by a stabilization phase. The predominant pore types observed in the samples are primarily open-necked holes or ink-bottle-shaped cavities.
Type H3: The adsorption curves of TL, DEP, and CP exhibit a narrow range of adsorption loops, resembling Type H3 behavior. At relative pressures (P/P0) below 0.8, the isothermal adsorption curves exhibit a gradual rise, characterized by an upper convex shape, signifying the transition from monolayer to multilayer adsorption. Subsequently, when the relative pressure surpasses 0.8, there is a rapid increase in gas adsorption capacity due to capillary condensation. In contrast, the desorption curves lack prominent inflection points but exhibit a steep decline in desorption quantity at higher relative pressures (above 0.85), forming a distinct concave shape. Following this, the amount of desorption decreases gradually in an approximately linear fashion. The predominant pore type observed in these samples consists of open slot-shaped pores, composed of non-rigid aggregates of plate-like particles.
3.3. Characterization of Pore Structure by AFM
Here we only list ×4000 nm image surface of samples for exhibition (Figure 6). In highly volatile bituminous coal (LSJ, DFS), linear parallel cracks develop, and their extension distance is relatively long. The surface is a banded structure with different widths. In middle-rank bituminous coal, GH and TL, the width of microcracks on its surface increases, and micro-cracks coexist with pores. In anthracite coal, DEP and CP, the surfaces present a fibrous structure, the structure tends to be compact, and the morphology becomes flat.
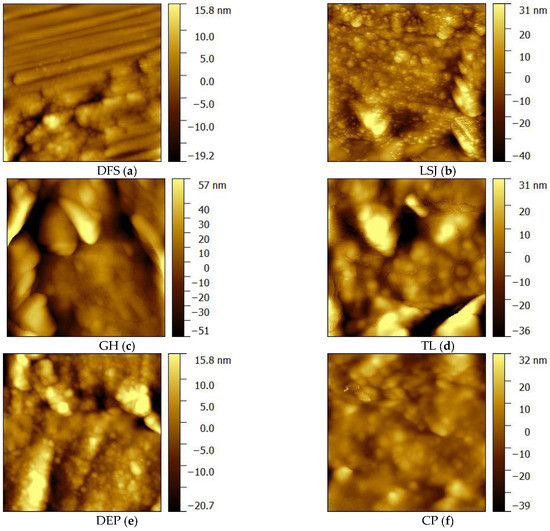
Figure 6.
Coal microscopic morphology obtained by AFM.
Cracks with a width of 500 nm are called micro-cracks [38]. A micro-crack is formed by the internal tension caused by physical and chemical changes in coal structure under the influence of temperature and pressure in the process of coalification [39]. Some particles are adsorbed in the cracks; the primary cracks give rise to secondary cracks. In the process of coalification, the side chains and functional groups of organic molecules in coal constantly break and fall off with the increase in temperature and pressure, resulting in a large number of volatile products. But the evolution of coalification does not follow a straight line. When Ro,max is about 1.3%, the second jump occurs, and a large amount of methane escapes from coal. The high fluid pressure caused by a large amount of fluid generation that is discharged over time is the main internal cause of crack generation. When Ro,max < 1.3%, the crack in coal increases with the increase in gas content. When Ro,max = 1.3%, the gas generation reaches its maximum, and the crack density in coal reaches its maximum at this stage (DFS and LSJ have obvious cracks). When Ro,max > 1.3%, the internal tension caused by devolatilizing decreases with the decrease in the amount of merperance, and the newly generated cracks are less. Meanwhile, under the action of increasing in situ stress, the existing cracks gradually close and disappear (the cracks of GH, TL, DEP, and CP become less and less). Therefore, when Ro,max > 1.3%, the density of cracks in coal decreases with the increase in metamorphism. Therefore, with the increase in metamorphism degree in bituminous coal, cracks in the coal gradually decrease. The crack is the foundation of fracturing, so the development of cracks is conducive to the generation of fractures after fracturing.
4. Discussion
4.1. Comparing Different Algorithms and Magnification Results of AFM
Table 4 and Table 5 present the statistical analysis of pore structure using different algorithms. It is important to note that the choice of algorithm and magnification can have a notable impact on coal pore volume and surface roughness. The Threshold and Chen’s algorithms exhibit significant influence on the results, while variations in magnification (×200 and ×4000) also yield different outcomes.

Table 4.
The pore parameters tested by AFM calculated by the Threshold algorithm for coal samples (×4000/×200).

Table 5.
The pore parameters tested by AFM calculated by Chen’s algorithm for coal samples (×4000/×200).
At an image magnification of ×200, the Threshold algorithm yields a range of pore numbers between 127 and 264, while Chen’s algorithm results in a range of pore numbers spanning from 154 to 288. On the other hand, at an image magnification of ×4000, the Threshold algorithm produces a pore number range of 494 to 1056, whereas Chen’s algorithm generates a pore number range of 563 to 1220. Whether the analysis is based on the Threshold algorithm or Chen’s algorithm, the smallest pore sizes characterized at ×4000 magnification are smaller than those observed at ×200 magnification. For instance, using the Threshold algorithm, the smallest pore sizes for DFS are 0.18 nm and 0.35 nm at ×4000 and ×200 magnifications, respectively. Similarly, with Chen’s algorithm, the smallest pore sizes for DFS are 0.12 nm and 0.28 nm at ×4000 and ×200 magnifications, respectively. However, there is not much variation observed in the maximum pore sizes characterized between different magnifications. For example, with the Threshold algorithm, the maximum pore sizes characterized are 220.4 nm and 219.2 nm at ×4000 and ×200 magnifications, respectively. Similarly, using Chen’s algorithm, the maximum pore sizes are 234.5 nm and 235.3 nm at ×4000 and ×200 magnifications, respectively. The porosity obtained by calculations based on different algorithms is shown in Figure 7.
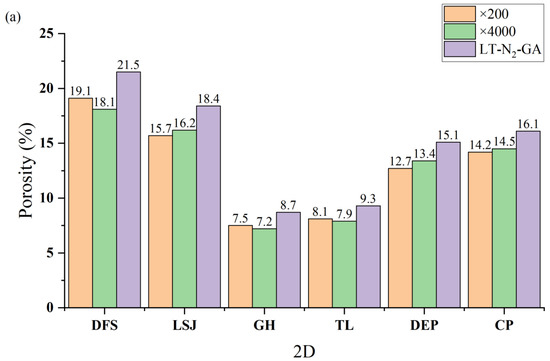
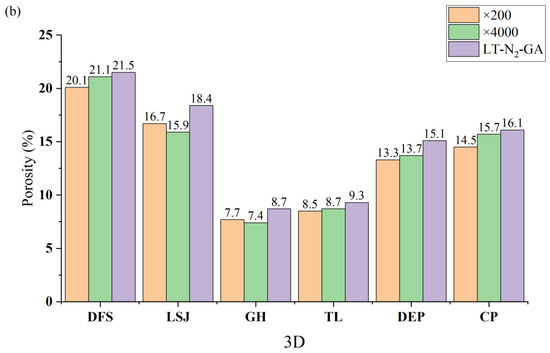
Figure 7.
Comparison of the porosity histograms of coal samples obtained by different algorithms and magnifications: (a) the Threshold algorithm and (b) Chen’s algorithm.
The discrepancy between the results obtained from the Threshold and Chen’s algorithms can be attributed primarily to variations in pore shape. The presence of numerous ink-bottle-shaped pores, characterized by a small orifice and a larger throat, leads to smaller pore size measurements with the Threshold algorithm compared to Chen’s algorithm. Regarding the disparities between ×200 and ×4000 images, they arise primarily from the inherent heterogeneity of coal. The broader scope of the AFM image captures a more pronounced manifestation of this heterogeneity. It is important to note that the absolute deviation between ×200 and ×4000 images is irregular, indicating that the strength of heterogeneity characteristics is contingent on the specific region selected within the AFM image.
Figure 7 presents the porosity values obtained using different methods. The porosity distribution obtained from the Threshold algorithm ranges from 7.2% to 19.1%, while Chen’s algorithm yields a porosity range of 7.4% to 21.1%. Conversely, the porosity values determined through LT-N2-GA measurements fall within the range of 8.7% to 21.5%. Notably, the results obtained from Chen’s algorithm exhibit a closer resemblance to the porosity values derived from LT-N2-GA measurements. Moreover, the difference in porosity between ×200 and ×4000 nm magnifications is similar, suggesting that the effect of magnification on porosity calculations can be considered negligible.
Table 6 provides the values of surface roughness for different magnifications. At ×200 magnification, the Rsk values range from −0.73 to 0.19, the Rku values range from 4.35 to 13.1, the Rq values range from 52.2 to 132, and the Ra values range from 30.7 to 107. On the other hand, at ×4000 magnification, the Rsk values range from −0.31 to 0.19, the Rku values range from 2.74 to 5.51, the Rq values range from 3.85 to 21.1, and the Ra values range from 2.60 to 16.23. Notably, the Rq and Ra values at ×200 magnification are 5–14 times higher than those at ×4000 magnification, with the largest difference observed in the LSJ sample. This can be attributed to the increased variation in height from the mean line as magnification increases, indicating a rougher outer surface. The differences in Rsk and Rku values between ×200 and ×4000 magnifications are relatively small. However, the Rsk and Rku values at ×4000 magnification are generally higher, suggesting a greater prominence of surface peaks at lower magnifications.

Table 6.
The surface roughness of samples.
4.2. Pore Structure Evolution on the Second Coalification Jump
In the LT-N2-GA images, high-volatile bituminous coals and middle-volatile bituminous coals exhibit a higher presence of cylindrical pores, while low-volatile bituminous coal displays a significant proportion of wedge-shaped pores. On the other hand, the AFM images reveal distinct characteristics for each coal type. In high-volatile bituminous coal, linear parallel cracks are prevalent, extending over considerable distances, and the surface exhibits a banded structure with varying widths. Middle-volatile bituminous coal exhibits an increase in the width of microcracks on the surface, along with the coexistence of microcracks and pores. Low-volatile bituminous coal displays a fibrous surface structure that tends to be compact and flattened in morphology. By combining the LT-N2-GA and AFM images, it becomes apparent that the pore structure of bituminous coal gradually narrows as the degree of metamorphism increases. Furthermore, larger cracks diminish while the presence of micro-cracks becomes more pronounced.
The pore volume, specific surface area (SSA), pore number, pore size, and porosity can be effectively determined through LT-CO2-GA, LT-N2-GA, and AFM experiments. These pore characteristics exhibit a close relationship with coal ranks. The SSA, PV, and pore number demonstrate a rapid decline within the Ro,max = 1.19%~1.29%, followed by a gradual increase within the Ro,max = 1.37%~1.97%. However, the observed values for pore size and porosity differ slightly. The maximum pore size exhibits irregular changes, while the minimum pore size increases between Ro,max = 1.19%~1.37% before gradually decreasing. Conversely, the variation in porosity follows an opposite trend, wherein it rapidly decreases within the Ro,max = 1.19%~1.37% and then increases.
The observed phenomenon can be attributed to the changes in the macromolecular structure during the second coalification jump. Low metamorphic coal exhibits an irregular molecular structure, with long side chains and numerous functional groups, leading to the formation of a relatively loose spatial structure with significant micropores SSA and PV. As coal rank increases, the presence of oxygen-containing functional groups and alkyl side chains decreases, while aromatic nuclei increase. This results in a compression of the coal structure, leading to the lowest values of SSA and PV at this stage (Ro,max = 1.19%~1.29%). With further coalification, a substantial number of aromatic layers are formed, enhancing the ordering of macromolecules and causing the aromatics to be arranged more closely. This arrangement leads to the formation of new cracks, resulting in an increase in micropores SSA and PV, and a decrease in pore size (Ro,max = 1.37%~1.97%). It is important to note that the changes in porosity and micropore characteristics are not identical.
This discrepancy can be attributed to the stage between Ro,max = 1.29%~1.6%, where the length of aliphatic chains decreases, leading to an increase in the aromatic system. However, the dehydrogenation of aromatic groups prevents the formation of a well-defined parallel structure in the enlarged aromatic system. As a result, the coal skeleton volume increases, causing a lag in porosity changes compared to micropore changes. Nonetheless, as the aromatic structure gradually arranges itself in a regular manner, the spacing between layers decreases, resulting in increased SSA, PV, and porosity.
4.3. Surface Roughness Evolution on the Second Coalification Jump
Surface roughness changed with the degree of coalification, relationships between surface roughness (Ra, Rq, Rsk, and Rku) and thermal maturity (Ro,max) of naturally matured coals are shown in Figure 8.
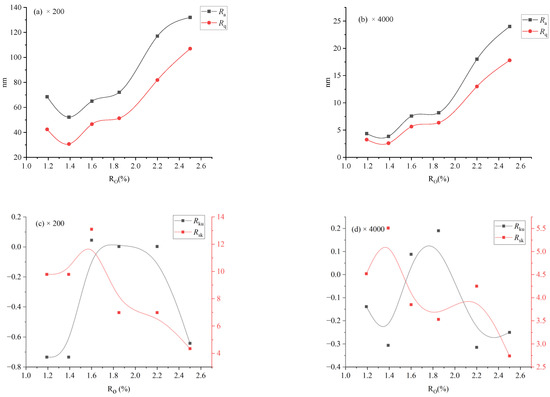
Figure 8.
The relationship of surface roughness and Ro,max.
As illustrated in Figure 8a,b, the evolution trends of Ra and Rq are clearly similar. As Ro,max = 1.19–1.39%, Ra and Rq decrease with increasing Ro,max, with minimum values at Ro, max = 1.39%; as Ro,max = 1.39–1.80%, Ra and Rq increase with increasing Ro,max slowly, and more rapidly when Ro,max = 1.8–2.5%. As illustrated in Figure 8c,d, no significant correlation was observed between Rsk or Rku and Ro,max, suggesting that Ro,max does not exert control over either Rsk or Rku. It is noteworthy that for GH and TL, the major surface heights are below the average (Rsk < 0), while for other samples, more surface heights are above the average (Rsk > 0). Additionally, all samples demonstrated Rku > 0, indicating a concentration of all sample surface heights around the average value.
The surface roughness of coal can be influenced by both its composition and nanopore development. In coals with relatively low thermal maturity (Ro,max < 1.3%), the surface roughness is primarily controlled by micro-composition and mineral composition. However, in coals with higher maturity (Ro,max > 1.3%), nanopore development plays a more dominant role [40]. Thus, the observed variation in surface roughness of bituminous coal with increasing coal rank aligns with the trend observed in nanopore development, wherein it initially decreases and then increases.
4.4. Effect of the 3D Pore Structure on CH4 Adsorption Capacity
To investigate the relationship between the 3D pore structure and the adsorption capacity of CH4, HP-CH4-GA was carried out on different samples. The Langmuir, Freundlich, and Sips adsorption models were employed to simulate the adsorption isotherms, as illustrated in Figure 9. The isotherm model constants and nonlinear regression parameters are summarized in Table 7. The adsorption isotherm data were analyzed using three different models: Langmuir, Freundlich, and Sips. Among these models, the Sips model demonstrated a strong fit to the experimental data, exhibiting a higher correlation coefficient (R2 = 0.9958). According to Milan et al. [35], the Sips model is well-suited for capturing the adsorption site interactions that occur on the heterogeneous surface of the adsorbent. In contrast, the Langmuir model assumes monolayer adsorption on a homogeneous surface. Given these considerations, the Sips model is considered more appropriate for accurately characterizing the isothermal adsorption behavior of bituminous coal. The Qmax of CH4 was 5.30~25.12 cm3/g according to the Sips adsorption model.
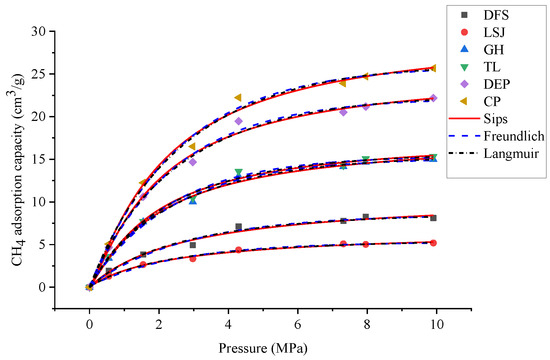
Figure 9.
The adsorption isotherms simulated by Langmuir, Freundlich, and Sips models.

Table 7.
The adsorption parameters of samples.
The adsorption effective capacities of the samples are presented in Figure 10. The results indicate that the adsorption capacity of bituminous coal exhibits a trend of initially decreasing and then increasing with increasing coal rank, and it also increases with higher pressure. The order of adsorption capacity from highest to lowest is CP > DEP > TL > GH > DFS > LSJ. The observed ordering of adsorption capacities aligns with the ordering of the Qmax.
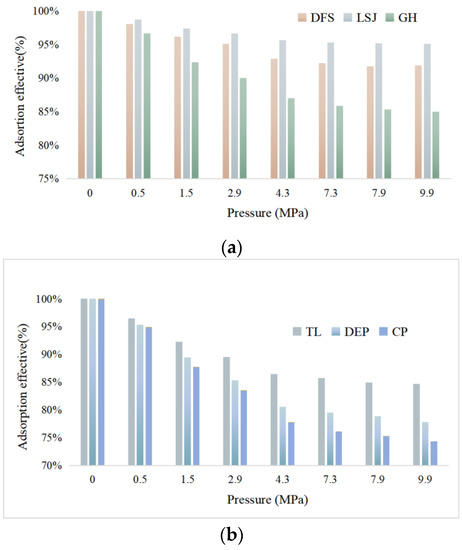
Figure 10.
The adsorption effectiveness of samples. (a) Adsorption effective of DFS, LSJ and GH; (b) Adsorption effective of TL, DEPand CP.
Previous studies commonly suggest that the gas adsorption capacity of coal is influenced by micro- and mesopores [41,42,43]. However, the analysis of pore structure in this study reveals that there is no evident linear relationship between porosity, micropore volume, or specific surface area and coal reservoir adsorption capacity, particularly for lignite and bituminous coal [44,45]. Notably, in this study, the micropore volume and specific surface area of DFS (43.354, 0.019) and TL (48.049, 0.019) are comparable. However, the Qmax and effective adsorption capacity of TL (9.98, 91%) are higher than those of DFS (17.95, 84%). Similarly, although the micropore volume and specific surface area of GH (43.354, 0.019) are smaller than those of TL (48.049, 0.019), the Qmax and effective adsorption capacity are comparable. These findings suggest that additional factors beyond micropore volume and specific surface area contribute to the adsorption capacity of coal reservoirs. Functional groups and surface roughness are recognized as factors that can influence adsorption capacity. While there have been numerous studies investigating the impact of functional groups on adsorption, the relationship between surface roughness and coal adsorption capacity has received relatively less attention.
Generally, it has been observed that samples exhibiting a lower surface roughness tend to have a smaller specific surface area and gas adsorption capacity, whereas samples with a higher surface roughness exhibit a larger specific surface area. This increased surface roughness provides more space for gas adsorption [46,47]. The relationship between 3D surface roughness and Qmax for all samples is presented in Figure 11. The Rsk and Rku values of the samples exhibit a positive correlation with Qmax, as they represent the degree of fluctuation in the sample’s storage space, reflecting the combined surface area and volume characteristics. According to the definitions of Rsk and Rku, a greater fluctuation in coal results in a higher gas storage capacity. Therefore, Rsk and Rku can be used as indicators of gas adsorption capacity. Similarly, the values of Ra and Rq also show a positive correlation with Qmax. Smaller values of Ra and Rq indicate a smoother coal surface, which leads to reduced friction between the gas and coal surfaces. This reduction in friction weakens the intermolecular forces between the gas and coal, resulting in stronger gas adsorption capacity. These findings highlight the significant influence of Ra, Rq, Rsk, and Rku values on the analysis of gas adsorption volume.

Figure 11.
The relationship of surface roughness and Qmax. (a) The relationship of Ra, Rq and Qmax; (b) The relationship of Rku, Rsk and Qmax.
5. Conclusions
- Upon conducting a comparative analysis of AFM images across various algorithms and scales, we posit that the pore calculation results derived from the 3D algorithm at ×4000 magnification are more accurate than those obtained through other algorithms. These results exhibit greater resemblance to the LP-CO2/N2-GA findings. Chen’s algorithm discerned a larger number of pores than the Threshold algorithm. For example, in the case of DFS, the numbers were 1220 (×4000) versus 1056 (×4000). Furthermore, Chen’s algorithm uncovered more micropores. The porosity determined by the 3D algorithm outperformed that of the Threshold algorithm and was closer to the LP-N2-GA results. When observed at a magnification of ×4000, more pores were identified than at ×200 (DFS: 1056 vs. 264 using the Threshold algorithm). However, the porosities observed at magnifications of ×200 and ×4000 nm were similar, rendering the effect of magnification inconsequential.
- AFM, employing Chen’s algorithm and a magnification of ×4000, can accurately analyze the 3D pore structure of bituminous coal. Based on this, the range of pore quantity in bituminous coal is found to be 563–1220, with the maximum value of CP and the minimum of DF. The range of the maximum pore size is 234.5–234.5 nm, while the range of the minimum pore size is 0.12–0.15 nm. These values show minimal variation with respect to coal rank. The variation range of porosity is 7.4% to 21.1%, with GH having the minimum value; Rsk ranges from −0.31 to 0.19, and Rku ranges from 2.74 to 5.51, with weak regularity in their variations. The range of Rq is 3.85–3.85, and Ra ranges from 2.60 to 17.8, with LSJ having the minimum value and CP having the maximum value. Among the different adsorption models, the Sips model exhibits the best fitting performance. The Qmax values for CH4 adsorption range from 5.30 to 25.12 cm3/g. The ordering of adsorption capacity from highest to lowest is CP > DEP > TL > GH > DFS > LSJ, which aligns with the observed ordering of Qmax.
- The second coalification transition exerts a significant impact on the coal’s pore structure. Over time, the structure evolves from linear, parallel cracks and cylindrical pores to microcracks and wedge-shaped pores. Simultaneously, coal’s pore volume and surface roughness initially decline before escalating, correlating with the coal rank. Ra and Rq decrease linearly with the increase; the Rku value increases, and the Rsk value is greater than 0 in the early stage and gradually becomes less than 0. This variation is predominantly attributed to the transformation of the coal’s macromolecular structure.
- Surface roughness significantly impacts the gas adsorption capacity of samples. A more pronounced fluctuation in coal structure corresponds to a higher gas storage capacity. As a result, Rsk and Rku serve as reliable indicators of gas adsorption potential. Furthermore, smaller Ra and Rq values, indicative of a smoother coal surface, result in diminished friction between the gas and coal surface, thereby enhancing gas adsorption.
Author Contributions
Validation, S.L.; Formal analysis, H.L.; Investigation, B.J. and K.W.; Resources, B.C.; Writing—original draft, K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [National Natural Science Foundation of China] grant number [42103047], [National Natural Science Foundation of China] grant number [52174207], and [Applied Basic Research Project of Shanxi Province] grant number [20210302124644].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lv, Y.; Tang, D.; Xu, H.; Luo, H. Production characteristics and the key factors in high-rank coalbed methane fields: A case study on the Fanzhuang Block, Southern Qinshui Basin, China. Int. J. Coal. Geol. 2012, 96–97, 93–108. [Google Scholar] [CrossRef]
- Chen, S.; Gong, Z.; Li, X.; Wang, H.; Wang, Y.; Zhang, Y. Pore structure and heterogeneity of shale gas reservoirs and its effect on gas storage capacity in the Qiongzhusi Formation. Geosci. Front. 2021, 12, 101244. [Google Scholar] [CrossRef]
- Tang, S.; Tang, D.; Li, S.; Xu, H.; Tao, S.; Geng, Y.; Ma, L.; Zhu, X. Fracture system identification of coal reservoir and the productivity differences of CBM wells with different coal structures: A case in the Yanchuannan Block, Ordos Basin. J. Pet. Sci. Eng. 2018, 161, 175–189. [Google Scholar] [CrossRef]
- Wang, F.; Yao, Y.; Wen, Z.; Sun, Q.; Yuan, X. Effect of water occurrences on methane adsorption capacity of coal: A comparison between bituminous coal and anthracite coal. Fuel 2020, 266, 117102. [Google Scholar] [CrossRef]
- Li, G.; Qin, Y.; Zhou, X.; Zhang, Y.; Hu, W. Comparative analysis of the pore structure of fusain in lignite and high-volatile bituminous coal. J. Nat. Gas Sci. Eng. 2021, 90, 103955. [Google Scholar] [CrossRef]
- Van, K. Coal-Typology, Chemistry, Physics, Constitution; Elsevier Science Publishing Company Inc.: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Liu, Y.; Paskevicius, M.; Sofianos, M.; Veronica, P.; Li, C. In situ SAXS studies of the pore development in biochar during gasification. Carbon 2020, 172, 454–462. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S. Laboratory study of cryogenic treatment induced pore-scale structural alterations of Illinois coal and their implications on gas sorption and diffusion behaviors. J. Pet. Sci. Eng. 2020, 194, 107507. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Lu, Z.; Pan, Z.J.; Zeng, M.; Du, X.; Xiao, S. The effect of subcritical and supercritical CO2 on the pore structure of bituminous coals. J. Nat. Gas. Sci. Eng. 2021, 94, 104132. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Ni, G.; Lu, J.; Lu, Y. Spectroscopic (FTIR, 1H NMR) and SEM investigation of physicochemical structure changes of coal subjected to microwave-assisted oxidant stimulation. Fuel 2022, 317, 123473. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, D.; Chen, Y.; Gao, Z.; Teng, J. Pore structure of different macroscopically distinguished components within low-rank coals and its methane desorption characteristics. Fuel 2021, 293, 120465. [Google Scholar] [CrossRef]
- Giergiel, M.; Zapotoczny, B.; Czyzynska, I.; Konior, J.; Szymonski, M. AFM image analysis of porous structures by means of neural networks. Biomed. Signal Process. 2021, 71, 103097. [Google Scholar] [CrossRef]
- Kong, L.; Hadavimoghaddam, F.; Li, C.; Liu, K.; Liu, B.; Semnani, A.; Ostadhassan, M. AFM vs. Nanoindentation: Nanomechanical properties of organic-rich Shale. Mar. Petol. Geol. 2021, 132, 105229. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Sun, Y.; Jiang, D.; Zhang, X. Investigation of coal components of Late Permian different ranks bark coal using AFM and Micro-FTIR. Fuel 2017, 187, 51–57. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, Y.; Ma, Z.; Huang, X. Quantitative study on coal and shale pore structure and surface roughness based on atomic force microscopy and image processing. Fuel 2019, 244, 78–90. [Google Scholar] [CrossRef]
- Liu, X.; Nie, B.; Wang, W.; Wang, Z.; Zhang, L. The use of AFM in quantitative analysis of pore characteristics in coal and coal-bearing shale. Mar. Petrol. Geol 2019, 105, 331–337. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Pan, Z.; Tong, W. Nanoscale pore structure and mechanical property analysis of coal: An insight combining AFM and SEM images. Fuel 2020, 260, 116352. [Google Scholar] [CrossRef]
- Chen, S.; Tao, S.; Tian, W.; Tang, D.; Zhang, B.; Liu, P. Hydrogeological control on the accumulation and production of coalbed methane in the Anze Block, southern Qinshui Basin, China. J. Pet. Sci. Eng. 2021, 198, 108138. [Google Scholar] [CrossRef]
- Song, S.-B.; Liu, J.-F.; Yang, D.-S.; Ni, H.-Y.; Huang, B.-X.; Zhang, K.; Mao, X.-B. Pore structure characterization and permeability prediction of coal samples based on SEM images. J. Nat. Gas. Sci. Eng. 2019, 67, 160–171. [Google Scholar] [CrossRef]
- GB/T 23561.1-2009; Methods for Determining the Physical and Mechanical Properties of Coal and Rock—Part 1: General Requirements for Sampling. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2009.
- GB/T 212-2008; Proximate Analysis of Coal. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2008.
- GB/T 6948-2008; Method of Determining Microscopically the Reflectance of Vitrinite in Coal. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2008.
- GB/T 21650.1-2008; Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption-Part 1: Mercury Porosimetry. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2008.
- GB/T 21650.3-2011; Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption-Part 3: Analysis of Micropores by Gas Adsorption. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2008.
- GB/T 19222-2003; Sampling of Coal Petrology. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2003.
- Huang, L.; Wang, M. Image thresholding by minimizing the measures of fuzziness. Pattern Recogn. 1995, 28, 41–51. [Google Scholar] [CrossRef]
- Kapur, J.; Sahoo, P.; Wong, A. A new method for gray-level picture thresholding using the entropy of the histogram. Comput. Vis. 1985, 29, 273–285. [Google Scholar]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Yen, J.; Chang, F.; Chang, S. A new criterion for automatic multilevel thresholding. IEEE Trans. Image Process. 1995, 4, 370–378. [Google Scholar] [PubMed]
- Wlanis, T.; Hammer, R.; Ecker, W.; Lhostis, S.; Sart, C.; Gallois-Garreignot, S.; Maier, G.A. Cu-SiO2 hybrid bonding simulation including surface roughness and viscoplastic material modeling: A critical comparison of 2D and 3D modeling approach. Microelectron. Reliab. 2018, 86, 1–9. [Google Scholar] [CrossRef]
- Su, D.; Wang, X.; Yang, H.; Hong, C. Roughness analysis of general-shape particles, from 2D closed outlines to 3D closed surfaces. Powder Technol. 2019, 356, 423–438. [Google Scholar] [CrossRef]
- Carvalho, A.; Maugeri, F.; Silva, V.; Hernandez, A.; Palacio, L.; Pradanos, P. AFM analysis of the surface of nanoporous membranes: Application to the nanofiltration of potassium clavulanate. J. Mater. Sci. 2011, 46, 3356–3369. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Shi, Z.; Wei, Y.; Zhou, H.; Deng, K. The Description of Shale Reservoir Pore Structure Based on Method of Moments Estimation. PLoS ONE 2016, 11, 151631. [Google Scholar] [CrossRef]
- Mustafa, A.; Mohamad, A.; Suriati, S.; Muhammad, B.; Usama, E.; Ahmad, M. High pressure CO2 adsorption onto Malaysian Mukah-Balingian coals: Adsorption isotherms, thermodynamic and kinetic investigations. Environ. Res. 2023, 218, 114905. [Google Scholar]
- Milan, B.; Ganesh, P.; Han, J. Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co-Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar]
- Hotot, B.B. Coal and Gas Outburst; Song, S.Z.; Wang, Y.A., Translators; China Coal Industry Publishing House: Beijing, China, 1961. [Google Scholar]
- Luo, C.; Liu, S.; Sun, W. Pore structure characterization of blacks shale In the Lower Cambrian Niutitang Formation in western Hubei and eastern Chongqing area. J. N. Pet. Univ. 2014, 38, 8–16. [Google Scholar]
- Wang, S.; Zhang, M.; Zhuang, X. The mechanism of microfractures and cleats of coal reservoirs and their implication for methane recovery. Earth Sci. 1996, 21, 637–640. [Google Scholar]
- Zhang, J.; Liu, G.; Torsaeter, O.; Tao, S.; Jiang, M.; Li, G.; Zhang, S. Pore-throat structure characteristics and its effect on flow behavior in Gaotaizi tight siltstone reservoir, northern Songliao Basin. Mar. Petrol. Geol. 2020, 122, 104651. [Google Scholar] [CrossRef]
- Kun, J.; Yao, S.; Zhang, K.; Hu, W.; Cao, J. The evolution of nanopores and surface roughness in naturally matured coals in south china: An atomic force microscopy and image processing study. Fuel 2018, 234, 1123–1131. [Google Scholar]
- Han, M.; Wei, X.; Zhang, J. Influence of structural damage on evaluation of microscopic pore structure in marine continental transitional shale of the Southern North China Basin: A method based on the low-temperature N_2 adsorption experiment. Pet. Sci. 2022, 19, 100–115. [Google Scholar]
- Jiao, T.; Fan, H.; Liu, S. A review on nitrogen transformation and conversion during coal pyrolysis and combustion based on quantum chemical calculation and experimental study. Chin. J. Chem. Eng. 2021, 35, 107–123. [Google Scholar] [CrossRef]
- Masoudian, S. Multiphysics of carbon dioxide sequestration in coalbeds: A review with a focus on geomechanical characteristics of coal. J. Rock Mech. Geotech. Eng. 2016, 8, 93–112. [Google Scholar] [CrossRef]
- Li, Z.; Lin, B.; Hao, Z.; Gao, Y. Characteristics of pore size distribution of coal and its impacts on gas adsorption. J. China Univ. Min. Technol. 2013, 42, 1047–1053. [Google Scholar]
- Han, Y.; Zhang, J.; Li, L.; An, Y. Research on coal pore characteristics and gas adsorption control mechanism in Pingdingshan mining area. China Coal 2017, 43, 34–37. [Google Scholar]
- Javadpour, F.; Moravvej, F. Atomicforce microscopy: A new tool for gas-shale characterization. J. Can. Petrol. Technol. 2012, 51, 236–243. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, M.; Yang, C. AFM based pore characterization of shales and its relation to the analytical gas. J. Jilin Univ. Earth Sci. Ed. 2016, 46, 1332–1341. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).