Diclofenac-Impregnated Mesoporous Carbon-Based Electrode Material for the Analysis of the Arsenic Drug Roxarsone
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. Synthesis of DF-CMK-3
2.4. GCE/DF-CMK-3/CTAB Preparation and ROX Analysis
3. Results and Discussion
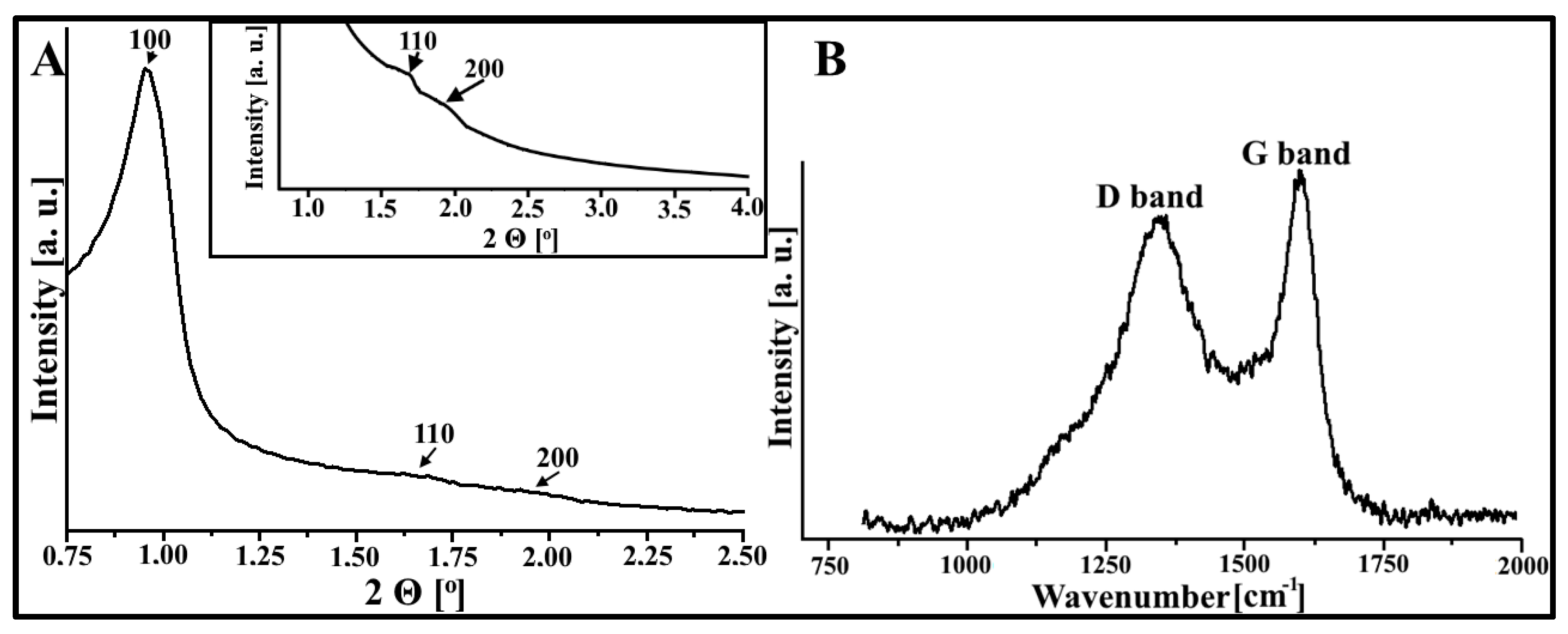
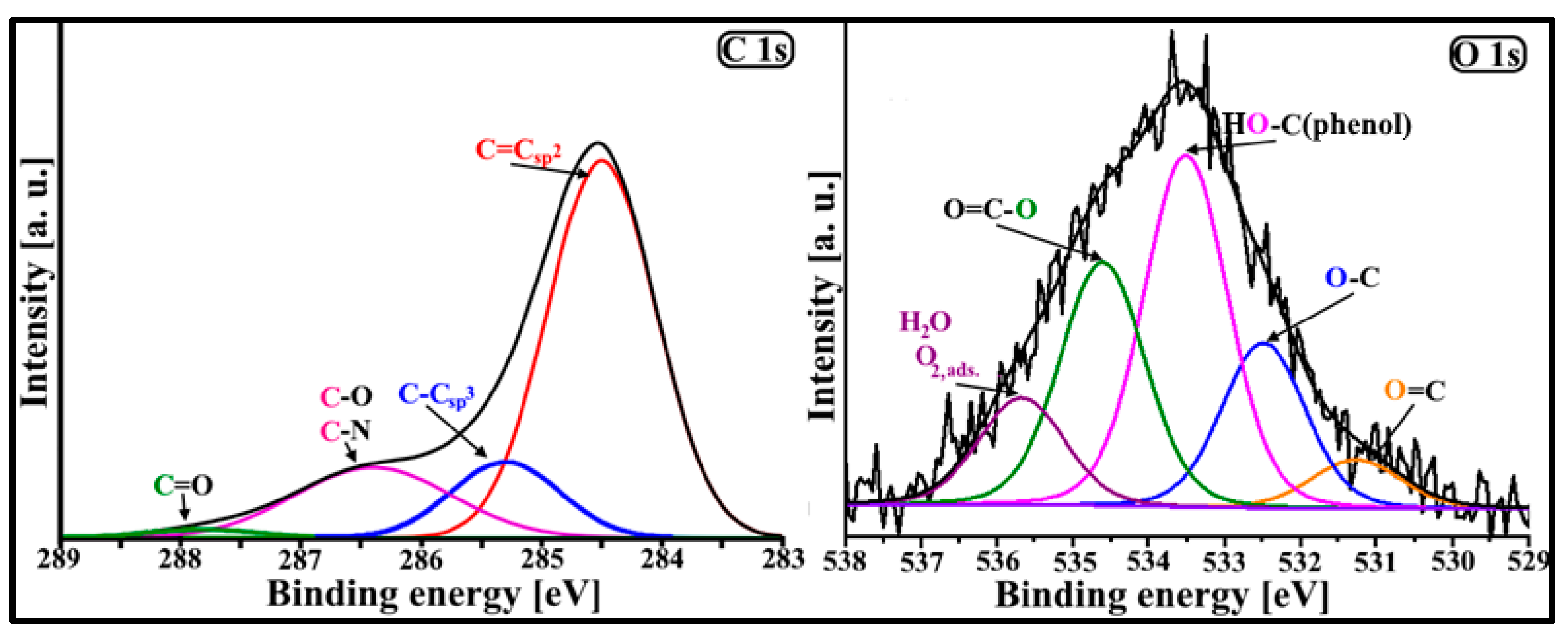
3.1. Physicochemical Characteristics of DF-CMK-3
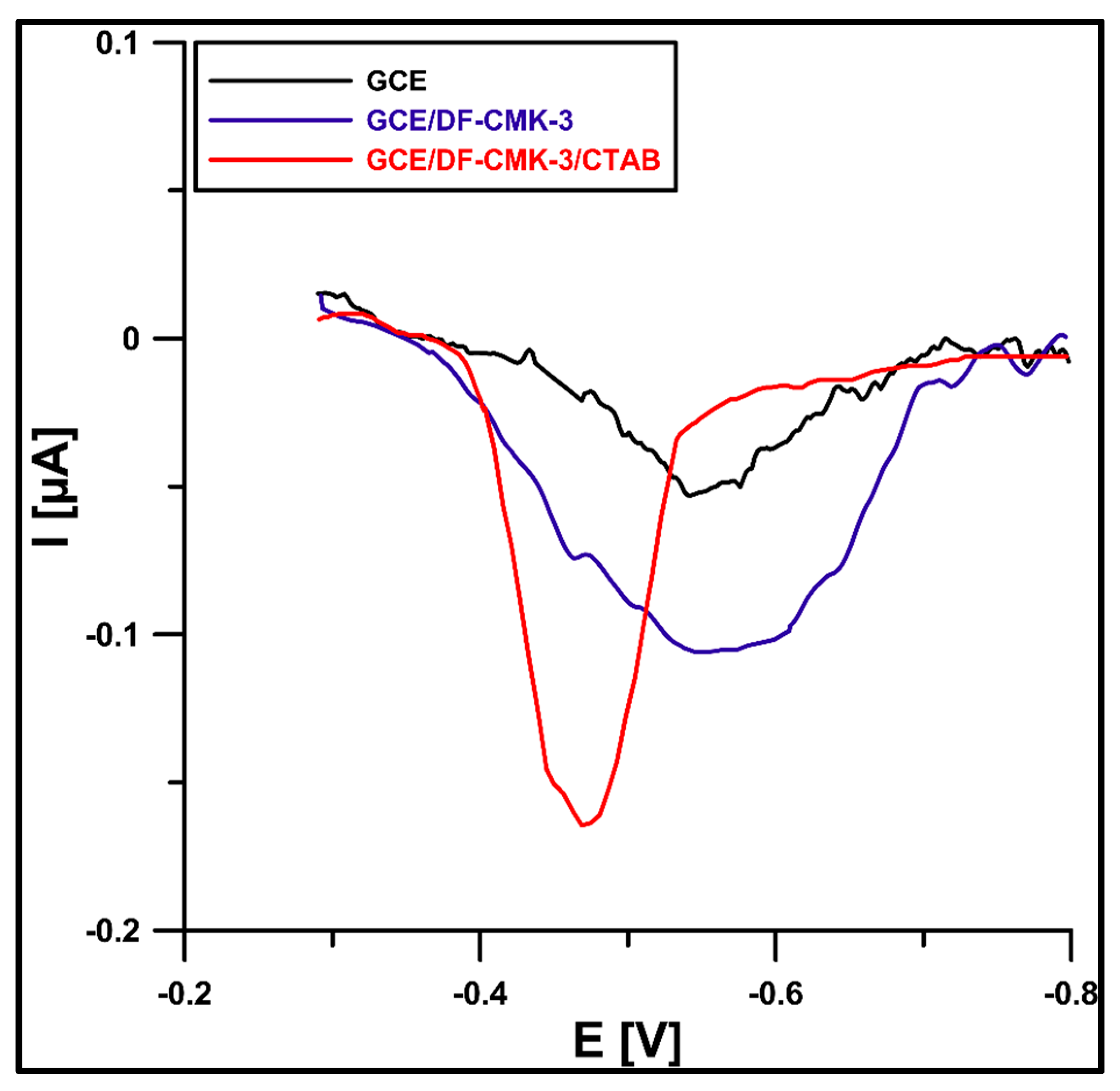
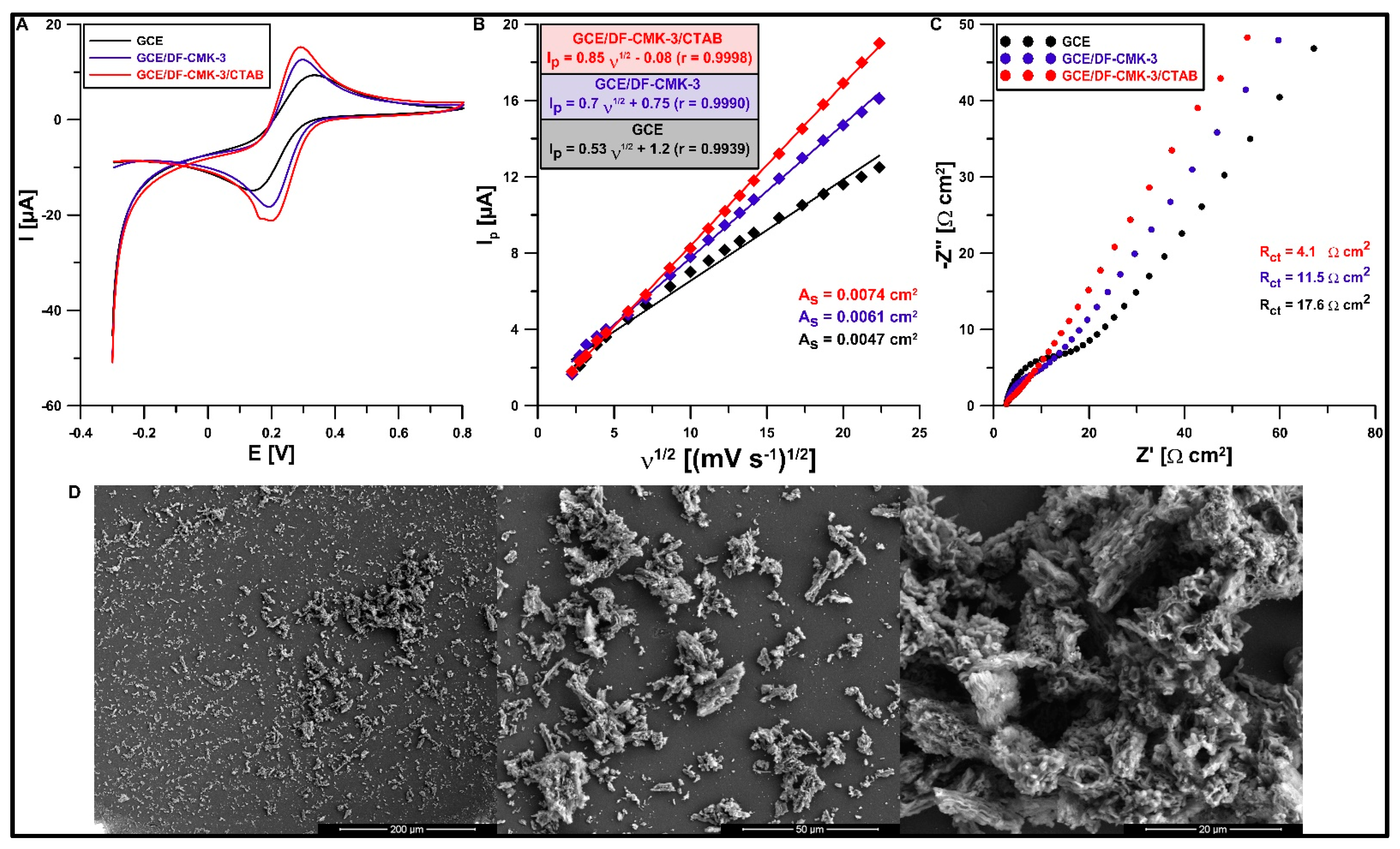
3.2. Characteristics of the GCE/CMK-3/CTAB Sensor
3.3. Optimization of the GCE/DF-CMK-3/CTAB Fabrication Procedure
3.4. Selection of Electrolyte and Study of ROX Behavior in It
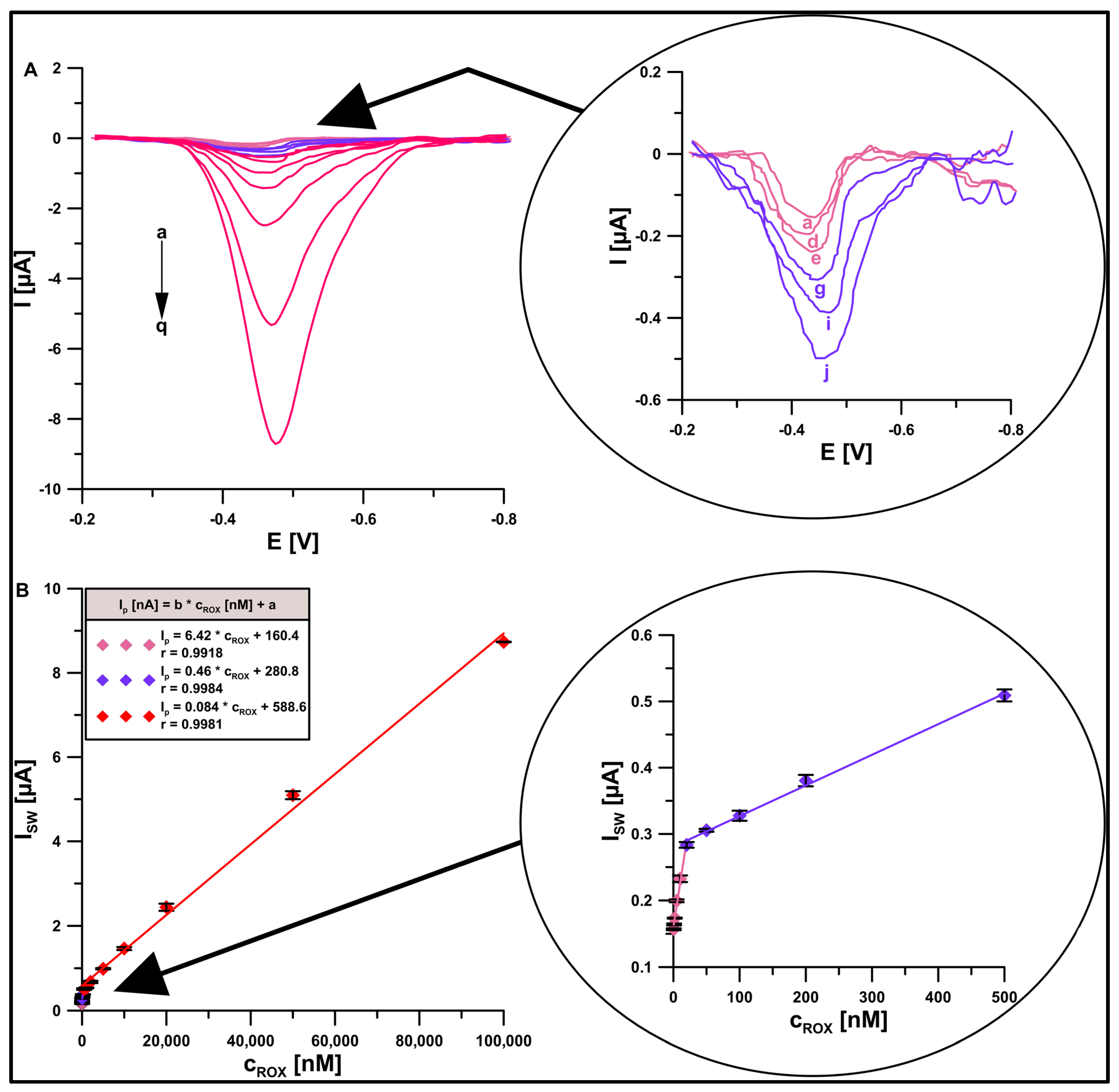
3.5. Optimization of Procedure Parameters
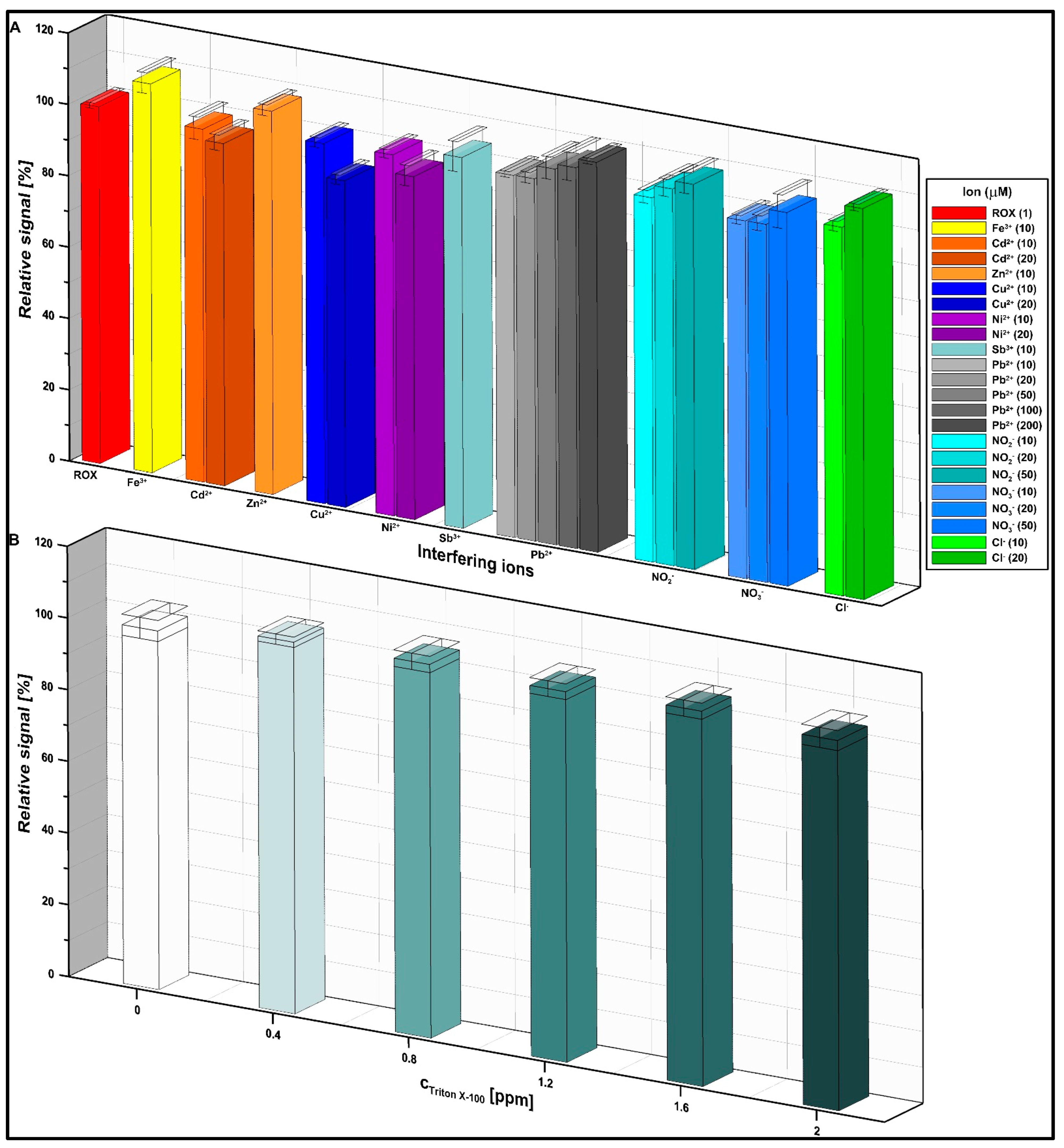
3.6. Selectivity and Analytical Characteristics
3.7. Practical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cortinas, I.; Field, J.A.; Kopplin, M.; Garbarino, J.R.; Gandolfi, A.J.; Sierra-Alvarez, R. Anaerobic biotransformation of roxarsone and related N-substituted phenylarsonic acids. Environ. Sci. Technol. 2006, 40, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.F.; Perera, E.; Kilonzo, B.; Kail, B.; Crable, B.; Fisher, E.; Ranganathan, M.; Wormer, L.; Basu, P. Biotransformation of 3-nitro-4-hydroxybenzene arsonic acid (roxarsone) and release of inorganic arsenic by Clostridium species. Environ. Sci. Technol. 2007, 41, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Alvarez, R.; Cortinas, I.; Field, J.A. Methanogenic inhibition by roxarsone (4-hydroxy-3-nitrophenylarsonic acid) and related aromatic arsenic compounds. J. Hazard. Mater. 2010, 175, 352–358. [Google Scholar]
- Huang, L.; Yao, L.; He, Z.; Zhou, C.; Li, G.; Yang, B.; Deng, X. Roxarsone and its metabolites in chicken manure significantly enhance the uptake of as species by vegetables. Chemosphere 2014, 100, 57–62. [Google Scholar] [PubMed]
- D’Angelo, E.; Zeigler, G.; Beck, E.G.; Grove, J.; Sikora, F. Arsenic species in broiler (Gallus gallus domesticus) litter, soils, maize (Zea mays L.), and groundwater from litter-amended fields. Sci. Total Environ. 2012, 438, 286–292. [Google Scholar]
- Hu, Y.; Cheng, H.; Tao, S.; Schnoor, J.L. China’s Ban on phenylarsonic feed additives, a major step toward reducing the human and ecosystem health risk from arsenic. Environ. Sci. Technol. 2019, 53, 12177–12187. [Google Scholar] [CrossRef]
- Frensemeier, L.M.; Büter, L.; Vogel, M.; Karst, U. Investigation of the oxidative transformation of roxarsone by electrochemistry coupled to hydrophilic interaction liquid chromatography mass spectrometry. J. Anal. At. Spectrom. 2017, 32, 153–292. [Google Scholar]
- Roerdink, A.R.; Aldstadt, J.H., III. Sensitive method for the determination of roxarsone using solid-phase microextraction with multi-detector gas chromatography. J. Chromatogr. A 2004, 1057, 177–183. [Google Scholar] [CrossRef]
- Cui, J.; Xiao, Y.-b.; Dai, L.; Zhao, X.-h.; Wang, Y. Speciation of organoarsenic species in food of animal origin using accelerated solvent extraction (ASE) with determination by HPLC-hydride generation-atomic fluorescence spectrometry (HG-AFS). Food Anal. Methods 2013, 6, 70–379. [Google Scholar]
- Govindasamy, M.; Wang, S.-F.; Jothiramalingam, R.; Ibrahim, S.N.; Al-lohedan, H.A. A screen-printed electrode modified with tungsten disulfide nanosheets for nanomolar detection of the arsenic drug roxarsone. Microchim. Acta 2019, 186, 420. [Google Scholar] [CrossRef]
- Chen, T.-W.; Rajaji, U.; Chena, S.-M.; Chinnapaiyan, S.; Ramalingam, R.J. Facile synthesis of mesoporous WS2 nanorods decorated N-doped RGO network modified electrode as portable electrochemical sensing platform for sensitive detection of toxic antibiotic in biological and pharmaceutical samples. Ultrason. Sonochem. 2019, 56, 430–436. [Google Scholar] [PubMed]
- Tamilalagan, E.; Akilarasan, M.; Chen, S.; Govindasamy, M.; Lin, K.; Alzahrani, F.; Alsaiari, N. Construction of perovskite structured ZnSnO3 embedded graphene oxide nanosheets for in-situ electrochemical quantification of organoarsenic roxarsone. Process Saf. Environ. Prot. 2023, 171, 705–716. [Google Scholar] [CrossRef]
- Waris, M.; Baig, J.A.; Sirajuddin; Kazi, T.G.; Solangi, I.B.; Siddiqui, S.; Afridi, H.I. Selective Electroanalytical Method for the Determination of Roxarsone in Poultry Feed and Litter. Food Anal. Methods 2016, 9, 2142–2151. [Google Scholar] [CrossRef]
- Ahamad, R.; Barek, J.; Yusoff, A.R.; Sinaga, S.M.; Zima, J. Determination of Roxarsone Using Carbon Paste and Amberlite LA2 Modified Carbon Paste Electrodes. Electroanalysis 2000, 12, 1220–1226. [Google Scholar]
- Tiwari, M.S.; Throat, R.G.; Borge, V.V.; Kadu, A.K. Voltammetric Quantification of the Antibiotic Roxarsone using a Surface-Modified Screen-Printed Carbon Electrode. ChemistrySelect 2023, 8, e202300283. [Google Scholar] [CrossRef]
- Srivastava, N.S.K.; Srivastava, A.; Srivastava, M.; Prakash, R. Electrochemical Sensing of Roxarsone on Natural Biomass Derived. Two-Dimensional Carbon Material as Promising Electrode Material. ACS Omega 2022, 7, 2908–2917. [Google Scholar]
- Chinnapaiyan, S.; Rajaji, U.; Chen, S.-M.; Liu, T.-Y.; Filho, J.d.O.; Chang, Y.-S. Fabrication of thulium metal–organic frameworks based smartphone sensor towards arsenical feed additive drug detection: Applicable in food safety analysis. Electrochim. Acta 2022, 401, 139487. [Google Scholar]
- Kokulnathan, T.; Rajagopal, V.; Wang, T.-J.; Huang, S.-J.; Ahmed, F. Electrochemical Behavior of Three-Dimensional Cobalt Manganate with Flowerlike Structures for Effective Roxarsone Sensing. Inorg. Chem. 2021, 60, 17986–17996. [Google Scholar] [CrossRef]
- Vinoth, S.; Govindasamy, M.; Wang, S.F.; Alothman, A.A.; Alshgari, R.A. Surface Engineering of Roselike Lanthanum Molybdate Electrocatalyst Modified Screen-Printed Carbon Electrode for Robust and Highly Sensitive Sensing of Antibiotic Drug. Microchem. J. 2021, 164, 106044. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Gorylewski, D. Glassy Carbon Modified with Cationic Surfactant (GCE/CTAB) as Electrode Material for Fast and Simple Analysis of the Arsenic Drug Roxarsone. Materials 2023, 16, 345. [Google Scholar]
- Sriram, B.; Baby, J.N.; Hsu, Y.F.; Wang, S.F.; George, M. Scheelite-type rare earth vanadates TVO4 (T = Ho, Y, Dy) electrocatalysts: Investigation and comparison of T site variations towards bifunctional electrochemical sensing application. Chem. Eng. J. 2023, 451, 138694. [Google Scholar]
- Manavalan, S.; Veerakumar, P.; Chen, S.M.; Karuppusamy, N. Development of robust multifunctional CrNiCo-P/GCN catalyst for oxygen evolution reaction, electrochemical sensing, and photodegradation of roxarsone. Nanoscale 2022, 14, 16233. [Google Scholar] [CrossRef] [PubMed]
- Rajaji, U.; Ganesh, P.-S.; Kim, S.-Y.; Govindasamy, M.; Alshgari, R.A.; Liu, T.-Y. MoS2 Sphere/2D S-Ti3C2 MXene Nanocatalysts on Laser-Induced Graphene Electrodes for Hazardous Aristolochic Acid and Roxarsone Electrochemical Detection. ACS Appl. Nano Mater. 2022, 5, 3252–3264. [Google Scholar]
- Olchowski, R.; Zįęba, E.; Giannakoudakis, D.A.; Anastopoulos, I.; Dobrowolski, R.; Barczak, M. Tailoring surface chemistry ofsugar-derived ordered mesoporous carbons towards efficient removal of diclofenac from aquatic environments. Materials 2020, 13, 1625. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.; Teng, N.; Long, D.; Ma, C.; Wei, Y.; Wang, J.; Ling, L. Simultaneous micropore development and nitrogen doping of ordered mesoporous carbons for enhanced supercapacitor and Li-S cathode performance. Electrochim. Acta 2016, 214, 231–240. [Google Scholar] [CrossRef]
- Olchowski, R.; Dobrowolski, R. Synthesis, properties and applications of CMK-3-type ordered mesoporous carbons. Ann. Univ. Mariae Curie-Sklodowska Sect. AA–Chem. 2018, 73, 11–30. [Google Scholar]
- Olchowski, R.; Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Zięba, E.; Dobrowolski, R.; Dobrzyńska, J. Arsenazo III removal from diagnostic laboratories wastewater by effective adsorption onto thermochemically modified ordered mesoporous carbon. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100607. [Google Scholar]
- Vinu, A.; Miyahara, M.; Ariga, K. Biomaterial Immobilization in Nanoporous Carbon Molecular Sieves: Influence of Solution pH, Pore Volume, and Pore Diameter. J. Phys. Chem. B 2005, 109, 6436–6441. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, F.; Bai, Y.; Li, Y.; Gong, Y.; Feng, X.; Li, Y.; Wang, X.; Wu, C. Advances in Carbon Materials for Sodium and Potassium Storage. Adv. Funct. Mater. 2022, 32, 2203117. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Olchowski, R.; Kozak, J.; Sekerzh-Zenkovich, O.; Dobrowolski, R. Modified carbon material (Pb-N-CMK-3) obtained by a hard-templating route, dicyandiamide impregnation and electrochemical lead particles deposition as an electrode material for the U(VI) ultratrace determination. Materials 2021, 14, 6490. [Google Scholar]
- Nayak, D.S.; Shetti, N.P. Voltammetric Response and Determination of an Anti-Inflammatory Drug at a Cationic Surfactant-Modified Glassy Carbon Electrode. J. Surfactants Deterg. 2016, 19, 1071–1079. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Amirmahmoodi, S.; Ghafari-Nazari, A. Taguchi optimization approach for Pb(II) and Hg(II) removal from aqueous solutions using modified mesoporous carbon. J. Hazard. Mater. 2011, 192, 1046–1055. [Google Scholar] [CrossRef]
- Kim, T.-W.; Park, I.-S.; Ryoo, R. A synthetic route to ordered mesoporous carbon materials with graphitic pore walls. Angew. Chem. Int. Ed. 2003, 42, 4375–4379. [Google Scholar] [CrossRef]
- Zieliński, W.; Rajca, A. Spectroscopic Methods and Their Application for Organic Compounds Identification, 2nd ed.; PWN: Warsaw, Poland, 2000. [Google Scholar]
- Fuente, E.; Menendez, J.A.; Diez, M.A.; Suarez, D.; Montes-Moran, M.A. Infrared Spectroscopy of carbon materials: A quantum chemical study of model compounds. J. Phys. Chem. B 2003, 107, 6350–6359. [Google Scholar]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar]
- Sun, P.; Liu, Y.; Mo, F.; Wu, M.; Xiao, Y.; Xiao, X.; Wang, W.; Dong, X. Efficient photocatalytic degradation of high-concentration moxifloxacin over dodecyl benzene sulfonate modified graphitic carbon nitride: Enhanced photogenerated charge separation and pollutant enrichment. J. Clean. Prod. 2023, 393, 136320. [Google Scholar]
- Koinuma, M.; Tateishi, H.; Hatakeyama, K.; Miyamoto, S.; Ogata, C.; Funatsu, A.; Taniguchi, T.; Matsumoto, Y. Analysis of reduced graphene oxides by X-ray photoelectron spectroscopy and electrochemical capacitance. Chem. Lett. 2013, 42, 924–926. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.H.; Park, S.B. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef]
- Barinov, A.; Malcioǧlu, O.B.; Fabris, S.; Sun, T.; Gregoratti, L.; Dalmiglio, M.; Kiskinova, M. Initial stages of oxidation on graphitic surfaces: Photoemission study and density functional theory calculations. J. Phys. Chem. C 2009, 113, 9009–9013. [Google Scholar]
- Beck, F. Cyclic voltammetry—Simulation and analysis of reaction mechanisms. By David K. Gosser, Jr., VCH, New York 1993, xi, 154 pp., hardcover, DM 124.00, ISBN 3-527-28226-2, disks included (5 1/4″ and 3 1/2″). Electroanalysis 1995, 7, 298. [Google Scholar]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques (Technical Report). Pure Appl. Chem. 1997, 6, 9297–9328. [Google Scholar]





| SBET (m2/g) | VT (cm3/g) | dBJH (nm) | ID:IG (au) | ζ (mV) | pH (au) |
|---|---|---|---|---|---|
| 690 ± 11 * | 0.79 ± 0.01 * | 3.4 ± 0.1 * | 0.87 ± 0.01 * | 12.3 ± 0.1 * | 7.77 ± 0.15 * |
| CHN | SEM-EDX # | XPS $ | |||||
|---|---|---|---|---|---|---|---|
| C (wt. %) | H (wt. %) | N (wt. %) | C (wt. %) | O (wt. %) | N (wt. %) | C (wt. %) | O (wt. %) |
| 93.5 ± 2.7 * | 0.63 ± 0.01 * | 0.87 ± 0.02 * | 92.9 ± 0.5 * | 4.6 ± 0.3 * | 1.81 ± 0.04 * | 91.3 ± 1.7 * | 7.4 ± 0.9 * |
| Technique (Sensor) | Linear Range (µmol L−1) | LOD (µmol L−1) | Matrix Type | Ref. |
|---|---|---|---|---|
| DPV (WS2NSs/SPCE) | 0.05–489.3 | 0.030 | meat | [10] |
| DPV (WS2 NRs/N-rGOs/SPCE) | 0.1–442.6 | 0.075 | human serum, urine, and pharmaceutical sample | [11] |
| DPV (ZnSnO3@GO/GCE) | 0.01–453.4 | 0.0043 | chicken and soil samples | [12] |
| DPAdSV (MCPME) | 3.8–190.0 | 0.19 | poultry feed and poultry litter | [13] |
| DPAdSV (CMCPE) | 0.1–1.0 | 0.10 | poultry drinks and veterinary products (tablets) | [14] |
| SWV (Au/ErGO/SPCE) | 1.0–1000.0 | 0.014 | meat | [15] |
| DPV (2D-AC/GCE) | 0.76–474.0 | 0.0015 | blood serum | [16] |
| DPV (Tm-BTC MOF/GCE) | 0.00015–770.0 | 0.0001 | food samples | [17] |
| DPV (CoMn2O4-500) | 0.01–0.84 and 0.84–1130.0 | 0.002 | river water | [18] |
| DPV (LaMoO) | 0.025–2650.0 | 0.012 | food analysis | [19] |
| SWAdSV (GCE/CTAB) | 0.001–0.02 and 0.02–20.0 | 0.00013 | river water | [20] |
| CV and DPV (DyVO4/SPCE) | 0.01–21.0 and 36.0–264.0 | 0.0009 | water, urine, chicken, and egg | [21] |
| DPV (CrNiCo-P/GCN/GCE) | 1.0–413.0 | 0.031 | chicken and swine meat | [22] |
| DPV (MoS2/S-Ti3C2/LGE) | 0.01–875.01 | 0.0023 | asarum sieboldii, urine, and blood serum | [23] |
| SWAdSV (GCE/DF-CMK-3/CTAB) | 0.0005–0.02, 0.02–0.5, and 0.5–100.0 | 0.000096 | river water and municipal wastewater | This work |
| Sample | Added (μmol L−1) | Determined ± SD (n = 3) (μmol L−1) | Coefficient of Variation (%) | Recovery (%) |
|---|---|---|---|---|
| Vistula River | 0.02 0.2 | 0.0204 ± 0.000743 0.192 ± 0.00753 | 3.64 3.92 | 102.0 96.0 |
| Purified municipal wastewater | 0.02 0.2 | 0.0199 ± 0.000666 0.204 ± 0.00675 | 3.35 3.31 | 99.5 102.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyszczuk-Rotko, K.; Gorylewski, D.; Olchowski, R.; Dobrowolski, R. Diclofenac-Impregnated Mesoporous Carbon-Based Electrode Material for the Analysis of the Arsenic Drug Roxarsone. Materials 2023, 16, 5420. https://doi.org/10.3390/ma16155420
Tyszczuk-Rotko K, Gorylewski D, Olchowski R, Dobrowolski R. Diclofenac-Impregnated Mesoporous Carbon-Based Electrode Material for the Analysis of the Arsenic Drug Roxarsone. Materials. 2023; 16(15):5420. https://doi.org/10.3390/ma16155420
Chicago/Turabian StyleTyszczuk-Rotko, Katarzyna, Damian Gorylewski, Rafał Olchowski, and Ryszard Dobrowolski. 2023. "Diclofenac-Impregnated Mesoporous Carbon-Based Electrode Material for the Analysis of the Arsenic Drug Roxarsone" Materials 16, no. 15: 5420. https://doi.org/10.3390/ma16155420
APA StyleTyszczuk-Rotko, K., Gorylewski, D., Olchowski, R., & Dobrowolski, R. (2023). Diclofenac-Impregnated Mesoporous Carbon-Based Electrode Material for the Analysis of the Arsenic Drug Roxarsone. Materials, 16(15), 5420. https://doi.org/10.3390/ma16155420






