Improved Visible Emission from ZnO Nanoparticles Synthesized via the Co-Precipitation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO Nanoparticles
2.2. Characterization of the ZnO Nanoparticles
3. Results and Discussion
3.1. Influence of the Solvent Used in the Co-Precipitation Method
3.2. Crystalline Structure of ZnO Nanoparticles
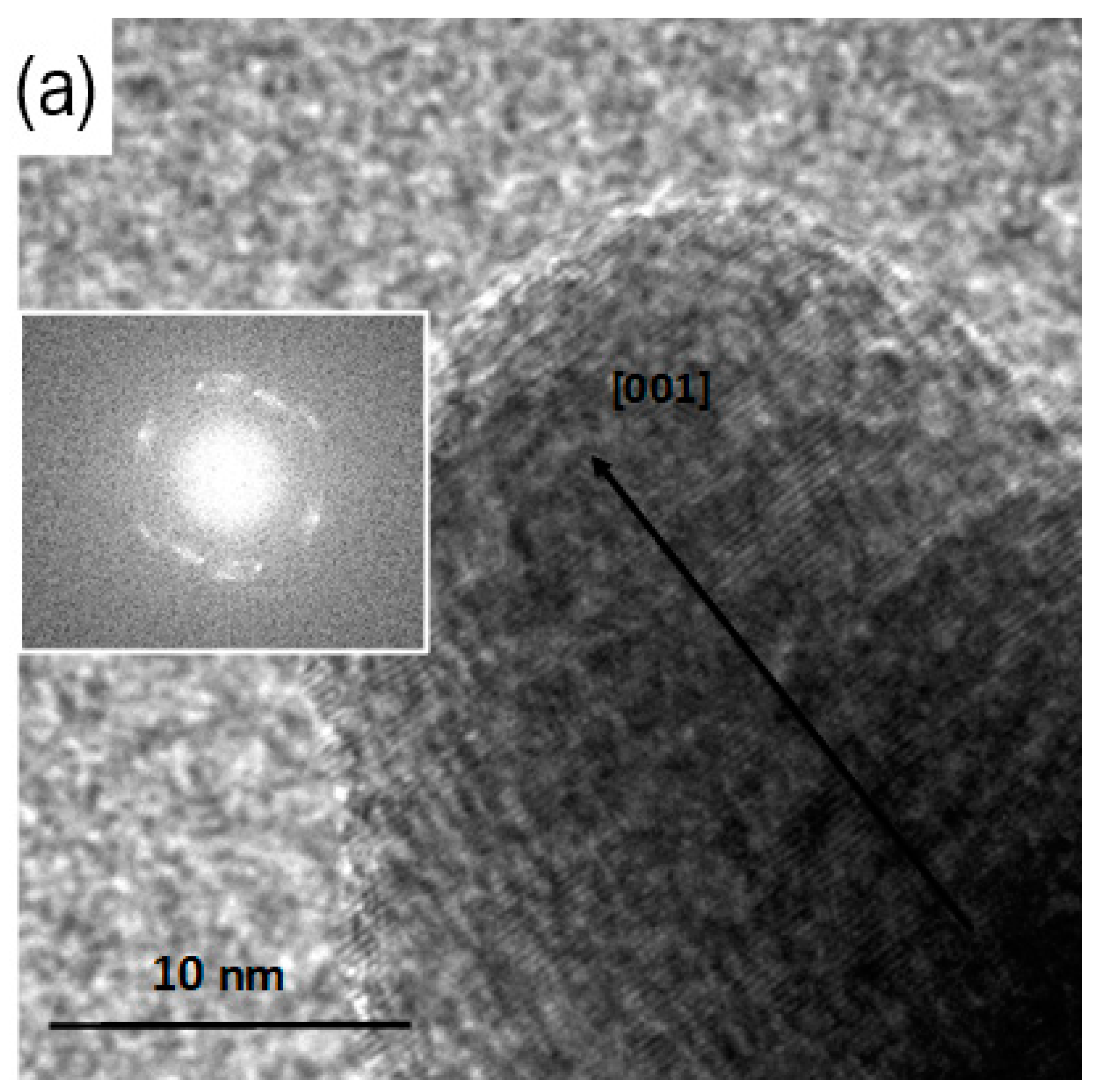
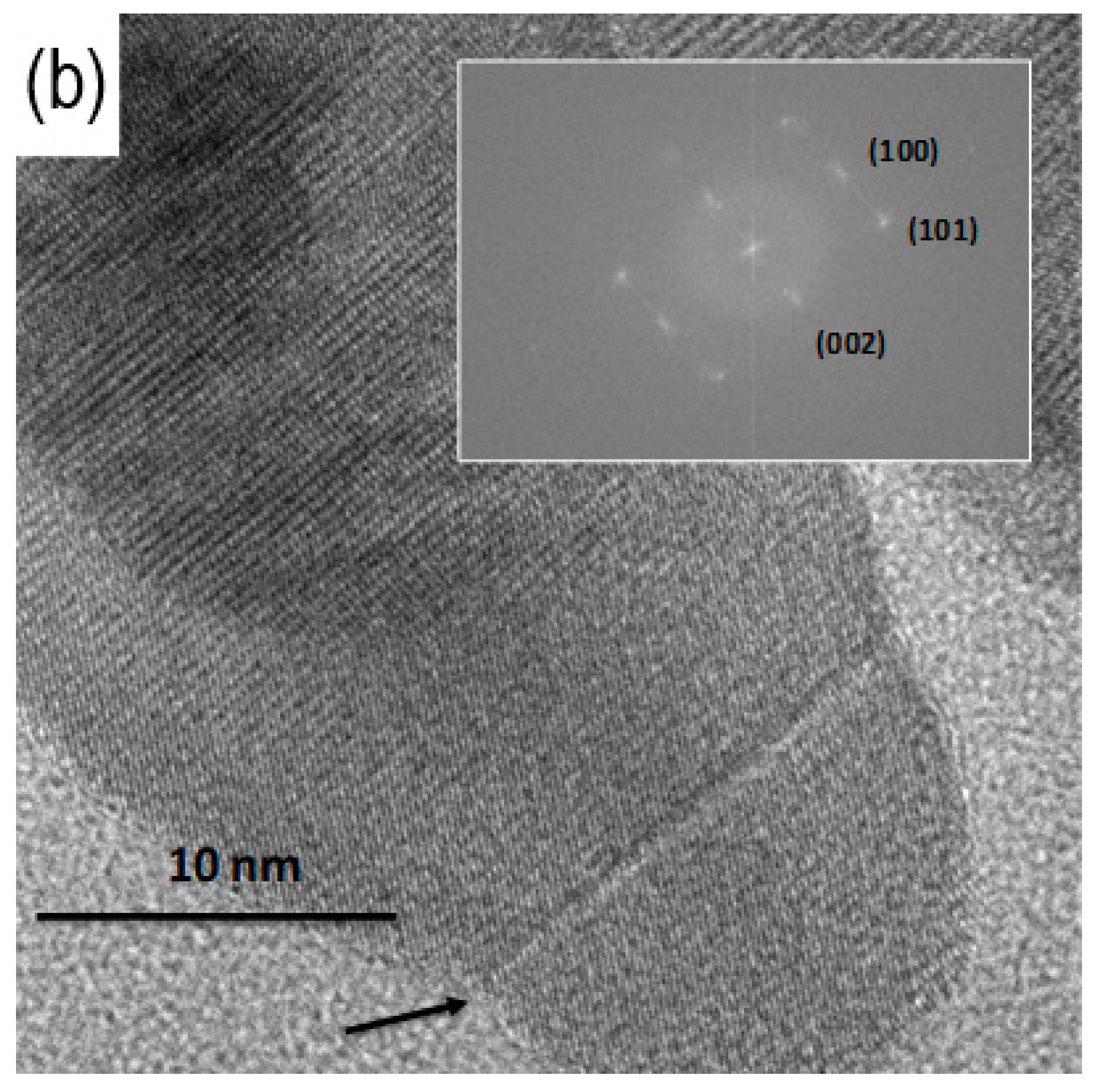
3.3. TEM Analysis
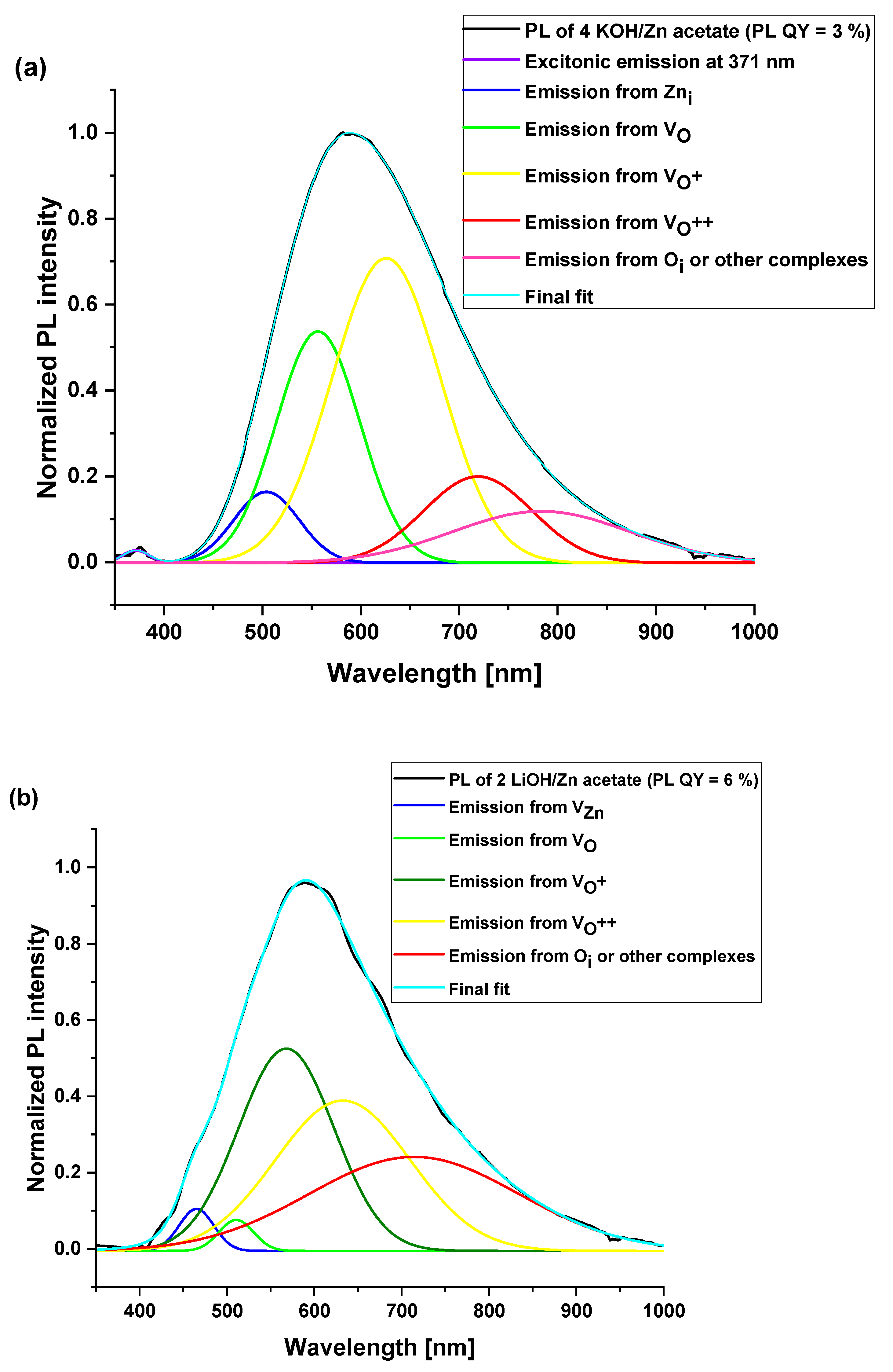
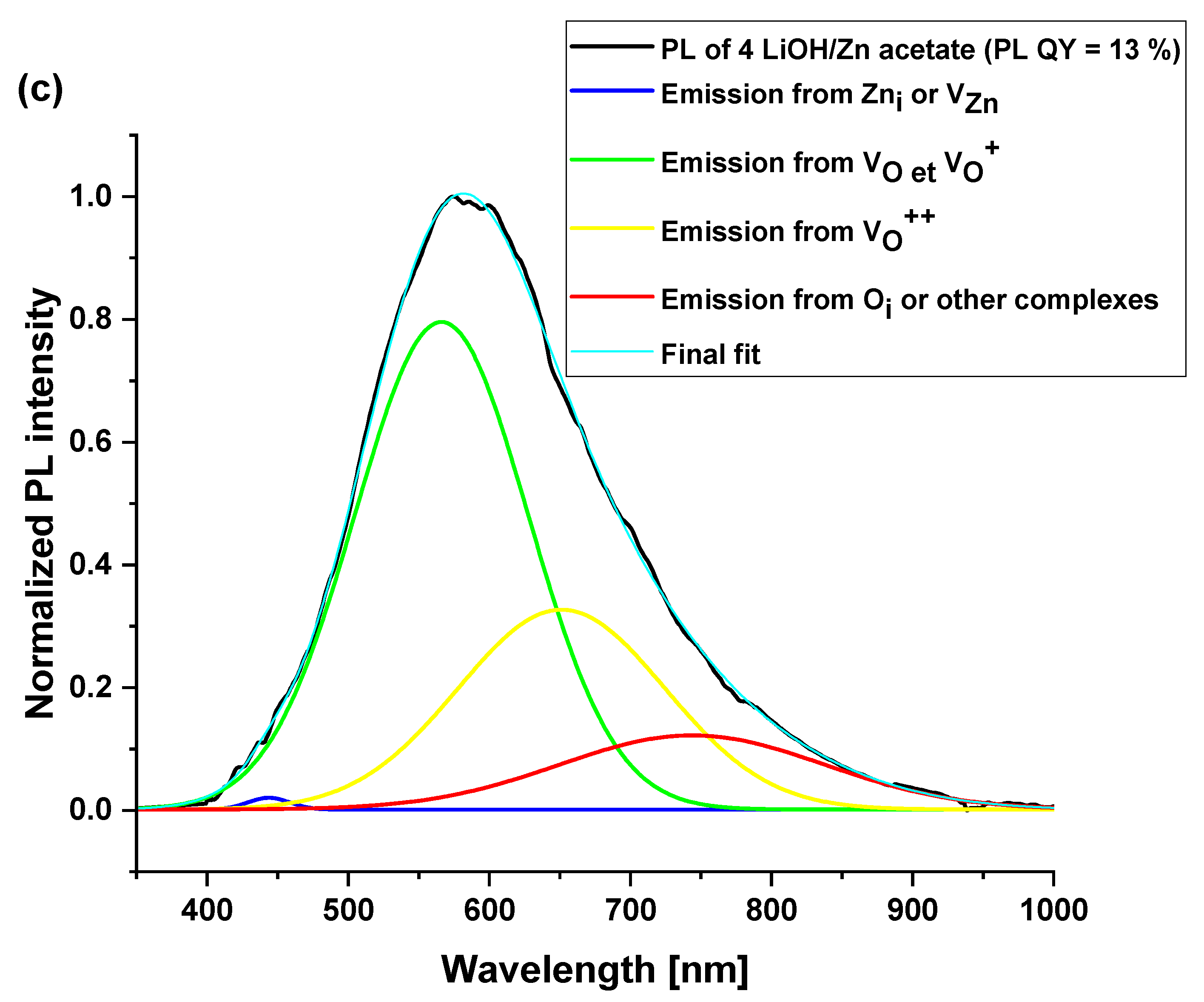
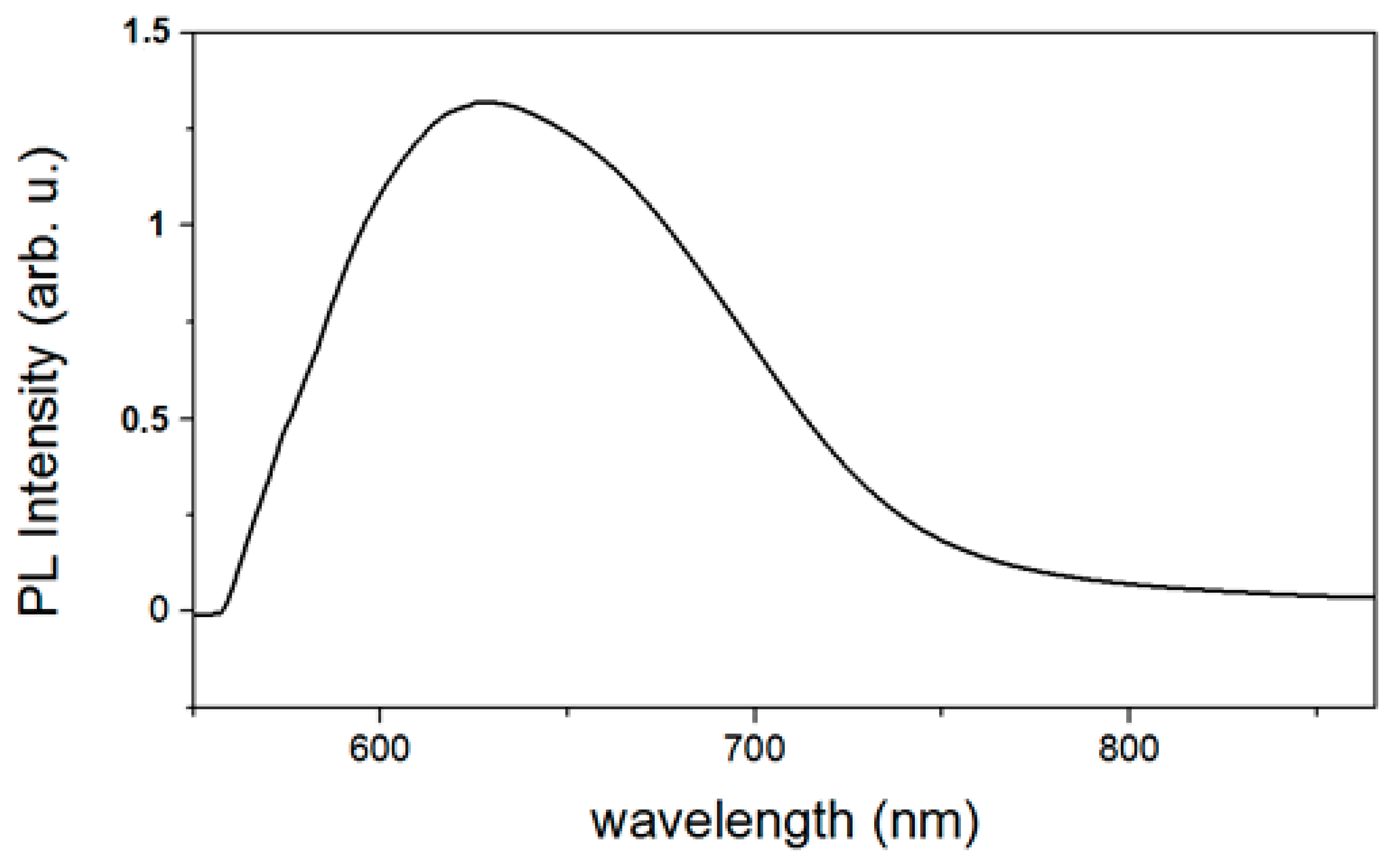
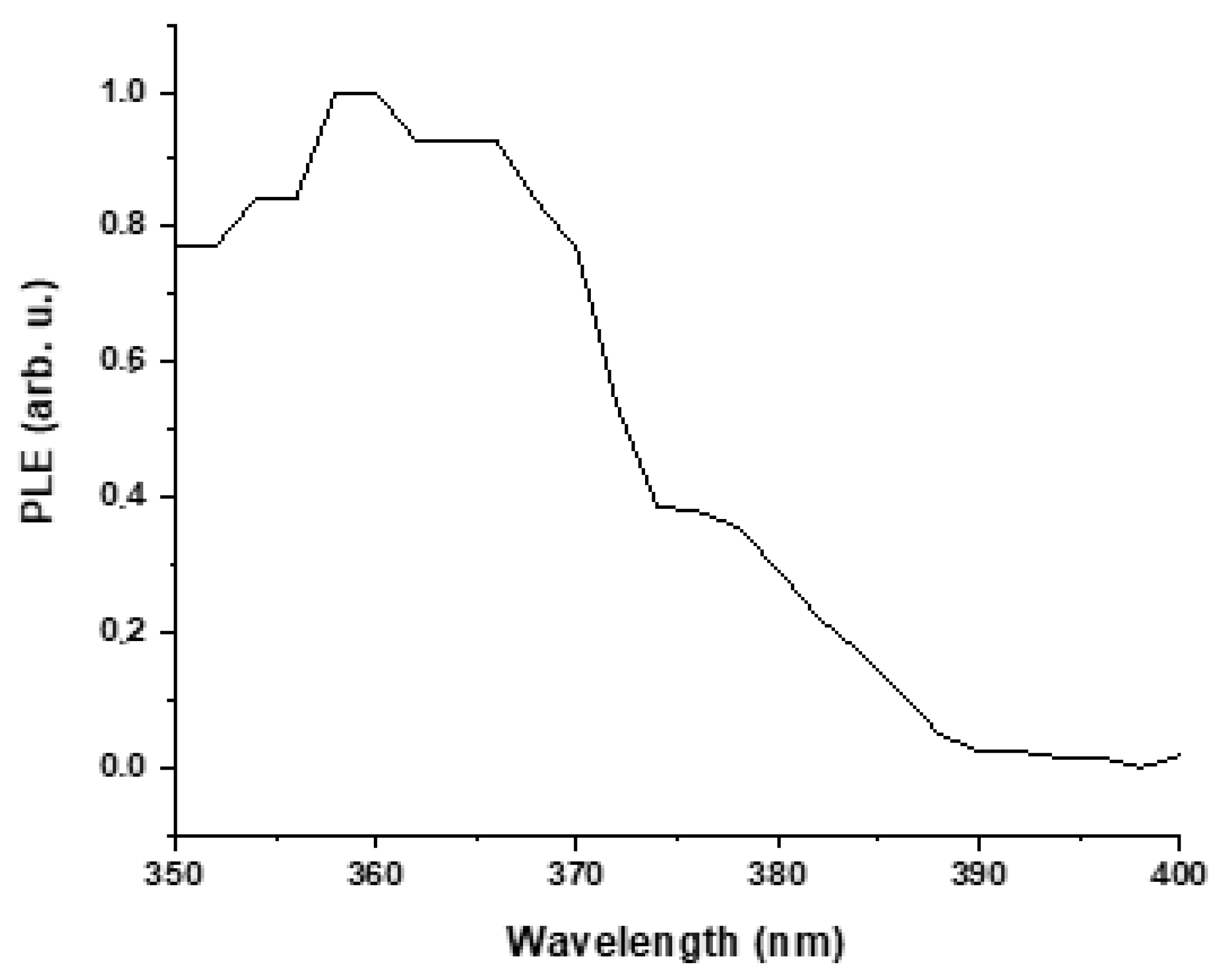
3.4. Optical Properties of ZnO Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 1301. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-based ultraviolet photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.X.; Liu, Y.; Lu, J.; Chen, Z.H.; Wang, J.; Zhang, L. Single-crystalline tower-like ZnO microrod UV lasers. J. Mater. Chem. C 2013, 1, 202–206. [Google Scholar] [CrossRef]
- Vinod, R.; Sajan, P.; Achary, S.R.; Tomas, C.M.; Muñoz-Sanjosé, V.; Bushiri, M.J. Enhanced UV emission from ZnO nanoflowers synthesized by the hydrothermal process. J. Phys. D Appl. Phys. 2012, 45, 425103. [Google Scholar] [CrossRef]
- Richters, J.P.; Voss, T.; Wischmeier, L.; Ruckmann, I.; Gutowski, J. Near-band-edge photoluminescence spectroscopy of ZnO nanowires embedded in polymers. J. Korean Phys. Soc. 2008, 53, 2844–2846. [Google Scholar] [CrossRef]
- Samanta, P.K.; Patra, S.K.; Ghosh, A.; Chaudhuri, P.R. Visible emission from ZnO nanorods synthesized by a simple wet chemical method. Int. J. Nanosci. Nanotechnol. 2009, 1, 81–90. [Google Scholar]
- Lv, Y.; Xiao, W.; Li, W.; Xue, J.; Ding, J. Controllable synthesis of ZnO nanoparticles with high intensity visible photoemission and investigation of its mechanism. Nanotechnology 2013, 24, 175702. [Google Scholar] [CrossRef]
- Apostoluk, A.; Zhu, Y.; Masenelli, B.; Delaunay, J.-J.; Sibiński, M.; Znajdek, K.; Focsa, A.; Kaliszewska, I. Improvement of the solar cell efficiency by the ZnO nanoparticle layer via the downshifting effect. Microelectron. Eng. 2014, 127, 51–56. [Google Scholar] [CrossRef]
- Bano, N.; Zaman, S.; Zainelabdin, A.; Hussain, S.; Hussain, I.; Nur, O.; Willander, M. ZnO-organic hybrid white light emitting diodes grown on flexible plastic using low temperature aqueous chemical method. J. Appl. Phys. 2010, 108, 043103. [Google Scholar] [CrossRef]
- Baruah, S.; Sinha, S.S.; Ghosh, B.; Pal, S.K.; Raychaudhuri, A.K.; Dutta, J. Photoreactivity of ZnO nanoparticles in visible light: Effect of surface states on electron transfer reaction. J. Appl. Phys. 2009, 105, 074308. [Google Scholar] [CrossRef]
- Zhu, Y.; Apostoluk, A.; Liu, S.B.; Daniele, S.; Masenelli, B. ZnO nanoparticles as a luminescent downshifting layer for photosensitive devices. J. Semicond. 2013, 34, 053005. [Google Scholar] [CrossRef]
- Morales-Flores, N.; Galeazzi, R.; Rosendo, E.; Díaz, T.; Velumani, S.; Pal, U. Morphology control and optical properties of ZnO nanostructures grown by ultrasonic synthesis. Adv. Nano. Res. 2013, 1, 59–70. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef] [PubMed]
- Rania, S.; Surib, P.; Shishodiac, P.K.; Mehra, R.M. Synthesis of nanocrystallineZnO powder via sol–gel route for dye-sensitized solar cells. Sol. Energ. Mat. Sol. Cells 2008, 92, 1639–1645. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Jurablu, S. Co-precipitation Synthesis of zinc oxide (ZnO) nanoparticles by zinc nitrate precursor. Int. J. Bio-Inorg. Hybrid Nanomater. 2014, 3, 179–184. [Google Scholar]
- Manzoor, U.; Islam, M.; Tabassam, L.; Rahman, S.U. Quantum confinement effect in ZnO nanoparticles synthesized by co-precipitate method. Phys. E 2009, 41, 1669–1672. [Google Scholar] [CrossRef]
- Singh, J.; Mittu, B.; Chauhan, A.; Sharma, A.; Singla, M.L. Role of alkali metal hydroxide in controlling the size of ZnO nanoparticles in non-aqueous medium. Int. J. Fundam. Appl. Sci. 2012, 1, 91–93. [Google Scholar]
- Xiong, H.M.; Ma, R.Z.; Wang, S.F.; Xia, Y.Y. Photoluminescent ZnO nanoparticles synthesized at the interface between air and triethylene glycol. J. Mater. Chem. 2011, 21, 3178–3182. [Google Scholar] [CrossRef]
- Rocha, M.; Araujo, F.P.; Castro-Lopes, S.; de Lima, I.S.; Cavalcanti Silva-Filho, E.; Anteveli Osajima, J.; Oliveira, C.S.; Viana, B.C.; Almeida, L.C.; Guerra, Y.; et al. Synthesis of Fe–Pr co-doped ZnO nanoparticles: Structural, optical and antibacterial properties, Ceram. Int. 2023, 49, 2282–2295. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Ghosh, S.; Gryzenhout, M.; Conradie, J. One-pot synthesis of zinc oxide nanoparticles via chemical precipitation for bromophenol blue adsorption and the antifungal activity against filamentous fungi. Sci. Rep. 2021, 11, 8305. [Google Scholar] [CrossRef]
- De Mello, J.C.; Wittmann, H.F.; Friend, R.H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 1997, 9, 230–232. [Google Scholar] [CrossRef]
- Damen, T.C.; Porto, S.P.S.; Tell, B. Raman effect in zinc oxide. Phys. Rev. 1966, 142, 570–574. [Google Scholar] [CrossRef]
- Spanhel, L. Colloidal ZnO nanostructures and functional coatings: A survey. J. Sol-Gel Sci. Technol. 2006, 39, 7–24. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Zhang, Y.; Kim, E.J.; Hahn, S.H.; Seong, S.G. Near-infrared photoluminescence from ZnO. Appl. Phys. Lett. 2012, 100, 101906. [Google Scholar] [CrossRef]
- Lin, W.W.; Chen, D.G.; Zhang, J.Y.; Lin, Z.; Huang, J.K.; Li, W.; Wang, Y.H.; Huang, F. Hydrothermal Growth of ZnO Single Crystals with High Carrier Mobility. Cryst. Growth Des. 2009, 9, 4378–4383. [Google Scholar] [CrossRef]
- Dem’yanets, L.N.; Kostomarov, D.V.; Kuz’mina, I.P. Chemistry and Kinetics of ZnO Growth from Alkaline Hydrothermal Solutions. Inorg. Mater. 2002, 38, 171–179. [Google Scholar]
- Pourrahimi, A.M.; Liu, D.; Pallon, L.K.H.; Andersson, R.L.; Abad, A.M.; Lagarón, J.M.; Hedenqvist, M.S.; Ström, V.; Gedde, U.W.; Olsson, R.T. Water-based synthesis and cleaning methods for high purity ZnO nanoparticles–comparing acetate; chloride; sulphate and nitrate zinc salt precursors. R. Soc. Chem. Adv. 2014, 4, 35568–35577. [Google Scholar] [CrossRef]
- Vempati, S.; Mitra, J.; Dawson, P. One-step synthesis of ZnO nanosheets: A blue-white fluorophore. Nanoscale Res. Lett. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18, 95702. [Google Scholar] [CrossRef]
- Felbier, P.; Yang, J.; Theis, J.; Liptak, R.W.; Wagner, A.; Lorke, A.; Bacher, G.; Kortshagen, U. Highly Luminescent ZnO Quantum Dots Made in a Nonthermal Plasma. Adv. Funct. Mater. 2014, 24, 1988–1993. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Morfa, A.J.; Gibson, B.C.; Karg, M.; Karle, T.J.; Greentree, A.D.; Mulvaney, P.; Tomljenovic-Hanic, S. Single-photon emission and quantum characterization of zinc oxide defects. Nano Lett. 2012, 12, 949–954. [Google Scholar] [CrossRef]
- Leung, Y.H.; Chen, X.Y.; Ng, A.M.C.; Guo, M.Y.; Liu, F.Z.; Djurišić, A.B.; Chan, W.K.; Shi, X.Q.; Van Hove, M.A. Green emission in ZnO nanostructures—Examination of the roles of oxygen and zinc vacancies. Appl. Surf. Sci. 2013, 271, 202–209. [Google Scholar] [CrossRef]
- Li, M.J.; Xing, G.C.; Xing, G.Z.; Wu, B.; Wu, T.; Zhang, X.H.; Sum, T.C. Origin of green emission and charge trapping dynamics in ZnO nanowires. Phys. Rev. B 2013, 87, 115309. [Google Scholar] [CrossRef]
- Li, D.; Leung, Y.H.; Djurišić, A.B.; Liu, Z.T.; Xie, M.H.; Shi, S.L.; Xu, S.J.; Chan, W.K. Different origins of visible luminescence in ZnO nanostructures fabricated by the chemical and evaporation methods. Appl. Phys. Lett. 2004, 85, 1601–1603. [Google Scholar] [CrossRef]
- An, W.; Wu, X.J.; Zeng, X.C. Adsorption of O2, H2, CO, NH3 and NO2 on ZnO nanotube: a density functional theory study. J. Phys. Chem. C 2008, 112, 5747–5755. [Google Scholar] [CrossRef]
- Awan, S.U.; Hasanain, S.K.; Hassnain, J.G.; Anjum, D.H.; Qurashi, U.S. Defects induced luminescence and tuning of bandgap energy narrowing in ZnO nanoparticles doped with Li ions. J. Appl. Phys. 2014, 116, 083510. [Google Scholar] [CrossRef]
- Sa’aedi, A.; Cheraghizade, M.; Aghaji, A.G.; Saedi, A. Effect of Li-Dope on Morphological and Optical Properties of ZnO Nanostructures. J. Basic Appl. Sci. Res. 2013, 3, 226–231. [Google Scholar]
- Ganesh, I.; Sekhar, P.S.C.; Padmanabham, G.G.; Sundararajan, G. Preparation and characterization of Li-doped ZnO nano-sized powders for photocatalytic applications. Mater. Sci. Forum 2013, 734, 90–116. [Google Scholar] [CrossRef]
- Nanda, S.; Chandni, D.; Patra, A.K.; Gupta, P.S.; Kumar, S. Lithium doping and photoluminescence properties of ZnO nanorods. AIP Adv. 2018, 8, 015306. [Google Scholar]
- Tanyawong, S.; Tang, I.-M.; Herng, T.S.; Thongmee, S. Enhancement of Virtual Magnetic Moment Formation in ZnO NPs by Li+ Ion Doping. J. Supercond. Nov. Magn. 2020, 33, 2851–2859. [Google Scholar] [CrossRef]
- Adama, R.E.; Pozinab, G.; Willandera, M.; Nura, O. Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photonics Nanostruct.-Fundam. Appl. 2018, 32, 11–18. [Google Scholar] [CrossRef]
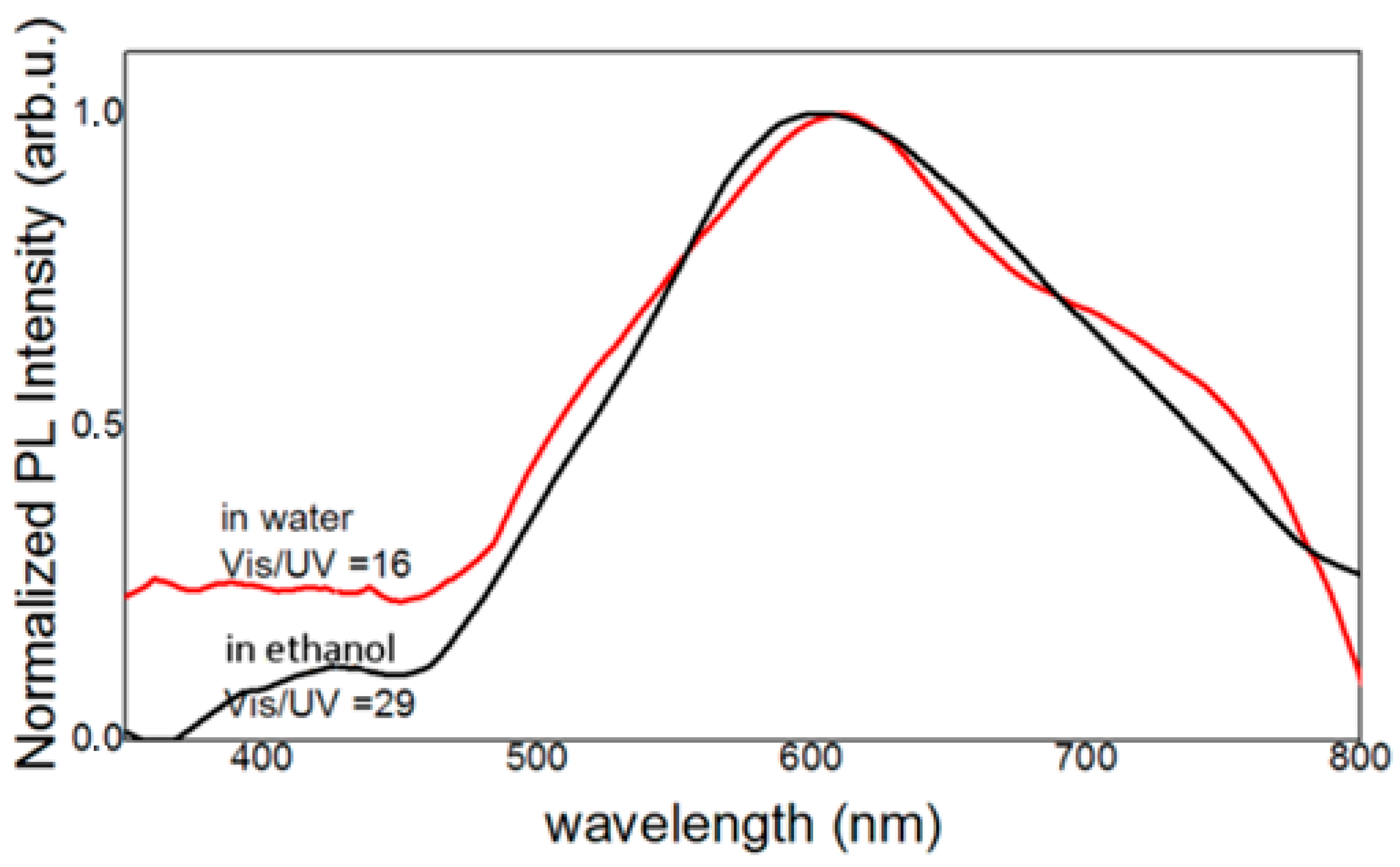
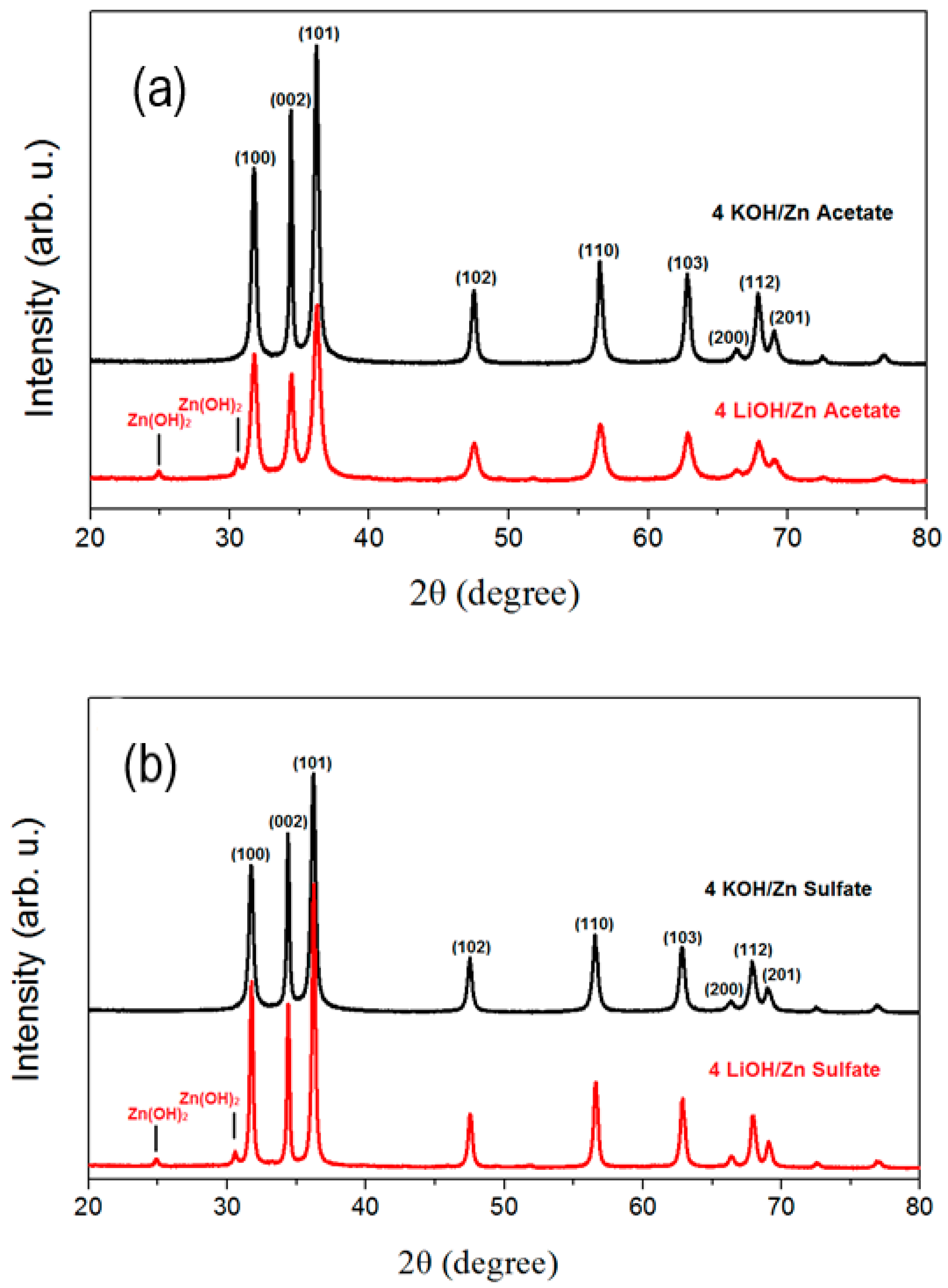

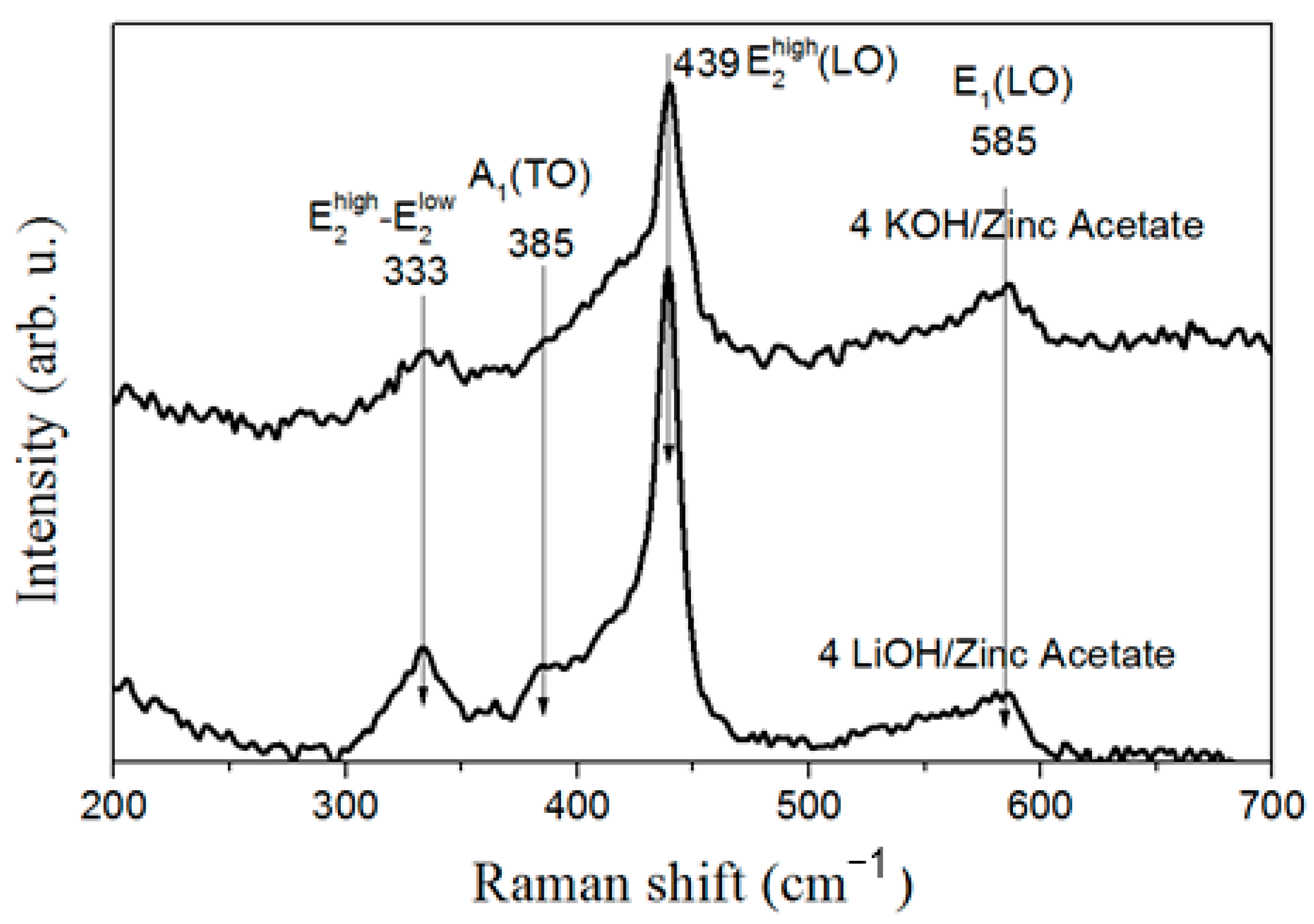
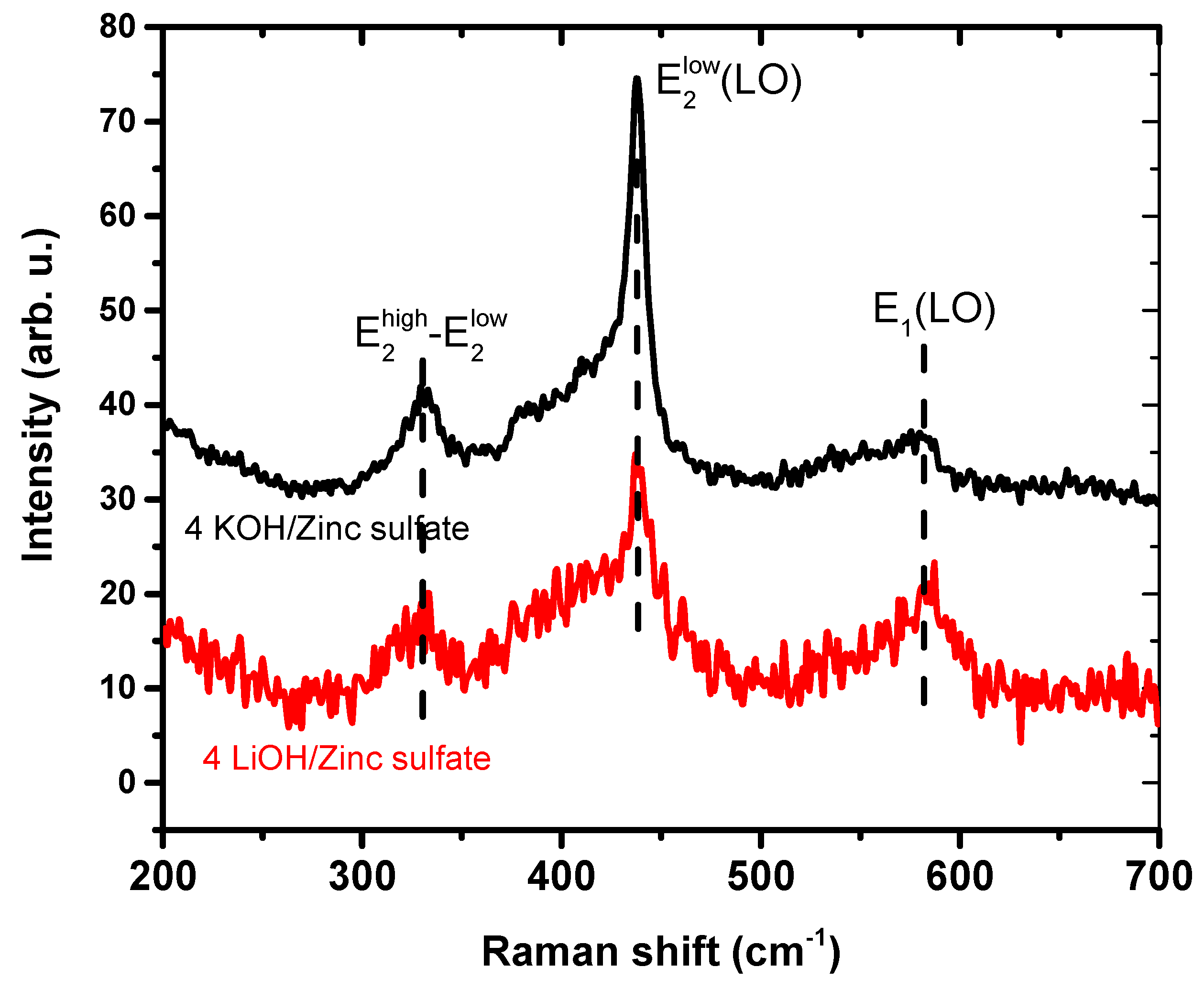
| Precursor | Hydroxide | ||
|---|---|---|---|
| KOH | LiOH | ||
| 4 eq. | 2 eq. | 4 eq. | |
| Zinc acetate | 20 nm | 21 nm | 13 nm |
| Zinc sulfate | 20 nm | 23 nm | 27 nm |
| Precursors | FWHM of (101) Peak and Resulting Crystalline Domain Size | FWHM of (002) Peak and Resulting Crystalline Domain Size |
|---|---|---|
| 4 LiOH/Zn acetate | 0.63° (13 nm) | 0.57° (15 nm) |
| 4 KOH/Zn acetate | 0.44° (20 nm) * | 0.36° (24 nm) |
| 4 LiOH/Zn sulfate | 0.32° (27 nm) | 0.31° (28 nm) |
| 4 KOH/Zn sulfate | 0.44° (20 nm) * | 0.33° (26 nm) |
| Surface Defects | Peak Range (nm) |
|---|---|
| VZn | 470–520 |
| VO | 520–570 |
| VO+ | 570–620 |
| VO++ | 620–670 |
| Oi | 670–720 |
| Sample Synthesis Condition | I1 (nm) | I2 (nm) | I3 (nm) | I4 (nm) | I5 (nm) | PL QY |
|---|---|---|---|---|---|---|
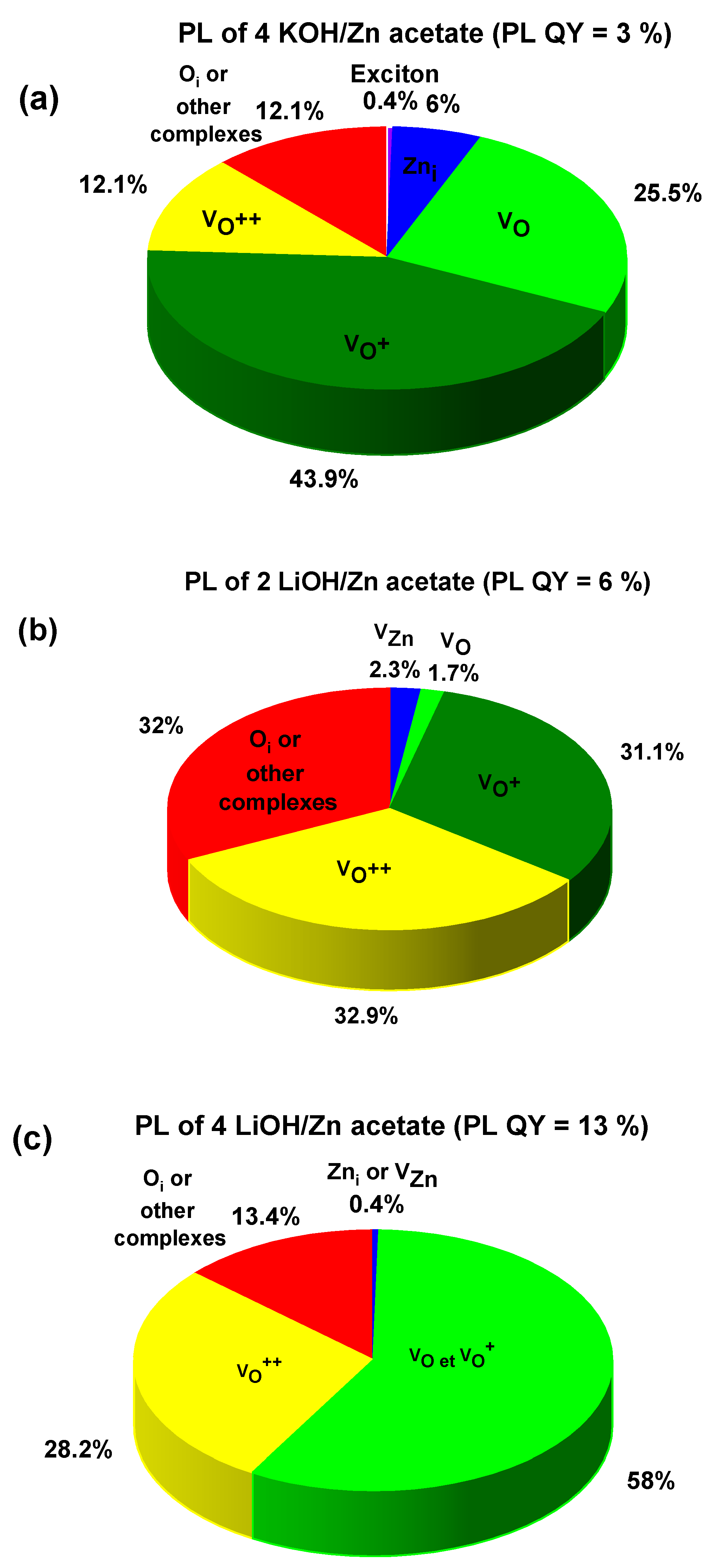
| 4 KOH/ Zn acetate | 371 (0.4) (exciton) and 504 (6) | 556 (25.5) | 625 (43.9) | 719 (12.1) | 783 (12.1) | 3 ± 1% |
| 2 LiOH/ Zn acetate | 465 (2.3) | 510 (1.7) | 567 (31.1) | 632 (32.9) | 714 (32) | 6 ± 1% |
| 4 LiOH/ Zn acetate | 443 (0.4) | 566 (58) | 566 * | 651 (28.2) | 743 (13.4) | 13 ± 1% |
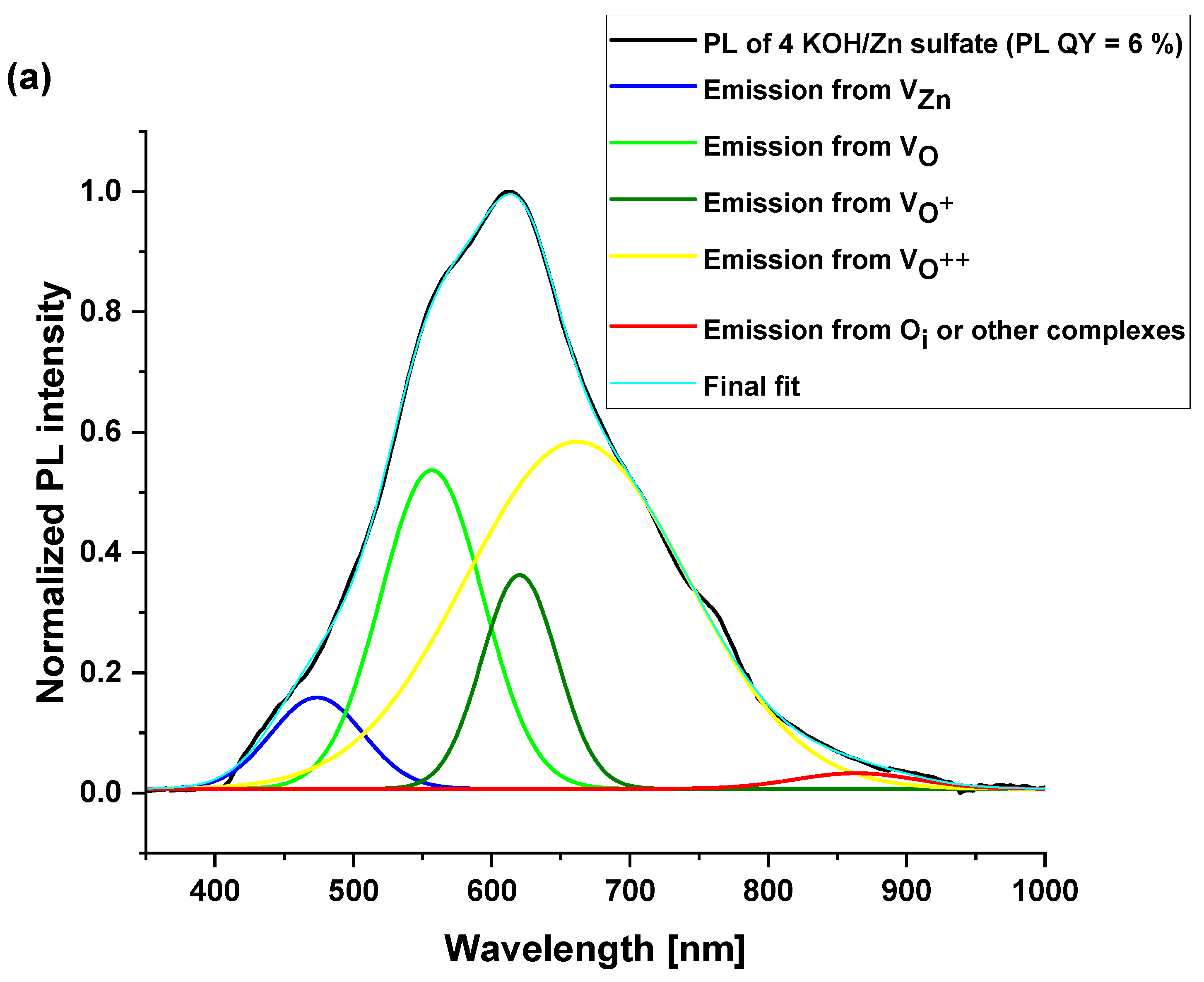
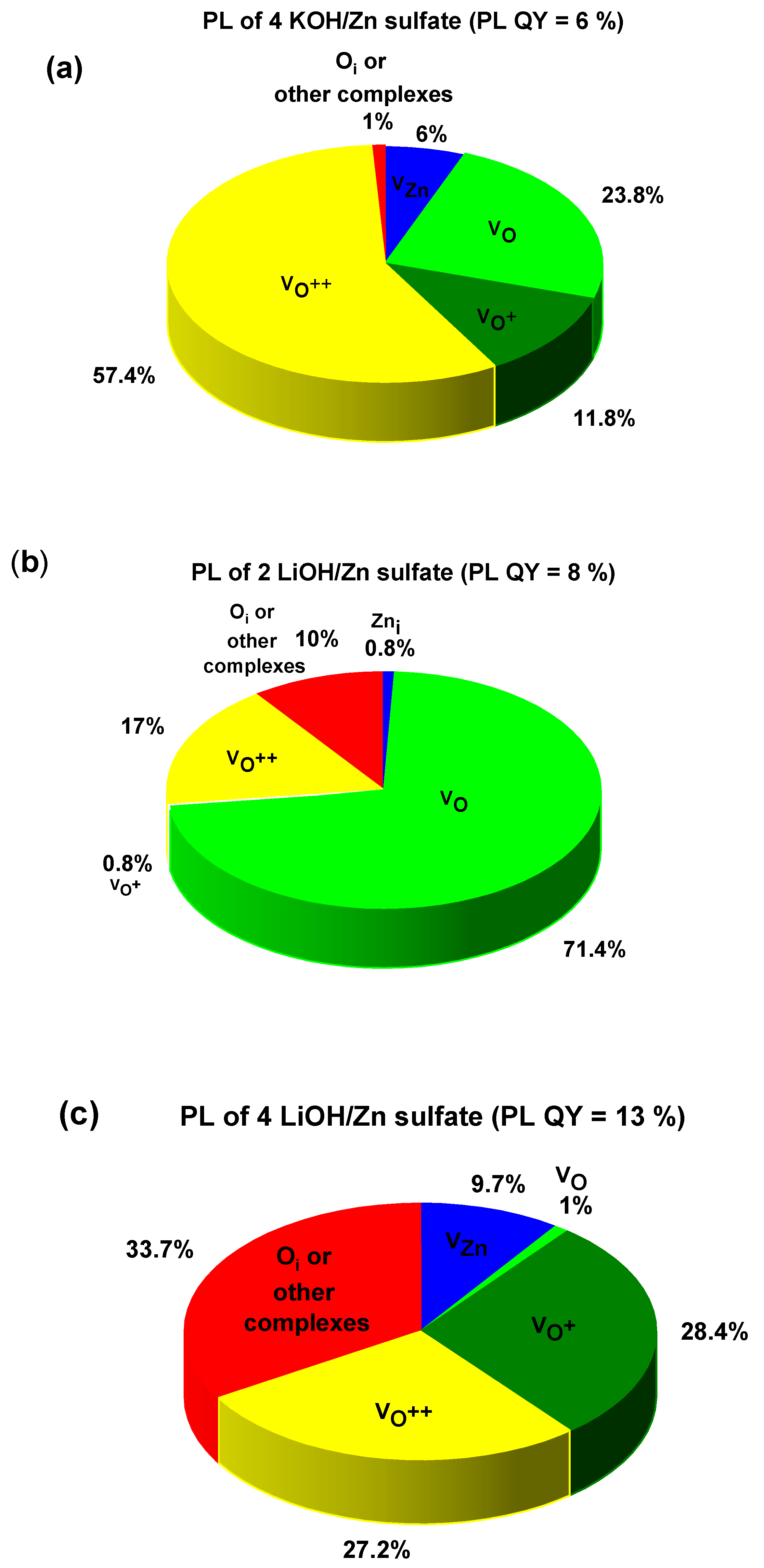
| 4 KOH/ Zn sulfate | 473 (6) | 556 (23.8) | 620 (11.8) | 661 (57.4) | 863 (1) | 6 ± 1% |
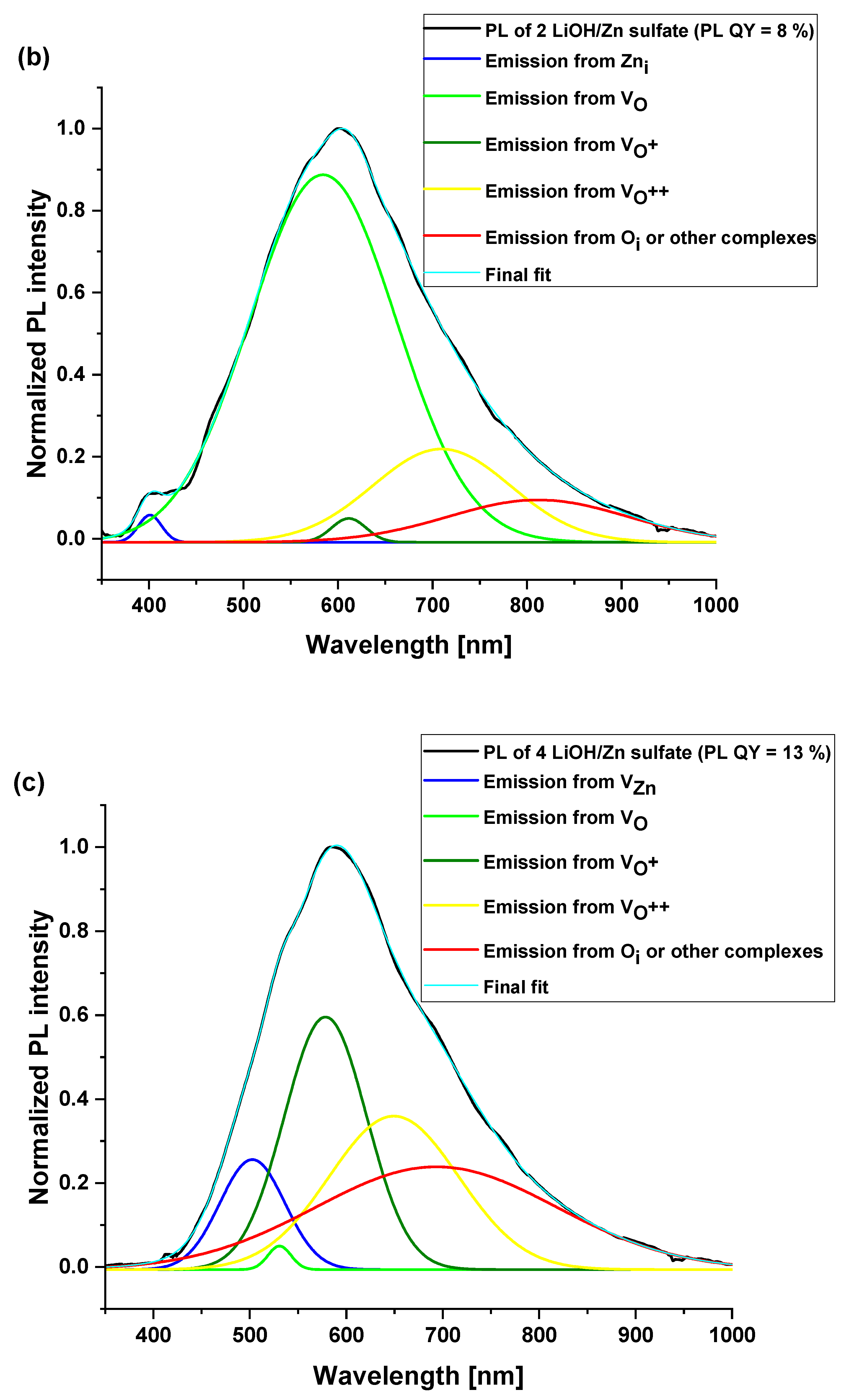
| 2 LiOH/ Zn sulfate | 401 (0.8) | 584 (71.4) | 611 (0.8) | 709 (17) | 811 (10) | 8 ± 1% |
| 4 LiOH/Zn sulfate | 502 (9.7) | 530 (1) | 578 (28.4) | 649 (27.2) | 693 (33.7) | 13 ± 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostoluk, A.; Zhu, Y.; Gautier, P.; Valette, A.; Bluet, J.-M.; Cornier, T.; Masenelli, B.; Daniele, S. Improved Visible Emission from ZnO Nanoparticles Synthesized via the Co-Precipitation Method. Materials 2023, 16, 5400. https://doi.org/10.3390/ma16155400
Apostoluk A, Zhu Y, Gautier P, Valette A, Bluet J-M, Cornier T, Masenelli B, Daniele S. Improved Visible Emission from ZnO Nanoparticles Synthesized via the Co-Precipitation Method. Materials. 2023; 16(15):5400. https://doi.org/10.3390/ma16155400
Chicago/Turabian StyleApostoluk, Alexandra, Yao Zhu, Pierrick Gautier, Audrey Valette, Jean-Marie Bluet, Thibaut Cornier, Bruno Masenelli, and Stephane Daniele. 2023. "Improved Visible Emission from ZnO Nanoparticles Synthesized via the Co-Precipitation Method" Materials 16, no. 15: 5400. https://doi.org/10.3390/ma16155400
APA StyleApostoluk, A., Zhu, Y., Gautier, P., Valette, A., Bluet, J.-M., Cornier, T., Masenelli, B., & Daniele, S. (2023). Improved Visible Emission from ZnO Nanoparticles Synthesized via the Co-Precipitation Method. Materials, 16(15), 5400. https://doi.org/10.3390/ma16155400








